Abstract
Cemento-ossifying fibroma (COF) is a fibro-osseous lesion or non-odontogenic tumour that affects craniofacial bones. These lesions are included in the spectrum of fibro-osseous lesions arising from periodontal ligament cells, which can deposit combination of cementum and bone surrounded by fibrous tissue. It clinically, macroscopically and radiologically resembles complex composite odontome and can be differentiated only on the basis of histopathology. They usually occur solitarily as a painless and expansile spherical or ovoid jawbone mass that may displace the roots of adjacent teeth. They predominantly occur in females in third and fourth decades of life. We present a case report of a 20-year-old man, with a mildly painful swelling in the mandible which was successfully treated with enucleation and diagnosed as COF. Its resemblance to complex composite odontome and unique surgical approach are highlighted in this paper.
Background
Cemento-ossifying fibroma (COF) is a relatively rare osteogenic tumour, which is seen in the maxillofacial region. There are many fibro-osseous and odontogenic entities, especially complex composites odontomes, which mimic it clinically, macroscopically and radiologically. Although the line of treatment for both these lesions is similar, it is important to differentiate them histologically as they have distinct features. Further to add to its uniqueness, the lesion was more on lingual cortex below the level of mylohyoid muscle which warranted extraoral approach for excision.
Case presentation
A 20-year-old man presented with mild pain and swelling over left lower third region of face since 1 month. The pain was dull aching and referred to the temporal region. There was a history of toothache and extractions of multiple teeth in the left mandibular posterior region 5 months before. There was no associated paraesthesia of the inferior alveolar nerve, no pus discharge or no any trismus. Extraoral inspection and palpation (figure 1) revealed a small stony hard nodule-like growth on the left mandibular body region more on the lingual side. Intraoral inspection revealed no abnormal findings. Based on clinical findings and history of multiple teeth extractions, a provisional diagnosis was made as focal sclerosing osteitis. To further locate the position of the lesion, radiographic investigations were carried out in the form of orthopantomogram (OPG) (figure 2), various CT and cone-beam CT (CBCT) scan sections (figures 3–5). They revealed a solitary relatively defined high-density sclerotic lesion with hypodense borders seen in mandible on left side measuring 2×2 cm. Lesion was also causing expansion of the same along with breach noted in the lingual cortex. The findings suggested it as a benign lesion. All preoperative haematological investigations were within normal limits, and the patient was deemed to be fit for surgery. The patient was operated under general anaesthesia. As the lesion was below the level of mylohyoid muscle, it was exposed extraorally via submandibular approach (figure 6). Expansion and perforation of lingual cortex were visualised, and the lesion was cleaved from normal bone. Complete enucleation of the lesion was performed. A dense sclerotic mass surrounded by a capsule of fibrous tissue was excised and was measuring ∼2×2 cm. After haemostasis, the wound was closed in layers with negative suction drain to prevent haematoma formation in the dead space. Tissue was sent for histopathological examination. On grossing (figure 7), findings revealed a hard gritty lesion with a fibrous capsule along with haemorrhagic areas. Histologically, decalcified H&E-stained and studied sections revealed (figure 8) dense lamellar bone with the presence of typical resting lines and numerous scattered basophilic cementum-like calcifications in the form of droplets with sparse cellular component. Correlating clinically and radiologically, histopathological features were suggestive of mature stage of COF.
Figure 1.
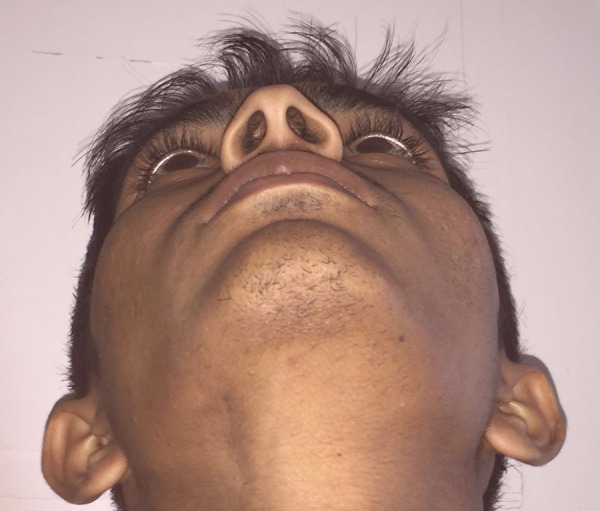
Extraoral photograph revealing slight bony enlargement on left junction of body and ramus of mandible.
