Abstract
Background
Several studies in rodents with complete spinal cord transections have demonstrated that treadmill training improves stepping movements. However, results from studies in incomplete spinal cord injured animals have been conflicting and questions regarding the training dosage after injury remain unresolved.
Objectives
To assess the effects of treadmill-training regimen (20 minutes daily, 5 days a week) for 3, 6 or 9 weeks on the recovery of locomotion in hemisected SCI mice.
Methods
A randomized and blinded controlled experimental trial used a mouse model of incomplete spinal cord injury (SCI). After a left hemisection at T10, adult male mice were randomized to trained or untrained groups. The trained group commenced treadmill training one week after surgery and continued for 3, 6 or 9 weeks. Quantitative kinematic gait analysis was used to assess the spatiotemporal characteristics of the left hindlimb prior to injury and at 1, 4, 7 and 10 weeks post-injury.
Results
One week after injury there was no movement of the left hindlimb and some animals dragged their foot. Treadmill training led to significant improvements in step duration, but had limited effect on the hindlimb movement pattern. Locomotor improvements in trained animals were most evident at the hip and knee joints whereas recovery of ankle movement was limited, even after 9 weeks of treadmill training.
Conclusion
These results demonstrate that treadmill training may lead to only modest improvement in recovery of hindlimb movement after incomplete spinal cord injury in mice.
Keywords: Spinal cord injury, Treadmill, Hemisection, Locomotion, Exercise
Introduction
The limited capacity of the adult mammalian CNS to regenerate after injury means that conditions such as traumatic SCI often result in severe and permanent locomotion deficits. Improvements in medical care after SCI have led to an increased proportion of patients living with incomplete SCIs.1 As these patients generally have some preservation of motor and sensory function, a degree of walking recovery, which is considered a high priority by people with SCI, is possible.2
Treadmill training is one type of intervention aimed at promoting and accelerating locomotor recovery following SCI. Treadmill training provides repetitive sensory input that activates and modulates spinal cord circuitry to generate rhythmic hindlimb movements, even in the absence of supraspinal input.3 Several studies in rodents with complete spinal transection show that treadmill training improves stepping movements.4,5 However, studies investigating the effects of treadmill training alone (i.e. without pharmacological or epidural stimulation) in animals with an incomplete SCI have produced conflicting results.6–8 This is important from a clinical perspective, as most SCI patients have incomplete lesions and show greater benefits from treadmill training compared to those with complete injuries.1,9,10
Questions regarding the most appropriate type and duration of training, remain unanswered.11 As clinical trials of locomotor training on SCI patients are extremely difficult (especially dose-response studies) preclinical investigations are necessary to further understand the benefits of locomotor training after SCI. Thus, the aim of this study was to investigate the effects of exercise training for 3, 6 and 9 weeks of recovery of locomotion in mice with incomplete SCI. We hypothesized that: (1) recovery of locomotion would be enhanced in treadmill trained compared to untrained mice and (2) 9 weeks of training would result the greatest improvement in locomotion.
Materials and methods
Experimental design
One week after an incomplete SCI, animals were randomly assigned to trained or untrained groups. Trained animals underwent 3, 6, or 9 weeks of treadmill training, while untrained animals remained in their home cages for the same duration as their trained counterparts. Videos of trained and untrained animals running on the treadmill were recorded pre-injury (week 0), one week post-injury (week 1) and then at 4, 7 and 10 weeks post-injury (i.e. end of each training period). Animals undergoing 6 weeks of treadmill training and their untrained comparison animals were also assessed at 4 weeks and animals on the 9 weeks training protocol at 4 and 7 weeks. Thus, there were more animals (and videos recorded) at the early time points of assessments. The higher mortality of animals on the 9 weeks protocol also contributed to the lower sample size in this group (Table 1).
Table 1.
Number of included animals
| Time of point assessment | Number of animals |
Total number of animals (trained, untrained) | ||
|---|---|---|---|---|
| 3 weeks training | 6 weeks training | 9 weeks training | ||
| Pre-injury (week 0) | 45 | 40 | 39 | 124 (60, 64) |
| Post-injury (week 1) | 41 | 37 | 33 | 111 (58, 53) |
| 4 weeks post-injury | 39 | 33 | 28 | 100 (54, 46) |
| 7 weeks post-injury | NA | 29 | 28 | 57 (27, 30) |
| 10 weeks post-injury | NA | NA | 26 | 26 (13,13) |
| Animal mortality | 13% | 27% | 33% | |
Spinal cord injury
All experiments were approved by the University of Newcastle Animal Care and Ethics Committee. Adult male C57BL/6 mice (9–10 weeks of age at surgery, bodyweight ∼20 g) underwent a lateral spinal cord hemisection between the T10 and T11 spinal roots, as described previously.12 Briefly, mice were anesthetized with isoflurane (5% induction, 1.5% to 2.5% maintenance) and medetomidine (0.03 mg/kg sc). The vertebral column was then exposed by a midline incision over T7 to L1 vertebrae. Fine forceps were used to remove the spinous processes and laminae of the T9 or T10 vertebra. An ophthalmic knife was then used to create a left hemisection at T10. The overlying muscle was then sutured and the skin incision sealed using staples. All procedures were conducted under aseptic conditions. Post-surgical analgesia was provided with buprenorphine (0.1 mg/kg sc, every 8 hours for 48 hours) and carprofen (5 mg/kg sc, every 24 hours for 5 days). Animals were closely observed for the next 24 hours for signs of pain or distress. The surgeon was blinded to experimental group allocation.
