Abstract
A 39-year-old man, born in India but resident in the UK for 10 years, was travelling in America when he became feverish with an altered mentation. He reported a 10-day history of fever, photophobia, headache and fatigue. His medical history included hypothyroidism and migraine. He was a non-smoker, did not consume alcohol and denied a history of drug use. He was transferred to the emergency department. Laboratory investigations confirmed hyponatraemia (sodium 128 mmol/L). A chest radiograph confirmed no focal consolidation. Further investigation with a CT brain was unremarkable. A lumbar puncture was suggestive of viral meningitis, with a raised white cell count, lymphocytosis, high protein and low glucose. His PCR was negative for enterovirus and herpes simplex virus. Further investigation with a CT thorax, abdomen and pelvis demonstrated bilateral upper-lobe infiltrations. A bronchoalveolar lavage was negative for acid alcohol fast bacilli (AAFB). A diagnosis of tuberculous meningitis was rendered following a repeat lumbar puncture. Gram stain revealed AAFB and PCR was also positive. He started antitubercular treatment and corticosteroids. A repeat CT brain demonstrated ventriculomegaly, suggestive of hydrocephalus and an MRI head revealed likely communicating hydrocephalus with basilar enhancement. He was repatriated to the UK. Eleven days post transfer, he became acutely confused and required external ventricular drain insertion. After surgical management of his hydrocephalus, there was no further neurological deterioration. He remains committed to his neurorehabilitation.
Background
Owing to increased immigration, tuberculous meningitis represents a serious problem, not only to developing countries endemic to tuberculosis, but also worldwide. According to the ‘Global Tuberculosis Report 2015’ published by the WHO, in 2014, tuberculosis (TB) for the first time surpassed HIV in total deaths per year with 1.5 million individuals dying from TB in 2014 (400 000 were HIV-positive). It has a high mortality, with 30% of patients dying despite antituberculous chemotherapy. Owing to non-specific symptoms, there is often a delay in initiating antitubercular treatment. Neurological deficits, including seizures, cognitive impairment, hydrocephalus and endocrinopathies, have been reported. The diagnosis is based on clinical features, cerebrospinal fluid (CSF) findings and imaging characteristics. The patient’s clinical stage at presentation is the most important prognostic factor. NICE guidelines recommend 12 months of antitubercular treatment and adjuvant corticosteroids. There is a paucity of research on TB meningitis and further randomised controlled trials are required. This paper discusses the clinical presentation, pathophysiology, investigations, complications and management of tubercular meningitis. This publication focuses on an important differential diagnosis in patients with lymphocytosis, high protein and low glucose in CSF. It is also an important clinical reminder to consider tuberculous meningitis in the differential diagnosis of a patient born in a country with a high endemic rate of TB, presenting with non-specific symptoms and recent travel to an endemic region.
Case presentation
A 39-year-old man, born in India but resident in the UK for 10 years, was travelling in the USA when he became feverish with an altered mentation. He reported a 10-day history of fevers (39°C), headache, photosensitivity and significant fatigue following his return from a trip to Dubai. His medical history included migraines and hypothyroidism. His medications included levothyroxine 75 μg. He was self-employed, a non-smoker, did not consume alcohol and denied taking drugs. In the emergency department, he was feverish (temperature 39°C) and tachycardic (heart rate 120). He denied chest pain or palpitations. No rash, bruises or petechiae were evident. Respiratory, cardiovascular and abdominal examination were unremarkable. Neurological examination confirmed normal power, tone and reflexes of his upper and lower limbs. His pupils were reactive to light. Extra-ocular eye movements were intact, although he reported some diplopia on downward gaze. No cerebellar signs, dysphasia or dysarthria were elicited.
