Abstract
Background
Sarcoptic mange is a contagious skin disease caused by the mite Sarcoptes scabiei, affecting different mammalian species worldwide including the Iberian ibex (Capra pyrenaica), in which mortalities over 90 % of the population have been reported. No efficient diagnostic methods are available for this disease, particularly when there are low mite numbers and mild or no clinical signs. In this study, three enzyme-linked immunosorbent assays (ELISA) developed for dog (ELISA A), Cantabrian chamois (Rupicapra pyrenaica parva) (ELISA B) and Alpine chamois (Rupicapra rupicapra) (ELISA C), were evaluated to detect specific antibodies (IgG) to sarcoptic mange in Iberian ibex sera.
Methods
Serum samples from 131 Iberian ibexes (86 healthy and 45 scabietic) were collected from 2005 to 2012 in the Sierra Nevada Natural and National Parks (southern Spain). Based on visual inspection, ibexes were classified into one of three categories, namely healthy (without scabietic compatible lesions), mildly affected (skin lesions over less than 50 % of the body surface) and severely affected (skin lesions over more than 50 % of the body surface). The optimal cut-off point, specificity, sensitivity and the area under the curve (AUC) were calculated, and the agreement between tests was determined. Moreover, differences in the optical density (OD) related to scabies severity have been evaluated for the best test.
Results
ELISA C showed better performance than the two other tests, reaching higher values of sensitivity (93.0 %) and specificity (93.5 %) against the visual estimation of the percentage of affected skin, chosen as the gold standard. Significantly higher concentrations of specific antibodies were observed with this test in the mildly and severely infested ibexes than in healthy ones.
Conclusions
Our results revealed that ELISA C was an optimal test to diagnose sarcoptic mange in the Iberian ibex. Further studies characterizing immune response during the course of the disease, including spontaneous or drug induced recovery, should follow in order to better understand sarcoptic mange in Iberian ibex populations.
Keywords: Capra pyrenaica, Diagnostic, ELISA, Sarcoptes scabiei, Serum
Background
Sarcoptic mange is a contagious skin disease caused by the mite Sarcoptes scabiei (Linnaeus, 1758). This parasite is worldwide distributed [1–3], causing disease in a wide range of mammalian species [2]. Wild ungulates are one of the most affected groups, as demonstrated by mange outbreak records in African and European ungulates [4–6]. All wild ruminant species in Spain have also been affected by sarcoptic mange, even causing fatal epizootics, e.g. Iberian ibex (Capra pyrenaica) [7–9], Cantabrian chamois (Rupicapra pyrenaica parva) [10] and aoudad (Ammotragus lervia) [11]. The clinical signs and lesions in individuals infected by S. scabiei are characterized by pruritus, drying, peeling, alopecia, hyperkeratosis and scab formation. In population terms, this parasite is able to cause severe demographic downturns, up to above 90 % on occasion [12]. In fact, stochastic simulations on population extinction have shown that the impact of sarcoptic mange on ungulate populations can be comparable to the impact observed for emerging viral diseases [13].
Iberian ibex is a mountain ungulate endemic to the Iberian Peninsula [14]. Andalusia populations (southern Spain) have suffered sarcoptic mange outbreaks since 1987 [7], and recently the eastern populations have been also affected by this disease. Different methods exist for the diagnosis of sarcoptic mange, although none of them showed the ideal specificity and sensitivity [15]. Amongst direct diagnostic methods, the gold standard is the microscopical detection of mites, exuviae, eggs or faeces in scrapings of infested skin. Although this method is deemed 100 % specific, low sensitivity was found at low mite densities [15, 16]. Visual diagnosis of scabies compatible lesions has also been used for monitoring the disease in free-ranging ibexes [17], and this disease has been included among those to check before translocating wild ruminants in Spain, visual inspection being the legally mandatory diagnostic method [18]. A number of methods have been developed or proposed in an attempt to overcome such diagnostic deficiency, including the adhesive tape test [16, 19], serological methods [20, 21], polymerase chain reaction (PCR) [22, 23], dermoscopy [16, 24, 25], termography [26], or trained disease-detector dogs [27].
Sarcoptes scabiei stimulate E and G immunoglobulin production in infested hosts [28–33], including Iberian ibex [34, 35]. Different commercial enzyme-linked immunosorbent assays (ELISA) have been evaluated to detect specific antibodies to S. scabiei in dogs [36, 37], pigs [38, 39], wild boar [40] or chamois [41]. However, the use of ELISA tests to diagnose scabies in Iberian ibex has not yet been evaluated.
In this study, three IgG indirect ELISA tests were compared as diagnostic tools of sarcoptic mange in ibexes showing different lesional severity. In particular, the objectives of this study are: (i) to estimate the optimal cut-off points, specificity and sensitivity of the ELISA tests in Iberian ibex; (ii) to determine the agreement between tests; and (iii) to determine if a correlation between mange severity and the detected levels of humoral immune response (IgG) exists.
Methods
Sample collection
Serum samples from 131 healthy and scabietic Iberian ibexes were collected from 2005 to 2012 in Sierra Nevada Natural and National Parks (36°00'–37°10'N, 2°34'–3°40'W) (Table 1). The ibexes were chemically immobilized using a combination of ketamine and xylazine (3.0 + 3.0 mg/kg) [42]. After induction, blood samples were collected from the jugular vein and kept at 4 °C in a cold box. The age of the ibexes was estimated by horn-segment counts [12], and ranged between 1 and 12 years. Based on the visual estimation of the percentage of scabietic skin, each individual was classified into one of three categories [17, 43]: healthy (although the authors are aware that the absence of scabietic-compatible lesions is not a synonym of “uninfested”, for the purpose of this study “healthy” will be used as “without visible scabietic-compatible lesions”), mildly infested (0–50 % of the body surface affected by sarcoptic mange), and severely infested (more than 50 % of the surface affected) (Table 2). This diagnostic method was considered as the “gold standard” to evaluate the three ELISA tests.
Table 1.