Treadmill training
A six-lane treadmill (Simple II, Columbus Instruments, OH, USA) was used to train the animals and to acquire kinematic data. For two weeks prior to surgery, all animals were familiarized with running on the treadmill at approximately 10 m/min for 10 mins, twice per day, five days per week. One week after SCI, animals were randomly assigned to treadmill training or untrained groups. The trained group then commenced the training regimen as before injury and continued for 3, 6 or 9 weeks (subgroups of animals were sacrificed at each time point for other investigations). Following injury, treadmill speed was initially 6 m/min, and gradually increased to 10–12 m/min over the course of training. Gentle tapping of the tail or hindlimb was used to encourage running if the animal stopped (body weight support was not provided during training or assessment).
Kinematic assessment
Animals were briefly anesthetized with 2% isoflurane and the skin over the lower back and left hindlimb was shaved. Hemispherical reflective markers (3 mm diameter) were attached over the iliac crest, head of femur, knee, ankle and 5th metatarsal (Figure 1). A high-speed video camera (100 frames/s; Nikon M3, Integrated Digital Technology, FL, USA) was used to capture movements of the left hindlimb in the sagittal plane. The camera was placed 1 m from the center of the treadmill with a field of view of approximately 32 cm × 24 cm (width × height). Several trials of the animal running on the treadmill (speed of 10 m/min pre-injury, 7 m/min 1 week post-injury and between 10–12 m/min at 4, 7 and 10 weeks assessment time points) were videotaped. Then a blinded assessor digitized three full-step cycles for each animal using the Peak Motus Motion Measurement System 9.2 software (Vicon Motion Systems, CO, USA). A full step cycle (swing and stance) was defined from toe-off to the next toe-off.
Figure 1.
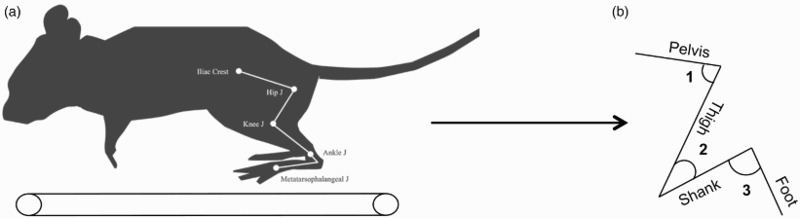
Kinematic data analysis. (A) Kinematics of the left hindlimb were analyzed from several step cycles of the animal walking on the treadmill with reflective markers placed on the iliac crest, hip, knee and ankle joints of the left hindlimb. Kinematic data analysis was performed using the reflective markers to generate the left hindlimb segments and joint angles. (B) Representation of the left hindlimb segments and joint angles. Hip angle (1), knee angle (2) and ankle angle (3).
Kinematic data analysis
Quantitative and qualitative kinematic gait outcomes of the left (affected) hindlimb were assessed in all animals. Videos were digitized and the raw coordinate dataset for each trial was imported into Igor Pro 6.0 software (WaveMetrics, Portland, OR) for analysis. The iliac crest, head of femur, knee, ankle and 5th metatarsal markers were used to measure joint angles, joint excursion (difference between the maximum and the minimum angle value within a step cycle), step duration (time taken to complete a full step cycle) and hip to toe maximum (the maximum distance that the animals brought the toe forward relative to the hip while stepping) (Figure 1).
Lesion extent measurements
After the survival periods of 4, 7 or 10 weeks post-surgery, the animals were re-anesthetised (100 mg/kg i.p. ketamine) and sacrificed by decapitation. Thereafter, the spinal cord was removed and sliced horizontally for electrophysiological recording. Horizontal spinal cord slices were prepared as previously described.13,14 The extent of the hemisection was measured on photos of the horizontal slices. According to Flynn et al.,14 lesion extent was defined as the distance between the medial apex of the lesion and the midline of the spinal cord. A value of 0 mm indicated that the lesion extended all the way to the midline of the cord. The lesion area that included the cavitation (i.e. missing tissue) and the glial/scar tissue was also measured. All the measurements were performed using Image J software.
Statistical analysis
Kinematic data between trained and untrained animals were compared using Analysis of Variance (ANOVA), with group and weeks as factors. Sheffé’s post-hoc test was used to determine which groups were significantly different from one another. Statistical analyses were performed using Data Desk, Version 6.3 software (Data Description Inc., NY, USA). All data are presented as means ± SEM (unless otherwise stated), and significance was set at p < 0.05.
Results
The lesion extent (distance between the medial apex of the lesion and the midline of the spinal cord) was similar between untrained and trained animals (0.21 ± 0.01 mm2 and 0.20 ± 0.02 mm2, respectively, p = 0.7). The lesion area (cavitation and missing tissue) was also similar between the two groups (0.31 ± 0.04 mm2 and 0.32 ± 0.02 mm2, respectively, p = 0.4). Thus, these data suggest that the hemisection lesion was similar between trained and untrained animals.
Gait pattern
The changes in joint angles of the hip, knee and ankle of the left (affected) hindlimb throughout a full step cycle at each assessment time point are shown in Figure 2. Prior to injury, the gait pattern was smooth, with a distinct change from swing to stance phase, particularly at the knee and ankle joints (Figure 2A). One week after SCI, all animals dragged their hindlimb, as indicated by the reduced excursions at all three joints and the decrease in the swing phase (Figure 2B). Changes in movement patterns after SCI were most evident at the knee and ankle (which were in an extended position) whereas there was comparatively little difference at the hip.
