Abstract
B cells mediate multiple functions that influence immune and inflammatory responses in rheumatoid arthritis. Production of a diverse array of autoantibodies can happen at different stages of the disease, and are important markers of disease outcome. In turn, the magnitude and quality of acquired humoral immune responses is strongly dependent on signals delivered by innate immune cells. Additionally, the milieu of cells and chemokines that constitute a niche for plasma cells rely strongly on signals provided by stromal cells at different anatomical locations and times. The chronic inflammatory state therefore importantly impacts the developing humoral immune response and its intensity and specificity. We focus this review on B cell biology and the role of the innate immune system in the development of autoimmunity in patients with rheumatoid arthritis.
Keywords: autoantibodies, B cells, inflammation, innate immunity, plasma cells
It is well established that B lymphocyte responses are dysregulated in autoimmune diseases, including rheumatoid arthritis (RA) [1]. B cells mediate disease pathogenesis by a number of mechanisms that include acting as APCs [2], functioning as source and/or sink of cytokines and chemokines [3,4], interacting with effector cells [5–7] and serving as a source of autoreactive antibodies [5,8]. RA shares certain B-cell-specific traits, cellular interactions and molecular pathways with other autoimmune diseases, but the precise role of B cells in the different clinically related conditions still remains under investigation. Innate immune cells and stromal cells can critically contribute to the generation of an inflammatory milieu that affects antigen presentation, B-cell development, B-cell/plasma cell (PC) survival and antibody production.
We will review the current knowledge regarding the contribution of B lymphocytes (with a special emphasis on innate-like B subsets) and PC to RA pathogenesis and discuss how signals from the innate immune cells and the stromal cell compartments contribute to altered B-cell responses. We will then discuss how inflammatory environments modify the behavior and function of all these cells, and the implications of these relationships to RA prevention and treatment.
RA pathogenesis
Overview
The current paradigm for RA pathogenesis reflects a multistep pathway beginning with genetic vulnerability [5,9]. HLA remains the most powerful genetic risk for development of RA. Non-HLA genes, often shared with other autoimmune diseases, affect T lymphocyte responsiveness and innate inflammatory programs [10,11]. Some environmental exposures are associated with chronic inflammation and increased expression of the peptidylarginine deiminase (PAD) enzymes that increase protein citrullination [12,13]. Those individuals with genetic vulnerabilities can lose tolerance to citrullinated protein motifs and develop anticitrullinated peptide antibodies (ACPAs). These appear to be T-cell-dependent, antigen-driven responses with autoantibodies undergoing class switching. Rheumatoid factors (RFs) also develop and may indicate the presence of immune complexes (IC) or other aspects of immune dysregulation. Long-lived antibody-producing cells and circulating autoantibodies may be present for many years before clinically detectable synovitis [9]. Patients tend to accumulate autoantibodies to additional citrullinated peptide antigens over time, suggesting epitope spreading. Events that are more proximal to the development of inflammatory arthritis involve systemic inflammation as detected by increased circulating proinflammatory cytokines. It is likely that exaggerated citrullination of proteins in the synovia [14], possibly as a result of PAD secretion by neutrophils and macrophages [15], triggers a local autoimmune response. All along these steps toward RA, B lymphocytes and long-lived PC play a role that is still incompletely understood. Furthermore, the role of innate immunity and stromal cells in shaping the acquired immune response remains under investigation.
Production of autoantibodies
The development and presence of autoantibodies is a hallmark of RA. A classical molecule that has been widely used with relative diagnostic value is the RF, which consists of anti-Fc antibodies that are self-reactive (anti-‘self-Fc’). These are typically IgM antibodies and are not highly specific for RA. They can be identified in many instances of hypergammaglobulinemia occurring in other autoimmune diseases (e.g., Sjögren’s syndrome) and hematologic malignancies. They are also commonly detected in clinical situations of chronic antigenemia and IC formation associated with viral (e.g., hepatitis C) and bacterial (e.g., endocarditis) infections [16,17]. Recent advances have helped identifying specific motifs of autoantigens in RA, which can lead to the production of disease-specific autoantibodies and increasing the current availability of detectable autoantibodies relevant to RA diagnosis.
ACPAs constitute the best categorized group of autoreactive antibodies in RA. Seropositivity for ACPAs strongly associates with high-risk alleles of HLA-DR genes and generally predicts a more aggressive disease course. Citrullination (conversion of arginine into citrulline) of certain peptides can occur in many proteins [18]. The citrullinated peptides can give rise to potential autoantibodies when seen within the context of inflammation, cell damage, dysregulated apoptosis and/or impaired apoptotic cell clearance [12,19]. These citrullinated autoantigens can be both intracellular and extracellular proteins. Among others, vimentin, α-enolase, aggrecan and fibrinogen have been identified as citrullinated proteins that are recognized by autoantibodies from RA patients [20–22]. It is noteworthy that although citrullination is a biochemical modification catalyzed by the PAD enzymes and occurs under healthy steady-state conditions, activity of the PAD enzymes and increased citrullination can be enhanced by certain external stimuli like cigarette smoke. So far, most ACPAs that have been characterized belong to the IgG and IgA isotypes [23,24], which underscores how autoreactive B cells must have been selected to reach a mature PC fate and have undergone isotype switching during the process. Interestingly, although there are also IgM ACPAs, the IgM ACPAs response displays a more restricted antigen recognition profile than IgG ACPA in RA patients [25]. Somatic hypermutation is likely to be involved in the development of ACPAs, as reversion of citrulline-specific antibodies cloned from the synovium B cells of RA patients to their corresponding germline configuration results in loss of citrulline-specific antigen binding [8]. To increase the level of complexity involved in this matter, the autoantibody specificity/pathogenicity can further evolve due to epitope spreading [26,27] or glycosylation of the Fc-portion of the autoantibody [28].
Citrullination is also increased at sites of inflammation including synovial tissues and atherosclerotic plaques, and it is thought that initial ACPA development may occur through priming at distant sites such as the lung or gingival tissues, while clinical RA and its complications may develop when citrullination occurs in RA target organs [29,30]. A highly relevant question, therefore, is the mechanism underlying citrullination in these tissues. Romero and colleagues recently reported hypercitrullination of cells in rheumatoid synovial fluid [15]. Their studies implicate neutrophils as the source of these hypercitrullinated proteins and only during extrinsic apoptosis in response to granzyme B/perforin or by activation of the complement pathway, not by intrinsic apoptosis, NETosis or autophagy. Neutrophils are the first immune cells to enter the arthritic joint [31], but it remains unclear what specific processes target neutrophils or cytotoxic cells to the joints. It is also plausible that IC involving either RF or ACPAs could locally activate complement leading to the establishment of a local autoimmune response involving hypercitrullinated proteins derived from neutrophils.
Anticarbamylated protein antibodies (anti-CarP), on the other hand, are directed towards peptides that are catalyzed by a nonenzymatic transformation that involves lysine and N-terminal addition of homocitrulline [32], which largely resembles citrulline but is one methylene group longer [33]. Interestingly, ACPA- patients can be anti-CarP+ and vice versa, with higher anti-CarP concentrations in ACPA- patients having a stronger predictive value for a more severe disease course as measured by radiological progression [32].
The extent to which ACPAs are causative agents of disease and not only epiphenomena of other immune dysregulation processes is still under debate. The relative contribution of predisposing HLA alleles to genetic risk has been estimated to be about 40% for ACPA+ patients and 2% for ACPA- patients [34]. Additionally, ACPAs can contribute to the generation of ICs, which are characteristic of other autoimmune disorders [35]. Very recently, Scally and colleagues demonstrated a molecular association between ACPAs and RA in humans. They showed that citrullinated aggrecan and vimentin epitopes bind to HLADRB1* 04:01/04 [36] by identifying larger numbers of recirculating tetramer-specific T cells in RA patients compared with healthy individuals. It is also noteworthy to say that first-degree relatives of RA patients who do not yet suffer from overt RA demonstrate reactivity to multiple ACPAs, and the presence of an increasing number of ACPAs (>8) correlates with joint inflammation [22].
One of the keys to understanding how the innate and adaptive immune responses interact relies in the roles of ICs and the complement system in RA. ICs can contain inflammatory molecules embedded in a cluster of antibodies, and it is possible that during disease progression some of the newly generated ICs contain autoantibodies and ACPAs. Additionally, the ICs can retain autoantigens and allow for their persistence, migration and presentation in places remote to their origin. Indeed, citrullinated fibrinogen-containing IC can stimulate TNF-α production in murine and human macrophages through their Fc receptors [35]. This suggests a potential role of citrullination in increasing the potency of an endogenous innate immune ligand. Whether ICs can have different impacts, depending not only on the composition of the proinflammatory and antigenic molecules they carry but also on the specificity of their antibodies, has not been rigorously investigated. Concomitant to the presence of autoantigens, autoantibodies, ICs and a proinflammatory environment, alterations in the complement activation system have been well described. In the serum transfer K/BxN mouse model of inflammatory arthritis, arthritogenic immunoglobulins act in a coordinated manner through Fc receptors and the alternative complement activation pathway to trigger disease onset [37].
The anatomical location where autoantibody production actually occurs can be difficult to track. Selection of B cells occurs in the bone marrow (BM) and spleen, but priming and further differentiation into distinct B cell subsets strongly differs in localization and costimulatory requirements. First, a B cell with a certain degree of self-reactivity has to be selected and receive the sufficient survival signals to escape negative selection. Additionally, that same B-cell clone needs to avoid becoming fully anergic (or if so, retain the capacity to revert from the functionally silenced state). Hence, breaking tolerance for B-cell responses in RA is highly likely to happen at more than one site and may need a combination of consecutive factors to be enabled at precise time points. The transition of a mature B cell into an autoreactive PC can occur in different ways as well. Secondary lymphoid organs (SLOs) harbor small numbers of PCs, and once RA is established, there is a gradual accumulation of PCs in the inflamed joints. Lymphoid neogenesis in the synovium is a well-characterized reversible process that is associated with inflammation during RA [38]. Humby and collaborators also demonstrated that ectopic lymphoid structures (ELS) found in the synovia of RA patients [39] were able to actively express activation-induced deaminase (AID) and support ongoing ACPA production by PC. More importantly, such AID expression was associated with increased mRNA levels of CXCL13 and LTβ but not APRIL and BAFF, suggesting that these structures support recruitment and class switch more than B-cell proliferation and survival. However, whether ELS-generated PCs are associated with circulating ACPA levels that are relevant for diagnostic purposes is still to be determined [40,41]. Not surprisingly, several of the features associated with ELS are also illustrated in other autoimmune diseases where tertiary lymphoid organs contain B cells and can promote B-cell selection, such as the pancreas during type 1 diabetes [42].