Number of Iberian ibexes (Capra pyrenaica) sampled in the Sierra Nevada Natural and National Parks according to their sex and sarcoptic mange status
| Sarcoptic mange status | Sex | Total | |
|---|---|---|---|
| Males | Females | ||
| Healthya | 59 | 27 | 86 |
| Mildly infestedb | 31 | 1 | 32 |
| Severely infestedc | 9 | 4 | 13 |
| Total | 99 | 32 | 131 |
aWithout scabietic compatible lesions
bLess than 50 % of the body surface affected
cMore than 50 % of the body surface affected
Table 2.
Number of Iberian ibexes (Capra pyrenaica) sampled in the Sierra Nevada Natural and National Parks according the ELISA results and the categories based on the visually estimated percentage of affected skin [17]
| Healthya | Infested | ||||
|---|---|---|---|---|---|
| Mildlyb | Severelyc | Total | |||
| ELISA A | Positive serology | 9 | 10 | 4 | 14 |
| Negative serology | 76 | 21 | 8 | 29 | |
| Total | 85 | 31 | 12 | 43 | |
| ELISA B | Positive serology | 16 | 13 | 12 | 25 |
| Negative serology | 58 | 17 | 1 | 18 | |
| Total | 74 | 30 | 13 | 43 | |
| ELISA C | Positive serology | 5 | 27 | 13 | 40 |
| Negative serology | 72 | 3 | 0 | 3 | |
| Total | 77 | 30 | 13 | 43 | |
aWithout scabietic compatible lesions
bLess than 50 % of the body surface affected
aMore than 50 % of the body surface affected
Serological analyses
Blood samples were allowed to clot at room temperature. Within 24 h from collection, serum was obtained by centrifugation at 4,750× g for ten minutes and stored at -20 °C until analysis.
The Iberian ibex sera were tested by three indirect ELISAs developed for dog (ELISA A, n = 128) [44], Cantabrian chamois (ELISA B, n = 117) [45] and Alpine chamois (ELISA C, n = 120) (in-house indirect ELISA, Istituto Zooprofilattico Sperimentale delle Venezie, modified from [41]). ELISA A is a commercial ELISA (Univet, Barcelona, Spain) developed for the diagnosis in dog. The plates are coated with S. scabiei var. canis antigen. To adapt the ELISA A for its use in ibex, specific antibodies for goat (Donkey antigoat IgG-HRP SC-2020, Santa Cruz Biotechnology, Dallas, Texas, USA) were used. ELISA B is based on a structural antigen of the mite (Ssλ20∆B3), whose encoding cDNA was identified by screening of S. scabiei var. hominis library using the sera from an infected chamois, and expressed in Escherichia coli as a unitary recombinant antigen [45]. ELISA C is an in-house method, developed by the Istituto Zooprofilattico Sperimentale delle Venezie and validated for lung extract. ELISA C is a modification from [41] by using commercial ELISA plates coated with S. scabiei var. suis antigen (Sarcoptes-Elisa 2001® PIG, AFOSA GmbH, Blankenfelde-Mahlow, Germany) instead of the red fox (Vulpes vulpes) S. scabiei antigens originally used [41]. This test uses the avidin-biotin detection system, as previously reported [41].
Samples were analyzed in duplicate, and in triplicate in ELISA C.
The optical density (OD) was read at 450 nm in ELISA A and B, and at 405 nm in ELISA C, and was expressed as Optical Density percentage (OD %) using the following formula:
where OD % is the optical density percentage; ODS is the mean optical density (OD) of the sample (two or three replicates); ODNcon is the mean OD of the negative control; and ODPcon is the mean OD of the positive control.
Ten sera from healthy Iberian ibexes (animals without skin lesions) without previous contact with mange were used as negative controls, whereas ten sera from actively sarcoptic mange-infested Iberian ibexes were used as positive controls. The same negative and positive control sera were used for the three ELISA tests, and all controls consistently showed low (negative controls) and high (positive controls) values for the three ELISA tests.
Statistical analysis
The optimal cut-off points between positive and negative samples was estimated using the Youden index, maximizing the difference between true positive rate (sensitivity) and false positive rate (1 - specificity). Thereby, the maximum of sensitivity and specificity is achieved [46, 47]. Once the optimal cut-off points were established and positive and negative individuals assigned, the specificity and sensitivity of the three ELISA tests were then established using the following formulae [48]:
After that, the area under a receiver operating characteristic (ROC) curve, a graph of true positive rate (sensitivity) versus false positive rate (1 - specificity), was used to determine the diagnosis accuracy of the ELISA tests [49, 50].
The agreement between the ELISA tests was evaluated by the Cohen’s Kappa coefficient [51] and Bland-Altman plots [52, 53]. The Cohen’s Kappa coefficient ranges between 0 and 1, and values close to 1 indicate high correlation. Bland-Altman plot describes the agreement between two quantitative measurements by constructing limits of agreement, which are calculated by using the mean and the standard deviation of the differences between two measurements. The difference of the two paired measurements is plotted against the mean of the two measurements.
Since ELISA results did not fit a normal distribution, as assayed by Agostino skewness test and Bonett-Seier test for Geary kurtosis, Kruskal-Wallis with Mann-Whitney tests with Bonferroni correction were used to evaluate whether optical density (a proxy for immunoglobulin G production) was influenced by mange severity (healthy, mildly and severely infested). This analysis was performed only with the optical density values from the ELISA test showing the highest sensitivity and specificity against the gold standard (ELISA C, see below).
Although sex and season determine sarcoptic mange severity and progression in Iberian ibex [17, 34, 54, 55], the effects of these two factors on ELISA performance could not be evaluated due to insufficient sample size. Statistical analyses were performed using the R software version 3.2.4 (R Development Core Team 2016, The R Foundation, Vienna, Austria). Skewness and kurtosis tests were performed with the “moments” package [56], Bland-Altman regressions with the “BlandAltmanLeh” package [57], the Kappa coefficients with the “irr” package [58], and the ROC-curves and the cut-off points were estimated with the “OptimalCutpoints” package [59].