Figure 2.
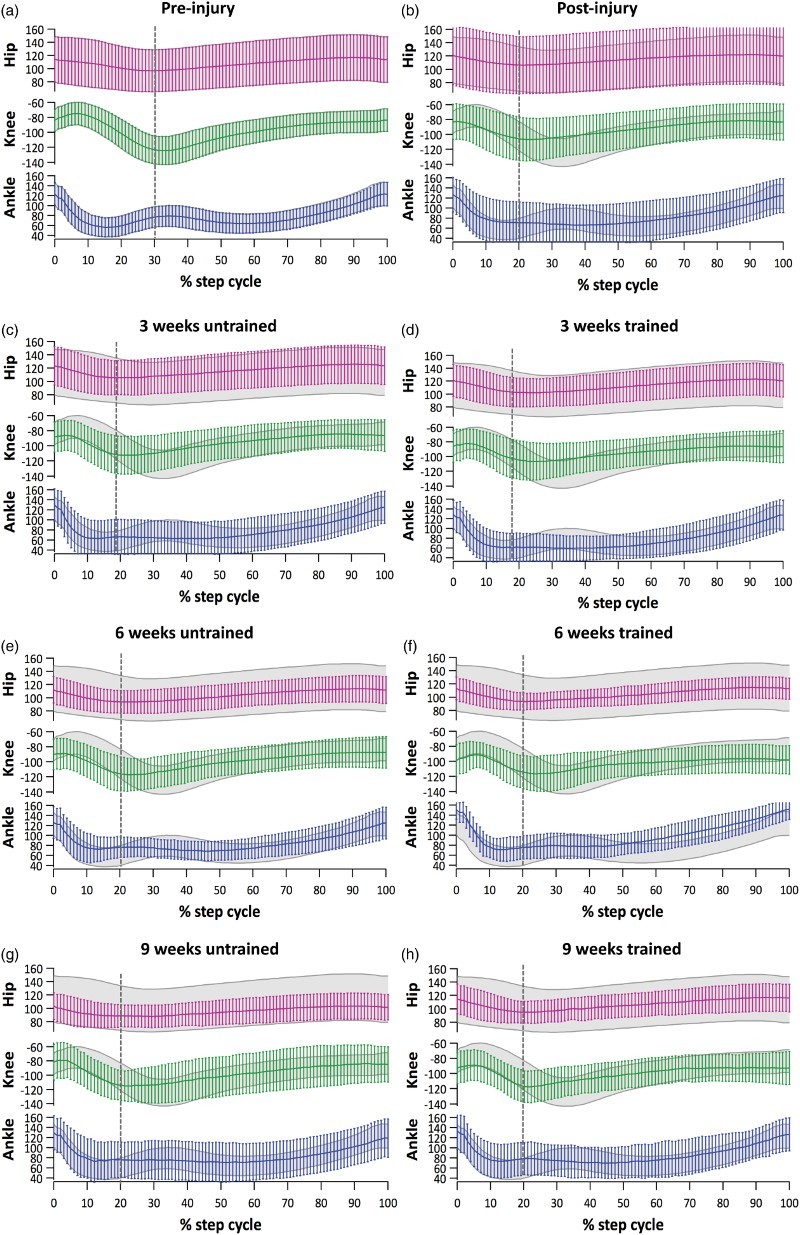
Kinematic analysis of gait cycle. (A) Average joint excursion during a complete normal step cycle of the left hindlimb (grey shadow) was used to compare data from untrained and 3, 6 and 9 weeks trained mice. A full step cycle was defined from toe off (beginning of swing phase) to the next toe off. The vertical dashed grey line indicates the end of swing phase (knee angle was used as a guide). (B) One week after SCI, animals dragged their hindlimb and had small joint excursions. The larger error bars observed at this time point may reflect hindlimb spasticity or step adaptations such as hopping. (C) At 4-weeks, untrained mice were not able to maintain their knee flexed during the swing phase. (D) At 4-weeks, the hip joint excursion of trained animals was similar to pre-injury whereas motion of knee and ankle joints remained limited. Results are expressed as mean (horizontal line) ± SD (vertical lines). Seven weeks after SCI, untrained (E) and trained (F) mice had joint angles that approached pre-injury values. However, untrained animals had smaller ankle angles compare to trained animals during toe off and swing. At 10-weeks, untrained (G) and trained (H) animals had gait a pattern that was similar to the pattern at the 7-weeks time point and that remained different to that of pre-injury. Results are expressed as mean (horizontal line) and ± SD (vertical lines).
At the 4-week assessment, both groups were able to flex the knee after toe off and during early swing phase, but only a small movement could be observed at this joint for the rest of the cycle. Trained animals did appear to maintain knee flexion for slightly longer during the swing phase (∼ 20% of step cycle vs. ∼ 10% of step cycle in the untrained group, Figure 2C and 2D). Motion at the ankle joint remained limited in both groups at 4 weeks.
At the 7-week assessment, both groups showed similar recovery of joint angles, although their gait patterns remained partially compromised compared to pre-injury (Figure 2E and 2F). Untrained animals had smaller ankle angles (mean ± SD = 125 ± 35°) compared to trained animals (mean ± SD = 150 ± 20°) during toe off and swing, suggesting that untrained animals maintained their ankle in dorsiflexion and were not weight-bearing throughout these phases.