Integration of the innate & acquired immune systems at the B-cell interphase
Although the presence of B cells and autoantibodies has been extensively investigated, the other potential roles of B cells in RA are less well understood. Additionally, among all cells with phagocytic and APC characteristics, only B cells possess the capacity to recognize a specific Ag through its clonally restricted BCR, and hence drive a BCR-mediated Ag capture and incorporation, and even concentrate antigens present at low level, all of which imbues them with the capacity to potentially become Ag-specific APCs. Different phenotypically defined B-cell subsets exist both in mice and humans. We will not discuss in detail the role of follicular B cells as we consider that it has been properly addressed in recent reviews [5,43]. Innate-like B cells include marginal zone (MZ) B cells, B1 B cells and other less defined B cell populations. When compared with canonical B2 B cells, all of them, to a different degree, populate specific niches, possess different reactivities to pathogenic (self or non-self) antigens, generate faster humoral responses and confer quick protective responses to certain microorganisms. Therefore, innate-like B cells constitute a link between innate and adaptive immune responses throughout evolution, anatomical locations and types of responses [44–46]. MZ B cells constitute a population of splenic B cells that are characterized by hyporesponsiveness to Ag-driven signals through their BCRs and CD40 but hyperresponsiveness to several innate-like and proinflammatory signals [47,48]. MZ B cells also respond rapidly to blood-borne antigens and can behave in both innate- and adaptive immune response fashions. Expansion of MZ B cells has been observed in many autoimmune diseases, and compared with follicular B cells they are more potent APCs when driving T-cell proliferation [6]. MZ B cells can also regulate antigen capture by MZ macrophages [49]. A different type of B cells, B1 cells, are also altered in many autoimmune diseases, and although their ontogeny, anatomical location and functional capacities are very different from all other B-cell populations, they share some characteristics with MZ B cells, including cell surface markers, responsiveness to toll-like receptor (TLR) ligands and certain pathogens, and partial overlap in the molecular pathways activated upon BCR stimulation [44]. Both MZ and B1 cells have distinctive phagocytic and Ag-presenting capacities [45,49,50]. All these characteristics suggest that MZ and B1 cells can become critical partners in misleading immune responses that may result in the development of autoimmunity, especially in the context of a chronic inflammatory environment. However, the role of MZ and B1 cells in RA is still a matter of debate.
B cells are currently also appreciated as significant contributors to the cytokine and chemokine milieu in given microenvironments. In normal homeostatic conditions, there is balance among pro- and anti-inflammatory B cells, but this equilibrium is disrupted during RA. B cells can secrete many cytokines that affect migration and/or recruitment of other lymphocytic populations (e.g., T cells) in an endocrine and paracrine manner. Some B-cell-specific cytokines also have autocrine effects, as is the case of IL-4, IL-6 and many of the cytokines that tightly regulate the maturation of naive B cells into plasmablasts or PCs [51,52]. Interestingly, some of the cytokines, such as IL-10, that are relatively decreased during RA either systemically or in the joint are considered anti-inflammatory or Th2-promoting cytokines [9,53]. A special mention is required for a newly redefined subset of B cells that fall under the term regulatory B cells (Bregs), which share several phenotypical markers with both MZ and B1 cells, although there is no absolute or exclusive overlap among all of them. The immunomodulatory capacity of Bregs depends critically on their competence to secrete IL-10, independently of how they are phenotypically characterized or named. Bregs can strongly suppress autoimmunity in many different murine models [54], and their suppressive capacity is not always exclusive to the endogenous production of IL-10. Bregs can alter the numbers of Tregs in the EAE mouse model independently of IL-10 [55], but also reduce Th17 cells in the CIA model [56] in an IL-10-dependent fashion. Interestingly, transient production of IL-10 occurs when B cells differentiate into antibody secreting cells (ASCs) [57]. Moreover, a defect in Breg function has been found in RA patients regarding their capacity to enhance Th1 bias [58,59], and patients with new-onset RA have decreased numbers of circulating Bregs [59]. IL-10-producing B cells are also diminished in ANCA-associated vasculitis [60] and can partially suppress proliferation of activated human Th cells [61]. However, despite these advances in understanding Bregs in RA, we still know little about what causes their functional impairment.
Inflammatory mediators of B cell & PC functions
RA is a systemic inflammatory disease. Some potent inflammatory stimuli occur locally at the affected joint and hence have more site-restricted effects. However, systemic inflammation results from circulating proinflammatory cytokines and other mediators that can be identified in blood. Pattern recognition receptors (PRRs) bind certain exogenous pathogen-associated molecular patterns (PAMPs) and endogenous damage-associated molecular patterns (DAMPs), molecules that elicit inflammation and also enhance immune responses, in part by stimulating B cells to undergo activation and differentiation. All these ‘danger signals’ can act on PRRs that can be categorized as TLRs, NOD-like receptors, C-type lectin receptors and RIG-I-like receptors, and all of them contribute to both innate and acquired immune responses. Binding of the molecules that activate these receptors can result in release of inflammatory mediators in the local microenvironment. Additionally, there is a well-documented link between autoimmunity and infections [62], and PAMPs and DAMPs are present in many adjuvants currently in use, although the precise mechanisms by which they contribute to the development of acquired immune responses are still largely unknown.
Given the importance of PRRs in shaping immunity, the role that the microbiota plays in autoimmunity is a matter of great recent interest. B cells shape the composition of the microbiota, especially through secretion of IgA and IgM, and have a deep and multifactorial impact in how the whole immune system interacts and reacts to the microbiota in order to establish microbial tolerance [63,64]. Alteration of the microflora composition and the presence of specific genus or even species can dramatically impact the development and presence of immune cells relevant to RA, as is the case for Th17 cells [65]. Discerning the composition of the intestinal and oral microbiota has also led to insight on how chronic or semichronic inflammatory insults can affect RA development. Analysis of the microbiota composition in the stool of new-onset RA patients, for example, revealed that the specific presence of Prevotella copri was strongly correlated with disease [66]. Additionally, colonization of mouse intestinal tract with P. copri rendered the mice more sensitive to chemically induced colitis, which for the first time links inflammatory bowel conditions to RA due to the colonization of a single bacterium species, which is interesting considering that some forms of arthritis like ankylosing spondylitis share a common genetic background with inflammatory bowel disease in some human populations. It is clear that the intestinal flora impacts the development of the immune system in mice, altering the expression of many spontaneous and induced autoimmune diseases [67,68], though further studies are necessary to identify whether a causal link occurs in humans, especially in RA.
B-cell subsets are differentially responsive to many of the PAMP/DAMP signals due to the expression pattern of a certain set of TLRs and NOD-like receptors. Different B-cell subsets display different levels of expression of TLRs, with MZ B cells and B1 cells showing higher expression of TLR3, TLR 7 and TLR9 than their follicular B-cell counterparts [69]. Additionally, TLR ligation promotes much stronger differentiation of B1 and MZ B cells into mature PC compared with follicular B cells, with the concomitant upregulation of the PC master regulator transcription factors BLIMP-1 and XBP-1 [70]. Naive B cells are well known to proliferate and differentiate in response to TLR4 (LPS) or TLR9 (CpG) ligands alone. There is, however, also a complex level of synergism that arises from the combination of BCR, CD40 (provided by T cells) and different TLR signals delivered to B cells. Ligation of CD40 leads to differential additive effects in inducing either both B-cell proliferation and activation (together with TLR3, 4 and 9) or differentiation into ASCs (TLR1/2, 2/6, 4 and 7). Moreover, addition of BCR signals to CD40L and either TLR3 or TLR9 does not induce differentiation of ASCs [71]. TLR triggering does not only regulate proliferation, activation and differentiation, but also enhances antibody production per se in human PC isolated from peripheral blood [72]. Additionally, autoreactive B cells that make RF can be activated by IgG2a-chromatin ICs by co-engaging the BCR and TLRs [73]. Signals emanating from endogenous PAMP/DAMP danger signals in RA deeply alter disease progression [74,75], but the extent of their impact on B cells during RA is still to be further investigated. Some proteins that activate the lectin-complement pathway may also play an important role in inflammatory responses. Ficolins and collectins, which can be found in sera and diverse tissues, contain both a collagen-like domain and a fibrinogen-like domain that allows them to bridge connections between the extracellular matrix and oligosaccharide structures in the surface of microorganisms [76]. Therefore, immune cells involved in activation of the lectin-complement pathway can also be part of the interactive conundrum of signals (‘danger signals’, complement and immunoglobulins/IC) that can be received by B cells in RA.
In addition to sensing foreign, ‘danger’ signals from microorganisms, B cells also respond to inflammatory cytokines strongly linked to RA. These include TNF-α and IL-1-β, along with other proinflammatory cytokines (IL-6, IL-12, IL-18 and IL-20), which are classical mediators of local and systemic inflammatory processes. TNF-α has very well-established properties as a lymphocyte activator, but is also a potent inducer of stromal and myelomonocytic cells in regards to inducing their production of cytokines, chemokines, matrix enzymes and adhesion molecules. Independently of that, it also contributes to RA pathogenesis by activating osteoclasts [5]. IL-6, on the other hand, which can be produced in response to IL-1-β and TNF-α stimulation, activates both B cells and osteoclasts. It also regulates B- cell hematopoiesis [77] and postgerminal center (GC) receptor editing [78]. In combination with IL-21, IL-6 can control the formation of T follicular helper cells, critical to mounting strong humoral T-cell-dependent (TD) responses [51]. Additionally, a positive feedback loop can be established in proinflammatory conditions between macrophages reacting to IC by producing IL-6, and responder B cells that enhance their survival and IgG production capacities [77]. Another molecule with strong inflammatory properties is the high-mobility group box 1 protein, which is released mostly due to impaired clearance of apoptotic or necrotic material and can act as cytokine and enhance inflammatory responses in RA [79]. Treatment with neutralizing anti-high-mobility group box 1 protein antibodies significantly ameliorates RA symptoms in the murine CIA model [80].
Prostaglandins and leukotrienes are also critical mediators of inflammatory responses, but it is not well understood how their quantity and quality alter B-cell responses. Prostaglandin E2 (PGE2) and (PG)I2(prostacyclin) are abundantly present in the synovial fluid of RA patients [81]. PGE2 is able to down-regulate B-cell proliferation through binding to one of its cognate receptors EP4 [82] and can also induce BCR-mediated cell death of immature B cells [83]. Surprisingly, PGE2, in combination with IL-21, can also induce selective apoptosis of B cells undergoing GC reactions [84,85]. Additionally, from all four known prostaglandin receptors, EP4 seems to be the most critical during RA, as EP4 receptor-deficient mice showed decreased incidence and severity of disease in murine CIA models, as well as reduced systemic IL-6 and dampened IL-1-β and IL-6 production from isolated macrophages [86]. However, EP2 also contributes to joint inflammation and CIA development in mice [87].
Further evidence of the critical importance of PG in the development of a humoral immune response comes from mice deficient in PG biosynthetic enzymes. Mice deficient for cyclooxygenase-2 and microsomal PGE synthase-1 (mPGES-1) are resistant to the development of CIA and have deficient ability to generate type II collagen antibodies. mPGES-1 null and heterozygous mice exhibit a decreased incidence and severity of arthritis, and this correlates with reduced levels of anti-type II collagen humoral responses [88]. Furthermore, mPGES1 null mice have impaired general TD humoral responses, although this effect is not B-cell intrinsic but mediated by the absence of mPGES-1 in the nonhematopoietic compartment [89].