Results
The cut-off points, sensitivity and specificity obtained for the three ELISA tests are shown in Table 3. The best results were obtained with ELISA C (cut-off = 92.0 % OD; sensitivity = 93.0 %; specificity = 93.5 %), followed by ELISA B (cut-off = 10.4 % OD; sensitivity = 58.1 %; specificity = 78.4 %) and ELISA A (cut-off = 45.5 % OD; sensitivity = 32.6 %; specificity = 89.4 %). Consequently, the area under the curve (AUC) obtained for ELISA C was close to 1 (AUC = 0.971), indicating an excellent diagnostic accuracy. Conversely, the AUC for ELISA A and B was lower (0.594 and 0.691, respectively), indicating a poorer diagnosis accuracy of these two tests (Fig. 1, Table 3). The false negative ibexes (i.e. ibexes with negative ELISA results but showing skin lesions compatible with sarcoptic mange) belonged to the mildly infested category in all cases (100 %, 3 out of 3) for ELISA C and most cases for ELISA B (94.4 %, 17 out of 18) and ELISA A (72.4 %, 21 out of 29) (Table 2). Furthermore, the three mildly infested false negative cases for ELISA C, 15 out of 17 for ELISA B, and 13 out of 21 for ELISA A had less than 25 % of the body surface affected, based on the visually estimated percentage of affected skin [17, 43].
Table 3.
Area under the curve (AUC) and its 95 % confidence interval (CI), cut-off, sensitivity and specificity values of the ELISA tests
| ELISA A | ELISA B | ELISA C | |
|---|---|---|---|
| AUC (95 % CI) | 0.594 (0.488–0.700) | 0.691 (0.590–0.793) | 0.971 (0.947–0.995) |
| Cut-off (% OD) | 45.5 | 10.4 | 92.0 |
| Sensitivity (%) | 32.6 | 58.1 | 93.0 |
| Specificity (%) | 89.4 | 78.4 | 93.5 |
Fig. 1.
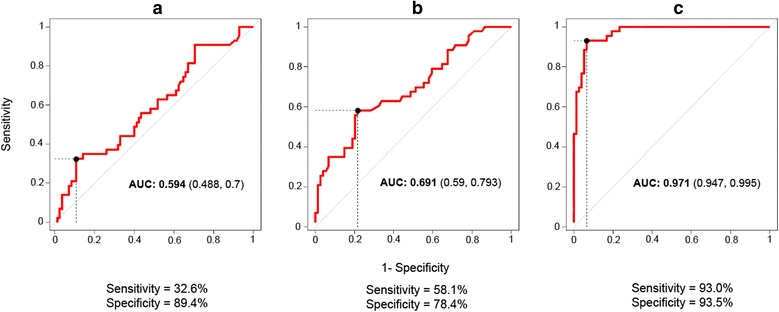
Receiver operating characteristic (ROC) curves for ELISA A (a), ELISA B (b) and ELISA C (c) for the detection of antibodies against Sarcoptes scabiei in Iberian ibex. The red line shows the mean area under the curve (AUC) plot, with the AUC value and the 95 % confidence intervals in parentheses. The sensitivity and specificity values correspond to the points in the plots
Bland-Altman plots show that the three ELISA tests are not interchangeable, especially at high values of OD (Fig. 2). Along the same lines, the Cohen’s Kappa coefficient (k) indicated that there is not a good strength of agreement between the three tests. The ELISA B and C showed the higher strength of agreement, which was only moderate, whereas the strength of agreement between the ELISA A and C was still lower (fair) and the ELISA A and B showed the lowest strength of agreement (slight) [60].
Fig. 2.
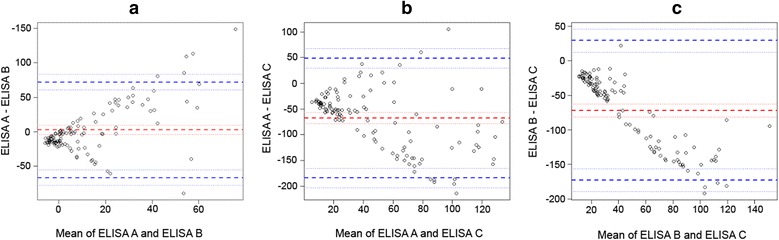
Bland-Altman plots comparing the ELISA tests. Dashed blue lines indicate the confidence interval limits for the agreement limits (± 1.96*standard deviation); dashed red lines indicate the confidence interval limits for the mean bias difference. a Comparison of results for ELISA A and ELISA B: differences tended to increase with increasing mean % OD values. b Comparison of results for ELISA A and ELISA C: differences tended to decrease with increasing mean % OD values. c Comparison of results for ELISA B and ELISA C: differences decreased with increasing mean % OD values
The relationship between sarcoptic mange severity and the % OD values was analyzed only for ELISA C, due to the better performance of this test as compared with the other two (Table 4). Significant differences in the % OD (Kruskal-Wallis test: χ 2 = 73.99, df = 2, P < 0.0001) among the three categories of sarcoptic mange status were found. The healthy ibexes showed statistically significant lower % OD values than the mildly (W = 90, P < 0.0001) and severely (W = 6, P < 0.0001) infested ibexes. The difference between the higher % OD of the severely infested ibexes and the lower % OD of the mildly infested ones was close to significance (W = 106, P = 0.058).
Table 4.
ELISA results (% OD) based on a visually estimated percentage of affected skin [17] in Iberian ibex (Capra pyrenaica) sampled in the Sierra Nevada Natural and National Parks
| Healthy | Mildly infested | Severely infested | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| ELISA A | 85 | 9.50 | 28.55 | 31 | 19.46 | 33.49 | 12 | 24.28 | 41.43 |
| ELISA B | 74 | 7.63 | 9.45 | 30 | 13.76 | 19.61 | 13 | 34.71 | 28.72 |
| ELISA C* | 77 | 47.61a | 26.45 | 30 | 137.86b | 41.59 | 13 | 168.16b | 25.62 |
*Kruskal-Wallis test: χ 2 = 73.99, df = 2, P < 0.0001; a,b means with different superscripts are statistically different
Abbreviation: SD standard deviation
Discussion
This study compared three ELISA tests for the detection of specific serum antibodies to S. scabiei in the Iberian ibex, which showed different performance in terms of specificity and sensitivity. Furthermore, the OD % varied among degrees of sarcoptic mange infestation for the best performing test.