At the 10-week assessment, both groups had hip and knee angle values similar to pre-injury (Figure 2G and 2H). The ankle angle pattern and values were different to those pre-injury, but similar between the two groups.
Joint excursions
In order to further investigate and quantify the differences in the knee and ankle angles observed in the gait pattern data, the excursions of these two joints were compared between groups at each time point. There was no significant difference between the mean knee and ankle excursion of trained and untrained animals in any time point of assessment (data not shown).
Step duration
Prior to injury, step duration was similar for both trained and untrained groups (Figure 3A). As expected, after injury animals exhibited considerably longer step duration than pre-injury values. Four and 7 weeks after injury, trained animals had significantly improved step duration compared to untrained. By week 10, both groups had step duration closer to pre-injury values and no significant difference was found between the two groups. These results suggest that treadmill training accelerated the recovery of step duration.
Figure 3.
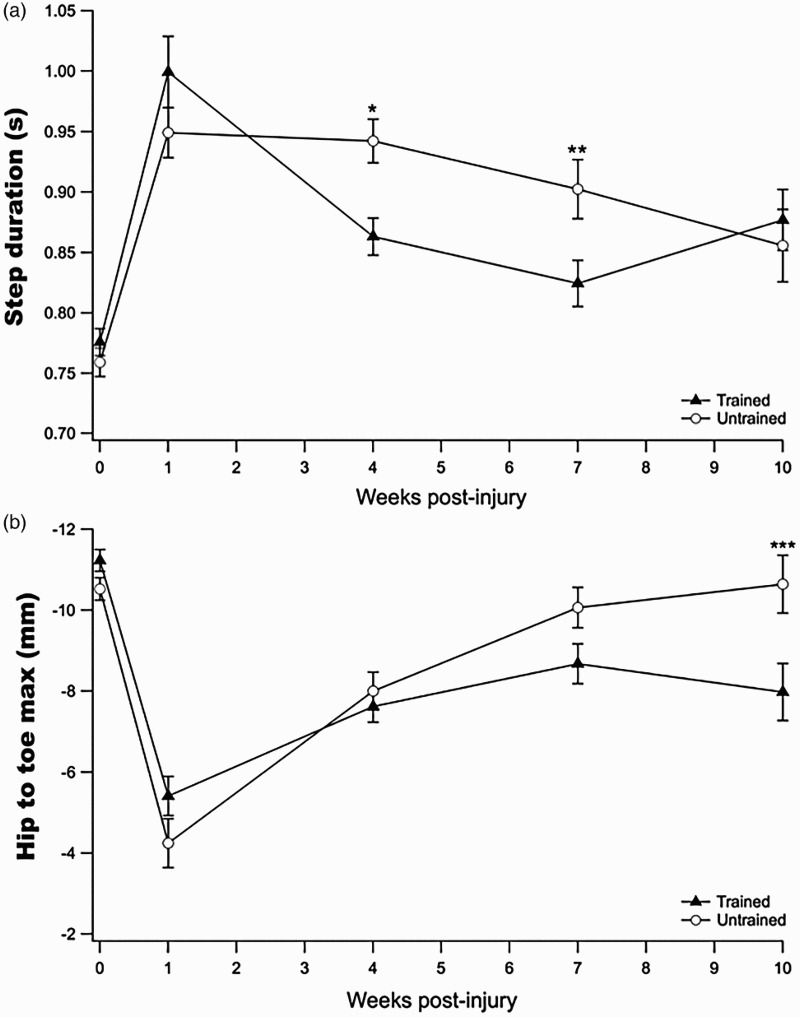
Kinematic gait outcomes. (A) Prior to injury (week 0) there was no difference in the step duration between untrained and trained. At 4-weeks there was a significant improvement in step duration for trained animals (* p = 0.002), which continued at week 7 post-injury (** p = 0.04). At 10-weeks post-injury, however, both animals had similar step duration values. (B) Hip to toe maximum (distance between the toe and hip in a step cycle) was the same for both groups prior to and after injury. No difference was found between groups at 4 and 7-weeks but at 10-weeks the mean hip to maximum of untrained animals was significantly different from trained animals (*** p = 0.05). Results are expressed as mean ± SEM.
Hip-to-toe maximum
The maximum distance that the animals brought the toe forward relative to the hip while stepping (hip-to-toe maximum) was used as an indicator of the degree to which the animals dragged their hindlimb following injury (Figure 3B). The mean hip-to-toe maximum was similar between trained and untrained animals prior to injury and after SCI, when their capacity to bring the toe forward decreased considerably. At the 4-week assessment, the hip-to-toe maximum was similar between the two groups. By the 7th week post-injury, the mean hip-to-toe maximum of the untrained animals tended to be closer to pre-injury values than trained animals (trained = −8.6 ± 0.5 mm and untrained = −10.0 ± 0.5 mm, p = 0.19). The difference between the two groups continued to increase so that by the 10-week assessment the difference was significant (trained = −7.9 ± 0.7 mm and untrained = −10.6 ± 0.7; p = 0.05).