Innate immune system regulation of B-cell responses
The paradigm of classifying humoral responses into T-independent (TI) and TD has recently been challenged by investigations demonstrating intricate interplay among different cells involved in shaping the final Ag-driven antibody response. Both TI and TD responses can be generated outside the spleen follicle, termed extrafollicular responses, and some of these extrafollicular responses are strongly linked to the development of autoreactivity in B cells [90]. The splenic MZ harbors several types of innate cells that can influence B-cell responses, which are generally TI. The nature of the antigen is critical with regard to which cell type will most efficiently capture, process and present the respective antigen. Also, innate cells that populate the spleen and other SLOs critically contribute to shape B-cell responses [91]. Most innate-like cells can function as APCs and express B-cell costimulatory molecules that can, in some instances, be more potent than the ones delivered by T cells. This fact, together with a biased antigen presentation repertoire, which might include autoantigens, could help to override B-cell tolerance.
Among innate cells, NKT cells have emerged as critical providers of B-cell help in mounting humoral responses to glycoproteins [92]. Invariant NKT cells can become activated and express high levels of CD40L, IL-4 and IFN-γ that induce differentiation of MZ B cells into low-affinity IgM or IgG plasmablasts [93]. Indeed, NKT cells can induce the formation of early GCs, although without the generation of long-lived PC [94]. Neutrophils can also support antibody production and class switching by activating MZ B cells, and their role in shaping B-cell responses is supported by the observation that neutropenic patients show fewer hypomutated MZ B cells [95]. Moreover, a process by which neutrophils capture Ag from neighboring tissue upon inflammation called ‘NETosis’ has been linked to RA and the generation of a limited repertoire of citrullinated antigens [30]. Neutrophils, along with monocytes/ macrophages, are also the only cells expressing FcγRIV in mice, and these play an important role in the development of arthritis in the K/BxN model [96], and FcγR expression on synovial macrophages from RA patients also impacts the production of TNF-α induced by ICs [97].
Innate lymphoid cells (ILCs) are a recently recognized category of heterogeneous innate cells that might impact B-cell responses. More precisely, group 3 ILCs, which include lymphoid-tissue inducer cells, are responsive to the proinflammatory IL-1-β and IL-23 signals. Group 1 ILCs, on the other hand, respond to other cytokines like IL-12 and IL-18 in order to produce IFN-γ, which can, in turn, enhance an inflammatory or cytotoxic response [98]. Other ILCs that can have an important role in B-cell responses are the lymphoid tissue organizer cells as they are a source of CXCL13, CCL19 and CCL21 to attract lymphoid-tissue inducer cells to a specific anatomical location.
When considering a TD response that involves the formation of a lymphoid follicle, follicular dendritic cells (fDCs) have proven to be master regulators [99]. fDCs, which are of mesenchymal origin, can potentiate the generation of PCs through production of CXCL13 and IL-21, which is interestingly also a critical cytokine for the development of Bregs [100]. Central to its potential implications in autoimmune diseases, fDCs are also able to capture ICs for Ag presentation [101] and acquire complement-coated ICs to bridge the antigen presentation from noncognate B cells to cognate B cells [102]. Some MZ-specialized DCs can also drive TD humoral responses that do not involve the formation of GC reactions [103]. Additionally, a different kind of DC, the plasmacytoid DCs, also populates the MZ, and it has been shown that human pDCs are capable of enhancing TD B-cell differentiation through a mechanism involving IFN-α [104].
Extrafollicular B-cell responses can develop in the MZ, as previously discussed. The splenic MZ is populated by very unique populations of macrophages (metallophilic macrophages), and NK cells can also locate there when mounting a response against blood-borne pathogens. MZ macrophages play a critical role in the clearance of apoptotic cells and can contribute to reduce the immunogenicity of autoantigens [105]. A good example of the implication of MZ macrophages in autoimmunity is illustrated by DBX2 mice, which suffer from a complex autoimmune syndrome that involves autoantibody production and development of arthritis in females with a 50% incidence after 8 months of age. Interestingly, the MZ macrophages from DBX2 mice are defective in their ability to clear apoptotic debris, which in turn drives follicular Ag transportation by MZ precursor B cells to stimulate an autoimmune response [106].
Apart from the spleen, other SLOs or even areas that generally do not harbor resident lymphocyte populations can influence B-cell responses and deeply impact RA pathogenesis. That is indeed the case in RA, where the formation of tertiary lymphoid structures can happen as a result of disease progression in the vicinity of target organs [107,108]. It is also characteristic of RA pathogenesis that the synovium recruits many innate cells.
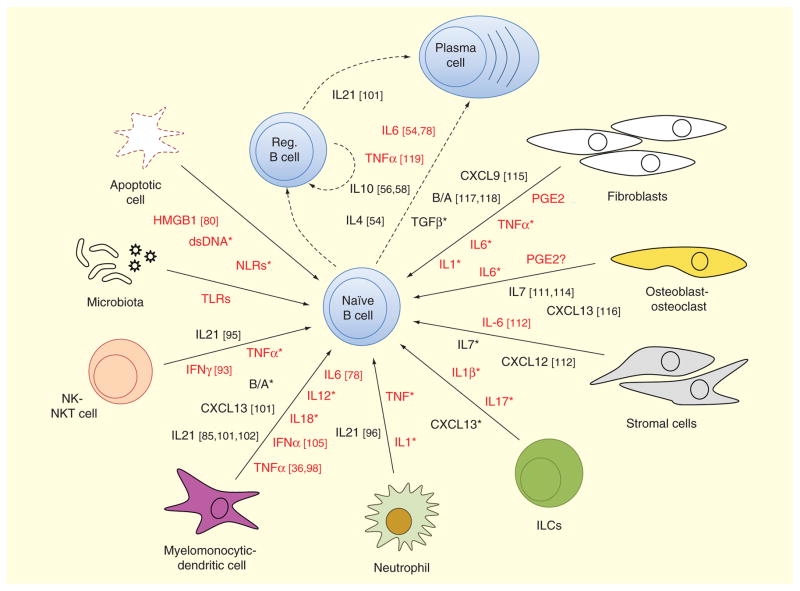
Altogether, innate cells produce an array of molecules that affect B-cell differentiation and function. Production of IL-6, IL-10, IL-21, BAFF, APRIL and CXCL13 has a critical role in the generation of plasmablasts that secrete IgM and also in the class-switch to the IgG and IgA isotypes [45,91]. Moreover, most innate cells express surface Fc receptors that allow them to react to antigen-bound antibodies and ICs, and trigger an activation program that contributes to a proinflammatory environment and dysregulation of B-cell responses. Hence, innate immune cells may play critically important roles in humoral immune dysregulation at different times during the pathogenesis of RA, as depicted in Figure 1.
Figure 1. Innate cell-mediated inflammatory signals influencing B-cell function.
A naive B cell can be influenced by many signals from their immediate environment to acquire a plasmablast/plasma cell or regulatory B-cell fate. Different cells can contribute to the milieu of signaling molecules at a given time. Cell types considered in this graph include either cells that are structurally inherent to a certain tissue (osteoblasts, fibroblasts, stromal cells) or cells of the innate immune system (neutrophils, myelomonocytic cells, DCs, ILCs, MDSCs, NK and NKT cells). Due to their potential contribution in an inflammatory environment, we have included molecules derived from apoptotic cells and the local microbiota. Despite their profound differences, for simplification purposes, myelomonocytic and dendritic cells, as well as NK and NKT cells, have been grouped. For the same reason, we have also omitted differentiating fDCs versus nonfollicular DCs or including MDSCs. Molecules labeled in red indicate a strong proinflammatory bias, while black ones represent molecules implicated in recruitment, survival and proliferation of cells or suppressors of inflammation. Molecules indicated with an asterisk are well described in previous reviews like [3,5,98]. For the purpose of clarity, not all cell types and mediator molecules mentioned in the text are depicted in the figure.
B/A: BAFF/APRIL; DC: Dendritic cells; HMGB1: High-mobility group box 1 protein; ILC: Innate lymphoid cell; MDSC: Myeloid-derived suppressor cell; NK: Natural-killer; NLR: NOD-like receptors; PGE2: Prostaglandin E2.
Contribution from stromal cells & other nonlymphoid cells
Cells that do not belong to any lymphoid lineage populate bone and joint tissues. These can affect and be affected by B cells and PCs. The synovium can not only attract but also contribute to the expansion and differentiation of PCs. It is in a constrained time frame and space that nonlymphoid cells can become critical players upon receiving inflammatory signals. B cells can become a dominant source of the osteoclast-promoting cytokine RANKL in the synovium of RA patients [109]. Reciprocally, osteoclast activity can also regulate B-cell function in the BM by producing CXCL12 and IL-7 [110,111], cytokines that both recruit and allow the expansion of B cells. Osteoblasts configure another essential part of bone and joint tissues. They can constitute a significant source of osteoprotegerin, VCAM-1, SDF-1 and IL-7, all of which impact B-cell development [112,113]. Synoviocytes, on the other hand, are the main fibroblast-like population in the joints. They are highly responsive to many inflammatory triggering molecules and can establish cross-talk with PCs expressing CXCR3 by secreting CXCL9 [114]. Interestingly, synovitis might be an important source of circulating CXCL13, as serum CXCL13 correlates with synovial CXCL13 measured at a single joint in RA patients that underwent rituximab treatment and showed repopulation of their B-cell compartment [115]. Independently of recruitment of B cells to the target joints, synovial fibroblasts from RA patients were shown to produce higher levels of BAFF/APRIL than those of osteoarthritis patients upon TLR3 stimulation, which increased AID expression and IgG/IgA class switching in autologous co-cultured B cells [116]. Even more relevant in an inflammatory environment, fibroblast-like synoviocytes stimulated with IFN-γ/TNF-α were shown to provide BAFF in vitro [117]. Irrelevant to their location, we summarize the signals from neighboring cells that shape the survival, differentiation and expansion of B cells in Figure 1.
The BM is not only the location where hematopoietic cell precursors reside, but it also constitutes the niche where recirculating PC and some memory B cells retreat after being generated. BM stromal cells are providers of specific signals that can promote B-cell survival, retention or migration, and also quiescence. The BM niche provides cell contact-dependent signals, chemokines (CXCL12, VCAM-1) and cytokines (APRIL, IL-6, IL-4 and IL-5) highly relevant to B-cell survival, migration and activity [3,111]. On the side of the cells receiving the signals generated in the BM niche, human BM PCs exhibit constitutive expression of CXCR4, CXCR6, CCR10 and CCR3, while CXCL12 can be differentially upregulated upon stimulation with IL-1-β and TNF-α [118]. Galectin-1, a glycoprotein known for its immunosuppressive roles, can also be produced by stromal cells from the BM and regulate pre-B-cell development [119]. Importantly, galectin-1 expression modulates PC homeostasis and regulates humoral responses in an autonomous manner [120]. It is possible that some BM stromal signals might be altered during inflammatory processes, which can contribute to a dysregulated humoral response in RA. Supporting this idea, it has been shown that the BM of RA patients can present subchondral lesions with concomitant lymphoid cell infiltration [121]. Additionally, Chu et al. have demonstrated that eosinophils play an important role in the maintenance of PCs in the BM by providing IL-6 and BAFF [122].
