Abstract
Drug fever caused by dalteparin-sodium (DS), a low-molecular-weight derivative of heparin, is neither listed in the official drug information and nor published as a case report until today. A preterm infant, born at 26 weeks of gestation, developed fever 2 days after starting a treatment with DS for an intracardial thrombus. The fever reverses soon after changing the treatment to unfractionated heparin and reappeared after reintroduction of DS. Once again, after discontinuing DS, the infant regained normothermia. Bacterial and viral infections, tissue damage, impaired liver or kidney function, preservative agents and comedications could be ruled out as fever origin. By using the Naranjo adverse drug reaction (ADR) probability scale and the Liverpool ADR causality assessment tool, this case can be classified as ‘probable ADR’ and ‘definite ADR’. This is the first case report of a drug fever caused by the low-molecular-weight heparin DS in a preterm infant.
Background
Drug fever is a condition where the body reacts with a febrile response to an administered drug in the absence of other underlying causes, for example, infection. The fever reverses once the offending drug is discontinued. A non-sensitised adult person receiving an offending drug for the first time usually takes a couple of days to develop the fever. When sensitised, the same person would react with a fever within hours when exposed to the same drug again.1
Febrile reactions in the preterm infants, in general, are rare events as the immune system is highly immature and hypothermia is the most common response to inflammatory events.
Unfractionated heparin (UFH) is a widely used drug in sick infants, mostly to prevent central-line-associated thrombosis. Using low doses of UFH continuously in central catheters is known to extend the usability of central lines.2 Using a low-molecular-weight heparin for treating thrombosis in a preterm infant is an off-label indication and a more seldom event on an NICU (neonatal intensive care unit).
UHF, in particular low-molecular-weight heparin, is until today not known to cause fever/hypertermia.1
Case presentation
The infant was born due to maternal contractions in 26+3 weeks of gestation by Caesarian section with uncomplicated postnatal transition.
During routine echocardiography on day of life (DOL) 5, a thrombus in the right ventricle of the heart was diagnosed.
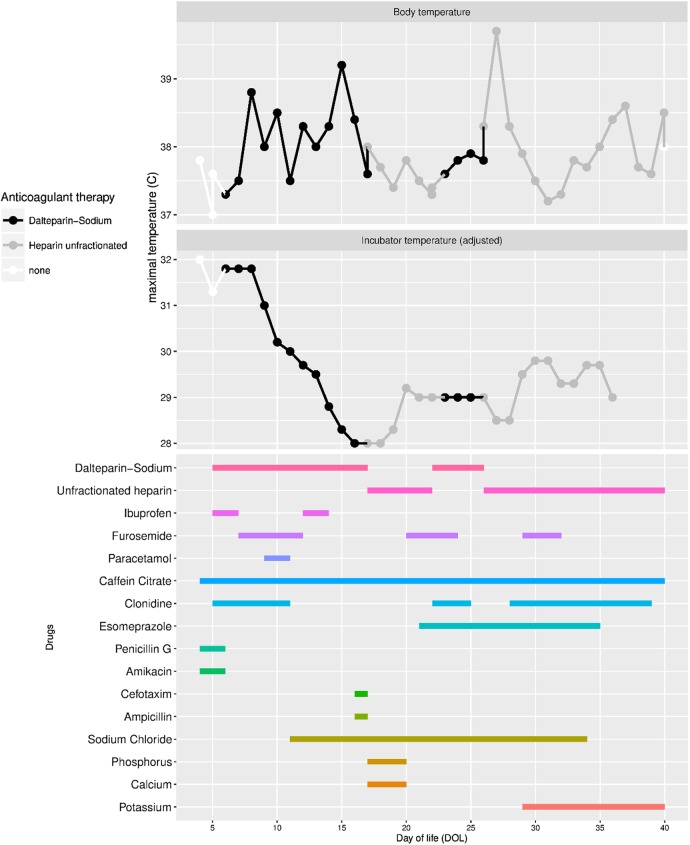
After screening for coagulation disorders, a treatment with dalteparin-sodium, Fragmin, (DS) was initiated the same day. DS (100 IU/kg) was given two times per day by injections via a subcutaneous Insuflon catheter (Unomedica, Lejre, Danmark). The insertion site was inspected for signs of infection, bleeding or dislocation at least three times a day. By our local routine, the catheter was replaced every third day. On DOL 7, the infant's body temperature increased. At the same time, the patient begun showing signs of discomfort and the heart rate increased. As body temperature in preterm babies is related to the thermal environment (temperature and humidity in the incubator), the temperature and humidity in the incubator were decreased much more rapidly as usual to maintain normothermia (figure 1). After excluding infection as a cause for the hyperthermia, medication was changed from DS to a continuous infusion of UFH on DOL 17. The infant regained normothermia, the incubator temperature could be increased and the signs of irritation and tachycardia disappeared.