Discussion
To our knowledge, this is the first study to assess the effects of treadmill-training alone for 3, 6 and 9 weeks on the recovery of locomotion in a mouse model of incomplete SCI. Because of the complexity of locomotor recovery, both qualitative and quantitative kinematic gait analysis were used to compare treadmill trained and untrained animals. Clinical trial design features such as randomization, blinding and allocation concealment were also employed to ensure that our data would be valid and unbiased. Our results show that treadmill training after incomplete SCI in mice had very limited effects on recovery of hindlimb movement, largely because the very substantial recovery of the untrained mice. Although the ability of both trained and untrained mice to swing their affected hindlimb was still compromised 10 weeks after SCI, there was some evidence of greater improvement in knee and ankle joints in treadmill trained compared to untrained mice. Treadmill training also accelerated the recovery of step duration.
In contrast to animals with complete SCI, the mechanism of recovery of locomotion in animals with incomplete SCI remains unclear. The normally symmetrical central pattern generator may be disorganized as a result of the loss of descending pathways on one side. Studies have shown that descending pathways are extremely important for spontaneous recovery after incomplete injuries15–17 and that treadmill training enhances this recovery by either promoting collateral sprouting of spared pathways and/or regeneration of damaged fibers.4,7,18 In addition, our group's electrophysiological investigations of spinal neurons from 3- and 6-weeks trained animals demonstrate that training enhances local synaptic activity in spinal cord interneurons and descending excitatory drive.12,14 The results of this study suggest such changes firstly occur in regions proximal to the lesion site as both trained and untrained groups initially exhibited improvements in hip movement followed by improvements in knee movement, whereas the ankle joint was still compromised seven weeks after injury. This pattern of proximal to distal recovery following SCI has been described previously.19 Goldshmit et al.7 found that hip movement improved most in treadmill-trained mice following spinal hemisection. The same proximal-distal motor recovery pattern has also been observed in other incomplete central nervous injuries such as stroke.20 Taken together, these findings support the notion that treadmill training improves hindlimb function by influencing plasticity in spinal circuitry and that recovery has both a temporal and spatial profile.
The present finding that treadmill trained mice had a significant acceleration in recovery of step duration despite no evidence of improved hindlimb kinematics may also reflect proximal-distal recovery. Trained animals had knee height values that were close to pre-injury values after 6 weeks of training. This suggests these animals had better knee control, which enabled them to swing the hindlimb even though they had limited ankle movement. Moreover, the fact that trained animals were not bringing their hindlimb forward to the same extent as pre-injury (hip-to-toe maximum data) suggests a shorter swing phase, which may also influence step duration. After SCI, the pathways involved in movement control (i.e. supra-spinal and intra-spinal circuitry) are changed considerably and this results in an altered or adapted motor output.21 It has been shown that the timing and proportion of swing and stance phases in SCI animals remain different from those of intact animals and that training can increase stance time, thus improving the ability of SCI animals to weight-bear on the affected limb.19,22 As shown in our study, the proportion of swing phase after SCI remained smaller compared to pre-injury (∼20% post-injury vs ∼30% pre-injury), however, treadmill training did not affect the proportion of swing/stance phase.
Our findings that the treadmill-training regimen used in this study provides limited benefit and is not sufficient to completely restore stepping function are in contrast to those found in studies in animals with complete SCI. Although the majority of studies in incomplete SCI animals report benefits of exercise training, 7,8,17,23–26 they vary considerably in the model of SCI, the intervention applied, and outcomes measured, making it difficult to compare results. Several of these studies used an incomplete SCI model similar to ours and reported a positive outcome. Goldshmit et al.7 reported that treadmill training for 5 weeks improved motor recovery in mice. It is important to note that, although those authors used a battery of tools to assess motor recovery, gait was only assessed qualitatively. In a recent study, quadrupedal treadmill training was shown to result in better hindlimb locomotion of hemisected SCI rats compared to when only the hindlimbs were trained.17 An earlier study found that 5 weeks of treadmill training in a rat model of dorsal hemisection did not lead to additional locomotor recovery.6 In cage voluntary wheel running has been shown to improve stepping in hemisected rats and that neurotrophins such as brain-derived neurotrophic factor, play an important role in motor recovery and plasticity following SCI.27, 28 These studies raise an important question about in-cage locomotor activity in incomplete SCI animal models. In complete SCI models, exercise training provides most of the locomotor stimulation, especially early after injury. In incomplete SCI models, in-cage activity levels are much higher and potentially provide a much greater “self-training” stimulus for recovery, and therefore limit the capacity of 20 min of structured treadmill training per day to affect the time course or extent of recovery. Much longer treadmill training sessions (or multiple training sessions per day) may be needed to have substantial impact when studying incomplete models of SCI. Kuerzi et al.37 also demonstrated a ceiling effect of training following incomplete SCI which the authors suggested was reflective of in-cage self-training to the extent that additional training became futile. The effect of in-cage activity on functional recovery after incomplete SCI has been assessed through hindlimb immobilization of contused SCI rats.29,30 In an elegant experiment, animals were placed in a wheelchair to immobilize their hindlimbs in an extended position for up to 18 hours a day for 8 weeks. The results demonstrate that wheelchair animals not only had worse locomotion compared to non-wheelchair animals, but also had significant deficits on gait kinematic analysis that persisted for weeks after wheelchair use was ceased, suggesting that hindlimb immobilization may have had a long-term effect on recovery. These data also suggest that in-cage self-training is an important potential explanation for the limited effect of training found in the present study, and is an important consideration for future studies.
