Abstract
The capacity to envision the future plays an important role in many aspects of cognition, including our ability to make optimal, adaptive choices. Past work has shown that the medial temporal lobe (MTL) is necessary for decisions that draw on episodic future thinking. By contrast, little is known about the role of the MTL in decisions that draw on semantic future thinking. Accordingly, the present study investigated whether the MTL contributes to one form of decision making, namely intertemporal choice, when such decisions depend on semantic consideration of the future. In an intertemporal choice task, participants must select either a smaller amount of money that is available in the present or a larger amount of money that would be available at a future date. Amnesic individuals with MTL damage and healthy control participants performed such a task in which, prior to making a choice, they engaged in a semantic generation exercise, wherein they generated items that they would purchase with the future reward. In experiment 1, we found that, relative to a baseline condition involving standard intertemporal choice, healthy individuals were more inclined to select a larger, later reward over a smaller, present reward after engaging in semantic future thinking. By contrast, amnesic participants were paradoxically less inclined to wait for a future reward following semantic future thinking. This finding suggests that amnesics may have had difficulty “tagging” the generated item(s) as belonging to the future. Critically, experiment 2 showed that when the generated items were presented alongside the intertemporal choices, both controls and amnesic participants shifted to more patient choices. These findings suggest that the MTL is not needed for making optimal decisions that draw on semantic future thinking as long as scaffolding is provided to support accurate time tagging. Together, these findings stand to better clarify the role of the MTL in decision making.
Keywords: hippocampus, amnesia, temporal discounting, delay discounting, semantic future thinking
1. Introduction
Accumulating evidence suggests that the medial temporal lobe (MTL) plays an important role in decision making—particularly in situations where decisions draw on memory for prior experiences (Gupta, et al., 2009; Gutbrod, et al., 2006; Lee, Ghim, Kim, Lee, & Jung, 2012; also see Palombo, Keane, & Verfaellie, 2015b for review). Moreover, there is evidence that the MTL may also be important for decision making when choices involve a consideration of future scenarios. For example, the human tendency to engage in temporal discounting (i.e., the propensity to choose a smaller present reward over a larger future reward) is attenuated when individuals first imagine consuming a reward in the context of a future event such as a being at a restaurant or a sporting event (i.e., when they engage in episodic future thinking; e.g., Benoit, Gilbert, & Burgess, 2011; Lin & Epstein, 2014; Liu, Feng, Chen, & Li, 2013; Peters & Büchel, 2010; Sasse, Peters, Buchel, & Brassen, 2015). This “episodic cueing” effect involves the MTL: The extent to which individuals attenuate their temporal discounting following episodic cueing is correlated with the magnitude of connectivity between the hippocampus and midline prefrontal regions (Benoit, et al., 2011; Peters & Büchel, 2010).
Corroborating this finding, we recently found that the attenuation in temporal discounting following episodic cueing that is observed in healthy individuals is not observed in amnesic individuals with damage to the MTL (Palombo, Keane, & Verfaellie, 2015c). That is, amnesic participants did not demonstrate the expected shift towards more “patient” choices after they imagined being at a specific event in the future. As expected, the imagined future events elicited from amnesic participants were severely impoverished, in accordance with previous observations (e.g., Maguire & Hassabis, 2011; Race, Keane, & Verfaellie, 2011; Tulving, 1985, but see Squire et al., 2010). Moreover, these findings were observed regardless of whether amnesic participants had larger MTL lesions or circumscribed hippocampal damage. By contrast, amnesics’ performance on a “standard” intertemporal choice task (i.e., one that does not involve episodic cueing) was similar to that of well-matched controls (Palombo, et al., 2015c), in line with previous work (Kwan, et al., 2012; Kwan, Craver, Green, Myerson, & Rosenbaum, 2013).
Intriguingly, a recent study by Kwan et al. (2015), also involving amnesic participants with damage to the MTL or related structures, reported a somewhat different pattern of findings using a similar paradigm. In Kwan et al. (2015), amnesic participants and healthy controls were asked to select events that were either planned or likely to occur in the future (e.g., my granddaughter's birthday party in 1 month); these events were then presented to participants in an intertemporal choice task, such that participants imagined the events prior to making their intertemporal choices. In contrast to Palombo et al. (2015c), they found that the episodic cueing effect in several of their amnesic participants was in the normal range (i.e., was indistinguishable from that of controls) despite impaired performance on an ancillary episodic future thinking task (Kwan, et al., 2015). In other words, notwithstanding deficient episodic future thinking, temporal discounting was nonetheless attenuated by episodic cueing to the same degree as controls in a number of their amnesic participants.