Figure 2.
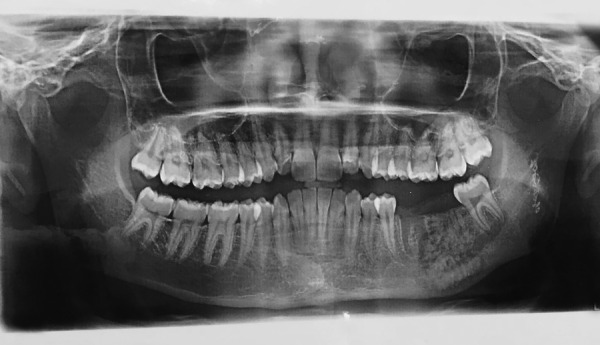
OPG reveals relatively defined heterogeneous radiopaque lesion, oval in shape with a surrounding radiolucent rim seen at the apical region of first and second molar edentulous area. OPG, orthopantomogram.
Figure 3.
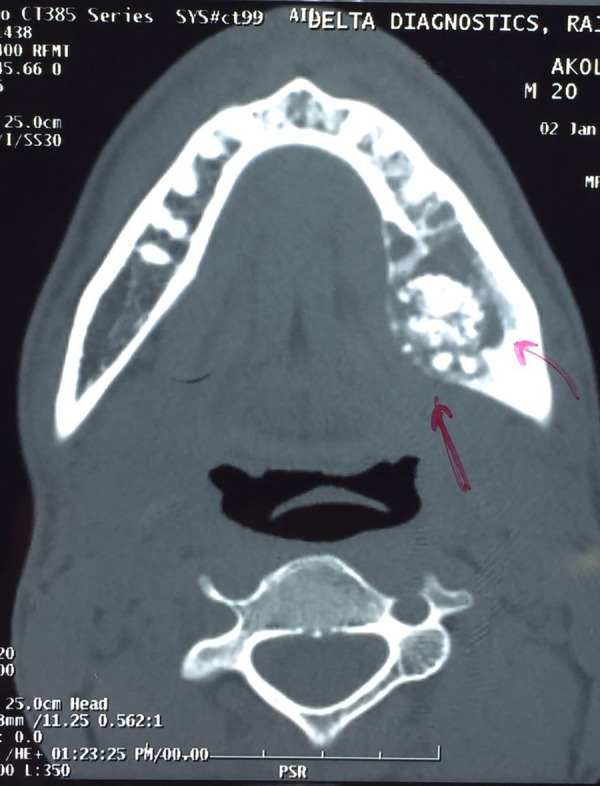
CT axial section revealing high-density relatively defined sclerotic lesion in mandibular periapical molar region.
Figure 4.
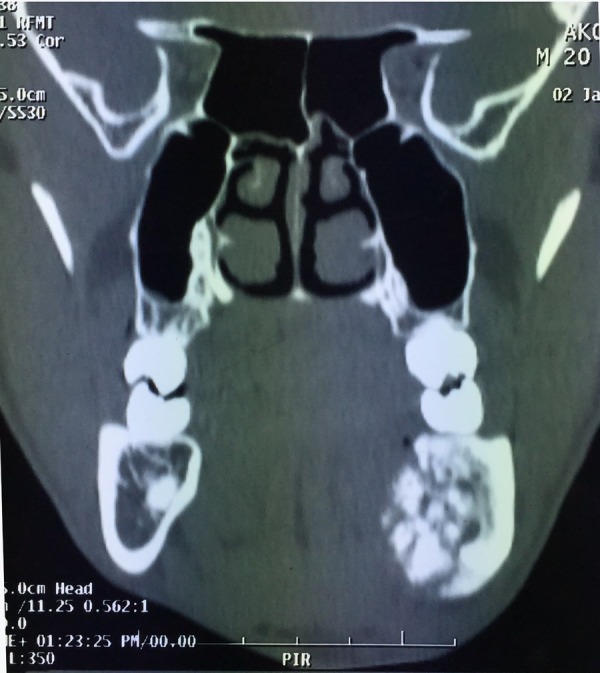
CT coronal section revealing sclerotic hyperdense lesion surrounded by hypodense rim causing expansion of surrounding bone in the mandibular periapical molar region.