There are several other factors such as duration, type and timing of training, which are key components of any training regimen and may also influence recovery. For example, training periods longer than 9 weeks may be required to further enhance behavioral recovery. This is supported by anatomical data from Bareyre et al.15 that demonstrate regenerating corticospinal synapses require up to 12 weeks to form appropriate connections with propriospinal neurons that bridge the lesion, allowing descending commands to engage spinal circuitry below the SCI.15 Importantly, behavioral recovery was still occurring 10 weeks after SCI in these animals. Another important factor that may influence recovery is whether body weight support is given during training. In this study, weight support was not provided, as in our hemisected animal model, less assistance is required during training compared to complete transection or contusion models of SCI. However, body weight support has been shown to play a crucial role in stepping recovery after complete SCI.5,31,32 Although the impact of body weight support is less clear in models of incomplete SCI, it is possible that the limited recovery found in this study may be related to the fact that body weight support was not provided during training.
With regard to the mechanism underlying behavioral recovery observed in this study, electrophysiological data from the same cohort of mice used in this work show that treadmill training for 3 and 6 weeks has little effect on the intrinsic properties of neurons surrounding the lesion site, but instead alters the synaptic properties of dorsal horn pathways (that include the corticospinal tract) and local interneuron connections.12,14 This suggests that treadmill training promotes functional recovery mainly through alteration of synaptic connections, rather than modification of intrinsic neuronal excitability. This also suggests an extended time-course may be required for clinically relevant functional recovery via treadmill training. However, as found in this study, long training periods may not be feasible for mouse models of SCI due to the effects of aging. Normal mice with a similar age to those included in this study (age at time of SCI: ∼ 2 months and end of experimental period: 5 months) are still considered young, but as SCI substantially accelerates aging processes, 33 our mice may have had degenerative changes found in aged mice (19 to 26 months old).33,34 Rats or other animal models of SCI may be more suitable for studies assessing behavioral changes after longer periods of treadmill training.
Although over-ground locomotion was not assessed, the limited ankle recovery observed in trained animals may be due to the lack of specificity of treadmill training. Task-specificity has emerged as an important aspect of motor re-learning after SCI. Spinal cats trained to stand develop motor patterns specific for standing and not for walking.35 Similarly, training to step on a treadmill does not necessarily lead to improvement in over-ground walking in people with SCI and appears to be a task insufficiently specific to result in functional locomotion.36 Swimming training and stepping in shallow water are other examples of task-specific training, with animals improving stepping in those environments but not in over-ground locomotion.37–39 Several studies have assessed the effect of different training paradigms that could potentially be superior to treadmill training or could be used as a bridging therapy between treadmill training and over-ground walking.40 Environmental enrichment (e.g. obstacles, ladders, stairs and etc.) may be an additional self-training paradigm, which takes advantage of the ability of incomplete SCI animals to move around their cages, providing potential additional benefits to recovery of over-ground stepping.
Limitations
One of the limitations of this study was the smaller sample size of animals in the 9-week training protocol. This was primarily due to the experimental sacrificing of animals at 4 and 7 weeks but was also because of the higher mortality of animals in the 9-week group. The higher mortality rate in this group may be due to urinary tract infection and other health problems (e.g. pulmonary embolism) that commonly affect animals with SCI in the first 3 months post-injury.41 These health issues can be difficult to detect and may not have been detected despite daily professional animal care. A second limitation of this study is the high variability in our data (reflected in the large error bars in Figure 2). Behavioral assessment of an SCI mouse model is challenging.42 The use of open-field testing enables a gross evaluation of motor function but it does not provide quantitative details on the gait pattern. For this reason we opted for kinematic gait analysis. However, small variations in marker placement and also marker movement may have contributed to the high variability in the present data. Some of these limitations in kinematic gait analysis have been addressed in the recent development of automated tracking markerless devices. For example, the MotorRater (TSE-Systems) system allows the evaluation of the overground walking kinematics and provides data on individual joint angles.43 More recently, a valid and sensitive automatic 3D kinematic measurement also using markerless motion tracking was developed specifically for mice with SCI. 44
Conclusions
The findings from this study demonstrate that 20 minutes of treadmill training five days a week has limited benefits in restoring normal hindlimb stepping after incomplete SCI in mice. Future pre-clinical SCI studies should carefully consider the type, intensity, duration of training sessions, training program duration, and in-cage activity levels, along with the SCI model. The data from this and previous studies have important clinical implications, suggesting that more than 9 weeks of treadmill training or a combination of different therapies, which require specific motor skills, may be vital to achieve better recovery following SCI.
Disclaimer statement
Conflict of interest There are no conflicts of interest to declare.