Aside from the BM and the joints, stromal cells from secondary and tertiary lymphoid organs can also be pivotal in mounting an acquired immune response by presenting local antigen to T and B cells, and could eventually act in synergy with more specialized APCs like DCs as has been shown in the thymus [123]. Additionally, stromal cells alone are sufficient to enforce T-cell tolerance [124]. CXCL13, as discussed earlier, can be produced by fDCs and several innate cells, but also from stromal cells. CXCL13 plays a central role in lymphoid neogenesis [107], and in regulating B-cell follicle structures within SLOs, which is defined by the localized expression of CXCL13 and its receptor CXCR5 [50]. CXCL13 is also expressed in the synovium, where it is central in assisting the formation of GC-like structures and AID expression [125,126]. A different kind of myelomonocytic cell with suppressor properties, the myeloid-derived suppressor cell (MDSC), has been shown to accumulate in the spleens of mice undergoing CIA, and autologous adoptive transfers of MDSCs can strongly reduce CIA severity, most likely due to their capacity to impair Th17 differentiation and/or accumulation in the draining lymph nodes [127]. However, whether MDSCs can affect B-cell responses or have a role in RA remains unexplored.
Therapeutic approaches targeting B cells
B-cell-depleting strategies
Elimination of B cells using specific monoclonal antibodies (mAbs) targeting B-cell-specific molecules has become a widespread second-line approach in RA since the advent of the anti-CD20 antibody, rituximab, was shown effective [128]. Concomitantly, renewed interest in B cells has been fueled by the use of rituximab in RA patients. Rituximab depletes pre-B cells, immature, mature, transitional and memory subsets, but not long-lived PCs, which do not express CD20 on their cell surface. However, rituximab does deplete short-lived autoreactive PCs in the K/BxN model mouse model of inflammatory arthritis [129]. The efficiency of B-cell depletion through anti-CD20 treatment is dependent on the potency of the antibody-dependent cell cytotoxicity triggered. In this regard, it has been recently identified that Kupfer cells are the major contributors to the scavenging and elimination of anti-CD20-coated B cells [130]. In an effort to increase the efficiency and reduce the side effects of anti-CD20 therapies, several strategies with different success rates in RA treatment have been developed in the last 10 years: anti-CD20 mAbs have been fully humanized [131], glyco-engineered to enhance complement-derived cell cytotoxicity [132] and their Fc-portion has been removed to avoid adverse effects like complement-derived cell cytotoxicity in single-chain variable fragment approaches [133]. However, the action of rituximab goes beyond the removal of B cells and the antibodies they produce. Removal of B cells has been shown to regulate the numbers of Tregs in the acute proteoglycan-induced arthritis model of RA in mice [134]. Additionally, rituximab decreases inflammatory Th17 responses in murine models [135]. Finally, elimination of B cells can lead to impairment in the proinflammatory cytokine profile of invariant NKT cells in systemic lupus erythematosus (SLE) in humans [7]. Moreover, therapy with B-cell-depleting agents, in spite of the improvements in clinical and radiographic responses in RA patients, leaves damaging inflammatory pathways in the rheumatoid synovium essentially unaffected [136].
Despite all of the clinical promise of anti-CD20 drugs, there is still an unmet demand for better B-cell-specific targets. Due to the pattern of surface CD20 expression during B-cell development and activation, it is precisely at the plasmablast/PC stage that several canonical B-cell markers are not present or downregulated, and therefore there are critical B-cell populations that cannot be removed that contribute to disease pathogenesis. Hence, other B-cell-specific surface molecules have arisen as potentially more effective targets. CD19 is more widely expressed during B-cell ontogeny and involves a different cellular removal mechanism, which makes it a more desirable candidate as it could also deplete antibody producing cells and precursors that escape targeting by rituximab, but the studies with these drugs in auto-immune diseases are more limited [137,138]. Interestingly, co-engaging the BCR and FcγRIIb in B cells with a novel anti-CD19 mAb (XmAb5871) in SCID mice engrafted with RA patients’ PBMCs suppressed humoral responses, which suggests potential suppressive activity in RA [139].
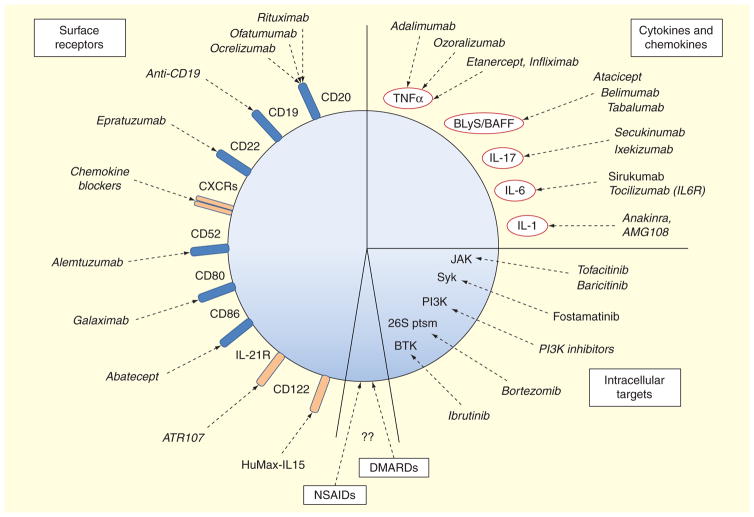
Another strategy to eliminate B cells in a more indirect fashion is the removal of B-cell survival factors. B-cell expression levels of BAFF and TACI are known to be disturbed in early RA patients, and during onset of RA BAFF receptor expression also changes [140]. BAFF-neutralizing strategies like the anti-BAFF mAb tabalumab have undergone clinical trials for RA patients refractory to anti-TNF-α treatment, but did not meet primary endpoint requirements, despite some efficacy shown at early time points [141]. RA patients receiving atacicept, a different anti-BAFF-APRIL antibody that blocks TACI signaling, showed a strong decrease in circulating levels of antibodies, especially in IgM and anti-RF IgG and IgM, although they did not show significant clinical improvement over the placebo group for that precise Phase II trial [142]. Another B-cell survival factor that reached Phase II trials and seems more promising in patients is the anti-BLyS belimumab, which is approved for treatment of certain patients with SLE [143]. Figure 2 summarizes the drugs targeting surface molecules that are either approved, in clinical trial phases for RA, or that we consider harbor potential to be used in RA treatment but are currently used for other autoimmune diseases.
Figure 2. Targeting B cells for current and potential therapeutic approaches in rheumatoid arthritis.
Considering a B cell as the sole target, we have gathered all current target molecules in three main groups: surface molecules, cytokines and chemokines, and intracellular signaling. Several of these drugs are not approved for their use in rheumatoid arthritis, but have been approved for treatment of other autoimmune diseases or B-cell cancers. We also consider that DMARDs and NSAIDs might have potential, albeit more limited effects on B-cell functions, although this is largely unexplored. Current drugs and the ones under development are indicated by their generic name.
DMARD: Disease-modifying antirheumatic drug; NSAID: Nonsteroidal anti-inflammatory drugs.
Bortezomib is currently approved only for the use in multiple myeloma and mantle cell lymphoma. Bortezomib represents a different therapeutic approach as it does not target surface molecules but the proteasome 26S subunit. In fact, bortezomib was proven to be highly effective in preventing murine SLE by depleting PC in a dose-dependent manner [144]. In contrast to other drugs, both murine PC generated during SLE and human myeloma lines depict a finer selective sensitivity to proteasome inhibition, and they show enhanced cell death that depends on modifying their unfolded protein response [145]. Bortezomib inhibits the release of TNF-α, IL-1-β, IL-6 and IL-10 in T cells from RA patients [146] and ameliorates disease severity in an adjuvant-induced rat model of arthritis, partially by reducing the invasiveness of fibroblast-like synoviocytes [147], and also attenuates murine CIA [148], but we are unaware of studies pursuing this treatment in RA.
It is also worth mentioning that B-cell depletion strategies can have an impact on other cells of the innate immune system. An increase in BAFF, IL-10 and CD86 mRNA in human monocyte-derived macrophages has been reported in patients undergoing rituximab treatment [149]. Additionally, a newly characterized murine B-cell population (CD11ahiFcgRIIIhi) is able to alter the phagocytic response of macrophages when challenged with certain pathogens. These latter B cells modify macrophage effector functions via IFN-γ production [150], and the response seems to be dependent on Bruton’s tyrosine kinase (Btk) signaling. These finding raise the possibility that B-cell depletion may have more widespread effects on innate immune cells than currently appreciated.
Altering B-cell responses without depletion
Engagement of certain surface proteins expressed on B cells does not result in depletion, but rather modulation of B-cell function and amelioration of disease in autoimmunity. Anti-CD22 mAbs have been used in Phase IIb clinical trials for SLE [151] and a Phase I/II study in Sjögren’s syndrome patients [152], but their potential in RA has been unassessed so far. Alternatively, targeting CD80 and CD86 with drugs like abatecept that bind to CTLA4 is currently an alternative line of therapy that is known to block T-cell costimulation, but it also alters monocytic migratory properties [153] and could concomitantly be modifying B-cell functions.
Circulating molecules that are direct mediators of inflammation constitute a distinct target class. Such is the case of TNF-α, blockade of which is broadly used in the treatment of RA by different drugs with different mechanisms of action. The effects of TNF-α on B cells are largely unknown, but it is clear that many of the downstream effects on TNF-α-responsive myelomonocytic and stromal cells can deeply alter B-cell responses. In a similar way, blocking the activity of soluble cytokines that affect B cells, such as the anti-IL-6 receptor tocilizumab, ameliorates RA either alone [154] or in combination with methotrexate [155].
A different series of drugs have been designed to directly target signaling pathways that are more selectively active in cell populations of the adaptive immune system, which contribute to pathogenesis. Such is the case of JAK and SYK inhibitors. The JAK inhibitor tofacitinib is approved for treatment of RA, and other, more specific, JAK inhibitors like baricitinib are in Phase III clinical trials [156]. Fostamatinib, a SYK inhibitor, despite its safety, does not seem to be effective enough to offer significant improvements over current treatments [157]. BTK is an intermediate molecule downstream of the BCR signaling pathway, which, in contrast to JAK and SYK, is more restricted to the B cell and myeloid compartments. Ibrutinib and other selective BTK inhibitors have shown promising results in limiting autoantibody production and decreasing disease pathogenesis in myeloid and FcγR-dependent autoantibody-induced arthritis in rodents [158,159]. However, ibrutinib, which is an irreversible inhibitor of the IL-2-inducible tyrosine kinase in T cells, is also able to subvert Th2 immunity [160], which might affect some humoral responses.