Investigations
Laboratory studies revealed the following: WCCs 5.6×109/L, haemoglobin 107 g/L, platelets 163×109/L, neutrophils 89.5%, lymphocytes 3.1%, sodium 128 mmol/L, potassium 4 mmol/L and glucose 13.3 mmol/L. Blood cultures revealed no growth. A chest radiograph revealed no focal consolidation (figure 1) and his CT brain was unremarkable. His lumbar puncture results were consistent with viral meningitis: increased white cell counts (58/μL) with predominant lymphocytes, red blood cells (16/μL), high protein (2.09 g/L) and low glucose (1.3 mmol/L). Gram stain showed evidence of some white cells, lymphocytic predominant. PCR was negative for enterovirus, herpes simplex virus and acid alcohol fast bacilli (AAFB). HIV serology and Cryptococcus CSF antigen were negative. He was admitted to the intensive care unit (ICU). He remained feverish (temperature 39.2°C), and further investigation with a CT thorax, abdomen and pelvis identified bilateral upper-lobe infiltrates. A bronchoscopy with broncho-alveolar lavage (BAL) was negative for AAFB, thus making a diagnosis of active pulmonary TB unlikely. He denied sputum production or haemoptysis. His viral respiratory panel was positive for Coronavirus HKU1. A repeat lumbar puncture 5 days later showed persistent inflammatory components, WCC (14.3×109/L), lymphocyte predominant (62%), red blood cells (724/μL), low glucose (1.7 mmol/L) and elevated protein (4.13 g/L). CSF cytology showed evidence of AAFB on special stains, suggesting a diagnosis of TB. His Quantiferon Gold test was also positive for Mycobacterium tuberculosis.
Figure 1.
Chest radiograph. A chest radiograph demonstrated no focal consolidation.
Differential diagnosis
He was diagnosed with tuberculous meningitis, severe hyponatraemia, thought to be consistent with his pulmonary process and Coronavirus. Furthermore, a left trochlear nerve palsy was also noted and an eye patch aided his diplopia.
Treatment
He started antitubercular treatment in the USA with isoniazid 300 mg, rifampicin 600 mg, ethambutol 1200 g, pyrazinamide 1500 mg and pyridoxine 50 mg. He started 12 weeks of 60 mg of prednisolone as per NICE guidelines. His headache persisted and a repeat CT brain 10 days post admission showed development of ventriculomegaly suggestive of obstructive hydrocephalus (figure 2). Further investigation with an MRI head showed likely communicating hydrocephalus with basilar enhancement (figure 3). Dilation of the third, fourth and lateral ventricles were noted (figure 4). His serum sodium levels decreased over several days (119 mmol/L). As his neurological status remained unchanged, there was no indication for a ventriculoperitoneal shunt or an external ventricular drain. He continued treatment with decadron 10 mg intravenous twice daily for management of his basilar inflammation and raised intracranial pressure.
Figure 2.
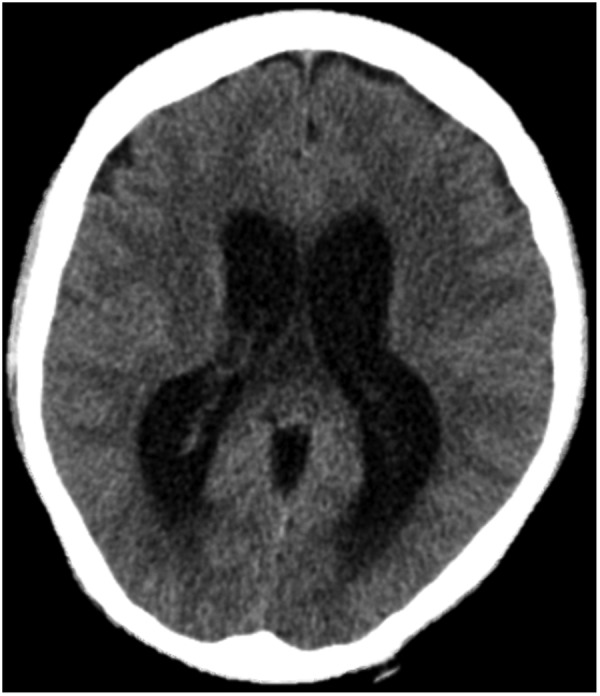
CT head. A CT head showed gross hydrocephalus.