To the best of our knowledge, this is the first time in which several ELISA tests are compared and evaluated for the diagnosis of sarcoptic mange in Iberian ibex. Based on results of this study, ELISA C was the best performing test to detect specific antibodies against S. scabiei in this species, showing higher sensitivity and specificity and an AUC close to 1. This test was validated for the detection of IgG in Alpine chamois lung extracts with a sensitivity of 100 % and a specificity of 98.6 % (data not published). Moreover, the ELISA C allowed the differentiation of healthy and mangy ibexes. ELISA B revealed lower specificity and sensitivity than previously reported in Cantabrian chamois and red deer, with a specificity of 97 %, and a sensitivity of 100 % and 75 % in chamois and red deer [45, 61], respectively. ELISA A had never been used before this study for the detection of antibodies against S. scabiei in wild ungulates, and the sensitivity and specificity of the test in dog are 92.1 % and 94.6 %, respectively.
The avidin-biotin detection system used in the ELISA C could be one of the reasons explaining the best performance of ELISA C as compared to ELISAs B and C. This method has the potential to increase the sensitivity of the indirect ELISA, since avidin has four binding sites for biotin, resulting in an essentially irreversible complex which is stable in later washes and incubations [62]. Although serum cross-reactivity against different S. scabiei strains exists, each host species responds differently to different mite varieties [63, 64]. Therefore, the differences in sensitivity among the three ELISA tests could also depend on the specific antigens used and the origin of mites.
False negative results (i.e. mangy ibexes in which no antibodies against S. scabiei were detected) could be explained by low levels of circulating IgG in recently infested ibexes or in chronic infestations. The clinical signs of sarcoptic mange may appear before IgG detection in different species [29, 30, 32, 38, 65], and in experimentally infected Iberian ibex, IgG were detected no earlier than 18−27 days post-infestation [34]. This time lapse may explain at least some of the false negative ibexes within the mildly infested category. On the other hand, maximum OD values indicating specific IgG peaks occur between 50 days and 12–16 weeks post infestation in domestic goats, pigs and Iberian ibexes, to decline slightly or plateauing afterwards [20, 32, 34]. This could explain some false negative results in chronically affected ibexes.
A low test specificity (i.e. positive ELISA results in healthy ibexes) can be explained by a lack of detection of minute skin lesions [17, 40], subclinical infestations [66], or cross reactions with antigens from other related parasites [67, 68]. Cross-reactivity against dust mites, ticks and Trombicula spp., a mite that affects the skin of different European wild ungulates like chamois [69], have been previously assessed and discarded for ELISA A, B and C, respectively [41, 44, 45]. Therefore, false positive results could be putatively related to cross-reactions with other parasites reported to subclinically infest wild Iberian ibex, such as other mites (Psoroptes sp., Trombicula sp.) [70] or different tick species [70, 71]. ELISA C showed lower specificity than previously reported in Alpine chamois probably due to the application of the test on two different types of samples with different antibodies amount (serum versus lung extracted). However, false positive ibexes could also be due to individuals having had previous contact with S. scabiei and having recovered from the clinical phase of the disease. Hosts infested with S. scabiei develop resistance to re-infestation [28] and healthy Iberian ibexes exposed to sarcoptic mange show detectable IgG levels [34, 35]. In Sierra Nevada, sarcoptic mange is endemic, thus some of the healthy Iberian ibexes of this study could have had previous contacts with the mite, thus developing an IgG response. However, the higher S. scabiei-positivity by ELISA in the severely infested ibexes suggests that IgGs are not protective against mange. In general, protection against mange has been associated with a cell-mediated immune response as well as with a humoral immune response [28, 29, 32, 65, 72, 73]. Therefore, further research appears to be needed to understand the specific role of both humoral and cellular immune responses. The significant differences in % OD found between healthy and infested ibexes for ELISA C are not consistent with a previous report, in which there were no % OD differences between infested and healthy ibexes from the same area [35]. However, results in this study fit the differences in two acute phase proteins (APP), namely alpha-1 acid glycoprotein (AGP) and serum amyloid A (SAA), observed in similarly ranked Iberian ibex. The higher APP level in severely affected ibexes was attributed to skin inflammation or the pathological secondary amyloidosis, leading to organ dysfunction in this category [74]. The increasing % OD values with increasing sarcoptic mange severity found in this study could be explained by a higher intensity of infestation in severely affected ibexes, which in turn would stimulate more intensely the immune system and elicit a higher antibody production. Scabies-specific IgG rapidly decline in parallel with the disappearance of mites from the skin in goats [32], and the percentage of mange-damaged skin and mite load are related in Iberian ibex [17]. Moreover, although in some species skin lesions are reflective of delayed hypersensitivity reaction associated with few or no mites, in Iberian ibex mites are definitely abundant in the skin in the generalized stage of the disease [2]. Therefore, the % OD results obtained with ELISA C test would not only indicate the presence of viable S. scabiei in the skin, but could also correlate with mite load.
The evaluation of the ELISA tests carried out in this study and the identification of ELISA C as a reliable tool for sarcoptic mange diagnosis in Iberian ibex will be useful in future research. Nevertheless, the limitations in sensitivity and specificity should be considered while assessing the results, and more studies on (i) the dynamics of the antibody response after S. scabiei infestation in Iberian ibex; (ii) the correlation between parasitic load, mange severity and immune response; and (iii) the influence of sex [34, 54] and season [17, 55] on the OD % are necessary in order to better understand the immune response to sarcoptic mange in the Iberian ibex.
Conclusions
In conclusion, the ELISA developed for diagnose sarcoptic mange in Alpine chamois (Istituto Zooprofilattico delle Venezie, data not published) has been validated in this study as an effective test to be similarly applied in Iberian ibex. This can be helpful for completing the legal requirements for the transport of wild ungulates [18] and for seroepidemiological studies.