Five-year view
Current therapeutic strategies for RA that specifically target B cells are focused on direct depletion. Drug developments directed at B cell/PC depletion are continuing with different antibody targets or antibody characteristics. However, alternative B-cell-specific approaches are also being developed by targeting distinct molecules with different degrees of B-cell specificity and mechanisms of action. Continuous administration of antibody-based drugs, whether for depletion or neutralization, can cause the development of antidrug antibodies that interfere with efficacy. In addition, long-term chronic use of B-cell-depleting agents reduces Ig levels and the consequences of this effect are likely to increase infectious complications related to the duration of drug administration. In contrast, limiting intrinsic B-cell functions without depletion is an alternative approach with some potential advantages. It does not depend on the elimination of endogenous B cells, which may preserve some important B-cell functions including antigen presentation and cytokine secretion. Additionally, nondepleting strategies would avoid the elimination of Bregs, which are critical in restricting autoimmune responses in many murine models. In our opinion, it is possible to discover therapeutic drugs that could abrogate B-cell localization or function in the GCs that drive the generation of high-affinity PCs, but it would also be possible to block the recruitment of already mature autoreactive B cells or PCs to the joints. This could be achieved by targeting the appropriate cytokine/chemokine combination. The caveat of this approach is that chemokines lack sharp delimitations regarding their cell type exclusivity, and many have pleiotropic effects difficult to restrict and predict. Finally, we should consider that autoreactive B cells may also exhibit different signaling properties that make them more susceptible to certain types of therapeutic intervention [161].
Another potential strategy would be to intervene with B cells or PCs in the preclinical stage of RA, where autoantibodies are present in the absence of clinically detectable synovitis. In this stage, preventing hypercitrullination, localization of IC to the joint, complement activation or even targeting RA-specific autoantibody-producing long-lived PCs for elimination could prevent or mitigate development of joint-specific autoimmunity. It may be possible to identify strategies that would inhibit the generation of antigen-specific autoimmune reactions relevant for RA as exemplified by strategies to induce tolerance towards RA-specific antigens [162]. Finally, enhancing endogenous regulatory mechanisms in the preclinical or early clinical phase of disease by enhancing innate immune mechanisms such as MDSCs or alternatively activated macrophages may help to halt the autoimmune process. Certain molecules, including PGE2, induce MDSCs and polarize other innate cells towards a suppressive phenotype. Developing drugs that facilitate suppressor polarization, likely in combination with other strategies, will increase the likelihood of prevention or improve long-term outcome of RA.
Expert commentary
Tremendous progress has been made in understanding RA pathogenesis. Genetic predisposition, epigenetic factors including post-translational protein modifications and environmental factors, can coalesce around a specific microenvironment to lead the generation and persistence of autoreactive B cells. The importance of B cells in RA is confirmed by the therapeutic efficacy of anti-B-cell therapies. Whatever the initial combination of instigating factors that trigger the development of RA is, and when and how B cells contribute to RA is still largely unknown.
The renewed interest in B-cell biology in the clinical field sparked by B-cell-depleting therapies has fostered a re-evaluation of some current concepts in B-cell biology. A plethora of new B-cell subsets has emerged in the last few years, including Bregs, which can suppress pathogenic inflammation, as it has been reported in several autoimmune mouse models. However, Bregs could also perpetuate disease by enhancing plasmablast/PC production or by suppressing desirable immune cytotoxic responses. Likewise, the canonical categorization of humoral responses is being revisited, mostly due to the contribution of innate immunity helper cells that were not considered until very recently. Not only the contribution of B cells as producers of antibodies and cytokines/chemokines, but also the character and function of the generated antibodies during the different phases of RA pathogenesis is being revisited. For instance, ICs containing pathogenic autoantibodies within a defined inflammatory environment might activate myelomonocytic cell populations in SLO and other restricted locations like the joints. In such situations, FcRγ binding of autoantibodies/ICs might trigger innate immune cells to reinforce a positive feedback effect not only in perpetuating an inflammatory process, but also in boosting biased and pathogenic antibody production.
Factors guiding pathogenic B-cell functions in RA include impaired elimination of autoreactive clones, failure in triggering anergy in peripheral autoreactive B cells, altered chemokine and cytokine production promoting the recruitment and retention of ASCs to pathogenic sites (e.g., to the synovium) and increased microenvironmental inflammatory signals leading to differentiation and expansion of PCs. Autoantibodies produced by dysregulated PCs could, at different stages of the pathogenesis of RA, strongly contribute to an amplification feedback network of proinflammatory signals, and hence both propagate and perpetuate RA complications. Novel treatment approaches will need to take into account the newly emerging understanding of B cells/PCs including the important interactions between innate immune mechanisms and B-cell development that may dramatically influence autoimmunity.
Key issues.
Inflammatory signals can alter many B-cell functions, from selection of autoreactive clones to survival of plasma cells.
B cells contribute to rheumatoid arthritis (RA) pathogenesis through production of autoantibodies, and these autoantibodies are important factors in the formation of immune complexes and triggering undesired activation of inflammatory cascades.
It is very likely that B cells play additional roles to capture, process and present antigen, as well as to alter the local cytokine/chemokine microenvironment.
Regulatory B cells have potent immunosuppressive capacities that can modulate RA development.
B cells can interact with many nonlymphoid cells. The innate immune system, with many newly identified cell types in the last few years, can shape the type and intensity of B-cell responses, especially the production of antibodies and autoantibodies.
Chronic inflammatory conditions lead to imbalanced immune system responses. Innate immune cells and stromal cells readily respond to many inflammatory signals, and their co-localization with B and T cells can support the amplification and perpetuation of inflammation.
The few existing therapies in RA targeting B cells are focused on depletion strategies. New therapies and approaches are under development targeting B-cell subsets and inhibiting B-cell functions without depletion.
Manipulating the interaction between inflammatory signals and innate immune responses could help to suppress undesired B-cell responses to block disease progression.
Acknowledgments
The authors thank Drs. Elizabeth M Johnson and David DiLillo for their input and comments.
Footnotes
Financial & competing interests disclosure
This work was supported by National Institutes of Health AR049010. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Silverman GJ, Carson DA. Roles of B cells in rheumatoid arthritis. Arthritis Res Ther. 2003;5(Suppl 4):S1–6. doi: 10.1186/ar1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson CL, Hine DW, Pradipta A, et al. Presentation of the candidate rheumatoid arthritis autoantigen aggrecan by antigen-specific B cells induces enhanced CD4(+) T helper type 1 subset differentiation. Immunology. 2012;135(4):344–54. doi: 10.1111/j.1365-2567.2011.03548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7(6):429–42. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 4.Burmester GR, Feist E, Dorner T. Emerging cell and cytokine targets in rheumatoid arthritis. Nat Rev Rheumatol. 2014;10(2):77–88. doi: 10.1038/nrrheum.2013.168. [DOI] [PubMed] [Google Scholar]
- 5.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205–19. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 6.Attanavanich K, Kearney JF. Marginal zone, but not follicular B cells, are potent activators of naive CD4 T cells. J Immunol. 2004;172(2):803–11. doi: 10.4049/jimmunol.172.2.803. [DOI] [PubMed] [Google Scholar]
- 7.Bosma A, Abdel-Gadir A, Isenberg DA, et al. Lipid-antigen presentation by CD1d (+) B cells is essential for the maintenance of invariant natural killer T cells. Immunity. 2012;36(3):477–90. doi: 10.1016/j.immuni.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amara K, Steen J, Murray F, et al. Monoclonal IgG antibodies generated from joint-derived B cells of RA patients have a strong bias toward citrullinated autoantigen recognition. J Exp Med. 2013;210(3):445–55. doi: 10.1084/jem.20121486. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Arend WP, Firestein GS. Pre-rheumatoid arthritis: predisposition and transition to clinical synovitis. Nat Rev Rheumatol. 2012;8(10):573–86. doi: 10.1038/nrrheum.2012.134. [DOI] [PubMed] [Google Scholar]
- 10.Diogo D, Kurreeman F, Stahl EA, et al. Rare, low-frequency, and common variants in the protein-coding sequence of biological candidate genes from GWASs contribute to risk of rheumatoid arthritis. Am J Hum Genet. 2013;92(1):15–27. doi: 10.1016/j.ajhg.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okada Y, Wu D, Trynka G, et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature. 2014;506(7488):376–81. doi: 10.1038/nature12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klareskog L, Malmstrom V, Lundberg K, et al. Smoking, citrullination and genetic variability in the immunopathogenesis of rheumatoid arthritis. Semin Immunol. 2011;23(2):92–8. doi: 10.1016/j.smim.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Haj Hensvold A, Magnusson PK, Joshua V, et al. Environmental and genetic factors in the development of anticitrullinated protein antibodies (ACPAs) and ACPA-positive rheumatoid arthritis: an epidemiological investigation in twins. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2013-203947. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.van Beers JJ, Schwarte CM, Stammen-Vogelzangs J, et al. The rheumatoid arthritis synovial fluid citrullinome reveals novel citrullinated epitopes in apolipoprotein E, myeloid nuclear differentiation antigen, and beta-actin. Arthritis Rheum. 2013;65(1):69–80. doi: 10.1002/art.37720. [DOI] [PubMed] [Google Scholar]
- 15.Romero V, Fert-Bober J, Nigrovic PA, et al. Immune-mediated pore-forming pathways induce cellular hypercitrullination and generate citrullinated autoantigens in rheumatoid arthritis. Sci Transl Med. 2013;5(209):209ra150. doi: 10.1126/scitranslmed.3006869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bojalil R, Mazon-Gonzalez B, Carrillo-Cordova JR, et al. Frequency and clinical significance of a variety of autoantibodies in patients with definite infective endocarditis. J Clin Rheumatol. 2012;18(2):67–70. doi: 10.1097/RHU.0b013e318247caf0. [DOI] [PubMed] [Google Scholar]
