Abstract
A 70-year-old man with a history of prostate cancer, previously submitted to surgical castration and trans-urethral resection of the prostate, was admitted to Accident and Emergency department. He had been suffering from osteoarticular and abdominal pain, and recent weight loss. An abdominal and a pelvic CT showed multiple hepatic metastases and a pelvic mass, but his prostate-specific antigen values were low (0.26 n/mL). A biopsy of a hepatic metastasis and of the pelvic mass revealed a small-cell neuroendocrine prostate cancer, a rare and aggressive androgen-independent form of prostate cancer with a poor prognosis. Our purpose was to report a clinical case of a rare and aggressive variant of a common disease. A high index of suspicion is required to make an early diagnosis and to ensure a proper therapeutic approach.
Background
Prostate cancer is the most frequent tumour in men, and the second cause of death by cancer.1 Prostate-specific antigen (PSA) is a non-specific marker of prostate cancer which is important for the follow-up of patients with prostate cancer and for treatment response evaluation.
Occasionally, metastatic progression of prostate cancer occurs even in the presence of continuously low PSA values. It is usually a sign of an undifferentiated prostate cancer, such as small-cell neuroendocrine prostate cancer.
Our goal is to report a case of a 70-year-old man with a history of prostate cancer who had visceral and bone metastases even though his PSA levels were unremarkable. A hepatic and a pelvic biopsies revealed a small-cell neuroendocrine prostate carcinoma, a rare and aggressive histological subtype of prostate carcinoma with a poor prognosis. Diagnosis and proper treatment for this condition may be delayed due to lack of awareness of its existence.
Case presentation
A 70-year-old man was admitted to Accident and Emergency department. He had been suffering from osteoarticular and abdominal pain for approximately a month. Moreover, he had lost 12 kg in the 3 months prior to hospital admission.
This patient had a history of arterial hypertension; diabetes mellitus type 2 treated with insulin; chronic renal failure stage II; and a prostate cancer which had been diagnosed 4 years before. A prostate biopsy had revealed an intermediate grade, Gleason 6 (3+3) prostate cancer. He had been submitted to surgical castration and trans-urethral resection of the prostate 4 years earlier, in order to relieve lower urinary symptoms. His last PSA value was 0.09 ng/mL.
The patient's blood tests showed elevated liver enzymes with a cholestasis pattern (elevated aspartate transaminase (AST), alanine transaminase (ALT), gamma-glutamyltransferase (GGT) and alkaline phosphatase).
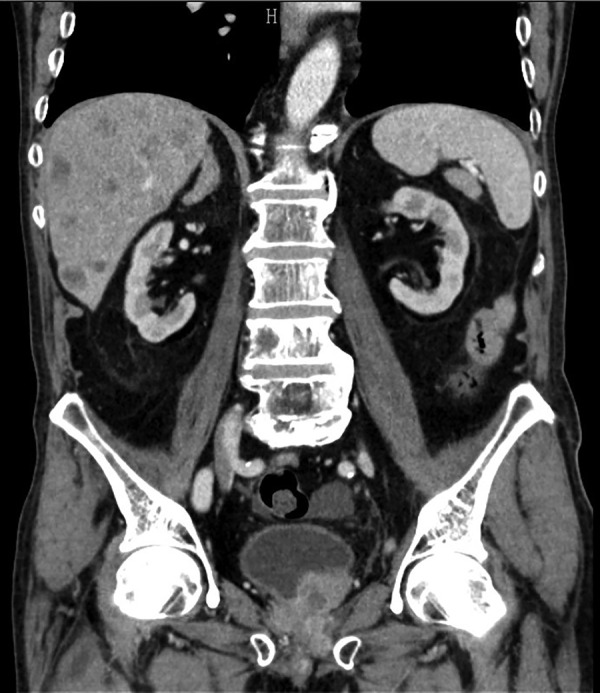
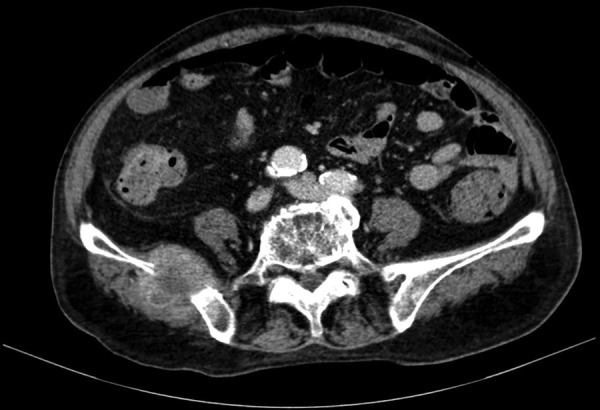
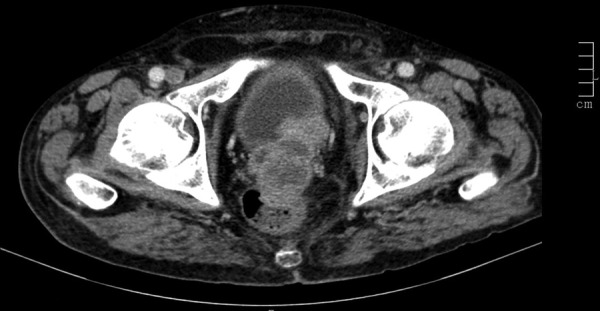
An abdominal CT was performed, which revealed a pelvic mass involving the bladder, the prostate and the rectum; and several hepatic lesions compatible with metastases (figure 1), multiple retroperitoneal lymphadenopathies and an iliac bone metastasis (figure 2).