Figure 1.
Body temperature (upper box) and temperature in the incubator (medial box) are shown. Treatment periods with dalteparin-sodium (black), unfractionated heparin (gray) and no heparin treatment (white). Concomitant drugs are shown in the lower box. DOL, day of life.
On DOL 22, the DS was started again and the UFH was stopped. Two days later, the body temperature increased again above 38°C and concurrent symptoms of irritation and tachycardia reoccurred. After discontinuing the low-molecular-weight heparin and re-inducing continuous treatment with UFH on DOL 26, the infant regained normal body temperature and the clinical symptoms disappeared. No clinical signs of allergic reaction were seen during the whole treatment period and treatment was continued until DOL 40 with normal body temperature. On DOL 37, the infant was transferred from incubator to a warming bed which resulted in slightly increased temperature (figure 1).
The intracardial thrombus diminished during the course of treatment with DS/UFH. During the treatment period, both drugs were used without any preservative agents. The timing of concomitant medications can be obtained from figure 1.
Naranjo et al3 published an algorithm to estimate the probability of an adverse drug reaction (ADR). Using this algorithm in the presented case, the ADR can be classified as a ‘probable ADR.’ In 2011 the Liverpool Adverse Drug Reaction Causality Assessment Tool was developed and tested. This tool takes the unique circumstances in paediatric patients into account when estimating the probability ADR in infants and children.4 Using this tool, the DS-ADR is classified as ‘definite’-ADR.
A report of an adverse drug reaction has been sent to the Medical Product Agency and has been classified as ADR.
Investigations
Screening for infection was started and antibiotic treatment initiated. C reactive protein was negative and the blood cell count was normal during DS and UHF treatment periods. Furthermore, liver enzymes (ALAT, ASAT) and renal parameters, including creatinine and BUN, remained normal. Negative blood cultures and molecular diagnostics for 15 different viruses could rule out a viral or bacterial infection as fever origin.
Outcome and follow-up
The infant was normal developed and was doing well, when seen for follow-up at the corrected age of 3 months.
Discussion
We present a case of drug-induced fever in a preterm infant which is probably/definitely caused by DS.
Fever, signs of irritation and tachycardia occurred 2 days after a treatment with DS was initiated and normalised after discontinuing the treatment. A rechallenge of the drug led to an increase in the patient's body temperature and decreased to normal after stopping the treatment.
Drug fever associated with DS is mentioned only once in a structured assessment of reported ADR in the French National Pharmacovigilance Database.5 Fifty-four cases of drug fever and dalteparin are reported in a WHO pharmacovigilance database (VigiBase http://www.who-umc.org/) and to the best of our knowledge, the drug-event is not highlighted from a statistical perspective. The VigiBase data do not represent the view of WHO and come from a variety of sources, and the likelihood that the suspected adverse reaction is drug-related is not the same in all cases. As this case is based on an off-label indication in the neonatal population, the response to a drug in this age group could be different from an infant, child and adult perspective. A safety assessment in infants and children did not find any case of drug fever.6 Furthermore, hyperthermia/fever is not described as side-effect in the official drug information provided by the manufacturer.
An infection causing increase in body temperature could be excluded by repetitive blood examinations. Blood culture and screening for virus infections remained negative. Clinical signs of infection where not observed. Furthermore, hyperthermia in preterm infants is a rare event as the immune system is highly immature which most commonly results in hypothermia as inflammatory response. Preservative agents as a cause of the hyperthermia can be excluded as both drugs were given only diluted in isotonic sodium chloride or sterile water. As the patient was treated with different drugs at the same time, it cannot be excluded that the fever could be caused of a different drug. Among those, furosemide and ibuprofen have a documented effect on hypertermia.7 Furthermore, an association between hyperthermia and the use of clonidine has been published in case reports.8 As shown in figure 1 these drugs were used only during short periods and the last cores of clonidine was even used when normothermia was re-established. The strongest time correlation was seen to DS treatment.