- 17.Charles ED, Orloff MI, Nishiuchi E, et al. Somatic hypermutations confer rheumatoid factor activity in hepatitis C virus-associated mixed cryoglobulinemia. Arthritis Rheum. 2013;65(9):2430–40. doi: 10.1002/art.38041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill JA, Southwood S, Sette A, et al. Cutting edge: the conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA-DRB1*0401 MHC class II molecule. J Immunol. 2003;171(2):538–41. doi: 10.4049/jimmunol.171.2.538. [DOI] [PubMed] [Google Scholar]
- 19.Elliott MR, Ravichandran KS. Clearance of apoptotic cells: implications in health and disease. J Cell Biol. 2010;189(7):1059–70. doi: 10.1083/jcb.201004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Law SC, Street S, Yu CH, et al. T-cell autoreactivity to citrullinated autoantigenic peptides in rheumatoid arthritis patients carrying HLA-DRB1 shared epitope alleles. Arthritis Res Ther. 2012;14(3):R118. doi: 10.1186/ar3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uysal H, Bockermann R, Nandakumar KS, et al. Structure and pathogenicity of antibodies specific for citrullinated collagen type II in experimental arthritis. J Exp Med. 2009;206(2):449–62. doi: 10.1084/jem.20081862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young KA, Deane KD, Derber LA, et al. Relatives without rheumatoid arthritis show reactivity to anti-citrullinated protein/ peptide antibodies that are associated with arthritis-related traits: studies of the etiology of rheumatoid arthritis. Arthritis Rheum. 2013;65(8):1995–2004. doi: 10.1002/art.38022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barra L, Scinocca M, Saunders S, et al. Anti-citrullinated protein antibodies in unaffected first-degree relatives of rheumatoid arthritis patients. Arthritis Rheum. 2013;65(6):1439–47. doi: 10.1002/art.37911. [DOI] [PubMed] [Google Scholar]
- 24.Kokkonen H, Mullazehi M, Berglin E, et al. Antibodies of IgG, IgA and IgM isotypes against cyclic citrullinated peptide precede the development of rheumatoid arthritis. Arthritis Res Ther. 2011;13(1):R13. doi: 10.1186/ar3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suwannalai P, Willemze A, van Toorn L, et al. The fine specificity of IgM anti-citrullinated protein antibodies (ACPA) is different from that of IgG ACPA. Arthritis Res Ther. 2011;13(6):R195. doi: 10.1186/ar3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sokolove J, Bromberg R, Deane KD, et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PloS One. 2012;7(5):e35296. doi: 10.1371/journal.pone.0035296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Woude D, Rantapaa-Dahlqvist S, Ioan-Facsinay A, et al. Epitope spreading of the anti-citrullinated protein antibody response occurs before disease onset and is associated with the disease course of early arthritis. Ann Rheum Dis. 2010;69(8):1554–61. doi: 10.1136/ard.2009.124537. [DOI] [PubMed] [Google Scholar]
- 28.Rombouts Y, Ewing E, van de Stadt LA, et al. Anti-citrullinated protein antibodies acquire a pro-inflammatory Fc glycosylation phenotype prior to the onset of rheumatoid arthritis. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2013-203565. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Kinloch A, Lundberg K, Wait R, et al. Synovial fluid is a site of citrullination of autoantigens in inflammatory arthritis. Arthritis Rheum. 2008;58(8):2287–95. doi: 10.1002/art.23618. [DOI] [PubMed] [Google Scholar]
- 30.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Science Transl Med. 2013;5(178):178ra140. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wipke BT, Allen PM. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J Immunol. 2001;167(3):1601–8. doi: 10.4049/jimmunol.167.3.1601. [DOI] [PubMed] [Google Scholar]
- 32.Shi J, Knevel R, Suwannalai P, et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc Natl Acad Sci USA. 2011;108(42):17372–7. doi: 10.1073/pnas.1114465108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mydel P, Wang Z, Brisslert M, et al. Carbamylation-dependent activation of T cells: a novel mechanism in the pathogenesis of autoimmune arthritis. J Immunol. 2010;184(12):6882–90. doi: 10.4049/jimmunol.1000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Vries RR, van der Woude D, Houwing JJ, et al. Genetics of ACPA-positive rheumatoid arthritis: the beginning of the end? Ann Rheum Dis. 2011;70(Suppl 1):i51–4. doi: 10.1136/ard.2010.138040. [DOI] [PubMed] [Google Scholar]
- 35.Sokolove J, Zhao X, Chandra PE, et al. Immune complexes containing citrullinated fibrinogen costimulate macrophages via Toll-like receptor 4 and Fcgamma receptor. Arthritis Rheum. 2011;63(1):53–62. doi: 10.1002/art.30081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scally SW, Petersen J, Law SC, et al. A molecular basis for the association of the HLA-DRB1 locus, citrullination, and rheumatoid arthritis. J Exp Med. 2013;210(12):2569–82. doi: 10.1084/jem.20131241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ji H, Ohmura K, Mahmood U, et al. Arthritis critically dependent on innate immune system players. Immunity. 2002;16(2):157–68. doi: 10.1016/s1074-7613(02)00275-3. [DOI] [PubMed] [Google Scholar]
- 38.Cantaert T, Kolln J, Timmer T, et al. B lymphocyte autoimmunity in rheumatoid synovitis is independent of ectopic lymphoid neogenesis. J Immunol. 2008;181(1):785–94. doi: 10.4049/jimmunol.181.1.785. [DOI] [PubMed] [Google Scholar]
- 39.Humby F, Bombardieri M, Manzo A, et al. Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium. PLoS Med. 2009;6(1):e1. doi: 10.1371/journal.pmed.0060001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosengren S, Wei N, Kalunian KC, et al. Elevated autoantibody content in rheumatoid arthritis synovia with lymphoid aggregates and the effect of rituximab. Arthritis Res Ther. 2008;10(5):R105. doi: 10.1186/ar2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van de Sande MG, de Hair MJ, van der Leij C, et al. Different stages of rheumatoid arthritis: features of the synovium in the preclinical phase. Ann Rheum Dis. 2011;70(5):772–7. doi: 10.1136/ard.2010.139527. [DOI] [PubMed] [Google Scholar]
- 42.Kendall PL, Yu G, Woodward EJ, et al. Tertiary lymphoid structures in the pancreas promote selection of B lymphocytes in autoimmune diabetes. J Immunol. 2007;178(9):5643–51. doi: 10.4049/jimmunol.178.9.5643. [DOI] [PubMed] [Google Scholar]
- 43.Marston B, Palanichamy A, Anolik JH. B cells in the pathogenesis and treatment of rheumatoid arthritis. Curr Opin Rheumatol. 2010;22(3):307–15. doi: 10.1097/BOR.0b013e3283369cb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11(1):34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 45.Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol. 2013;13(2):118–32. doi: 10.1038/nri3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kearney JF. Innate-like B cells. Springer Semin Immunopathol. 2005;26(4):377–83. doi: 10.1007/s00281-004-0184-0. [DOI] [PubMed] [Google Scholar]
- 47.Guerrier T, Youinou P, Pers JO, et al. TLR9 drives the development of transitional B cells towards the marginal zone pathway and promotes autoimmunity. J Autoimmun. 2012;39(3):173–9. doi: 10.1016/j.jaut.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 48.Oliver AM, Martin F, Gartland GL, et al. Marginal zone B cells exhibit unique activation, proliferative and immunoglobulin secretory responses. Eur J Immunol. 1997;27(9):2366–74. doi: 10.1002/eji.1830270935. [DOI] [PubMed] [Google Scholar]
- 49.You Y, Myers RC, Freeberg L, et al. Marginal zone B cells regulate antigen capture by marginal zone macrophages. J Immunol. 2011;186(4):2172–81. doi: 10.4049/jimmunol.1002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cinamon G, Zachariah MA, Lam OM, et al. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat Immunol. 2008;9(1):54–62. doi: 10.1038/ni1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eto D, Lao C, DiToro D, et al. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PloS One. 2011;6(3):e17739. doi: 10.1371/journal.pone.0017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wurster AL, Rodgers VL, White MF, et al. Interleukin-4-mediated protection of primary B cells from apoptosis through Stat6-dependent up-regulation of Bcl-xL. J Biol Chem. 2002;277(30):27169–75. doi: 10.1074/jbc.M201207200. [DOI] [PubMed] [Google Scholar]
- 53.Stahl EA, Raychaudhuri S, Remmers EF, et al. Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat Genet. 2010;42(6):508–14. doi: 10.1038/ng.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci. 2010;1183:38–57. doi: 10.1111/j.1749-6632.2009.05137.x. [DOI] [PubMed] [Google Scholar]
- 55.Ray A, Basu S, Williams CB, et al. A novel IL-10-independent regulatory role for B cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. J Immunol. 2012;188(7):3188–98. doi: 10.4049/jimmunol.1103354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang M, Deng J, Liu Y, et al. IL-10-producing regulatory B10 cells ameliorate collagen-induced arthritis via suppressing Th17 cell generation. Am J Pathol. 2012;180(6):2375–85. doi: 10.1016/j.ajpath.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 57.Maseda D, Smith SH, DiLillo DJ, et al. Regulatory B10 cells differentiate into antibody-secreting cells after transient IL-10 production in vivo. J Immunol. 2012;188(3):1036–48. doi: 10.4049/jimmunol.1102500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Flores-Borja F, Bosma A, Ng D, et al. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci Transl Med. 2013;5(173):173ra123. doi: 10.1126/scitranslmed.3005407. [DOI] [PubMed] [Google Scholar]
- 59.Ma L, Liu B, Jiang Z, et al. Reduced numbers of regulatory B cells are negatively correlated with disease activity in patients with new-onset rheumatoid arthritis. Clin Rheumatol. 2014;33(2):187–95. doi: 10.1007/s10067-013-2359-3. [DOI] [PubMed] [Google Scholar]
- 60.Wilde B, Thewissen M, Damoiseaux J, et al. Regulatory B cells in ANCA-associated vasculitis. Ann Rheum Dis. 2013;72(8):1416–19. doi: 10.1136/annrheumdis-2012-202986. [DOI] [PubMed] [Google Scholar]
- 61.Vadasz Z, Haj T, Kessel A, et al. B-regulatory cells in autoimmunity and immune mediated inflammation. FEBS Lett. 2013;587(13):2074–8. doi: 10.1016/j.febslet.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 62.Ercolini AM, Miller SD. The role of infections in autoimmune disease. Clin Exp Immunol. 2009;155(1):1–15. doi: 10.1111/j.1365-2249.2008.03834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shulzhenko N, Morgun A, Hsiao W, et al. Crosstalk between B lymphocytes, microbiota and the intestinal epithelium governs immunity versus metabolism in the gut. Nat Med. 2011;17(12):1585–93. doi: 10.1038/nm.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kamada N, Seo SU, Chen GY, et al. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13(5):321–35. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 65.Wu HJ, Ivanov II, Darce J, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32(6):815–27. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scher JU, Sczesnak A, Longman RS, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife. 2013;2:e01202. doi: 10.7554/eLife.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chervonsky AV. Influence of microbial environment on autoimmunity. Nat Immunol. 2010;11(1):28–35. doi: 10.1038/ni.1801. [DOI] [PubMed] [Google Scholar]