Figure 1.
Sagittal CT image showing several hepatic metastasis and a pelvic mass with invasion of the bladder trigone and posterior wall.
Figure 2.
Coronal CT image through the lower abdomen revealing a soft tissue mass metastasis in the right iliac bone.
The patient was admitted to the department of internal medicine for treatment and further study.
Investigations
Tumour markers alpha-fetoprotein, carcinoembryonic antigen, cancer antigen 19.9 and cancer antigen 125 were within the normal range. PSA was 0.26 ng/mL.
A thoracic and a pelvic CT were performed. They revealed a small bilateral pleural effusion and a 7-mm node in the right middle lobe. A roughly defined pelvic mass which included the prostate and involved the bladder and pelvic muscle floor muscles was described in the pelvic CT (figure 3).
Figure 3.
Coronal CT image through the pelvis showing a pelvic mass with origin in the prostate and involving the posterior wall of the prostate and the rectum.
The patient was submitted to an endoscopy that showed no significant findings and a colonoscopy that did not reveal any significant findings in the colon besides the protrusion of the pelvic mass through the anterior rectal wall. In addition, a trans-rectal biopsy of the pelvic mass was performed.
Afterwards the patient underwent an ultrasound-guided biopsy of a hepatic metastasis.
The pelvic mass biopsy revealed normal colonic mucosa with infiltration by a small-cell neuroendocrine cancer (figure 4) with strong reactivity to synaptophysin (figure 5). The hepatic biopsy revealed a malignant neoplasia made up of small cells (figure 6) with strong reactivity to synaptophysin (figure 7) and focal reactivity to chromogranine A. Both samples were negative for the PSA marker.
Figure 4.
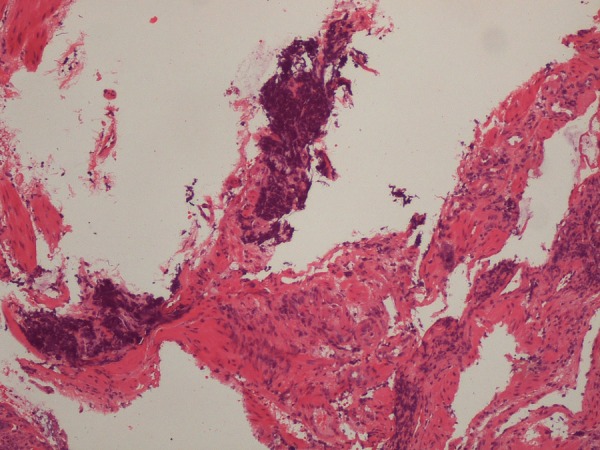
Trans-rectal biopsy of the pelvic mass. H&E stain at ×400 magnification. The superficial colonic mucosa showed no alterations. There is infiltration of the submucosa by small neoplastic cells with round, small and pleomorphic nuclei. Courtesy of Helena Garcia, MD.
Figure 5.
