Abstract
Objective:
The aim of this study is to compare the discriminant function of multiple organ dysfunction score (MODS) and sequential organ failure assessment (SOFA) components in predicting the Intensive Care Unit (ICU) mortality and neurologic outcome.
Materials and Methods:
A descriptive–analytic study was conducted at a level I trauma center. Data were collected from patients with severe traumatic brain injury admitted to the neurosurgical ICU. Basic demographic data, SOFA and MOD scores were recorded daily for all patients. Odd's ratios (ORs) were calculated to determine the relationship of each component score to mortality, and area under receiver operating characteristic (AUROC) curve was used to compare the discriminative ability of two tools with respect to ICU mortality.
Results:
The most common organ failure observed was respiratory detected by SOFA of 26% and MODS of 13%, and the second common was cardiovascular detected by SOFA of 18% and MODS of 13%. No hepatic or renal failure occurred, and coagulation failure reported as 2.5% by SOFA and MODS. Cardiovascular failure defined by both tools had a correlation to ICU mortality and it was more significant for SOFA (OR = 6.9, CI = 3.6–13.3, P < 0.05 for SOFA; OR = 5, CI = 3–8.3, P < 0.05 for MODS; AUROC = 0.82 for SOFA; AUROC = 0.73 for MODS). The relationship of cardiovascular failure to dichotomized neurologic outcome was not significant statistically. ICU mortality was not associated with respiratory or coagulation failure.
Conclusion:
Cardiovascular failure defined by either tool significantly related to ICU mortality. Compared to MODS, SOFA-defined cardiovascular failure was a stronger predictor of death. ICU mortality was not affected by respiratory or coagulation failures.
Keywords: Multiple organ dysfunction syndrome, organ failure, sequential organ failure assessment, traumatic brain injury
Introduction
Multiple organ dysfunction syndrome (MODS) is the most common cause of death in patients admitted to Intensive Care Unit (ICU) settings.[1,2]
The development of MODS among patients with severe traumatic brain injury (TBI) is of significant importance. MODS could occur in this subtype of patients independent of any inflammation or infection. In addition to neurogenic injury which results in nonneurologic organ dysfunction, the procedure of treatment itself can lead to multiple organ damage. Catecholamine release following TBI might result in cardiopulmonary dysfunction.[3]
Neurocortical care involves using barbiturates and hypothermia to manage cerebral hypertension. This type of treatment affects the immune system and may cause different types of infection including pneumonia.[4,5]
SOFA and MODS are two of the most commonly used MOD scoring systems in ICU settings. Both predicting tools include six components, evaluating cardiovascular, renal, hepatic, respiratory, hematologic, and neurologic systems. The main difference between two tools is the cardiovascular part which is calculated based on pressure-adjusted heart rate in MODS and thus is independent of treatment. Cardiovascular section of SOFA is based on mean arterial pressure and dosage of inotrope used. Parameters applied to evaluate organ damages other than cardiovascular system are almost the same for both tools, differing in the range of parameter which classifies the score into dysfunction or failure.[6]
The aim of our study was to describe the scores of all five sections and to compare them component by component, and to determine the relationship of death and unfavorable neurologic outcome to each component score.
Materials and Methods
This descriptive–analytic study was conducted at a level I trauma center. All patients with severe TBI who admitted to neurosurgery ICU from November 2012 to December 2013 were enrolled. Severe TBI was defined as a TBI causing at least one of the followings: An initial resuscitated Glasgow Coma Scale (GCS) of 8 or less at admission; a postresuscitation GCS at presentation to the trauma center of 8 or less in the absence of any type of sedation; indication for intracranial pressure monitoring; or the presence of a clinical herniation syndrome as verified by a neurosurgeon.[7] Patients who were excluded from the study were patients with concomitant chest, abdominal and pelvic trauma resulted in vital organ damage, patients with previous history of vital organ involvement, and patients with unknown past medical history. APACHE II score was calculated for all patients during the first 24 h of admission. SOFA and MOD scores were collected every day for all patients based on original guidelines.[8,9]
For data analysis, we used SPSS for windows, Version 22.0 (Armonk, Ny:IBM Corp). Odd's ratio (OR) and Fisher's exact test were used. For both tools, the frequency of each component score was defined, and the relationship of each component score to outcome was calculated using OR. Contradictory results were reported. Glasgow outcome scale (GOS) was dichotomized into favorable (GOS: 4, 5) and unfavorable (GOS: 1, 2, 3). Areas under receiver operating characteristic curve (AUROC) were used to define the discriminant function of component scores in predicting the ICU mortality. All tests were two-sided, and P < 0.05 considered statistically significant.