One might suspect cross-sensitivity between UHF and DS, but there are some significant pharmacological differences that could explain the patient's different reactions to each drug as well as the different route of administration.
UFH is an unfractionated acid mucopolysaccharide and its effect on the coagulation mechanism is ascribed to its activation of antithrombin III which then deactivates thrombin and factor Xa. It is administered intravenously and has a dose-dependent half life, averaging 1–3 hours in neonates.
DS is a fractionated low-molecular-weight derivative of heparin. It is given subcutaneously, has a greater bioavailability and a more pronounced effect on the inhibition of Xa. It is eliminated through the kidneys with a half life of 3–5 hours.
Speculating about a mechanism explaining hyperthermia in our case is challenging as the immune system is highly immature but develops rapidly. DS has no known effect on the thermoregulation and a hypersensitivity reaction is implausible with regards to the delay of the second reaction (48 hours). A possible mechanism could be an idiosyncratic effect or a local effect, caused by the subcutaneous deposit of the drug.9
In summary, there are differences in hypersensitivity or the mode of administration that could be related to tissue damage. In our case, DS was administered two times per day via a subcutaneous catheter that was changed every third day. No signs of tissue damage or local infection were observed during the treatment period.
Learning points.
Several drugs have the potential to cause drug fever in patients, but until today no case of drug fever caused by dalteparin-sodium (DS) (Fragmin) has been published. Drug fever is not mentioned in the official drug information.
In the presented case, DS caused drug fever in a preterm infant.
This novel insight should be considered when treating infants off-label with this drug and preferentially will lead to increased recognition and reporting.
Footnotes
Contributors: DW and SO recognised the adverse event, collected the data and did the literature research. PN provided essential background information and did the research in the pharmacovigilance databases. DW, SO and PN wrote the case report.
Funding: Financial support was provided through Regional Agreement on Medical Training and Clinical Research (ALF), project 20130324, between the Stockholm County Council and Karolinska Institutet.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Patel RA, Gallagher JC. Drug fever. Pharmacotherapy 2010;30:57–69. 10.1592/phco.30.1.57 [DOI] [PubMed] [Google Scholar]
- 2.Shah PS, Shah VS. Continuous heparin infusion to prevent thrombosis and catheter occlusion in neonates with peripherally placed percutaneous central venous catheters. Cochrane Database Syst Rev 2008;(2):CD002772 10.1002/14651858.CD002772.pub3 [DOI] [PubMed] [Google Scholar]
- 3.Naranjo CA, Busto U, Sellers EM et al. . A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239–45. [DOI] [PubMed] [Google Scholar]
- 4.Gallagher RM, Kirkham JJ, Mason JR et al. . Development and inter-rater reliability of the Liverpool adverse drug reaction causality assessment tool. PLoS ONE 2011;6:e28096 10.1371/journal.pone.0028096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vodovar D, LeBeller C, Mégarbane B et al. . Drug Fever: a descriptive cohort study from the French national pharmacovigilance database. Drug Saf 2012;35:759–67. 10.2165/11630640-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 6.O'Brien SH, Kulkarni R, Wallace A et al. . Multicenter dose-finding and efficacy and safety outcomes in neonates and children treated with dalteparin for acute venous thromboembolism. J Thromb Haemost JTH 2014;12:1822–5. [DOI] [PubMed] [Google Scholar]
- 7.Clegg HW, Riopel DA. Furosemide-associated fever. J Pediatr 1995;126:817–8. [DOI] [PubMed] [Google Scholar]
- 8.Kelesidis T, Kelesidis I. Unexplained high fever in an elderly patient treated with clonidine, duloxetine, and atorvastatin. Clin Ther 2009;31:2894–9. 10.1016/j.clinthera.2009.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson DH, Cunha BA. Drug fever. Infect Dis Clin North Am 1996;10:85–91. [DOI] [PubMed] [Google Scholar]