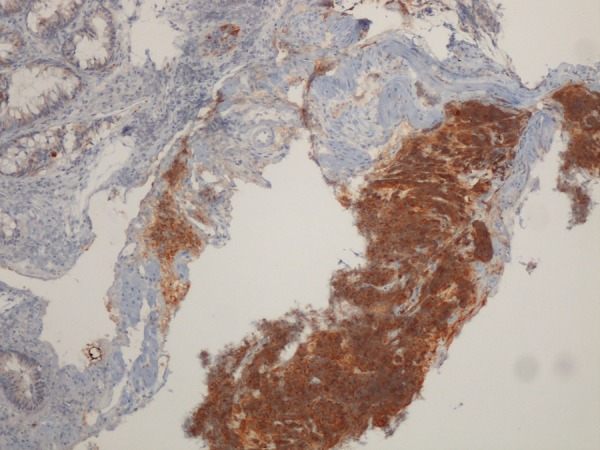
Trans-rectal biopsy of the pelvic mass. Immunostaining for synaptophysin at ×4 magnification. Cellular elements are strongly reactive to neuroendocrine marker synaptophysin. Courtesy of Helena Garcia, MD.
Figure 6.
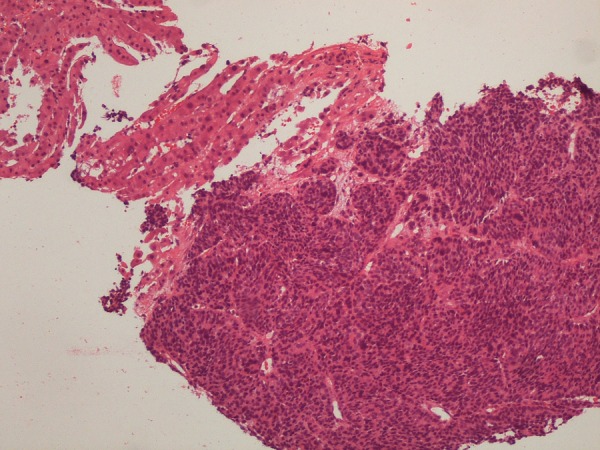
Ultrasound-guided biopsy of a hepatic metastasis. H&E stain at ×100 magnification. Normal hepatic parenchyma can be observed in the picture, but most of the core is occupied by a malignant neoplasia composed by small cells with large nuclei. Courtesy of Helena Garcia, MD.
Figure 7.
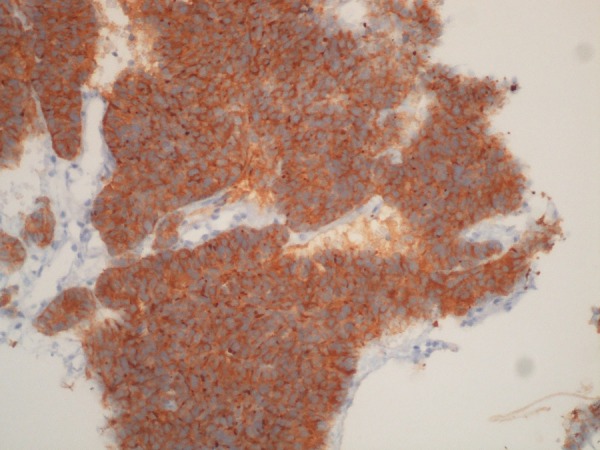
Ultrasound-guided biopsy of a hepatic metastasis. Immunostaining for synaptophysin at ×200 magnification. A strong reaction to the neuroendocrine marker synaptophysin is present. Courtesy of Helena Garcia, MD.
Differential diagnosis
Colon cancer
Small-cell lung cancer
Neuroendocrine prostate cancer
Treatment
The case was discussed with medical oncology. This aggressive tumour, comorbidities and the patient's general condition were taken into consideration. This patient was not considered a suitable candidate for chemotherapy. Best supportive care was started.
Outcome and follow-up
The patient was eventually discharged from the department of internal medicine. He died ∼2 months after having been diagnosed with a neuroendocrine prostate cancer.
Discussion
The first case of small-cell neuroendocrine prostate cancer was described in 1977 by Wenk et al.2 It is a rare and aggressive form of prostate cancer, which is usually androgen independent and it carries a dismal prognosis.
In recent years, there has been a great interest in the neuroendocrine differentiation of prostate cancer. Neuroendocrine differentiation is a common feature of prostatic carcinoma, which is usually present in primary high Gleason-grade tumours of the prostate heralding a more aggressive clinical behaviour and requiring a different treatment approach.3 4 However, there is still conflicting data reported in the literature regarding the prognostic significance of neuroendocrine tissue markers in prostate cancer. Although some authors have shown a significant correlation between neuroendocrine differentiation, tumour grade and poor prognosis, other groups of researchers did not find such a strong correlation.5
Furthermore, strong evidence is lacking concerning its potential independent prognostic value in hormone-naïve prostate cancer, refraining its use in routine clinical practice.6
The neuroendocrine component usually coexists with acinar prostate cancer component, displaying a variable extension of these two components, potentially ranging from 1% to 99%.