Figure 5.
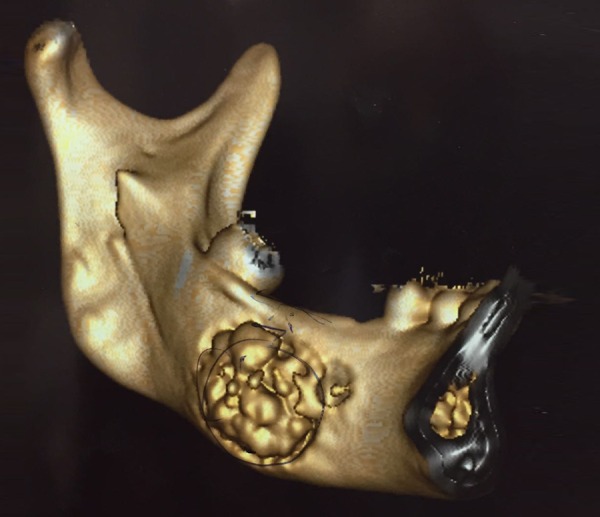
3D reconstruction CT revealing multiple high-density sclerotic lesions in the mandibular molar region.
Figure 6.
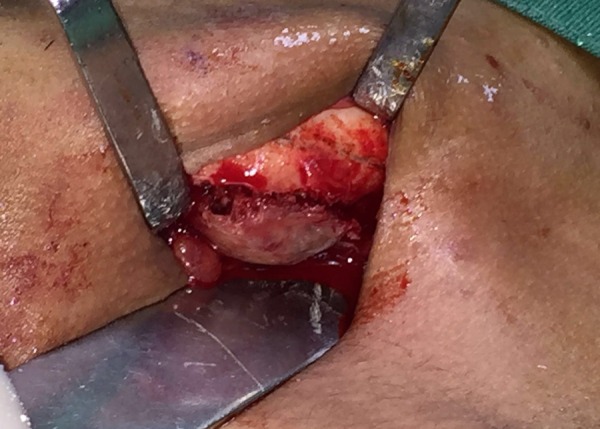
Intraoperative findings revealing capsulated lesion in the left mandibular body region.
Figure 7.
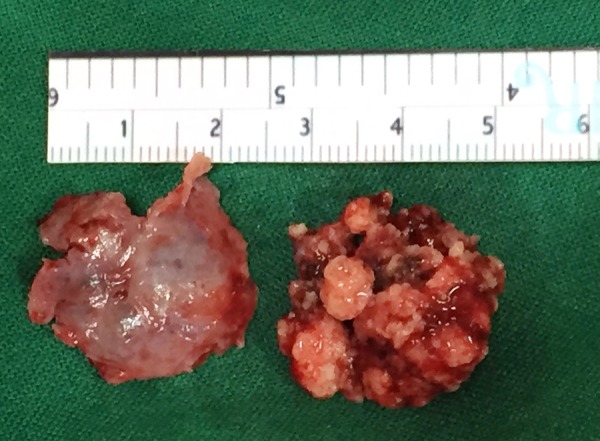
Grossing photo revealing multiple hard gritty fragments surrounded by haemorrhagic areas along with capsule.
Figure 8.
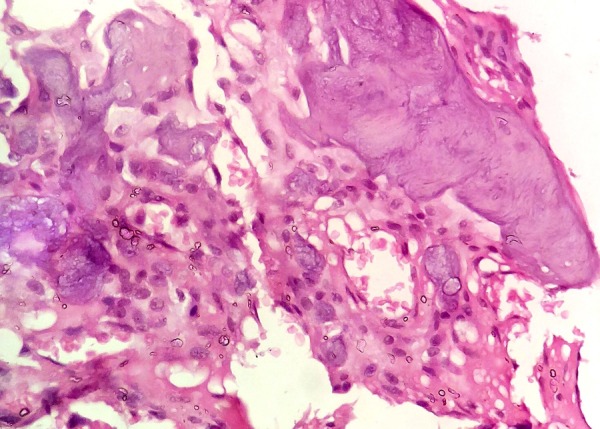
40× H&E decalcified section revealed lamellar woven bone surrounded with numerous scattered droplet-shaped basophilic cementum-like calcification.