Acknowledgements
The authors are grateful to the institutional authorities of the Parque Nacional de Sierra Nevada for their support to this study. Ibex capture and sample collection would have not been possible without the aid of Apolo Sánchez, José López, Isidro Puga, Elías Martínez, Manuela Cárdenas, Francisco Casado and Antonio Rodríguez, to whom the authors are deeply indebted. The authors are also indebted to Emanuela Cristelli, Francesca Denardi and Silvia Simonato for their support in the laboratory analyses.
Funding
The Consejería de Medio Ambiente y Ordenación del Territorio of the Junta de Andalucía supported sample collection and conservation through the projects 676/2006/A/00, 1571/2007/M/00, 173/2009/M/00 and 861/2011/M/00, as did the PAIDI Research Group RNM18 from the Junta de Andalucía. The Ministerio de Economía y Competitividad of the Spanish Government funded the study through the research projects CGL2012-40043-C02-02 and CGL2016-80543-P, and a predoctoral grant (BES-2013-063931) to JE. ES was supported by the Fundação para a Ciência ea Tecnologia (Portugal), through the postdoctoral program (SFRH/BPD/96637/2013), and the University of Aveiro (Department of Biology) and FCT/MEC (CESAM RU UID/AMB/50017), co-financed by the FEDER within the PT2020 Partnership Agreement. LR, DD and CC were supported by the Italian National PRIN Program “Genomics and host-pathogen interaction: a study model in the One-Health perspective”.
Availability of data and materials
Data supporting the conclusions of this article are included within the article.
Authors’ contributions
Designed the study: JRLO, JEG, RCS, JMP. Performed ibex sampling: JEG, FJCM, PF, JE, AR. Laboratory analyses: DD, RC, LR, AP, CC. Analyzed the data: AR, ES, JRLO. Wrote the paper: AR, JRLO, ES. All authors read and approved the final version of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval
This study accomplished with all Andalusian, Spanish and European legal requirements and guidelines regarding experimentation and animal welfare. It was approved by the Ethics on Animal Welfare Committee of the Universidad de Jaén and authorized by the Dirección General de Producción Agrícola y Ganadera of the Consejería de Agricultura, Pesca y Medio Ambiente of the Junta de Andalucía.
Contributor Information
Arián Ráez-Bravo, Email: arian.raez@gmail.com.
José Enrique Granados, Email: josee.granados.ext@juntadeandalucia.es.
Emmanuel Serrano, Email: emmanuel.serrano.ferron@gmail.com.
Debora Dellamaria, Email: ddellamaria@izsvenezie.it.
Rosa Casais, Email: rosacg@serida.org.
Luca Rossi, Email: luca.rossi@unito.it.
Anna Puigdemont, Email: anna.puigdemont@uab.cat.
Francisco Javier Cano-Manuel, Email: franciscoj.canomanuel@juntadeandalucia.es.
Paulino Fandos, Email: pfandos@agenciamedioambienteyagua.es.
Jesús María Pérez, Email: jperez@ujaen.es.
José Espinosa, Email: jcerrato@ujaen.es.
Ramón Casimiro Soriguer, Email: soriguer@ebd.csic.es.
Carlo Citterio, Email: ccitterio@izsvenezie.it.
Jorge Ramón López-Olvera, Email: Jordi.Lopez.Olvera@uab.cat.
References
- 1.Bornstein S, Mörner T, Samuel WM. Sarcoptes scabiei and sarcoptic mange. In: Samuel WM, Pybus MJ, Kocan AA, editors. Parasitic Diseases of Wild Mammals. 2. Ames: Iowa State University Press; 2001. pp. 107–19. [Google Scholar]
- 2.Pence DB, Ueckermann E. Sarcoptic mange in wildlife. Rev Sci Tech. 2002;21:385–98. [PubMed] [Google Scholar]
- 3.Walton SF, Holt DC, Currie BJ, Kemp DJ. Scabies: new future for a neglected disease. Adv Parasitol. 2004;57:309–76. doi: 10.1016/S0065-308X(04)57005-7. [DOI] [PubMed] [Google Scholar]
- 4.Rossi L, Fraquelli C, Vesco U, Permunian R, Sommavilla GM, Carmignola G, et al. Descriptive epidemiology of a scabies epidemic in chamois in the Dolomite Alps, Italy. Eur J Wildl Res. 2007;53:131–41. doi: 10.1007/s10344-006-0067-x. [DOI] [Google Scholar]
- 5.Gakuya F, Ombui J, Maingi N, Muchemi G, Ogara W, Soriguer RC, et al. Sarcoptic mange and cheetah conservation in Masai Mara (Kenya): epidemiological study in a wildlife/livestock system. Parasitology. 2012;139:1587–95. doi: 10.1017/S0031182012000935. [DOI] [PubMed] [Google Scholar]
- 6.Gakuya F, Ombui J, Heukelbach J, Maingi N, Muchemi G, Ogara W, et al. Knowledge of mange among Masai pastoralists in Kenya. PLoS ONE. 2012;7:1–8. doi: 10.1371/journal.pone.0043342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.León-Vizcaíno L, de Ybáñez MR R, Cubero MJ, Ortíz JM, Espinosa J, Pérez L, et al. Sarcoptic mange in Spanish ibex from Spain. J Wildl Dis. 1999;35:647–59. doi: 10.7589/0090-3558-35.4.647. [DOI] [PubMed] [Google Scholar]
- 8.León-Vizcaíno L, Astorga R, Escos J, Alonso F, Alados C, Contreras A, et al. Epidemiología de la sarna sarcóptica en el Parque Natural de las Sierras de Cazorla, Segura y Las Villas. In: Proceedings of the International Congress on the Genus Capra in Europe. Sevilla: Junta Rectora del Parque Natural Sierra de las Nieves, Consejería de Medio Ambiente, Junta de Andalucía; 1992. pp. 95–9. [Google Scholar]
- 9.León-Vizcaíno L. Patología de la sarna en la cabra montés en Cazorla. Quercus. 1990;50:22. [Google Scholar]
- 10.Fernández-Morán J, Gómez S, Ballesteros F, Quirós P, Benito J, Feliu C, et al. Epizootiology of sarcoptic mange in a population of cantabrian chamois (Rupicapra pyrenaica parva) in Northwestern Spain. Vet Parasitol. 1997;73:163–71. doi: 10.1016/S0304-4017(97)00061-7. [DOI] [PubMed] [Google Scholar]
- 11.González-Candela M, León-Vizcaíno L, Cubero-Pablo MJ. Population effects of sarcoptic mange in Barbary sheep (Ammotragus lervia) from Sierra Espuña Regional Park, Spain. J Wildl Dis. 2004;40:456–65. doi: 10.7589/0090-3558-40.3.456. [DOI] [PubMed] [Google Scholar]