- 68.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336(6086):1268–73. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gururajan M, Jacob J, Pulendran B. Toll-like receptor expression and responsiveness of distinct murine splenic and mucosal B-cell subsets. PLoS ONE. 2007;2(9):e863. doi: 10.1371/journal.pone.0000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Genestier L, Taillardet M, Mondiere P, et al. TLR agonists selectively promote terminal plasma cell differentiation of B cell subsets specialized in thymus-independent responses. J Immunol. 2007;178(12):7779–86. doi: 10.4049/jimmunol.178.12.7779. [DOI] [PubMed] [Google Scholar]
- 71.Boeglin E, Smulski CR, Brun S, et al. Toll-like receptor agonists synergize with CD40L to induce either proliferation or plasma cell differentiation of mouse B cells. PLoS ONE. 2011;6(10):e25542. doi: 10.1371/journal.pone.0025542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dorner M, Brandt S, Tinguely M, et al. Plasma cell toll-like receptor (TLR) expression differs from that of B cells, and plasma cell TLR triggering enhances immunoglobulin production. Immunology. 2009;128(4):573–9. doi: 10.1111/j.1365-2567.2009.03143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leadbetter EA, Rifkin IR, Hohlbaum AM, et al. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416(6881):603–7. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 74.Goh FG, Midwood KS. Intrinsic danger: activation of Toll-like receptors in rheumatoid arthritis. Rheumatology. 2012;51(1):7–23. doi: 10.1093/rheumatology/ker257. [DOI] [PubMed] [Google Scholar]
- 75.Huang Q, Pope RM. Toll-like receptor signaling: a potential link among rheumatoid arthritis, systemic lupus, and atherosclerosis. J Leukoc Biol. 2010;88(2):253–62. doi: 10.1189/jlb.0310126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lu J, Teh C, Kishore U, et al. Collectins and ficolins: sugar pattern recognition molecules of the mammalian innate immune system. Biochim Biophys Acta. 2002;1572(2–3):387–400. doi: 10.1016/s0304-4165(02)00320-3. [DOI] [PubMed] [Google Scholar]
- 77.Maeda K, Mehta H, Drevets DA, et al. IL-6 increases B-cell IgG production in a feed-forward proinflammatory mechanism to skew hematopoiesis and elevate myeloid production. Blood. 2010;115(23):4699–706. doi: 10.1182/blood-2009-07-230631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yan Y, Wang YH, Diamond B. IL-6 contributes to an immune tolerance checkpoint in post germinal center B cells. J Autoimmun. 2012;38(1):1–9. doi: 10.1016/j.jaut.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Taniguchi N, Kawahara K, Yone K, et al. High mobility group box chromosomal protein 1 plays a role in the pathogenesis of rheumatoid arthritis as a novel cytokine. Arthritis Rheum. 2003;48(4):971–81. doi: 10.1002/art.10859. [DOI] [PubMed] [Google Scholar]
- 80.Kokkola R, Li J, Sundberg E, et al. Successful treatment of collagen-induced arthritis in mice and rats by targeting extracellular high mobility group box chromosomal protein 1 activity. Arthritis Rheum. 2003;48(7):2052–8. doi: 10.1002/art.11161. [DOI] [PubMed] [Google Scholar]
- 81.Crofford LJ, Lipsky PE, Brooks P, et al. Basic biology and clinical application of specific cyclooxygenase-2 inhibitors. Arthritis Rheum. 2000;43(1):4–13. doi: 10.1002/1529-0131(200001)43:1<4::AID-ANR2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 82.Murn J, Alibert O, Wu N, et al. Prostaglandin E2 regulates B cell proliferation through a candidate tumor suppressor, Ptger4. J Exp Med. 2008;205(13):3091–103. doi: 10.1084/jem.20081163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Prijatelj M, Celhar T, Mlinaric-Rascan I. Prostaglandin EP4 receptor enhances BCR-induced apoptosis of immature B cells. Prostaglandins Other Lipid Mediat. 2011;95(1–4):19–26. doi: 10.1016/j.prostaglandins.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 84.Magari M, Nishikawa Y, Fujii Y, et al. IL-21-dependent B cell death driven by prostaglandin E2, a product secreted from follicular dendritic cells. J Immunol. 2011;187(8):4210–18. doi: 10.4049/jimmunol.1100934. [DOI] [PubMed] [Google Scholar]
- 85.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282(16):11613–17. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 86.McCoy JM, Wicks JR, Audoly LP. The role of prostaglandin E2 receptors in the pathogenesis of rheumatoid arthritis. J Clin Invest. 2002;110(5):651–8. doi: 10.1172/JCI15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Honda T, Segi-Nishida E, Miyachi Y, et al. Prostacyclin-IP signaling and prostaglandin E2-EP2/EP4 signaling both mediate joint inflammation in mouse collagen-induced arthritis. J Exp Med. 2006;203(2):325–35. doi: 10.1084/jem.20051310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cantaert T, Teitsma C, Tak PP, et al. Presence and role of anti-citrullinated protein antibodies in experimental arthritis models. Arthritis Rheum. 2013;65(4):939–48. doi: 10.1002/art.37839. [DOI] [PubMed] [Google Scholar]
- 89.Kojima F, Frolov A, Matnani R, et al. Reduced T Cell-dependent humoral immune response in microsomal prostaglandin E synthase-1 null mice is mediated by nonhematopoietic cells. J Immunol. 2013;191(10):4979–88. doi: 10.4049/jimmunol.1301942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shlomchik MJ. Sites and stages of autoreactive B cell activation and regulation. Immunity. 2008;28(1):18–28. doi: 10.1016/j.immuni.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 91.Vinuesa CG, Chang PP. Innate B cell helpers reveal novel types of antibody responses. Nat Immunol. 2013;14(2):119–26. doi: 10.1038/ni.2511. [DOI] [PubMed] [Google Scholar]
- 92.Leadbetter EA, Brigl M, Illarionov P, et al. NK T cells provide lipid antigen-specific cognate help for B cells. Proc Natl Acad Sci USA. 2008;105(24):8339–44. doi: 10.1073/pnas.0801375105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Barral P, Eckl-Dorna J, Harwood NE, et al. B cell receptor-mediated uptake of CD1d-restricted antigen augments antibody responses by recruiting invariant NKT cell help in vivo. Proc Natl Acad Sci USA. 2008;105(24):8345–50. doi: 10.1073/pnas.0802968105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chang PP, Barral P, Fitch J, et al. Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat Immunol. 2012;13(1):35–43. doi: 10.1038/ni.2166. [DOI] [PubMed] [Google Scholar]
- 95.Puga I, Cols M, Barra CM, et al. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol. 2012;13(2):170–80. doi: 10.1038/ni.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mancardi DA, Jonsson F, Iannascoli B, et al. Cutting edge: the murine high-affinity IgG receptor FcgammaRIV is sufficient for autoantibody-induced arthritis. J Immunol. 2011;186(4):1899–903. doi: 10.4049/jimmunol.1003642. [DOI] [PubMed] [Google Scholar]
- 97.Laurent L, Clavel C, Lemaire O, et al. Fcgamma receptor profile of monocytes and macrophages from rheumatoid arthritis patients and their response to immune complexes formed with autoantibodies to citrullinated proteins. Ann Rheum Dis. 2011;70(6):1052–9. doi: 10.1136/ard.2010.142091. [DOI] [PubMed] [Google Scholar]
- 98.Spits H, Artis D, Colonna M, et al. Innate lymphoid cells–a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13(2):145–9. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 99.Vinuesa CG, Linterman MA, Goodnow CC, et al. T cells and follicular dendritic cells in germinal center B-cell formation and selection. Immunol Rev. 2010;237(1):72–89. doi: 10.1111/j.1600-065X.2010.00937.x. [DOI] [PubMed] [Google Scholar]
- 100.Yoshizaki A, Miyagaki T, DiLillo DJ, et al. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature. 2012;491(7423):264–8. doi: 10.1038/nature11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Allen CD, Cyster JG. Follicular dendritic cell networks of primary follicles and germinal centers: phenotype and function. Semin Immunol. 2008;20(1):14–25. doi: 10.1016/j.smim.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Heesters BA, Chatterjee P, Kim YA, et al. Endocytosis and recycling of immune complexes by follicular dendritic cells enhances B cell antigen binding and activation. Immunity. 2013;38(6):1164–75. doi: 10.1016/j.immuni.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chappell CP, Draves KE, Giltiay NV, et al. Extrafollicular B cell activation by marginal zone dendritic cells drives T cell-dependent antibody responses. J Exp Med. 2012;209(10):1825–40. doi: 10.1084/jem.20120774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gujer C, Sandgren KJ, Douagi I, et al. IFN-alpha produced by human plasmacytoid dendritic cells enhances T cell-dependent naive B cell differentiation. J Leukoc Biol. 2011;89(6):811–21. doi: 10.1189/jlb.0810460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McGaha TL, Chen Y, Ravishankar B, et al. Marginal zone macrophages suppress innate and adaptive immunity to apoptotic cells in the spleen. Blood. 2011;117(20):5403–12. doi: 10.1182/blood-2010-11-320028. [DOI] [PubMed] [Google Scholar]
- 106.Li H, Wu Q, Li J, et al. Cutting Edge: defective follicular exclusion of apoptotic antigens due to marginal zone macrophage defects in autoimmune BXD2 mice. J Immunol. 2013;190(9):4465–9. doi: 10.4049/jimmunol.1300041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Takemura S, Braun A, Crowson C, et al. Lymphoid neogenesis in rheumatoid synovitis. J Immunol. 2001;167(2):1072–80. doi: 10.4049/jimmunol.167.2.1072. [DOI] [PubMed] [Google Scholar]
- 108.Wengner AM, Hopken UE, Petrow PK, et al. CXCR5- and CCR7-dependent lymphoid neogenesis in a murine model of chronic antigen-induced arthritis. Arthritis Rheum. 2007;56(10):3271–83. doi: 10.1002/art.22939. [DOI] [PubMed] [Google Scholar]
- 109.Yeo L, Toellner KM, Salmon M, et al. Cytokine mRNA profiling identifies B cells as a major source of RANKL in rheumatoid arthritis. Ann Rheum Dis. 2011;70(11):2022–8. doi: 10.1136/ard.2011.153312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mansour A, Anginot A, Mancini SJ, et al. Osteoclast activity modulates B-cell development in the bone marrow. Cell Res. 2011;21(7):1102–15. doi: 10.1038/cr.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tokoyoda K, Egawa T, Sugiyama T, et al. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20(6):707–18. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 112.Yun TJ, Tallquist MD, Aicher A, et al. Osteoprotegerin, a crucial regulator of bone metabolism, also regulates B cell development and function. J Immunol. 2001;166(3):1482–91. doi: 10.4049/jimmunol.166.3.1482. [DOI] [PubMed] [Google Scholar]
- 113.Zhu J, Garrett R, Jung Y, et al. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. 2007;109(9):3706–12. doi: 10.1182/blood-2006-08-041384. [DOI] [PubMed] [Google Scholar]
- 114.Tsubaki T, Takegawa S, Hanamoto H, et al. Accumulation of plasma cells expressing CXCR3 in the synovial sublining regions of early rheumatoid arthritis in association with production of Mig/ CXCL9 by synovial fibroblasts. Clin Exp Immunol. 2005;141(2):363–71. doi: 10.1111/j.1365-2249.2005.02850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rosengren S, Wei N, Kalunian KC, et al. CXCL13: a novel biomarker of B-cell return following rituximab treatment and synovitis in patients with rheumatoid arthritis. Rheumatology. 2011;50(3):603–10. doi: 10.1093/rheumatology/keq337. [DOI] [PubMed] [Google Scholar]