Figure 3.
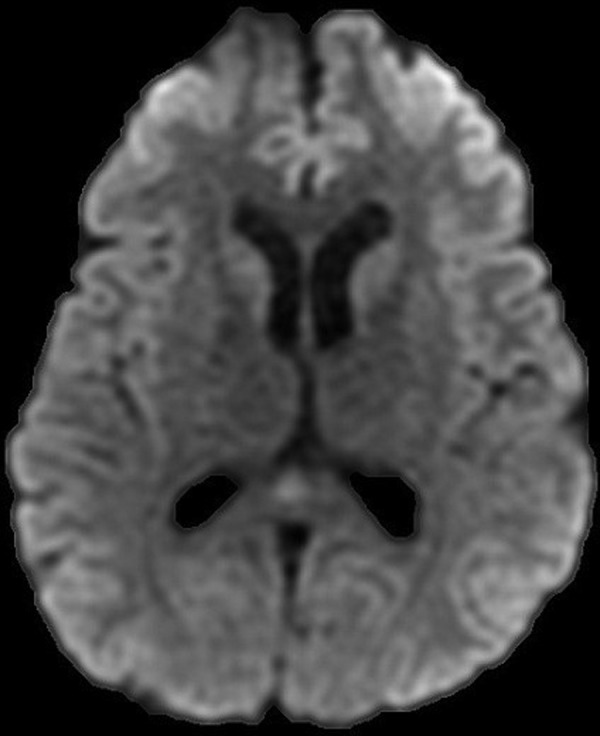
MRI head (DWI sequence). Further investigation with an MRI head demonstrated likely communicating hydrocephalus with basilar enhancement.
Figure 4.
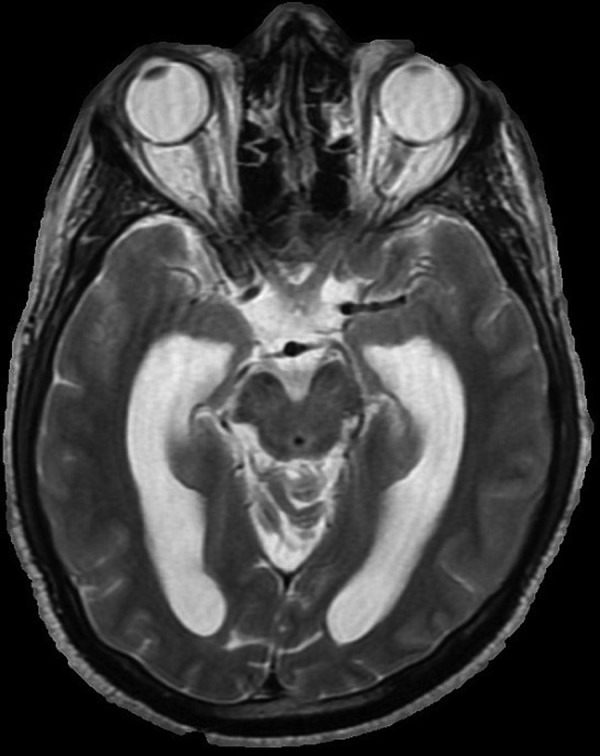
MRI head (AX T2 Image). Dilation of the third, fourth and lateral ventricles were noted.