Investigations
Serum biochemical markers like alkaline phosphatase, calcium and phosphate levels were within normal limits. This ruled out any systemic bony pathology.
To ascertain the exact nature of pathology, histopathological examination was advised, which revealed characteristic features of COF in its mature stage.
Differential diagnosis
As the patient had history of multiple teeth extractions in mandibular molar region and was presenting with mild pain and swelling, a working diagnosis of focal sclerosing osteitis was made. Clinical, radiological and histological differential diagnoses and exclusion criteria for other closely related pathologies are mentioned below.
Ossifying fibroma: This is common in women in their third to fourth decades of life. They usually present as slow asymptomatic growth leading to painless enlargement of the affected bone.1 Radiographically ossifying fibroma (OF) ranges from radiolucent stage to mixed stage and eventually sclerotic stage. Histological features range from three subtypes, ie, first equal amount of calcified material (retiform bony trabeculae) and fibroblastic stroma, second contains storiform pattern of stroma intermixed with few calcified components, and finally the third variant is combination of the first two.2
Focal osseous dysplasia: This term is used when the lesion is associated with the tooth apex of vital tooth or previous extraction site preferably in mandibular molar areas.3 Focal cemento-osseous dysplasia (FCOD) lesion is seen commonly in women with a mean age in mid-30s. They are commonly asymptomatic and are detected only on radiographic examination. Based on stage of lesion, radiographically the interpretation of the lesion varies from early radiolucent stage to mixed and finally radiopaque mature structure. Histologically, these lesions contain a combination of spherical calcifications believed to be of cemental origin (cementicles) and randomly oriented osseous structures resembling detached fragments of trabecular bone.4
Complex odontome: It represents hamartomatous malformation5 having an irregular-shaped calcification, which is slow growing, expanding and painless. Pain and inflammation are seen in rare cases. Most of these cases are seen in end of the second decade involving mandibular molar areas. They do not resemble tooth structure unlike compound composite odontomes. Cut sections of big lesion reveal calcified masses ranging in colour from white to yellowish hard surface covered with capsule of collagenous tissue. Radiologically, their size may vary from small to very large and may be associated with an impacted or supernumerary tooth. Their initial stage shows radiolucency followed by mixed stage and eventually sclerotic opacity surrounded by radiolucent rim. Histologically, tooth-like calcification is seen intermixed with odontogenic epithelium.6
Idiopathic osteosclerosis: It develops during a healing process, and it is not due to inflammation. It is seen most commonly in the periapical region of vital mandibular molars and the premolar region with infected root canal. Most commonly patients in their first and second decades of life are involved.7 Clinically, it is asymptomatic, and it is not associated with cortical expansion. Radiographically, this lesion is characterised by a well-defined, rounded or elliptical radiodense mass without radiolucent rim. Histopathologically, lamellar bone with scant fibrofatty tissue is seen.8
The present case was considered as mature stage of COF because clinically it was causing slight expansion of body of mandible. During enucleation, it was surrounded by well-defined capsule, and also radiologically it gave dense sclerotic radiopacity surrounded by radiolucent rim. Histopathological findings showed the presence of patternless fibrous stroma intermixed with tiny drop-like cementum structures.
Treatment
Decision of treatment was based on the radiographic and clinical findings, which showed a well-defined sclerotic lesion surrounded by radiolucent rim without any soft tissue component. Hence, surgical enucleation was performed.
Outcome and follow-up
Postoperative recovery was uneventful, and wound healed primarily with minimal scar. There was no paraesthesia of lingual or inferior alveolar nerve, and periodic follow-up of 1, 3 and 6 months postoperatively showed neither residual problems nor any signs of recurrence.