Results
Of all patients, 83 patients were eligible to enter the study. The mean age of participants was 33 years and 78 of them were males. The mean ICU length of stay was 12 days. During the study, 23 patients (27.7%) died. Demographic data are shown in Table 1.
Table 1.
Basic characteristic features of the study population

The proportion of patients who had any type of nonneurologic organ failure based on SOFA and MOD scoring system is presented in Table 2. The percentage of patients who had cardiovascular and respiratory failure based on SOFA was more than that of MOD scoring system. In terms of detecting other organ failures, their performance was the same. Based on both tools, none of our patients had renal or hepatic failure. Table 3 demonstrates the relationship of ICU mortality to nonneurologic organ failure/dysfunction defined by component scores of SOFA and MODS. Of 83 patients, 85.5% had SOFA-based abnormal cardiovascular system, 78.8% known to have cardiovascular dysfunction and 21.1% categorized as patients with cardiovascular failure. All of those who had SOFA-based cardiovascular failure and 14.3% of those with SOFA-defined cardiovascular dysfunction have died. Similar results show the relationship between the MOD-defined cardiovascular failure and ICU mortality. The cardiovascular failure defined by both tools was significantly related to mortality (SOFA: OR = 6.9, 95% CI = 3.6–13.3, P < 0.05, MODS: OR = 5, 95% CI = 3.01–8.3, P < 0.05) and is presented in Figure 1, that relation is more significant about SOFA than MODS (AUROC curve 0.82 vs. 0.73). The cardiovascular failure defined by both tools presented as good predictors of mortality (discriminant function for SOFA = 32.6% and for MODS: 23/9%). There was no significant relation between other organ failures defined by tools to mortality.
Table 2.
Frequency of organ failure detected by multiple organ dysfunction syndrome and sequential organ failure assessment
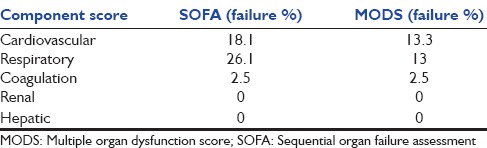
Table 3.
The relationship of organ dysfunction and failure defined by sequential organ failure assessment and multiple organ dysfunction syndrome with mortality
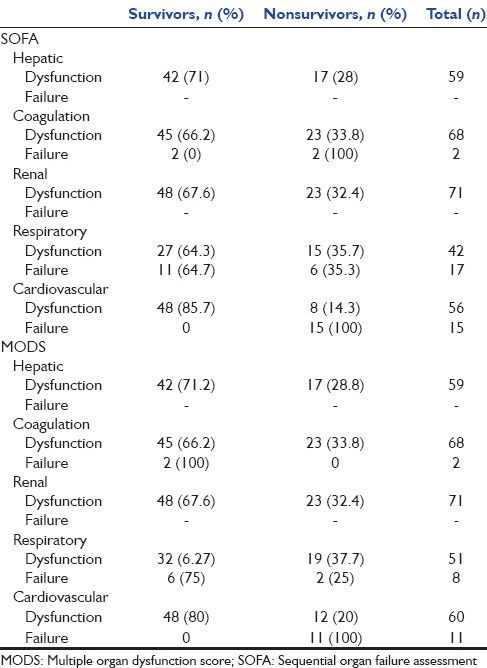
Figure 1.