Outcome and follow-up
He was repatriated to Milton Keynes University Hospital (MKH) on day 14 on his current antitubercular medications. A CT head (figure 5) and a non-gadolinium-enhanced MRI head 2 days later showed no interval change. A repeat lumbar puncture demonstrated macroscopic yellow turbid fluid. The total WCCs were 180/μL, red blood cells 25/μL, polymorphs 10% and lymphocytes 90%. No organisms were seen on Gram stain. There was no growth after 48 hours incubation. PCR (Genexpert) detected M. tuberculosis. Rifampicin resistance was not observed. On Ziehl Neelsen staining, no AAFB were seen. His four antituberculous medications were combined into a ‘4 in 1’ combination, Voractiv 5 tablets one time a day. His dexamethasone was increased to 6 mg four times a day. He was reviewed by ophthalmology and a resolving fourth cranial nerve palsy was noted. No choroidal tubercles, colour desaturation or nystagmus was observed. Diplopia was present in all positions, worse on the left. Eleven days post admission, he became acutely confused, with a GCS of 14/15 requiring ICU admission. A lumbar puncture showed an opening pressure of 34 cm CSF and a closing pressure of 13 cm CSF. Haematological investigations revealed: WCC 4.8×109/L, CRP <2 mg/L, sodium 123 mmol/L and potassium 3.6 mmol/L. His CT head showed abnormal dilatation of the ventricular system in keeping with hydrocephalus. His lumbar puncture demonstrated a WCC of 47 iu/L, an elevated total CSF protein 4.48 g/L (0.15–0.45) and lymphocytes 100%. No AAFB were isolated and there was no growth after 48 hours incubation. He was discussed with the Neurosurgical Team at Oxford and he was accepted for consideration of Ventriculo-Peritoneal shunt placement. Owing to the high protein content of his CSF and the risk of shunt blockage, an external ventricular drain was inserted. He was repatriated to MKH's Stroke Medicine Ward for further rehabilitation.
Figure 5.
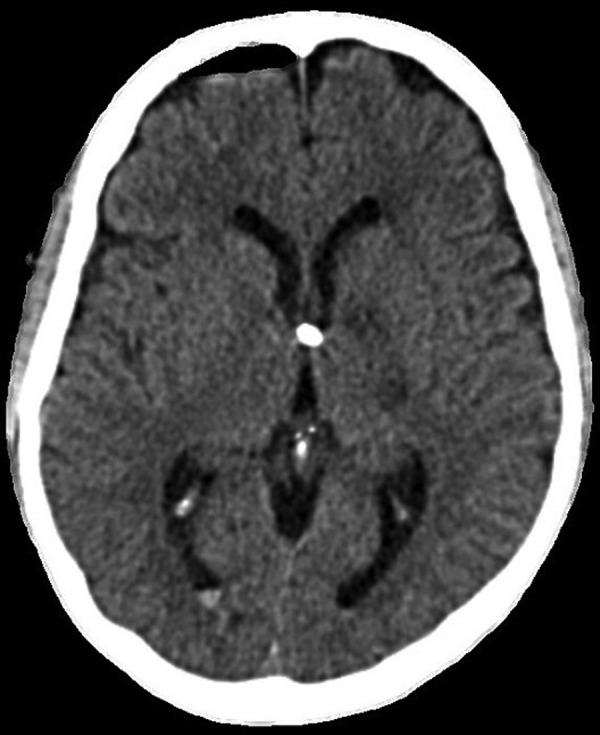
CT head. A CT head was performed, which demonstrated no interval change. Hydrocephalus was affecting all four ventricles. The sulci were effaced. There was no evidence of a surface collection or haemorrhage.