- 116.Bombardieri M, Kam NW, Brentano F, et al. A BAFF/APRIL-dependent TLR3-stimulated pathway enhances the capacity of rheumatoid synovial fibroblasts to induce AID expression and Ig class-switching in B cells. Ann Rheum Dis. 2011;70(10):1857–65. doi: 10.1136/ard.2011.150219. [DOI] [PubMed] [Google Scholar]
- 117.Ohata J, Zvaifler NJ, Nishio M, et al. Fibroblast-like synoviocytes of mesenchymal origin express functional B cell-activating factor of the TNF family in response to proinflammatory cytokines. J Immunol. 2005;174(2):864–70. doi: 10.4049/jimmunol.174.2.864. [DOI] [PubMed] [Google Scholar]
- 118.Nakayama T, Hieshima K, Izawa D, et al. Cutting edge: profile of chemokine receptor expression on human plasma cells accounts for their efficient recruitment to target tissues. J Immunol. 2003;170(3):1136–40. doi: 10.4049/jimmunol.170.3.1136. [DOI] [PubMed] [Google Scholar]
- 119.Mourcin F, Breton C, Tellier J, et al. Galectin-1-expressing stromal cells constitute a specific niche for pre-BII cell development in mouse bone marrow. Blood. 2011;117(24):6552–61. doi: 10.1182/blood-2010-12-323113. [DOI] [PubMed] [Google Scholar]
- 120.Anginot A, Espeli M, Chasson L, et al. Galectin 1 modulates plasma cell homeostasis and regulates the humoral immune response. J Immunol. 2013;190(11):5526–33. doi: 10.4049/jimmunol.1201885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bugatti S, Manzo A, Caporali R, et al. Inflammatory lesions in the bone marrow of rheumatoid arthritis patients: a morphological perspective. Arthritis Res Ther. 2012;14(6):229. doi: 10.1186/ar4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chu VT, Frohlich A, Steinhauser G, et al. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat Immunol. 2011;12(2):151–9. doi: 10.1038/ni.1981. [DOI] [PubMed] [Google Scholar]
- 123.Koble C, Kyewski B. The thymic medulla: a unique microenvironment for intercellular self-antigen transfer. J Exp Med. 2009;206(7):1505–13. doi: 10.1084/jem.20082449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lukacs-Kornek V, Turley SJ. Self-antigen presentation by dendritic cells and lymphoid stroma and its implications for autoimmunity. Curr Opin Immunol. 2011;23(1):138–45. doi: 10.1016/j.coi.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Manzo A, Bombardieri M, Humby F, et al. Secondary and ectopic lymphoid tissue responses in rheumatoid arthritis: from inflammation to autoimmunity and tissue damage/remodeling. Immunol Rev. 2010;233(1):267–85. doi: 10.1111/j.0105-2896.2009.00861.x. [DOI] [PubMed] [Google Scholar]
- 126.Weyand CM, Kang YM, Kurtin PJ, et al. The power of the third dimension: tissue architecture and autoimmunity in rheumatoid arthritis. Curr Opin Rheumatol. 2003;15(3):259–66. doi: 10.1097/00002281-200305000-00013. [DOI] [PubMed] [Google Scholar]
- 127.Fujii W, Ashihara E, Hirai H, et al. Myeloid-derived suppressor cells play crucial roles in the regulation of mouse collagen-induced arthritis. J Immunol. 2013;191(3):1073–81. doi: 10.4049/jimmunol.1203535. [DOI] [PubMed] [Google Scholar]
- 128.Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54(9):2793–806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- 129.Huang H, Benoist C, Mathis D. Rituximab specifically depletes short-lived autoreactive plasma cells in a mouse model of inflammatory arthritis. Proc Natl Acad Sci USA. 2010;107(10):4658–63. doi: 10.1073/pnas.1001074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Montalvao F, Garcia Z, Celli S, et al. The mechanism of anti-CD20-mediated B cell depletion revealed by intravital imaging. J Clin Invest. 2013;123(12):5098–103. doi: 10.1172/JCI70972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Taylor PC, Quattrocchi E, Mallett S, et al. Ofatumumab, a fully human anti-CD20 monoclonal antibody, in biological-naive, rheumatoid arthritis patients with an inadequate response to methotrexate: a randomised, double-blind, placebo-controlled clinical trial. Ann Rheum Dis. 2011;70(12):2119–25. doi: 10.1136/ard.2011.151522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bologna L, Gotti E, Manganini M, et al. Mechanism of action of type II, glycoengineered, anti-CD20 monoclonal antibody GA101 in B-chronic lymphocytic leukemia whole blood assays in comparison with rituximab and alemtuzumab. J Immunol. 2011;186(6):3762–9. doi: 10.4049/jimmunol.1000303. [DOI] [PubMed] [Google Scholar]
- 133.Geng SS, Feng J, Li Y, et al. Binding activity difference of anti-CD20 scFv-Fc fusion protein derived from variable domain exchange. Cell Mol Immunol. 2006;3(6):439–43. [PubMed] [Google Scholar]
- 134.Hamel KM, Cao Y, Ashaye S, et al. B cell depletion enhances T regulatory cell activity essential in the suppression of arthritis. J Immunol. 2011;187(9):4900–6. doi: 10.4049/jimmunol.1101844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.van de Veerdonk FL, Lauwerys B, Marijnissen RJ, et al. The anti-CD20 antibody rituximab reduces the Th17 cell response. Arthritis Rheum. 2011;63(6):1507–16. doi: 10.1002/art.30314. [DOI] [PubMed] [Google Scholar]
- 136.Gheorghe KR, Thurlings RM, Westman M, et al. Prostaglandin E2 synthesizing enzymes in rheumatoid arthritis B cells and the effects of B cell depleting therapy on enzyme expression. PLoS ONE. 2011;6(1):e16378. doi: 10.1371/journal.pone.0016378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tedder TF. CD19: a promising B cell target for rheumatoid arthritis. Nat Rev Rheumatol. 2009;5(10):572–7. doi: 10.1038/nrrheum.2009.184. [DOI] [PubMed] [Google Scholar]
- 138.Mei HE, Schmidt S, Dorner T. Rationale of anti-CD19 immunotherapy: an option to target autoreactive plasma cells in autoimmunity. Arthritis Res Ther. 2012;14(Suppl 5):S1. doi: 10.1186/ar3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chu SY, Yeter K, Kotha R, et al. Suppression of rheumatoid arthritis B cells by XmAb5871, an anti-CD19 antibody that coengages B cell antigen receptor and FcgammaRIIb inhibitory receptor. Arthritis Rheum. 2013 doi: 10.1002/art.38334. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 140.Moura RA, Canhao H, Polido-Pereira J, et al. BAFF and TACI gene expression are increased in patients with untreated very early rheumatoid arthritis. J Rheumatol. 2013;40(8):1293–302. doi: 10.3899/jrheum.121110. [DOI] [PubMed] [Google Scholar]
- 141.Genovese MC, Fleischmann RM, Greenwald M, et al. Tabalumab, an anti-BAFF monoclonal antibody, in patients with active rheumatoid arthritis with an inadequate response to TNF inhibitors. Ann Rheum Dis. 2013;72(9):1461–8. doi: 10.1136/annrheumdis-2012-202775. [DOI] [PubMed] [Google Scholar]
- 142.van Vollenhoven RF, Kinnman N, Vincent E, et al. Atacicept in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase II, randomized, placebo-controlled trial. Arthritis Rheum. 2011;63(7):1782–92. doi: 10.1002/art.30372. [DOI] [PubMed] [Google Scholar]
- 143.Stohl W, Merrill JT, McKay JD, et al. Efficacy and safety of belimumab in patients with rheumatoid arthritis: a phase II, randomized, double-blind, placebo-controlled, dose-ranging Study. J Rheumatol. 2013;40(5):579–89. doi: 10.3899/jrheum.120886. [DOI] [PubMed] [Google Scholar]
- 144.Neubert K, Meister S, Moser K, et al. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat Med. 2008;14(7):748–55. doi: 10.1038/nm1763. [DOI] [PubMed] [Google Scholar]
- 145.Meister S, Schubert U, Neubert K, et al. Extensive immunoglobulin production sensitizes myeloma cells for proteasome inhibition. Cancer Res. 2007;67(4):1783–92. doi: 10.1158/0008-5472.CAN-06-2258. [DOI] [PubMed] [Google Scholar]
- 146.van der Heijden JW, Oerlemans R, Lems WF, et al. The proteasome inhibitor bortezomib inhibits the release of NFkappaB-inducible cytokines and induces apoptosis of activated T cells from rheumatoid arthritis patients. Clin Exp Rheumatol. 2009;27(1):92–8. [PubMed] [Google Scholar]
- 147.Yannaki E, Papadopoulou A, Athanasiou E, et al. The proteasome inhibitor bortezomib drastically affects inflammation and bone disease in adjuvant-induced arthritis in rats. Arthritis Rheum. 2010;62(11):3277–88. doi: 10.1002/art.27690. [DOI] [PubMed] [Google Scholar]
- 148.Lee SW, Kim JH, Park YB, et al. Bortezomib attenuates murine collagen-induced arthritis. Ann Rheum Dis. 2009;68(11):1761–7. doi: 10.1136/ard.2008.097709. [DOI] [PubMed] [Google Scholar]
- 149.Toubi E, Kessel A, Slobodin G, et al. Changes in macrophage function after rituximab treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66(6):818–20. doi: 10.1136/ard.2006.062505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bao Y, Liu X, Han C, et al. Identification of IFN-gamma-producing innate B cells. Cell Res. 2014;24(2):161–76. doi: 10.1038/cr.2013.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wallace DJ, Kalunian K, Petri MA, et al. Efficacy and safety of epratuzumab in patients with moderate/severe active systemic lupus erythematosus: results from EMBLEM, a phase IIb, randomised, double-blind, placebo-controlled, multicentre study. Ann Rheum Dis. 2014;73(1):183–90. doi: 10.1136/annrheumdis-2012-202760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Steinfeld SD, Tant L, Burmester GR, et al. Epratuzumab (humanised anti-CD22 antibody) in primary Sjogren’s syndrome: an open-label phase I/II study. Arthritis Res Ther. 2006;8(4):R129. doi: 10.1186/ar2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bonelli M, Ferner E, Goschl L, et al. Abatacept (CTLA-4IG) treatment reduces the migratory capacity of monocytes in patients with rheumatoid arthritis. Arthritis Rheum. 2013;65(3):599–607. doi: 10.1002/art.37787. [DOI] [PubMed] [Google Scholar]
- 154.Jones G, Sebba A, Gu J, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis. 2010;69(1):88–96. doi: 10.1136/ard.2008.105197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kremer JM, Blanco R, Brzosko M, et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum. 2011;63(3):609–21. doi: 10.1002/art.30158. [DOI] [PubMed] [Google Scholar]
- 156.A Study in Moderate to Severe Rheumatoid Arthritis (RA-BEAM) Available from: http://clinicaltrials.gov/show/NCT01710358.
- 157.Evaluation of efficacy and safety of fostamatinib monotherapy compared with adalimumab monotherapy in patients with rheumatoid arthritis (RA) (OSKIRA -4) Available from: http://clinicaltrials.gov/show/NCT01264770.
- 158.Liu L, Di Paolo J, Barbosa J, et al. Antiarthritis effect of a novel Bruton’s tyrosine kinase (BTK) inhibitor in rat collagen-induced arthritis and mechanism-based pharmacokinetic/pharmacodynamic modeling: relationships between inhibition of BTK phosphorylation and efficacy. J Pharmacol Exp Ther. 2011;338(1):154–63. doi: 10.1124/jpet.111.181545. [DOI] [PubMed] [Google Scholar]
- 159.Di Paolo JA, Huang T, Balazs M, et al. Specific Btk inhibition suppresses B cell-and myeloid cell-mediated arthritis. Nat Chem Biol. 2011;7(1):41–50. doi: 10.1038/nchembio.481. [DOI] [PubMed] [Google Scholar]
- 160.Dubovsky JA, Beckwith KA, Natarajan G, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122(15):2539–49. doi: 10.1182/blood-2013-06-507947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bonami RHSA, Case JB, Hoek KL, et al. Bruton’s tyrosine kinase promotes persistence of mature anti-insulin B cells. J Immunol. 2014;192(4):1459–70. doi: 10.4049/jimmunol.1300125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Thomas R. Dendritic cells and the promise of antigen-specific therapy in rheumatoid arthritis. Arthritis Res Ther. 2013;15(1):204. doi: 10.1186/ar4130. [DOI] [PMC free article] [PubMed] [Google Scholar]