In neuroendocrine prostate cancer, the neuroendocrine component usually varies between 5% and 30% of tumour mass.4
Aetiology of small-cell prostatic cancer (SCPC) is still a matter of discussion. Focal neuroendocrine cells are widely distributed in the prostate, and they are members of the diffuse amine precursor uptake and decarboxylation (APUD) cell system. It is believed that they originate from pluripotent prostatic stem cells.
Fine divided prostate cancer's neuroendocrine differentiation into three major categories. It can appear as focal neuroendocrine differentiation amidst typical prostate cancer or as a more indolent prostate carcinoid tumour. In other cases, it is classified as high-grade neuroendocrine carcinoma, the small-cell carcinoma of the prostate. Approximately 50% of the SCPC is a composite tumour with conventional prostate cancer.7
Several studies have looked into the effect of androgen-deprivation therapy in the induction of neuroendocrine differentiation in prostate cancer. Ahlgren et al8 showed that a 3-month regimen of neoadjuvant hormonal treatment with luteinising hormone-releasing hormone analogue induced an increase in the number of Chromogranin A-positive cells and the proportion of neuroendocrine-positive tumours when compared with surgery alone. In fact, neuroendocrine differentiation is an androgen-independent phenomenon that allows tumour cell growth under androgen-deprivation therapy and may represent a viable option to escape hormone therapy and especially in castration-resistant prostate cancer.6
It is estimated that SCPC has an incidence of 0.5–2% of all primary prostatic cancers.9 However, small-cell cancer is rare outside the lung; ∼10% of extrapulmonary small-cell cancers occur in the prostate, making it one of the most common extrapulmonary sites.3
The diagnosis of SCPC is not straightforward and immediate. Low serum PSA value and clinical history can help clinicians but immunohistological confirmation is necessary. The measurement of serum neuroendocrine markers and immunohistological positivity for neuroendocrine markers, such as Chromogranin A, neuron-specific enolase and synaptophysin, support the diagnosis.4 Chromogranin A appears to be the most sensitive marker. It is more specific than neuron-specific enolase and it is the most frequently used marker for detecting neuroendocrine phenotype.6
Similar to its extra-urinary counterparts, especially in the lung, paraneoplastic manifestations of SCPC, such as Cushing disease or hypercalcaemia, are rare but have been reported. SCPC is an aggressive subtype and median survival time following diagnosis is 10 months. Fewer than 5% of patients survive 2 years.10
SCPC of the prostate should be considered in patients with a high disease volume, low serum PSA, poorly differentiated disease, and poor or insufficient response to hormonal treatment.11
The presence of a paraneoplastic syndrome may be a poor prognostic factor. In a retrospective study by Spiess et al,12 high serum lactate dehydrogenase and a low albumin level at the time of diagnosis are predictive of inferior disease-related outcome.
Similar to extra-urinary neuroendocrine small-cell cancer, prostatic lesions are usually diagnosed at an advanced stage. They can be unresectable, visceral metastases are common, and survival is in most cases poor.
The presence of metastases in unusual locations such as axillary lymph nodes, the omentum or the pericardium, or widespread visceral metastases are more commonly seen with SCPC than with acinar adenocarcinoma.13
Owing to its relative rarity and under-reporting, there are no large series reported or strong evidence supporting optimal management of SCPC. Small modern series have suggested managing SCPC with androgen-deprivation therapy combined with cisplatin-based chemotherapy combined with adjuvant radiotherapy and salvage surgery.7 A recent phase II trial with 120 patients with castration-resistant prostate cancer with neuroendocrine differentiation evaluated the efficacy of platinum-containing chemotherapy. Encouraging results consisting of a response rate of more than 30% and median OS of 16 months were reported, supporting the use of platinum-containing chemotherapy in this clinical setting.14 Docetaxel-based chemotherapy regimens have proved ineffective in the treatment of neuroendocrine-differentiated prostate cancer.6
There is some evidence supporting the administration of somatostatin analogues and radiolabelled somatostin analogues in the neuroendocrine prostate cancer, although larger prospective phase III trials are lacking.4 Furthermore, new potential therapeutic strategies are emerging involving mTOR inhibitors, anti-EGFR and anti-angiogenic drugs, or suppressors of relaxin receptor RXFP1. There is, however, limited evidence supporting these approaches.