- 12.Fandos P. La cabra montés (Capra pyrenaica) en el Parque Natural de Las Sierras de Cazorla, Segura y Las Villas. Madrid: Icona-CSIC Colección Técnica; 1991. [Google Scholar]
- 13.Serrano E, Colom-Cadena A, Gilot-Fromont E, Garel M, Cabezón O, Velarde R, et al. Border disease virus: an exceptional driver of chamois populations among other threats. Front Microbiol. 2015;6:1–9. doi: 10.3389/fmicb.2015.01307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pérez JM, Granados JE, Soriguer RC, Fandos P, Márquez FJ, Crampe JP. Distribution, status and conservation problems of the Spanish ibex, Capra pyrenaica (Mammalia: Artiodactyla) Mamm Rev. 2002;32:26–39. doi: 10.1046/j.1365-2907.2002.00097.x. [DOI] [Google Scholar]
- 15.Walton SF, Currie BJ. Problems in diagnosing scabies, a global disease in human and animal populations. Clin Microbiol Rev. 2007;20:268–79. doi: 10.1128/CMR.00042-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walter B, Heukelbach J, Fengler G, Worth C, Hengge U, Feldmeier H. Comparison of dermoscopy, skin scraping, and the adhesive tape test for the diagnosis of scabies in a resource-poor setting. Arch Dermatol. 2011;147:468–73. doi: 10.1001/archdermatol.2011.51. [DOI] [PubMed] [Google Scholar]
- 17.Pérez JM, Granados JE, Sarasa M, Serrano E. Usefulness of estimated surface area of damaged skin as a proxy of mite load in the monitoring of sarcoptic mange in free-ranging populations of Iberian wild goat, Capra pyrenaica. Vet Parasitol. 2011;176:258–64. doi: 10.1016/j.vetpar.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 18.España Real Decreto 1082/2009, de 3 de julio, por el que se establecen los requisitos de sanidad animal para el movimiento de animales de explotaciones cinegéticas, de acuicultura continental y de núcleos zoológicos, así como de animales de fauna silvestre. BOE. 2009;177:62851–61. [Google Scholar]
- 19.Katsumata K, Katsumata K. Simple method of detecting Sarcoptes scabiei var hominis mites among bedridden elderly patients suffering from severe scabies infestation using an adhesive-tape. Intern Med. 2006;45:857–59. doi: 10.2169/internalmedicine.45.1707. [DOI] [PubMed] [Google Scholar]
- 20.Rampton M, Walton SF, Holt DC, Pasay C, Kelly A, Currie BJ, et al. Antibody responses to Sarcoptes scabiei apolipoprotein in a porcine model: relevance to immunodiagnosis of recent infection. PLoS ONE. 2013;8:e65354. doi: 10.1371/journal.pone.0065354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jayaraj R, Hales B, Viberg L, Pizzuto S, Holt D, Rolland JM, et al. A diagnostic test for scabies: IgE specificity for a recombinant allergen of Sarcoptes scabiei. Diagn Microbiol Infect Dis. 2011;71:403–7. doi: 10.1016/j.diagmicrobio.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Fukuyama S, Nishimura T, Yotsumoto H, Gushi A, Tsuji M, Kanekura T, et al. Diagnostic usefulness of a nested polymerase chain reaction assay for detecting Sarcoptes scabiei DNA in skin scrapings from clinically suspected scabies. Br J Dermatol. 2010;163:892–94. doi: 10.1111/j.1365-2133.2010.09913.x. [DOI] [PubMed] [Google Scholar]
- 23.Angelone-Alasaad S, Min AM, Pasquetti M, Alagaili AN, Amelio SD, Berrilli F, et al. Universal conventional and real-time PCR diagnosis tools for Sarcoptes scabiei. Parasit Vectors. 2015;8:587–93. doi: 10.1186/s13071-015-1204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dupuy A, Dehen L, Bourrat E, Lacroix C, Benderdouche M, Dubertret L, et al. Accuracy of standard dermoscopy for diagnosing scabies. J Am Acad Dermatol. 2007;56:53–62. doi: 10.1016/j.jaad.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 25.Park JH, Kim CW, Kim SS. The diagnostic accuracy of dermoscopy for scabies. Ann Dermatol. 2012;24:194–9. doi: 10.5021/ad.2012.24.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arenas AJ, Gómez F, Salas R, Carrasco P, Borge C, Maldonado A, et al. An evaluation of the application of infrared thermal imaging to the tele-diagnosis of sarcoptic mange in the Spanish ibex (Capra pyrenaica) Vet Parasitol. 2002;109:111–7. doi: 10.1016/S0304-4017(02)00248-0. [DOI] [PubMed] [Google Scholar]
- 27.Alasaad S, Permunian R, Gakuya F, Mutinda M, Soriguer RC, Rossi L. Sarcoptic-mange detector dogs used to identify infected animals during outbreaks in wildlife. BMC Vet Res. 2012;8:110. doi: 10.1186/1746-6148-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arlian LG, Morgan MS, Vyszenskimoher DL, Stemmer BL. Sarcoptes scabiei: The circulating antibody response and induced immunity to scabies. Exp Parasitol. 1994;78:37–50. doi: 10.1006/expr.1994.1004. [DOI] [PubMed] [Google Scholar]
- 29.Arlian LG, Morgan MS, Rapp CM, Vyszenski-Moher DL. The development of protective immunity in canine scabies. Vet Parasitol. 1996;62:133–42. doi: 10.1016/0304-4017(95)00854-3. [DOI] [PubMed] [Google Scholar]
- 30.Bornstein S, Zakrisson G, Thebo P. Clinical picture and antibody response to experimental Sarcoptes scabiei var. vulpes infection in red foxes (Vulpes vulpes) Acta Vet Scand. 1995;36:509–19. doi: 10.1186/BF03547665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bornstein S, Zakrisson G. Humoral antibody response to experimental Sarcoptes scabiei var. vulpes infection in the dog. Vet Dermatol. 1993;4:107–10. doi: 10.1111/j.1365-3164.1993.tb00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tarigan S. Antibody responses in naïve and sensitised goats infested by Sarcoptes scabiei. Indones J Anim Vet Sci. 2004;9:258–65. [Google Scholar]