Discussion
Fibro-osseous lesions include a myriad of developmental, dysplastic and neoplastic pathologies exhibiting diverse clinical, radiological and histological characteristics. One of the variants, ie, COFs, are single focal lesions, which are selectively seen in maxillofacial bones.9 It contains fibrous tissue intermixed with variable quantity of mineralised material resembling bone and/or cementum for that it is called cemento-ossifying fibroma.10 When tumours contain bone and cementum-like material, with or without psammoma-like bodies, and are well circumscribed radiographically, a diagnosis of COF is made.6 There are four types of OFs affecting craniofacial skeleton: (i) OF of odontogenic origin (COF), (ii) trabecular juvenile ossifying fibroma (JOF), psammomatoid JOF and (4) extragnathic adult OF.11 The terminology cemento-ossifying fibroma is used because there is a spectrum of fibro-osseous lesions that arise from the periodontal ligament, ranging from those with only deposition of cementum to those with only deposition of bone.12
Review of the literature says that it occurs mostly in the second and fourth decades of life, with a 1:5 male:female ratio. Mandibular premolar and molar area is the most frequent location for this pathology.10 A more aggressive form of OF that occurs in younger individuals has been designated as JOF showing more aggressive features clinically and more vascular at pathological examination. JOF usually involves maxilla and paranasal sinuses, which is contradictory to COF which occurs in the mandible in 70–80% cases.13 14 COF commonly affects mandibular molar region causing painless expansion and is seen in first to second decade of life. Based on radiographic findings, MacDonald-Jankowski described three stages of COF: an initial radiolucent stage, then a mixed stage and eventually, a sclerotic appearing stage,15 and Lu et al reported four radiographic patterns of OF, namely, cystic radiolucency, ground glass pattern, sclerotic change and mixed type.16 According to the study of Su et al on 54 cases of COF,17 size of OF ranges from 0.2 to 15 cm. In their early stage or formative phase, these are commonly radiolucent since the osseous component is non-calcified osteoid. Over time, the tumours become progressively radiopaque as more matrix calcifies. Very few molecular investigations are reported in the literature for OF, and mutation in HRPT2 gene has been identified to cause its production.18 As the pathology was showing radiopaque sclerotic lesion surrounded by radiolucent halo in the left body of mandible, eventually radiological differential diagnosis as radiopaque OF, complex odontoma and idiopathic osteosclerosis was made.
Odontome is the most common odontogenic tumour seen in the second decade of life.19 They are classified into two types: compound composite and complex composite. The former resembles tooth-like structure, and the later mimics only calcified structure. Location of compound composite odontome is anterior maxilla. Most cases of complex composite odontome are seen in posterior mandible as suspected in our case of 20-year-old male patient. Aetiology of odontome includes trauma and infection.19 We anticipate the same aetiology as there was history of multiple teeth extraction in our case. Macroscopically cut section of complex composite odontome is a hard calcified mass covered with collagenous capsule as seen in the present case. Radiologically, odontomes are associated with impacted or supernumerary teeth and sometimes in the periapical region in between roots. They give a dense sclerotic radiopacity surrounded by radiolucent rim19 as seen in the present case. COF is differentiated from complex odontome mainly based on histopathology as complex odontome has mixture of mineralised masses of dentinoid substance, small spaces of pulp tissue, enamel matrix and epithelial remnants, which is absent in COF.