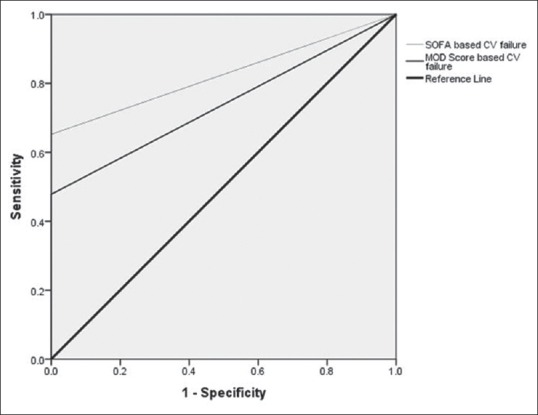
The relationship of cardiovascular score defined by multiple organ dysfunction score and sequential organ failure assessment to Intensive Care Unit mortality
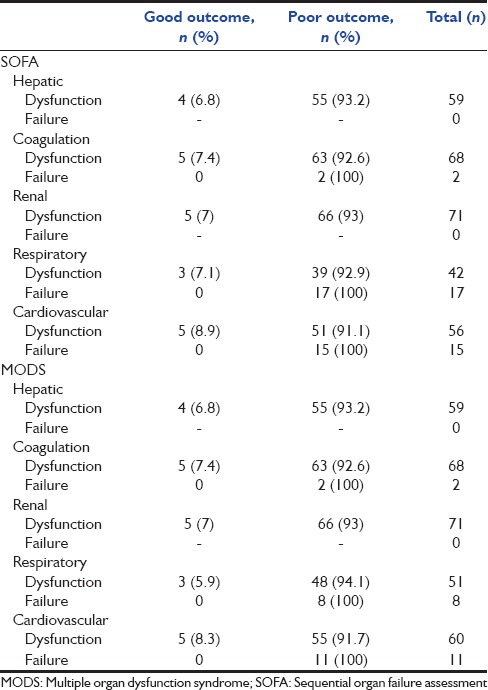
Although 100% of patients with cardiovascular failure defined by SOFA or MODS died, surprisingly there was no correlation between cardiovascular or respiratory failure and neurologic outcome [Table 4].
Table 4.
The relationship between organ dysfunction and failure defined by sequential organ failure assessment and multiple organ dysfunction syndrome with neurologic outcome
Discussion
Neurologic injury, as a pro-inflammatory state, could result in multiple organ failure. Organ failure deteriorates the neurologic outcome and increases the mortality independently.[10] Organ failure had a very high incidence among patients with brain injury and reported in literature up to 80%–90% of cases.[11]
In this study, 46.7% of patients developed a failure at least in one organ. None of the patients developed renal or hepatic failure and the incidence of hematologic failure was as low as 2.5%. The most common organ failure reported was cardiovascular and respiratory. The incidence of SOFA-defined respiratory failure was twice higher of the same component presented by MODS.
SOFA-based cardiovascular component, comparing to MODS, showed a stronger predictability of ICU death.
Zygun et al. described a population of 209 severe TBI patients with a single organ failure incidence of 35%. The most common reported was respiratory (23%), followed by cardiovascular (18%), coagulation (8%), and one case of renal failure with no cases of hepatic failure.[5]
A cohort study done by Ulvik et al. reported 28% of single organ failure in trauma patients admitted to ICU. The most common was respiratory failure (57%), followed by cardiovascular (3%), renal (2%), and hepatic failure (1%), defined by SOFA.[12]
Zygun et al. conducted a trial on patients with severe head trauma or SAH to define the proportion of patients with subsequent nonneurologic organ failure. SOFA was calculated retrospectively for 55 patients with severe TBI. They reported 82% and 80% of cardiac and respiratory failure, respectively. No renal or hepatic failure developed and three cases defined as having hematologic failure.[13]
Based on Zygun et al.‘s trial, study on patients with severe TBI revealed nonsignificant incidence of hepatic, renal, or hematologic failure, but 56% and 18% of SOFA and MODS defined cardiovascular failure.[7] They reported 43% of respiratory failure detected by SOFA and 23% by MODS.[9]
As SOFA cardiovascular score rises due to more inotrope use, the component score is going toward to be classified as failure. Inotrope use in patients with severe TBI has different indications. Ordering sedatives in ICU setting is very common for the management of intracranial hypertension and mechanical ventilation.[14,15] All types of sedatives may cause the patients to get hemodynamically unstable and for maintaining blood pressure inotropes are used. In this subtype of patients, as well as management of intracranial hypertension, trying to maintain the cerebral perfusion pressure above 60 mmHg is consequential. Treatment of vasospasms and prevention of secondary ischemia are achieved by inotropes, vasopressors, and volume expanders.