- 33.Millán J, Casais R, Colomar V, Bach E, Prieto JM, Velarde R. Experimental infection of wild-caught European rabbits (Oryctolagus cuniculus) with Sarcoptes scabiei from a naturally infected wild rabbit. Med Vet Entomol. 2013;27:232–35. doi: 10.1111/j.1365-2915.2012.01035.x. [DOI] [PubMed] [Google Scholar]
- 34.Sarasa M, Rambozzi L, Rossi L, Meneguz PG, Serrano E, Granados JE, et al. Sarcoptes scabiei: Specific immune response to sarcoptic mange in the Iberian ibex Capra pyrenaica depends on previous exposure and sex. Exp Parasitol. 2010;124:265–71. doi: 10.1016/j.exppara.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Lastras ME, Pastor J, Marco I, Ruiz M, Viñas L, Lavín S. Effects of sarcoptic mange on serum proteins and immunoglobulin G levels in chamois (Rupicapra pyrenaica) and Spanish ibex (Capra pyrenaica) Vet Parasitol. 2000;88:313–19. doi: 10.1016/S0304-4017(99)00221-6. [DOI] [PubMed] [Google Scholar]
- 36.Bornstein S, Thebo P, Zakrisson G. Evaluation of an enzyme-linked immunosorbent assay (ELISA) for the serological diagnosis of canine sarcoptic mange. Vet Dermatol. 1996;7:21–8. doi: 10.1111/j.1365-3164.1996.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 37.Lower KS, Medleau LM, Hnilica K, Bigler B. Evaluation of an enzyme-linked immunosorbant assay (ELISA) for the serological diagnosis of sarcoptic mange in dogs. Vet Dermatol. 2001;12:315–20. doi: 10.1046/j.0959-4493.2001.00265.x. [DOI] [PubMed] [Google Scholar]
- 38.Van Der Heijden HMJF, Rambags PGM, Elbers ARW, Van Maanen C, Hunneman WA. Validation of ELISAs for the detection of antibodies to Sarcoptes scabiei in pigs. Vet Parasitol. 2000;89:95–107. doi: 10.1016/S0304-4017(00)00197-7. [DOI] [PubMed] [Google Scholar]
- 39.Hollanders W, Vercruysse J, Raes S, Bornstein S. Evaluation of an enzyme-linked immunosorbent assay (ELISA) for the serological diagnosis of sarcoptic mange in swine. Vet Parasitol. 1997;69:117–23. doi: 10.1016/S0304-4017(96)01117-X. [DOI] [PubMed] [Google Scholar]
- 40.Haas C, Rossi S, Meier R, Ryser-Degiorgis MP. Evaluation of a commercial ELISA for the detection of antibodies to Sarcoptes scabiei in wild boar (Sus scrofa) J Wildl Dis. 2015;51:729–33. doi: 10.7589/2014-09-222. [DOI] [PubMed] [Google Scholar]
- 41.Rambozzi L, Menzana A, Lavín S, Rossi L. Biotin-avidin amplified ELISA for detection of antibodies to Sarcoptes scabiei in chamois (Rupicapra spp.) Vet Res. 2004;35:701–8. doi: 10.1051/vetres:2004039. [DOI] [PubMed] [Google Scholar]
- 42.Casas-Díaz E, Marco I, López-Olvera JR, Mentaberre G, Lavín S. Comparison of xylazine-ketamine and medetomidine-ketamine anaesthesia in the Iberian ibex (Capra pyrenaica) Eur J Wildl Res. 2011;57:887–93. doi: 10.1007/s10344-011-0500-7. [DOI] [Google Scholar]
- 43.González-Quirós P, Solano S. Monitoring of an outbreak of sarcoptic mange in Cantabrian chamois (Rupicapra pyrenaica parva) in the game reserves of Asturias (north of Spain) In: Pérez-Barbería FJ, Palacios B, editors. El rebeco cantábrico Rupicapra pyrenaica parva: conservación y gestión de sus poblaciones. Madrid: Ministerio de Medio Ambiente y Medio Rural y Marino; 2009. pp. 292–319. [Google Scholar]
- 44.Puigdemont A, Brazís P, Fondati S, Ferrer L. Diagnóstico serológico de la sarna sarcóptica en el perro. Consult Difusión Vet. 2002;89:71–3. [Google Scholar]
- 45.Casais R, Prieto M, Balseiro A, Solano P, Parra F, Martín Alonso JM. Identification and heterologous expression of a Sarcoptes scabiei cDNA encoding a structural antigen with immunodiagnostic potential. Vet Res. 2007;38:435–50. doi: 10.1051/vetres:2007007. [DOI] [PubMed] [Google Scholar]
- 46.Yin J, Tian L. Joint inference about sensitivity and specificity at the optimal cut-off point associated with Youden index. Comput Stat Data Anal. 2014;77:1–13. doi: 10.1016/j.csda.2014.01.021. [DOI] [Google Scholar]
- 47.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–5. doi: 10.1002/1097-0142(1950)3:1<32::AID-CNCR2820030106>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 48.Parikh R, Mathai A, Parikh S, Sekhar GC, Thomas R. Understanding and using sensitivity, specificity and predictive values. Indian J Opthalmology. 2008;56:45–50. doi: 10.4103/0301-4738.37595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 50.Zou KH, O’Malley AJ, Mauri L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation. 2007;115:654–7. doi: 10.1161/CIRCULATIONAHA.105.594929. [DOI] [PubMed] [Google Scholar]
- 51.Cohen J. A Coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. doi: 10.1177/001316446002000104. [DOI] [Google Scholar]
- 52.Altman DG, Bland JM. Measurement in medicine: the analysis of method comparison studies. Statistician. 1983;32:307–17. doi: 10.2307/2987937. [DOI] [Google Scholar]
- 53.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–60. doi: 10.1191/096228099673819272. [DOI] [PubMed] [Google Scholar]
- 54.López-Olvera JR, Serrano E, Armenteros A, Pérez JM, Fandos P, Carvalho J, et al. Sex-biased severity of sarcoptic mange at the same biological cost in a sexually dimorphic ungulate. Parasit Vectors. 2015;8:2–8. doi: 10.1186/s13071-015-1186-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carvalho J, Granados JE, López-Olvera JR, Cano-Manuel FJ, Pérez JM, Fandos P, et al. Sarcoptic mange breaks up bottom-up regulation of body condition in a large herbivore population. Parasit Vectors. 2015;8:572. doi: 10.1186/s13071-015-1188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Komsta L, Novomestky F. Moments: Moments, cumulants, skewness, kurtosis and related tests. R package version 0.14. 2015.