References
- 1.Devivo MJ. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord. 2012;50(5):365–72. doi: 10.1038/sc.2011.178 [DOI] [PubMed] [Google Scholar]
- 2.Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21(10):1371–83. doi: 10.1089/neu.2004.21.1371 [DOI] [PubMed] [Google Scholar]
- 3.Edgerton VR, Tillakaratne NJK, Bigbee AJ, de Leon RD, Roy RR. Plasticity of the spinal neural circuitry after injury. Annu Rev Neurosci. 2004;27:145–67. doi: 10.1146/annurev.neuro.27.070203.144308 [DOI] [PubMed] [Google Scholar]
- 4.Martinez M, Delivet-Mongrain H, Leblond H, Rossignol S. Effect of locomotor training in completely spinalized cats previously submitted to a spinal hemisection. J Neurosci. 2012;32(32):10961–70. doi: 10.1523/JNEUROSCI.1578-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Leon RD, Hodgson JA, Roy RR, Edgerton VR. Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. J Neurophysiol. 1998;79(3):1329–40. [DOI] [PubMed] [Google Scholar]
- 6.Fouad K, Metz GA, Merkler D, Dietz V, Schwab ME. Treadmill training in incomplete spinal cord injured rats. Behav Brain Res. 2000;115(1):107–13. doi: 10.1016/S0166-4328(00)00244-8 [DOI] [PubMed] [Google Scholar]
- 7.Goldshmit Y, Lythgo N, Galea MP, Turnley AM. Treadmill training after spinal cord hemisection in mice promotes axonal sprouting and synapse formation and improves motor recovery. J Neurotrauma. 2008;25(5):449–65. doi: 10.1089/neu.2007.0392 [DOI] [PubMed] [Google Scholar]
- 8.Heng C, de Leon RD. Treadmill training enhances the recovery of normal stepping patterns in spinal cord contused rats. Exp Neurol. 2009;216(1):139–47. doi: 10.1016/j.expneurol.2008.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobkin B, Apple D, Barbeau H, Basso M, Behrman A, Deforge D, et al. . Weight-supported treadmill vs over-ground training for walking after acute incomplete SCI. Neurology. 2006;66(4):484–93. doi: 10.1212/01.wnl.0000202600.72018.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Field-Fote EC, Roach KE. Influence of a locomotor training approach on walking speed and distance in people with chronic spinal cord injury: a randomized clinical trial. Phys Ther. 2011;91(1):48–60. doi: 10.2522/ptj.20090359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hillen BK, Abbas JJ, Jung R. Accelerating locomotor recovery after incomplete spinal injury. Ann N Y Acad Sci. 2013;1279:164–74. doi: 10.1111/nyas.12061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rank MM, Flynn JR, Battistuzzo CR, Galea MP, Callister R, Callister RJ. Functional changes in deep dorsal horn interneurons following spinal cord injury are enhanced with different durations of exercise training. J Physiol. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flynn JR, Brichta AM, Galea MP, Callister RJ, Graham BA. A horizontal slice preparation for examining the functional connectivity of dorsal column fibres in mouse spinal cord. J Neurosci Methods. 2011;200(2):113–20. doi: 10.1016/j.jneumeth.2011.06.017 [DOI] [PubMed] [Google Scholar]
- 14.Flynn JR, Dunn LR, Galea MP, Callister R, Callister RJ, Rank MM. Exercise training after spinal cord injury selectively alters synaptic properties in neurons in adult mouse spinal cord. J Neurotrauma. 2013;30(10):891–6. doi: 10.1089/neu.2012.2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nature Neurosci. 2004;7(3):269–77. doi: 10.1038/nn1195 [DOI] [PubMed] [Google Scholar]
- 16.Flynn JR, Graham BA, Galea MP, Callister RJ. The role of propriospinal interneurons in recovery from spinal cord injury. Neuropharmacoly. 2011;60(5):809–22. doi: 10.1016/j.neuropharm.2011.01.016 [DOI] [PubMed] [Google Scholar]
- 17.Shah PK, Garcia-Alias G, Choe J, Gad P, Gerasimenko Y, Tillakaratne N, et al. . Use of quadrupedal step training to re-engage spinal interneuronal networks and improve locomotor function after spinal cord injury. Brain. 2013;136:3362–77. doi: 10.1093/brain/awt265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh A, Balasubramanian S, Murray M, Lemay M, Houle J. Role of spared pathways in locomotor recovery after body-weight-supported treadmill training in contused rats. J Neurotrauma. 2011;28(12):2405–16. doi: 10.1089/neu.2010.1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thota A, Carlson S, Jung R. Recovery of locomotor function after treadmill training of incomplete spinal cord injured rats. Biomed Sci Instrum. 2001;37:63–7. [PubMed] [Google Scholar]
- 20.Colebatch JG, Gandevia SC. The distribution of muscular weakness in upper motor neuron lesions affecting the arm. Brain. 1989;112(Pt3):749–63. doi: 10.1093/brain/112.3.749 [DOI] [PubMed] [Google Scholar]
- 21.Edgerton VR, Leon RD, Harkema SJ, Hodgson JA, London N, Reinkensmeyer DJ, et al. . Retraining the injured spinal cord. J Physiol. 2001;533(1):15–22. doi: 10.1111/j.1469-7793.2001.0015b.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fong AJ, Cai LL, Otoshi CK, Reinkensmeyer DJ, Burdick JW, Roy RR, et al. . Spinal cord-transected mice learn to step in response to quipazine treatment and robotic training. J Neurosci. 2005;25(50):11738–47. doi: 10.1523/JNEUROSCI.1523-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez M, Delivet-Mongrain H, Leblond H, Rossignol S. Recovery of hindlimb locomotion after incomplete spinal cord injury in the cat involves spontaneous compensatory changes within the spinal locomotor circuitry. J Neurophysiol. 2011;106(4):1969–84. doi: 10.1152/jn.00368.2011 [DOI] [PubMed] [Google Scholar]