Learning points.
Prostate cancer is the most common tumour in men and the second oncological cause of death in men.
Prostate-specific antigen (PSA) is a non-specific, but sensitive tumour marker of prostatic carcinoma. It can be reliably used to monitor and follow-up disease progression.
Occasionally patients with prostate cancer may have metastatic progression of the prostate cancer with low PSA levels, especially in undifferentiated tumours.
Small-cell prostate carcinoma is a rare, aggressive and androgen-independent form of neuroendocrine prostate cancer, which can evolve from low-grade acinar prostate carcinoma.
Footnotes
Twitter: Follow Hugo Coelho at @hugompcoelho
Contributors: DA collected and analysed the data and contributed to writing the manuscript. MEC contributed to writing the manuscript and reviewed it. RS and HC conducted scientific research and reviewed the manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10–29. 10.3322/caac.20138 [DOI] [PubMed] [Google Scholar]
- 2.Wenk RE, Bhagavan BS, Levy R et al. Ectopic ACTH, prostatic oat cell carcinoma, and marked hypernatremia. Cancer 1977;40:773–8. [DOI] [PubMed] [Google Scholar]
- 3.Furtado P, Lima MV, Nogueira C et al. Review of small cell carcinomas of the prostate. Prostate Cancer 2011;2011:543272 10.1155/2011/543272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conteduca V, Aieta M, Amadori D et al. Neuroendocrine differentiation in prostate cancer: current and emerging therapy strategies. Crit Rev Oncol Hematol 2014;92:11–24. 10.1016/j.critrevonc.2014.05.008 [DOI] [PubMed] [Google Scholar]
- 5.Vashchenko N, Abrahamsson PA. Neuroendocrine differentiation in prostate cancer: implications for new treatment modalities. Eur Urol 2005;47:147–55. 10.1016/j.eururo.2004.09.007 [DOI] [PubMed] [Google Scholar]
- 6.Surcel CI, van Oort IM, Sooriakumaran P et al. Prognostic effect of neuroendocrine differentiation in prostate cancer: a critical review. Urol Oncol 2015;33:265.e1–7. 10.1016/j.urolonc.2014.08.007 [DOI] [PubMed] [Google Scholar]
- 7.Fine SW. Neuroendocrine lesions of the genitourinary tract. Adv Anat Pathol 2007;14:286–96. 10.1097/PAP.0b013e3180ca8a89 [DOI] [PubMed] [Google Scholar]
- 8.Ahlgren G, Pedersen K, Lundberg S et al. Regressive changes and neuroendocrine differentiation in prostate cancer after neoadjuvant hormonal treatment. Prostate 2000;42:274–9. [DOI] [PubMed] [Google Scholar]
- 9.Yashi M, Terauchi F, Nukui A et al. Small-cell neuroendocrine carcinoma as a variant form of prostate cancer recurrence: a case report and short literature review. Urol Oncol 2006;24:313–17. 10.1016/j.urolonc.2005.08.022 [DOI] [PubMed] [Google Scholar]
- 10.Umar SA, Umar SA, MacLennan GT. Small cell carcinoma of the prostate. J Urol 2009;181:838–9. 10.1016/j.juro.2008.11.006 [DOI] [PubMed] [Google Scholar]
- 11.Adshead F, De Graeff A, Mansi JL et al. Small cell carcinoma of the prostate: implications for management. Br J Urol 1991;67:217–18. 10.1111/j.1464-410X.1991.tb15118.x [DOI] [PubMed] [Google Scholar]
- 12.Spiess PE, Pettaway CA, Vakar-Lopez F et al. Treatment outcomes of small cell carcinoma of the prostate: a single-center study. Cancer 2007;110:1729–37. 10.1002/cncr.22971 [DOI] [PubMed] [Google Scholar]
- 13.Kotb AF, Ismail Ad. Small cell carcinoma of the genitourinary system. Turk J Urol 2010;36:344–9. 10.5152/tud.2010.042 [DOI] [Google Scholar]
- 14.Aparicio AM, Harzstark AL, Corn PG et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clin Cancer Res 2013;19:3621–30. 10.1158/1078-0432.CCR-12-3791 [DOI] [PMC free article] [PubMed] [Google Scholar]


