To the Editor
The use of model antigens such as ovalbumin (OVA) has been extremely beneficial to the field of immunology, and the availability of transgenic mice that have a T cell repertoire with a defined specificity has provided an important tool with which to study the selection, activation, motility and memory responses of T cells. One example of this is a transgenic strain of mice with CD8+ T cells specific for the OVA peptide of amino acids 257–264 (SIINFEKL) in the context of H-2Kb (ref. 1). These SIINFEKL-specific CD8+ T cells, commonly referred to as ‘OT-I T cells’, have a T cell antigen receptor that consists of α-chain variable region 2 (Vα2) and β-chain variable region 5 (Vβ5), which are inherited via a single transgene1. These have been used for studies of thymic selection2, autoimmunity and tolerance3 and vaccine-induced T cell responses4, and in situations in which pathogens or tumor cells have been engineered to express OVA, they can be used as surrogates of endogenous T cell responses5-7. Consequently, OT-I mice have become widely used, and there are more than 1,700 publications that cite the original description of these mice.
One practical consideration of maintaining the OT-I mouse strain is that the transgene should be inherited as a whole, and therefore genotyping through the use of flow cytometry to detect either the Vα2 chain or the Vβ5 chain provides an efficient and cost-effective alternative to the use of major histocompatibility complex (MHC) class I tetramers. However, our collective experience indicates that reliance solely on this approach can have substantial drawbacks. For example, here we provide a comparison of a typical stain using specific tetramers to detect the OT-I T cells (Fig. 1a) versus staining of Vα2, which is routinely used in the laboratory of C.A.H. (Fig. 1b). When the CD8+ T cells from the second mouse (Fig. 1b) were stained with the specific MHC class I tetramer, they failed to bind this tetramer (Fig. 1c), and when used in an experimental situation, they failed to respond in vivo to immunization of the mouse with OVA (data not shown). Thus, unlike true OT-I cells, which bind to a SIINFEKL-loaded MHC class I tetramer, these Vα2+ cells could not be detected through the use of this tetramer and did not respond to OVA. This unanticipated result led to re-screening of our colonies (those of G.H.P. and C.A.H.) and the recognition that there was loss of the Vβ5 chain in many of the progeny. Whether this loss was a result of lack of expression of the transgene or deletion of the transgene is unclear. In addition, exclusion of the Vα2 chain has occurred independently in several of our laboratories (those of M.S., S.C.J. and K.A.H.), as well as another laboratory (F. Carbone (University of Melbourne), personal communication).
Figure 1.
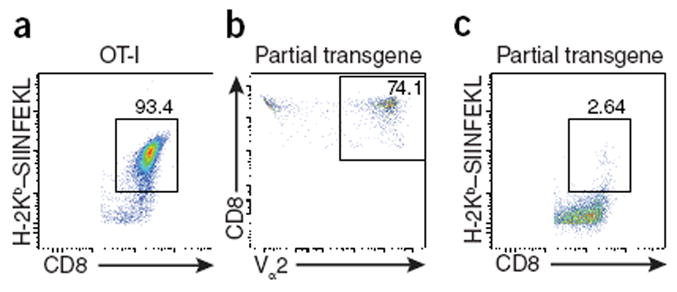
Mice can inherit Vα2 but not recognize SIINFEKL. (a) Flow cytometry analyzing binding of the H-2Kb–SIINFEKL tetramer by CD45.2+B220–CD8+ cells in blood from a ‘true’ OT-I mouse. (b) Flow cytometry analyzing Vα2 staining on CD8+ cells in blood from a mouse with partial loss of the OT-I transgene. (c) Flow cytometry as in a of cells from the mouse in b. Numbers adjacent to outlined areas indicate percent tetramer-positive CD8+ cells (a,c) or CD8+Vα2+ cells (b). Cells were stained with antibody to CD45.2 (anti-CD45.2) (104; BioLegend), anti-CD8a (56-7.8; BioLegend), anti-B220 (RA3-6B2; BioLegend) and anti-Vα2 (B20.1; eBioscience). The H-2Kb–SIINFEKL tetramer was made in baculovirus as described8.
The Jackson Laboratory routinely uses a PCR-based approach to screen for the transgene encoding Vα2 and Vβ5 and, on the basis of a retrospective analysis of ~50,000 genotyping samples, estimates that transgene loss occurs in 0.1% of pups screened, with 80% experiencing loss of the β-chain and 20% experiencing loss of the α-chain. Even at this frequency, fixation of either of these spontaneous mutations within the breeding colony would result in progeny unable to respond to the OVA peptide. It should be noted that it is not known whether maintenance of the OT-I mouse strain on a background sufficient or deficient in the RAG recombinase affects loss of expression of the α- or β-chain. Nevertheless, these observations from multiple independent laboratories suggest that this is not due to a founder effect and indicate that periodic verification of the ability of these transgenically expressed T cell antigen receptors to bind to a SIINFEKL-loaded MHC class I tetramer or staining for both the Vα2 chain and Vβ5 chain (for example, before new breeding pairs are set up) might be needed to minimize spontaneous partial loss of the OT-I transgene.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare competing financial interests: details are available in the online version of the paper.
CFI statement
M.S. is an employee of The Jackson Laboratory.
References
- 1.Hogquist KA, et al. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 2.Von Boehmer H. Front Immunol . 2014;5:424. doi: 10.3389/fimmu.2014.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyagawa F, Gutermuth J, Zhang H, Katz SI. J Autoimmun. 2010;35:192–198. doi: 10.1016/j.jaut.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harty JT, Badovinac VP. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 5.Condotta SA, Richer MJ, Badovinac VP, Harty JT. Adv Immunol. 2012;113:51–80. doi: 10.1016/B978-0-12-394590-7.00005-1. [DOI] [PubMed] [Google Scholar]
- 6.Wilson EH, et al. Immunity. 2009;30:300–311. doi: 10.1016/j.immuni.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pope C, et al. J Immunol. 2001;166:3402–3409. doi: 10.4049/jimmunol.166.5.3402. [DOI] [PubMed] [Google Scholar]
- 8.Kedl RM, et al. J Exp Med. 2000;192:1105–1113. doi: 10.1084/jem.192.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]