- 24.Martinez M, Delivet-Mongrain H, Rossignol S. Treadmill training promotes spinal changes leading to locomotor recovery after partial spinal cord injury in cats. J Neurophysiol. 2013;109(12):2909–22. doi: 10.1152/jn.01044.2012 [DOI] [PubMed] [Google Scholar]
- 25.Rossignol S, Chau C, Brustein E, Belanger M, Barbeau H, Drew T. Locomotor capacities after complete and partial lesions of the spinal cord. Acta Neurobiol Exp. 1996;56(1):449–63. [DOI] [PubMed] [Google Scholar]
- 26.Battistuzzo CR, Callister RJ, Callister R, Galea MP. A systematic review of exercise training to promote locomotor recovery in animal models of spinal cord injury. J Neurotrauma. 2012;29(8):1600–13. doi: 10.1089/neu.2011.2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ying Z, Roy RR, Edgerton VR, Gomez-Pinilla F. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp Neurology. 2005;193(2):411–9. doi: 10.1016/j.expneurol.2005.01.015 [DOI] [PubMed] [Google Scholar]
- 28.Ying Z, Roy RR, Zhong H, Zdunowski S, Edgerton VR, Gomez-Pinilla F. BDNF-exercise interactions in the recovery of symmetrical stepping after a cervical hemisection in rats. Neuroscience. 2008;155(4):1070–8. doi: 10.1016/j.neuroscience.2008.06.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caudle KL, Brown EH, Shum-Siu A, Burke DA, Magnuson TSG, Voor MJ, et al. . Hindlimb immobilization in a wheelchair alters functional recovery following contusive spinal cord injury in the adult rat. Neurorehab Neural Repair. 2011;25(8):729–39. doi: 10.1177/1545968311407519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Starkey ML, Bleul C, Maier IC, Schwab ME. Rehabilitative training following unilateral pyramidotomy in adult rats improves forelimb function in a non-task-specific way. Exp Neurol. 2011;232(1):81–9. doi: 10.1016/j.expneurol.2011.08.006 [DOI] [PubMed] [Google Scholar]
- 31.Timoszyk WK, Nessler JA, Acosta C, Roy RR, Edgerton VR, Reinkensmeyer DJ, et al. . Hindlimb loading determines stepping quantity and quality following spinal cord transection. Brain Res. 2005;1050(1–2):180–9. doi: 10.1016/j.brainres.2005.05.041 [DOI] [PubMed] [Google Scholar]
- 32.Ung RV, Lapointe NP, Rouleau P, Guertin PA. Non-assisted treadmill training does not improve motor recovery and body composition in spinal cord-transected mice. Spinal Cord. 2010;48(10):750–5. doi: 10.1038/sc.2010.19 [DOI] [PubMed] [Google Scholar]
- 33.Gorgey AS, Dolbow DR, Dolbow JD, Khalil RK, C. C, D.R. G . Effects of spinal cord injury on body composition and metabolic profile—Part I. J Spinal Cord Med. 2014; Jul 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433(7027):760–4. doi: 10.1038/nature03260 [DOI] [PubMed] [Google Scholar]
- 35.De Leon RD, Hodgson JA, Roy RR, Edgerton VR. Full weight-bearing hindlimb standing following stand training in the adult spinal cat. J Neurophysiol. 1998;80(1):83–91. [DOI] [PubMed] [Google Scholar]
- 36.Wessels M, Lucas C, Eriks I, de Groot S. Body weight-supported gait training for restoration of walking in people with an incomplete spinal cord injury: a systematic review. J Rehabil Med. 2010;42(6):513–9. doi: 10.2340/16501977-0525 [DOI] [PubMed] [Google Scholar]
- 37.Kuerzi J, Brown EH, Shum-Siu A, Siu A, Burke D, Morehouse J, et al. . Task-specificity vs. ceiling effect: step-training in shallow water after spinal cord injury. Exp Neurol. 2010;224(1):178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magnuson DSK, Smith RR, Brown EH, Enzmann G, Angeli C, Quesada PM, et al. . Swimming as a model of task-specific locomotor retraining after spinal cord injury in the rat. Neurorehab Neural Repair. 2009;23(6):535–45. doi: 10.1177/1545968308331147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith RR, Shum-Siu A, Baltzley R, Bunger M, Baldini A, Burke DA, et al. . Effects of swimming on functional recovery after incomplete spinal cord injury in rats. J Neurotrauma. 2006;23(6):908–19. doi: 10.1089/neu.2006.23.908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh A, Murray M, Houle JD. A training paradigm to enhance motor recovery in contused rats: effects of staircase training. Neurorehabil Neural Repair. 2011;25(1):24–34. doi: 10.1177/1545968310378510 [DOI] [PubMed] [Google Scholar]
- 41.Santos-Benito FF, Munoz-Quiles C, Ramon-Cueto A. Long-term care of paraplegic laboratory mammals. J Neurotrauma. 2006;23(3–4):521–36. doi: 10.1089/neu.2006.23.521 [DOI] [PubMed] [Google Scholar]
- 42.Basso DM. Behavioral testing after spinal cord injury: congruities, complexities, and controversies. J Neurotrauma. 2004;21(4):395–404. doi: 10.1089/089771504323004548 [DOI] [PubMed] [Google Scholar]
- 43.Zorner B, Filli L, Starkey ML, Gonzenbach R, Kasper H, Rothlisberger M, et al. . Profiling locomotor recovery: comprehensive quantification of impairments after CNS damage in rodents. Nature methods. 2010;7(9):701–8. doi: 10.1038/nmeth.1484 [DOI] [PubMed] [Google Scholar]
- 44.Sheets AL, Lai P-L, Fisher LC, Basso DM. Quantitative evaluation of 3D mouse behaviors and motor function in the open-field after spinal cord injury using markerless motion tracking. Plos One. 2013;8(9):e74536. doi: 10.1371/journal.pone.0074536 [DOI] [PMC free article] [PubMed] [Google Scholar]