Inotrope use is also recommended by the current guidelines for short-term management of cardiac decompensation.[16] Myocardial dysfunction is a known result of neurologic insult. Different neurogenic pathways such as sympathetic overactivity hypothesized to play a role.[17,18]
The score of SOFA cardiovascular component presents the state of cardiac failure with the use of inotropes, but without considering the indication. Thus, differentiating between a case with low cardiac ejection fraction and a case with normal cardiac ejection fraction but low cerebral perfusion pressure or cerebral vasospasm who is also in need of inotropes is not possible.[19] Based on using pressure-adjusted heart rate, a treatment independent indicator by MODS, it seems to be advantageous over SOFA cardiovascular component.[6,20]
Because SOFA takes into account not only patients with cardiac failure but also patients with cerebrovascular indications for inotrope use, it is expected to detect more patients than MODS as having cardiovascular failure. As presented in Table 2, in this study, 18% of patients were detected by SOFA as having cardiovascular failure and 15% were detected by MODS. Similar to other studies, respiratory failure reported by SOFA is about twice of that reported by MODS. It is consistent with other studies and could be explained by various cutoff values used by SOFA and MODS.[9]
In this trial, cardiovascular component score of both tools represented as significant predictors of death. Patients with SOFA- and MODS-defined cardiovascular failure had a 6.9- and 5-fold increase in mortality, respectively. In comparison between two tools, AUROC curve gave a good discrimination ability for SOFA but just acceptable for MODS (0.82 vs. 0.73). Unexpectedly, the relation of cardiovascular failure with neurologic outcome was out of any statistical significance.
Although we found differences in proportion of patients with respiratory failure defined by SOFA and MODS, no statistically significant correlation was found between respiratory failure and mortality, neither between respiratory failure and neurologic outcome. Zygun et al. found no correlation between mortality of patients with head injury or SAH and organ dysfunction on admission or during the days of ICU stay. They studied population of 55 patients with closed head injury or SAH, evaluated by modified SOFA for organ dysfunction. This was the first work of this group.[20]
Peres Bota et al. found no differences between MODS and SOFA in the prediction of mortality of ICU patient and both tools were introduced as reliable predictors of mortality. Cardiovascular component of SOFA has shown to be superior in terms of discriminating the mortality (AUROC = 0.82 for SOFA, AUROC = 75 for MODS).[1]
Holland et al. conducted a prospective 4-year study on patients with head trauma. Acute lung injury developed in 31% of patients with severe TBI. Thirty-eight percent of those with ALI and 15% of those without ALI died. The correlation between developing ALI and mortality was significant. ALI presented as an independent risk factor for mortality and long-term neurologic outcome.[21] Gonzalvo et al. reported similar results.[19]
Corral et al. enrolled 224 patients in a cohort trial to study the impact of nonneurologic complications on mortality. They reported 44% of hypotension, 41% of respiratory failure, and 8% of AKI. Independent of neurologic status, a 2-fold increase in mortality was observed in patients with AKI. Hypotension had an effect only on mortality of patients with initial GCS of 3–5. Similar to our results, they reported no association between respiratory failure and mortality.[22]
As well as treatment-related side effects, neurogenic insult is the main cause of the development of nonneurologic organ dysfunction.
Hypothermia used in neurocortical care for decreasing the ICP and prevention of secondary brain injury improves neurological outcome and decreases the mortality rate.[15] Many trials have shown hypothermia to associate with increased rates of infection and pneumonia.[23] Barbiturates applied for prevention of posttraumatic seizures, a common and potentially fatal complication of head injury, increase the rate of pneumonia by immunosuppression.[24] Although the pathophysiology was unclear, recent trials proposed interaction mechanisms that could explain neurogenic induced organ failure.[22] Respiratory failure followed by severe TBI is not only the result of sympathetic hyperactivity and increased vasoconstriction and permeability but also neuroinflammatory cascades leading by cytokines and neutrophil aggregation and dysregulated endothelial function, also known to play a part in the development of cardiopulmonary failure.[25] Myocardial dysfunction, neurogenic pulmonary edema, and DIC are also introduced as a consequence of high levels of catecholamine release and the toxic adrenergic effect following neurogenic insult.[26,27] In this study, only cardiovascular failure defined by SOFA and MODS elucidated a significant relation to mortality. Sample size of study population and patient selection only from an ICU of one center is a limitation of this study.