Pathogenesis for COF is unknown and controversial. Embryonic nests and ectopic periodontal membrane are the aetiological factors, as recommended by Cakir and Karadayi for extraosseous variant where there is no periodontal tissue, as suggested by Elarbi et al in their cytogenetic studies.20 WHO classifies COF among non-odontogenic tumours derived from the mesenchymal blast cells of the periodontal ligament, which are pluripotent to form fibrous tissue, bone and cement or combination of such components. Perhaps there is controversy over such an origin since tumours of similar histology have been reported in ethmoid bone, frontal bone or even long bones of body that lack periodontal ligament cells.21 22 Bernier and Thompson have speculated that infection-induced inflammation and fibrosis of the periapical area might stimulate the periodontal membrane. After trauma, such as tooth extraction, the remaining periodontal tissue attached to the wall of the alveolus may serve as the origin of COF.23 In the present case, COF might have developed from the remnants of periodontal ligament cells. Histopathological findings of COF mirror the radiographic findings. COF consists of fibrous tissue that exhibits varying degrees of cellularity and contains mineralised material in the form of bone and cementum.24 More radio-opaque lesions are composed of more mineralised component in the form of lamellar bone or woven bone intermixed with numerous tiny droplets and less of fibrous component as seen in our case. Untreated COF lesions expand slowly and progressively, which threatens the surrounding tissue. Recurrence rate for COF is reported as 28%, and there is a remote possibility of malignant transformation, so treatment ranges from surgical curettage or enucleation to resection and radical surgery followed by long-term observation.16 25 26 Since the present case is a well-demarcated lesion separated from normal bone, an amenable approach of enucleation was contemplated and carried out with follow-up of 1, 3, and 6 months.
Patient's perspective.
I came to this hospital because of pain and swelling in my jaw. After getting operated, I am completely alright. I am in regular follow-up with my doctor.
Learning points.
Histopathologically cemento-ossifying fibroma (COF) and focal cemento-osseous dysplasia (FCOD) look alike in their matured stage. They can be only differentiated as ossifying fibroma causes expansion of bone and it is covered by capsule, which is absent in FCOD.
COF commonly presents as unilocular radiolucency, and variations from routine radiologic features may mimic other ossifying and odontogenic pathologies and may be confused with periapical pathologies, which should be differentiated with histopathology for definite diagnosis.
Majority of fibro-osseous lesions can be approached intraorally, but this particular lesion was localised below the mylohyoid muscle breaching lingual cortex. Extraoral approach was taken not only for ease of surgical access but also to prevent damage to important anatomical structures like inferior alveolar nerve, lingual nerve, sublingual vessels and Wharton's duct, which may become afflicted on intraoral access.
Footnotes
Twitter: Follow Sumit Bahl at @Dr.Sumit Bahl
Contributors: All of the authors have equally contributed to this report. HSD discussed, planned and designed the study and interpreted data. SKD conducted and designed the study and interpreted data. SB and PTP participated in discussion and planning of the study and acquisition and interpretation of data.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Deliverska E, Rubiev M. Ossifying fibroma of the mandible—case report and literature review. Int J Sci Res 2016;5:300–3. [Google Scholar]
- 2.Ossifying fibroma. In: Riechart PA, Philipsen HP. Odontogenic tumours and allied lesions. 2nd edn London: Quintessence Publication, 2004;32:277–8. [Google Scholar]
- 3.Summerlin DJ, Tomich CE. Focal cement-osseous dysplasia: a clinic-pathologic study of 221 cases. Oral Surg Oral Med Oral Pathol 1994;78:611–20. [DOI] [PubMed] [Google Scholar]
- 4.Bone lesions. In: Sapp JP, Eversole LR, Wysocki GP. Contemporary oral & maxillofacial pathology. 4th edn China: Mosby Publication, 2004:95. [Google Scholar]