- 57.Lehnert B. BlandAltmanLeh: Plots (slightly extended) Bland-Altman plots. R package version 0.1.0. 2014.
- 58.Gamer M, Lemon J, Fellows I, Singh P. Irr: Various coefficients of interrater reliability and agreement. R package version 0.84. 2012.
- 59.López-Ratón M, Rodríguez-Álvarez MX, Cadarso-Suarez C, Gude-Sampedro F. OptimalCutpoints: An R package for selecting optimal cutpoints in diagnostic tests. J Stat Softw. 2014;61:1–36. doi: 10.18637/jss.v061.i08. [DOI] [Google Scholar]
- 60.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 61.Oleaga A, Casais R, González-Quirós P, Prieto M, Gortazar C. Sarcoptic mange in red deer from Spain: Improved surveillance or disease emergence? Vet Parasitol. 2008;154:103–13. doi: 10.1016/j.vetpar.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 62.Kendall C, Ionescu-Matiu I, Dreesman GR. Utilization of the biotin/avidin system to amplify the sensitivity of the enzyme-linked immunosorbent assay (ELISA) J Immunol Methods. 1983;56:329–39. doi: 10.1016/S0022-1759(83)80022-2. [DOI] [PubMed] [Google Scholar]
- 63.Arlian LG, Morgan MS, Arends JJ. Immunologic cross-reactivity among various strains of Sarcoptes scabiei. J Parasitol. 1996;82:66–72. doi: 10.2307/3284117. [DOI] [PubMed] [Google Scholar]
- 64.Haas N, Wagemann B, Hermes B, Henz BM, Heile C, Schein E. Crossreacting IgG antibodies against fox mite antigens in human scabies. Arch Dermatol Res. 2005;296:327–31. doi: 10.1007/s00403-004-0524-x. [DOI] [PubMed] [Google Scholar]
- 65.Casais R, Dalton KP, Millán J, Balseiro A, Oleaga Á, Solano P, et al. Primary and secondary experimental infestation of rabbits (Oryctolagus cuniculus) with Sarcoptes scabiei from a wild rabbit: Factors determining resistance to reinfestation. Vet Parasitol. 2014;203:173–83. doi: 10.1016/j.vetpar.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 66.Ippen R, Nickel S, Schröder H. Krankheiten des jagdbaren Wildes. Deutscher Landwirtschaftsverlag Berlin. 1995:189–95
- 67.Arlian LG, Morgan MS. Serum antibody to Sarcoptes scabiei and house dust mite prior to and during infestation with S. scabiei. Vet Parasitol. 2000;90:315–26. doi: 10.1016/S0304-4017(00)00251-X. [DOI] [PubMed] [Google Scholar]
- 68.Arlian LG, Vyszenski-Moher L, Ahmed SG, Estes SA. Cross-antigenicity between the scabieis mite, Sarcoptes scabiei, and the house dust mite, Dermatophagoides pteronyssinus. J Invest Dermatol. 1991;96:349–54. doi: 10.1111/1523-1747.ep12465257. [DOI] [PubMed] [Google Scholar]
- 69.Walzer C. Diseases of chamois. In: Fowler ME, Miller RE, editors. Zoo and Wild Animal Medicine. Current therapy 6. St Louis, MO: Sauders Elsevier; 2008. pp. 408–15. [Google Scholar]
- 70.Antón JM, Boticario D, Granados JE, Habela MA, Márquez FJ, Meana A, et al. Estudio de las enfermedades parasitarias de la cabra montés. In: Pérez JM, et al., editors. Distribución, genética y status sanitario de las poblaciones andaluzas de cabra montés. Jaén, Spain: Universidad de Jaén - Consejería de Medio Ambiente de la Junta de Andalucía; 2002. pp. 117–53. [Google Scholar]
- 71.Hueli LE, Díaz V. Ixódidos (Acarina: Ixodidae) parásitos de Capra pyrenaica en la provincia de Granada. In: Resúmenes del VI Congreso Nacional y I Congreso Ibérico de Parasitología. Cáceres, Spain; 1989. p. 223.
- 72.Lalli PN, Morgan MS, Arlian LG. Skewed Th1 / Th2 immune response to Sarcoptes scabiei. J Parasitol. 2004;90:711–14. doi: 10.1645/GE-214R. [DOI] [PubMed] [Google Scholar]
- 73.Walton SF. The immunology of susceptibility and resistance to scabies. Parasite Immunol. 2010;32:532–40. doi: 10.1111/j.1365-3024.2010.01218.x. [DOI] [PubMed] [Google Scholar]
- 74.Ráez-Bravo A, Granados JE, Cerón JJ, Cano-Manuel FJ, Fandos P, Pérez JM, et al. Acute phase proteins increase with sarcoptic mange status and severity in Iberian ibex (Capra pyrenaica Schinz, 1838) Parasitol Res. 2015;114:4005–10. doi: 10.1007/s00436-015-4628-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the conclusions of this article are included within the article.