Discussion
TB remains one of the world's deadliest communicable diseases and according to the ‘Global Tuberculosis Report 2015’ published by the WHO, TB for the first time surpassed HIV in total deaths per year with 1.5 million individuals dying from TB in 2014 (400 000 were HIV-positive).1 TB continues to disproportionately affect the most deprived communities, with 70% of all TB cases coming from the 40% most deprived areas.2 Over the last decade, the proportion of cases with extrapulmonary disease has increased slightly from 40.9% to 47.9%. Central nervous system (CNS) TB includes three clinical categories: TB meningitis, intracranial tuberculoma and spinal tuberculous arachnoiditis.3 The aforementioned are encountered frequently in regions of the world where the incidence of TB is high and the prevalence of post primary dissemination is common among children and young adults. Rates of TB are highest in patients born in countries of high endemic rates, even after moving to areas with low endemic rates. Admittedly, this should have been a ‘red flag’ in the social history of our patient. According to the ‘Tuberculosis in the UK, 2014 Report’, there were 172 cases reported of CNS meningitis in 2013, demonstrating an increase in cases from the year 2000, when 94 cases were reported.4 Tuberculous meningitis causes significant morbidity and death or disabling residual neurological deficit may ensue. A history of recent TB contact should be sought as well as travel to TB endemic regions. Homelessness, drug and alcohol abuse, malnutrition and immunosuppression should raise the clinical index of suspicion. Patients with tuberculous meningitis typically present with a subacute febrile illness, with low-grade fever, headache, fatigue and myalgia lasting 2–8 weeks prior to the development of the meningitic phase, with more pronounced neurological features such as vomiting, confusion and neck stiffness. The average duration between symptom onset and clinical presentation is between 5 and 30 days. As the disease process progresses, the paralytic phase supervenes with confusion, myoclonic jerks and ataxia giving way to stupor, coma and seizures. Vision loss may occur due to an optic nerve granuloma, optochiasmatic arachnoiditis or third ventricular compression of the optic chiasm. About one-third of patients on presentation have underlying miliary TB. Careful funduscopy may reveal choroidal tubercles, offering a valuable clue to the aetiology. Tuberculomas, cerebral infarctions, hydrocephalus and arteritis result in the pathophysiological changes of cerebral oedema, meningeal irritation and raised intracranial pressure. Almost 150 years ago, in 1882, Robert Koch first described the tubercle bacillus during histopathological examination. TB meningitis is pathologically characterised by the presence of thick basilar exudates predominantly present around the Circle of Willis. As far back as 1933, Rich and McCordock, following their observational research on the pathogenesis of tubercular meningitis at the John Hopkins Hospital, suggested that CNS TB develops when small tuberculous lesions, otherwise known as Rich's foci, develop in the CNS, either during primary tuberculous infection or shortly afterwards. These tuberculous lesions may remain dormant for several years. Rupture of these small tuberculous lesions, perhaps by immunological mechanisms, into the subarachnoid space results in meningitis, with the acute form presenting with fever, headache, radiating root pains and myelopathy.5 Various diagnostic algorithms have been published to aid clinical diagnosis of TB meningitis, but they have not been validated for use in low TB prevalence settings. The British Medical Research Council classifies tuberculous meningitis into three stages based on its severity (table 1). Stage 1 (mild) describes patients with no focal neurological signs. Stage 2 patients exhibit moderate neurological deficits such as cranial nerve palsies or hemiparesis. Stage 3 represents comatose patients, those with delirium, stupor, seizures, multiple cranial nerve palsies, hemiplegia, paraplegia or both.6
Table 1.
MRC staging of tuberculous meningitis
| Stage 1 | Prodromal phase with no definite neurological symptoms |
| Stage 2 | Signs of meningeal irritation with slight or no clouding of sensorium and minor cranial nerve palsies, or no neurological deficit |
| Stage 3 | Severe clouding of sensorium, convulsions, focal neurological deficit and involuntary movements |
The British Medical Research Council classifies tuberculous meningitis into three stages based on its severity.
MRC, Medical Research Council.