Conclusion
High incidence of nonneurologic organ failure in patients with head injury necessitates application of prognostic scoring systems with good discrimination and calibration with all components. Routine use of these systems helps clinicians to guide their treatment based on prognosis and most importantly can provide guidance for prevention, and in equivocal cases serve as a guide to limit the implementation of life support services.[28]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Peres Bota D, Melot C, Lopes Ferreira F, Nguyen Ba V, Vincent JL. The multiple organ dysfunction score (MODS) versus the sequential organ failure assessment (SOFA) score in outcome prediction. Intensive Care Med. 2002;28:1619–24. doi: 10.1007/s00134-002-1491-3. [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Ma S. The cytokine storm and factors determining the sequence and severity of organ dysfunction in multiple organ dysfunction syndrome. Am J Emerg Med. 2008;26:711–5. doi: 10.1016/j.ajem.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 3.Gadkary CS, Alderson P, Signorini DF. Therapeutic hypothermia for head injury. Cochrane Database Syst Rev. 2002;1:CD001048. doi: 10.1002/14651858.CD001048. [DOI] [PubMed] [Google Scholar]
- 4.Lim HB, Smith M. Systemic complications after head injury: A clinical review. Anaesthesia. 2007;62:474–82. doi: 10.1111/j.1365-2044.2007.04998.x. [DOI] [PubMed] [Google Scholar]
- 5.Zygun DA, Kortbeek JB, Fick GH, Laupland KB, Doig CJ. Non-neurologic organ dysfunction in severe traumatic brain injury. Crit Care Med. 2005;33:654–60. doi: 10.1097/01.ccm.0000155911.01844.54. [DOI] [PubMed] [Google Scholar]
- 6.Khwannimit B. A comparison of three organ dysfunction scores: MODS, SOFA and LOD for predicting ICU mortality in critically ill patients. J Med Assoc Thai. 2007;90:1074–81. [PubMed] [Google Scholar]
- 7.Zygun D, Berthiaume L, Laupland K, Kortbeek J, Doig C. SOFA is superior to MOD score for the determination of non-neurologic organ dysfunction in patients with severe traumatic brain injury: A cohort study. Crit Care. 2006;10:R115. doi: 10.1186/cc5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marshall J. In: Multiple organ dysfunction syndrome. Clinical Trials for the Treatment of Sepsis. 2nd ed. Sibbald WJ, Vincent JL, editors. Berlin: Springer-Verlag Press; 2012. pp. 122–39. [Google Scholar]
- 9.Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in Intensive Care Units: Results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European society of intensive care medicine. Crit Care Med. 1998;26:1793–800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Berthiaume L, Zygun D. Non-neurologic organ dysfunction in acute brain injury. Crit Care Clin. 2006;22:753–66. doi: 10.1016/j.ccc.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Zygun D. Non-neurological organ dysfunction in neurocritical care: Impact on outcome and etiological considerations. Curr Opin Crit Care. 2005;11:139–43. doi: 10.1097/01.ccx.0000155356.86241.c0. [DOI] [PubMed] [Google Scholar]
- 12.Ulvik A, Kvåle R, Wentzel-Larsen T, Flaatten H. Multiple organ failure after trauma affects even long-term survival and functional status. Crit Care. 2007;11:R95. doi: 10.1186/cc6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zygun DA, Doig CJ, Gupta AK, Whiting G, Nicholas C, Shepherd E, et al. Non-neurological organ dysfunction in neurocritical care. J Crit Care. 2003;18:238–44. doi: 10.1016/j.jcrc.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Haddad SH, Arabi YM. Critical care management of severe traumatic brain injury in adults. Scand J Trauma Resusc Emerg Med. 2012;20:12. doi: 10.1186/1757-7241-20-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts DJ, Hall RI, Kramer AH, Robertson HL, Gallagher CN, Zygun DA. Sedation for critically ill adults with severe traumatic brain injury: A systematic review of randomized controlled trials. Crit Care Med. 2011;39:2743–51. doi: 10.1097/CCM.0b013e318228236f. [DOI] [PubMed] [Google Scholar]