- 5.Rajendran R. Odontogenic cysts and tumours. In: Shafer WG, Hine MK, Levy BM, eds. Shafers textbook of oral pathology. 5th edn Noida: Elsevier Publication, 2006:404. [Google Scholar]
- 6.Complex odontome. In: Riechart PA, Philipsen HP. Odontogenic tumours and allied lesions. 2nd edn London: Quintessence Publication, 2004;15:142–5. [Google Scholar]
- 7.Wood NK. Periapical radioopacities. In: Wood NK, Gaoz PW. Differential diagnosis of ORAL and maxillofacial lesions. 5th edn New Delhi: MOSBY Publication, 2007:458. [Google Scholar]
- 8.Waldron CA. Bone pathology. In: Neville BW, Damm DD, Allen CM, et al. Oral & maxillofacial pathology. 2nd edn New Delhi: Elsevier publication, 2005:540. [Google Scholar]
- 9.Kenney JN, Kaugars GE, Abbey LM. Comparison between the peripheral ossifying fibroma and peripheral odontogenic fibroma. J Oral Maxillofac Surg 1989;47:378–82. [DOI] [PubMed] [Google Scholar]
- 10.Trijolet JP, Parmentier J, Sury F et al. Cemento-ossifying fibroma of the mandible. Eur Ann Otorhinolaryngol Head Neck Dis 2011;128:30–3. 10.1016/j.anorl.2010.06.005 [DOI] [PubMed] [Google Scholar]
- 11.Samir K. El- Mofty. Gnepp- Fibro-osseous lesions. In: Gnepp DR. Diagnostic surgical pathology of the head and neck. 2nd edn. Philadelphia (PA): Elsevier. Publication, 2002:766–769. [Google Scholar]
- 12.Kuta AJ, Worley CM, Kaugars GE. Central cementoossifying fibroma of the maxillary sinus: a review of six cases. AJNR 1995;16:1282–6. [PMC free article] [PubMed] [Google Scholar]
- 13.Slootweg PJ, Panders AK, Koopmans R et al. Juvenile ossifying fibroma. An analysis of 33 cases with an emphasis on the histological aspects. J Oral Pathol Med 1994;23:385–8. [DOI] [PubMed] [Google Scholar]
- 14.Khatua S, Banerjee A, Bandyopadhyay A et al. A rare case of Juvenile cemento-ossifying fibroma: diagnosed by cytological and radiological correlation. Ann Clin Pathol 2014;2:1025. [Google Scholar]
- 15.MacDonald-Jankowski DS. Cemento-ossifying fibromas in the jaws of the Hong Kong Chinese. Dentomaxillofac Radiol 1998;27:298–304. 10.1038/sj/dmfr/4600378 [DOI] [PubMed] [Google Scholar]
- 16.Saikrishna D, Shetty S, Ramya S. Massive ossifying fibroma of mandible. Ann Maxillofac Surg 2014;4:81–4. 10.4103/2231-0746.133075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su L, Weathers DR, Waldron CA. Distinguishing features of focal cernento-osseus dysplasia and cemento-ossifying fibro mas. II. A clinical and radiologic spectrum of 316 cases. Oral Surg Oral Med Oral Pathol Oral Radia l Endo d 1997;84:540–9. [DOI] [PubMed] [Google Scholar]
- 18.Eversole R, Su L, ElMofty S. Benign fibro-osseous lesions of the craniofacial complex a review. Head Neck Pathol 2008;2:177–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen DM, Bhattacharyya I. Ameloblastic fibroma, ameloblastic fibro-odontoma, and odontome. Oral Maxillofacial Surg Clin N Am 2004;16:375–84. [DOI] [PubMed] [Google Scholar]
- 20.Elarbi M, Azuzz L. Cemento-ossifying fibroma of the mandible case report . Rev Cir Traumatol Buco-Maxilo-Fac, Camaragibe 2014;14(4):41–4. [Google Scholar]
- 21.Ram R, Singhal A, Singhal P. Cemento-ossifying fibroma. Contemp Clin Dent 2012;3:83–5. 10.4103/0976-237X.94553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rangil JS, Silvestre FJ, Bernal JR. Cementoossifying fibroma of the mandible: presentation of a case and review of the literature. J Clin Exp Dent 2011;3:e66–9. [Google Scholar]
- 23.Bernier JL, Thompson HC. The histogenesis of the cementoma. Am J Orthod Oral Surg 1946;32:543–55. [DOI] [PubMed] [Google Scholar]
- 24.Waldron CA. Bone pathology. In: Neville BW, Damm DD, Allen CA, Bouquot JE. Oral & maxillofacial pathology. 2nd edn New Delhi: Saunders Elsevier Publication, 2002:563. [Google Scholar]
- 25.MacDonald-Jankowski DS. Ossifying fibroma: a systematic review. Dentomaxillofac Radiol 2009;38:495–513. 10.1259/dmfr/70933621 [DOI] [PubMed] [Google Scholar]
- 26.Oukabli M, Akhaddar A, Qamouss O et al. Nasoethmoidal psammomatoid cementoossifiying fibroma with intraorbital extension. Rev Stomatol Chir Maxillofac 2009;111:43–5. 10.1016/j.stomax.2009.05.002 [DOI] [PubMed] [Google Scholar]