The diagnosis of TBM can be challenging; maintaining a high degree of suspicion is vital in order to initiate antituberculous therapy promptly. The importance of repeated, careful, examination and culture of CSF specimens for Mycobacterium tuberculosis cannot be over emphasised. Definitive diagnosis of tuberculous meningitis depends on the detection of the tubercle bacilli in the CSF, either by smear examination or by bacterial culture in someone with symptoms or signs suggestive of the disease. Typically, the CSF shows an elevated protein level, a predominant lymphocytosis and a decreased glucose concentration7 (table 2). CSF protein ranges from 100 to 500 mg/dL in most patients. A minimum of three serial lumbar punctures, removing large volumes (10–15 mL), should be performed at daily intervals. In addition, it takes 6–8 weeks before the culture is positive for mycobacterium bacilli, thus adding to the diagnostic challenge. The intradermal tuberculin skin test is helpful when positive and where available, the GeneXpert assay, recommended by the World Health uses real-time PCR to amplify and detect Mycobacterium tuberculosis, should be used immediately in CSF samples of patients suspected of TB.8 This assay is an easy, rapid method that is sensitive (81%) and highly specific (98%) in initial studies performed in patients with pulmonary TB.8 Adenosine deaminase levels (ADA) are often raised in body fluids, including CSF, in patients with active TB.
Table 2.
The abnormalities seen in the cerebrospinal fluid in patients with meningitis
| Parameter | Normal | Acute bacterial | Viral | Tubercular |
|---|---|---|---|---|
| Opening pressure | <20 cm H2O | Increased | Normal/increased | Increased |
| Appearance | Clear | Cloudy, turbid, purulent | Clear | Clear/cloudy/yellow |
| Cells/mm3 | <5/mm3 | High—very high 1000–50 000 |
Normal—high | Increased 5–500 |
| Cell differential | 0 polymorphs | Neutrophils | Lymphocytes | Lymphocytes |
| Protein | <0.5 g/L | High>1 g/L | Normal—high 0.5–1 g/L | High—very high >1 g/L |
In recent years, CT and MRI have greatly improved characterisation and management. MRI can define the presence and extent of basilar arachnoiditis, hydrocephalus, infarcts, granulomas and exudates. Thick gelatinous exudates around the basal cisterns and sylvian fissures appear as intensely enhancing areas with a ‘spider-leg’ appearance.9 On neuroimaging, the ‘trapped temporal horn’ sign is suggestive of focal obstructive hydrocephalus at the foramen of Munro.10 The differential diagnosis of tuberculous meningitis includes cryptococcal meningitis and occasionally other deep-seated granulomatous fungal infections, brucellosis and neurosyphilis. Similar CSF findings may be exhibited in viral meningoencephalitis, cerebral malaria, parasitic meningitis and malignancy, such as lymphoma.
According to the NICE guidelines, patients with active meningeal TB should be offered a treatment regimen initially lasting 12 months, comprising isoniazid, pyrazinamide, rifampicin and a fourth drug, for example, ethambutol for the first 2 months, followed by isoniazid and rifampicin for the rest of the treatment period. The use of corticosteroids remains a controversial issue, but most authorities recommend adjunctive corticosteroids in stage 2 and stage 3 disease. The NICE guidelines advocate the use of a glucocorticoid; prednisolone 20–40 mg if on rifampicin, otherwise 10–20 mg, with a gradual withdrawal recommended 2–3 weeks after initiation. The response to steroids may be dramatic with rapid clearing of sensorium, regression of abnormalities of CSF and relief of headache.11 Recent studies have shown that steroids improve survival rate and neurological outcome.11 12