- 16.Hasenfuss G, Teerlink JR. Cardiac inotropes: Current agents and future directions. Eur Heart J. 2011;32:1838–45. doi: 10.1093/eurheartj/ehr026. [DOI] [PubMed] [Google Scholar]
- 17.Cotton BA, Snodgrass KB, Fleming SB, Carpenter RO, Kemp CD, Arbogast PG, et al. Beta-blocker exposure is associated with improved survival after severe traumatic brain injury. J Trauma. 2007;62:26–33. doi: 10.1097/TA.0b013e31802d02d0. [DOI] [PubMed] [Google Scholar]
- 18.Dujardin KS, McCully RB, Wijdicks EF, Tazelaar HD, Seward JB, McGregor CG, et al. Myocardial dysfunction associated with brain death: Clinical, echocardiographic, and pathologic features. J Heart Lung Transplant. 2001;20:350–7. doi: 10.1016/s1053-2498(00)00193-5. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalvo R, Martí-Sistac O, Blanch L, López-Aguilar J. Bench-to-bedside review: Brain-lung interaction in the critically ill - a pending issue revisited. Crit Care. 2007;11:216. doi: 10.1186/cc5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diringer MN, Axelrod Y. Hemodynamic manipulation in the neuro-Intensive Care Unit: Cerebral perfusion pressure therapy in head injury and hemodynamic augmentation for cerebral vasospasm. Curr Opin Crit Care. 2007;13:156–62. doi: 10.1097/MCC.0b013e32807f2aa5. [DOI] [PubMed] [Google Scholar]
- 21.Holland MC, Mackersie RC, Morabito D, Campbell AR, Kivett VA, Patel R, et al. The development of acute lung injury is associated with worse neurologic outcome in patients with severe traumatic brain injury. J Trauma. 2003;55:106–11. doi: 10.1097/01.TA.0000071620.27375.BE. [DOI] [PubMed] [Google Scholar]
- 22.Corral L, Javierre CF, Ventura JL, Marcos P, Herrero JI, Mañez R. Impact of non-neurological complications in severe traumatic brain injury outcome. J Crit Care. 2012;16:R44. doi: 10.1186/cc11243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henderson WR, Dhingra VK, Chittock DR, Fenwick JC, Ronco JJ. Hypothermia in the management of traumatic brain injury. A systematic review and meta-analysis. Intensive Care Med. 2003;29:1637–44. doi: 10.1007/s00134-003-1848-2. [DOI] [PubMed] [Google Scholar]
- 24.Bronchard R, Albaladejo P, Brezac G, Geffroy A, Seince PF, Morris W, et al. Early onset pneumonia: Risk factors and consequences in head trauma patients. Anesthesiology. 2004;100:234–9. doi: 10.1097/00000542-200402000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Mascia L. Acute lung injury in patients with severe brain injury: A double hit model. Neurocrit Care. 2009;11:417–26. doi: 10.1007/s12028-009-9242-8. [DOI] [PubMed] [Google Scholar]
- 26.Larson BE, Stockwell DW, Boas S, Andrews T, Wellman GC, Lockette W, et al. Cardiac reactive oxygen species after traumatic brain injury. J Surg Res. 2012;173:e73–81. doi: 10.1016/j.jss.2011.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ley EJ, Berry C, Mirocha J, Salim A. Mortality is reduced for heart rate 80 to 89 after traumatic brain injury. J Surg Res. 2010;163:142–5. doi: 10.1016/j.jss.2010.04.046. [DOI] [PubMed] [Google Scholar]
- 28.Vincent JL, Moreno R. Clinical review: Scoring systems in the critically ill. J Crit Care. 2010;14:207. doi: 10.1186/cc8204. [DOI] [PMC free article] [PubMed] [Google Scholar]