Furthermore, Mandell’s Principles and Practice of Infectious Diseases advocates corticosteroids in severely debilitated patients and those with marked constitutional symptoms, beginning with a daily 60–80 mg daily dose, tapered after 1–2 weeks and stopped by 4–6 weeks depending on symptoms. Prompt symptomatic improvement in fever, anaemia and hypoalbuminaemia is noted.13 A review of the Cochrane Database demonstrated 7 trials with 1140 participants establishing that adjunctive corticosteroids reduce death and disability by 30% in TB meningitis.14 A randomised trial, published in the New England Journal of Medicine, included 545 adolescents and adults with TB meningitis in Vietnam. A reduced mortality was evident in those with stage 1 and II disease who received dexamethasone.15 Schoeman noted reduced mortality in a randomised trial of 141 children with stage III disease who received prednisolone for the first month of treatment.16 Ethionamide and prothionamide, both thioamides, have proven efficacy in clinical studies in the management of TB meningitis and multidrug-resistant TB in adults and children.17
Sixty years ago, the Medical Research Council in their paper on ‘Streptomycin in Tuberculosis Trials’ published in the Lancet stated that the single most important determinant of outcome is the stage of tuberculous meningitis at which treatment is initiated; this remains true today.18 Hydrocephalus, described by Udani and Dastur in 1970, is one of the most common complications of CNS meningitis and it occurs in two types: obstructive and communicating.19 Obstructive hydrocephalus develops when the fourth ventricular outlets are blocked by the basal exudates and leptomeningeal inflammation, or when there is an obstruction in the aqueduct. Communicating hydrocephalus, which is much more frequent, develops when either there is overproduction of CSF or there is defective absorption of CSF in the subarachnoid space. The Vellore grading system is used to classify TBM and hydrocephalus and is a useful prognostic indicator. Mild to moderate hydrocephalus responds well to medical treatment with steroids, mannitol, furosemide and acetazolamide to reduce CSF production. However, ventriculo-peritoneal shunt insertion or a third ventriculostomy may be required to effectively manage raised intracranial pressure. Complications can occur, including shunt obstruction and infection, and further revision may be required. Endocrinopathies, including hyponatraemia, hypogonadism, Frohlich syndrome, diabetes insipidus and growth retardation, have been described.7
Admittedly, there is a paucity of research on TB meningitis in the UK and there are no randomised, controlled trials to establish the optimal drug combination. Standardised diagnostic criteria have not yet been established, thus making the comparison of research findings difficult. Understanding the quality of life after an illness improves our understanding of the disease. The ‘Brain Infections Group’, at the Institute of Infection and Global Health, are aiming to assess the applicability of the Vietnam algorithm in the UK population.20 Further randomised controlled trials are required to establish the best management. This case report reinforces the importance of considering tuberculous meningitis in the differential diagnosis of a patient presenting with non-specific symptoms, including fever and headache returning from a TB endemic region.
Learning points.
The cornerstone of diagnosis of tuberculous meningitis is examination of the cerebrospinal fluid (CSF). The cell count ranges from 0 to 1500/mm3, the protein is usually moderately elevated and the CSF glucose is said to be characteristically low. A lymphocytic predominance is usual.
Owing to the diagnostic challenge, clinicians must have the knowledge to recognise the clinical manifestations of tuberculous meningitis. Identifying tubercle bacilli requires a high index of clinical suspicion with examination of large volumes of CSF from repeated lumbar punctures. PCR is also helpful. CSF cultures take 6–8 weeks and are positive in less than two-thirds of cases.
According to the NICE guidelines, patients with active meningeal tuberculosis (TB) should be offered a treatment regimen initially lasting 12 months, comprising isoniazid, pyrazinamide, rifampicin and a fourth drug, for example, ethambutol for the first 2 months, followed by isoniazid and rifampicin for the rest of the treatment period. Adjunctive treatment with glucocorticoid therapy is recommended for all adults and children.
Ventricular shunting may be beneficial if symptomatic hydrocephalus supervenes.
Untreated TB is fatal. However, most of the world’s burden of TB is asymptomatic latent disease.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.WHO. Global tuberculosis report 2015. Switzerland: WHO Press. [Google Scholar]
- 2.Tuberculosis in the UK 2014 Report. Public Health England. Protecting and improving the Nation's Health. https://www.gov.uk/…/system/,,,/TB_Annual_report_4_0_300914.pdf
- 3.Christensen AS, Roed C, Omland LH et al. Long-term mortality in patients with tuberculous meningitis: a Danish nationwide cohort study. PLoS ONE 2011;6:e27900 10.1371/journal.pone.0027900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kruijshaar ME, Abubakar I. Increase in extrapulmonary tuberculosis in England and Wales 1999–2006. Thorax 2009;64:1090–5. 10.1136/thx.2009.118133 [DOI] [PubMed] [Google Scholar]
- 5.Jinkins JR, Gupta R, Chang KH et al. MR imaging of central nervous system tuberculosis. Radiol Clin North Am 1995;33:771–86. [PubMed] [Google Scholar]
- 6.MRC 1948 Medical Research Council Report. Streptomycin treatment of tuberculous meningitis. Lancet 1948;1:582–96. [PubMed] [Google Scholar]
- 7.Leonard JM, Des Prez RM. Tuberculous meningitis. Infect Dis Clin North Am 1990;4:769–87. [PubMed] [Google Scholar]
- 8.Helb D, Jones M, Story E et al. Rapid detection of Mycobacterium tuberculosis and rifampicin resistance by use of on-demand, near-patient technology. J Clin Microbiol 2010;48:229–37. 10.1128/JCM.01463-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahuja GK, Mohan KK, Prasad K et al. Diagnostic criteria for tuberculous meningitis and their validation. Tubercle Lung Dis 1994;75:149–52. 10.1016/0962-8479(94)90045-0 [DOI] [PubMed] [Google Scholar]
- 10.Berger JR. Tuberculous meningitis. Curr Opin Neurol 1994;7:191–200. 10.1097/00019052-199406000-00004 [DOI] [PubMed] [Google Scholar]
- 11.Holdiness MR. Management of tuberculous meningitis. Drugs 1990;39:224–33. 10.2165/00003495-199039020-00006 [DOI] [PubMed] [Google Scholar]
- 12.Kumarvelu S, Prasad K, Khosla A et al. Randomized controlled trial of dexamethasone in tuberculous meningitis. Tubercle Lung Dis 1994;75:203 10.1016/0962-8479(94)90009-4 [DOI] [PubMed] [Google Scholar]
- 13.Mandell, Douglas and Bennett's principles and practice of infectious diseases. In: Mycobacterium tuberculosis. 6th edn Vol 2 Churchill Livingstone, 2005:2853–86. [Google Scholar]
- 14.Prasad K, Singh MB. Corticosteroids for managing tuberculous meningitis. Cochrane Database Syst Rev 2008;(1):CD002244 10.1002/14651858.CD002244.pub3 [DOI] [PubMed] [Google Scholar]
- 15.Thwaites GE, Nguyen DB, Nguyen HD et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med 2004;351:1741–51. 10.1056/NEJMoa040573 [DOI] [PubMed] [Google Scholar]
- 16.Schoeman JF, Van Zyl LE, Laubscher JA et al. Effect of corticosteroids on intra cranial pressure, computed tomographic findings and clinical outcome in young children with tuberculous meningitis. Paediatrics 1997;99:226. [DOI] [PubMed] [Google Scholar]
- 17.Thee S, Garcia-Prats AJ, Donald PR et al. A review of the use of ethionamide and prothionamide in childhood tuberculosis. Tuberculosis (Edinb) 2016;97:126–36. 10.1016/j.tube.2015.09.007 [DOI] [PubMed] [Google Scholar]
- 18.Medical Research Council. Streptomycin in tuberculosis trials committee. Streptomycin treatment of tuberculous meningitis. Lancet 1948;i:582–96. [Google Scholar]
- 19.Udani PM, Dastur DK. Tuberculous encephalopathy with and without meningitis. Clinical features and pathological correlations. J Neurol Sci 1970;10:541–61. [DOI] [PubMed] [Google Scholar]
- 20.UK TB Meningitis Study. Institute of Infection and Global Health. The University of Liverpool. http://www.liv.ac.uk/infection-and-global-health/