This discrepancy in findings between the two studies cannot be accounted for by demographic, neuropsychological, or neuroanatomical characteristics; amnesic groups in the two studies were similar in these respects (also see Palombo, Keane, & Verfaellie, 2015a for discussion). Kwan et al. (2015) propose that these differences may instead arise from the nature of the cues used to evoke episodic future thinking: Whereas in Kwan et al. (2015), participants imagined real-life events that were either planned for the future or likely to occur (e.g., being at your granddaughter's upcoming birthday party in 1 month from now), in Palombo et al. (2015c), participants imagined generic future events (e.g., being at a street fair in 1 month from now). Thus in Palombo et al. (2015c), the events did not involve pre-determined plans that amnesic participants had for the future. The highly personal nature of the cues used by Kwan et al. (2015) may have enabled amnesic participants to draw on another form of future thinking, namely, semantic future thinking (Atance & O'Neill, 2001). That is, it is possible that even in the absence of episodic future thinking abilities, amnesics could still draw on personal knowledge and reasoning to construct a situation in the future, based on what Klein and colleagues (Klein, 2013; Klein, Loftus, & Kihlstrom, 2002) refer to as “known time” (as oppose to “lived time”), akin to the difference in memory between “knowing” and “remembering” (Tulving, 1985). For example, when cued with “imagine your granddaughter's birthday party in 1 month,” amnesic participants may have been able to reason semantically (e.g., based on schema-based knowledge) that this event would require the purchasing of a birthday present for their granddaughter or that bringing their granddaughter a gift would make her happy (because she loves gifts), even if they were not capable of picturing the birthday party unfolding as an event per se.1 We acknowledge that amnesics would also need to use semantic knowledge to construct a generic future event such as attending a street fair (in Palombo et al., 2015c), but a critical difference is that the personal nature of the Kwan et al., (2015) cues likely fostered the generation of future-oriented information that was more self relevant to amnesics. To the extent that such self-relevant information would involve a greater personal investment in the future-oriented information, it could make the future reward more appealing, increasing the likelihood of amnesic participants selecting the future reward and yielding an attenuation in temporal discounting similar to that observed in the control group.
What follows from this interpretation is the proposal that although either episodic or semantic future thinking can influence decisions (also see Klein, 2013; Schacter, et al., 2012), only the former requires the MTL. Indeed, there is some evidence to suggest that amnesics retain some capacity to envision the future semantically (Klein, et al., 2002), albeit not to the level of detail of healthy controls (Race, Keane, & Verfaellie, 2013). Nonetheless, if amnesics can consider the future semantically, even if at a coarser level, this may be sufficient to elicit greater patience for a future reward in the context of intertemporal choices and may account for the findings of Kwan et al (2015).
To address this possibility, here we directly examined the effect of semantic future thinking on intertemporal choice in amnesic participants and a comparison group of healthy controls. We designed a novel intertemporal choice paradigm in which future choices were “baited” by using personal semantic cues. More specifically, participants were asked to generate specific items that they would realistically either need or want to purchase in the future (e.g., “If you received $42 in 4 months what items would you buy with that money?”). Although this type of cue was selected because it does not require imagining a specific event, it is nonetheless possible that healthy, neurologically intact individuals may draw to some extent on episodic processes, as no future thinking task is process pure. However, the goal of the present report was to determine if a shift toward emphasis on personal semantic future thinking could successfully induce more patient choice behavior in amnesics who are otherwise unable to richly engage in episodic future thinking. In light of Kwan et al. (2015), we hypothesized that the use of future-oriented personal semantic cues would attenuate temporal discounting in amnesic participants with MTL damage (as well as in healthy control participants).
2. Experiment 1
2.1. Method
2.1.1. Participants
Nine patients with amnesia (3 women) participated in experiment 1 (see Table 1 for demographic and neuropsychological data). Each amnesic participant's neuropsychological profile indicated severe impairment limited to the domain of memory. Etiology of amnesia included ischemia or anoxia (7 amnesic participants), status epilepticus followed by temporal lobectomy (1 amnesic participant), and encephalitis (1 amnesic participant). Four amnesic participants (P03, P04, P06, P08) had lesions restricted to the hippocampus (see Table 1), one amnesic participant (P01) had a lesion that included the hippocampus and MTL cortices, and two amnesic participants (P02 an P09) had lesions that extended beyond the MTL into anterolateral temporal cortex. Amnesic participants’ lesions are presented in Figure 1, either on CT or MRI scans. Two amnesic participants (P05, P07), who had suffered from cardiac arrest, could not be scanned due to medical contraindications and thus are not included in the figure. MTL pathology for these individuals was inferred based on etiology and neuropsychological profile. As shown in Table 1, volumetric data for the hippocampus and MTL cortices was available for 5 of the 9 amnesics that participated in experiment 1 (P02, P03, P04, P06, P08), using previously reported methodology (Kan, Giovanello, Schnyer, Makris, & Verfaellie, 2007).
Table 1.
Demographic and Neuropsychological Characteristics of Amnesic Participants
| WAIS III |
WMS III |
Volume Loss (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amnesic | Etiology | Age | Edu | VIQ | WMI | GM | VD | AD | Hippocampal | Subhippocampal | Experiment |
| P01 | Anoxia/Ischemia | 65 | 12 | 83 | 84 | 52 | 56 | 55 | N/A | N/A | Exp 1 + 2 |
| P02 | Anoxia + Left Temporal lobectomy | 51 | 16 | 86 | 84 | 49 | 53 | 52 | 63% | 60%a | Exp 1 + 2 |
| P03 | Anoxia | 56 | 14 | 90 | 99 | 45 | 53 | 52 | 70% | - | Exp 1 |
| P04 | CO poisoning | 59 | 14 | 111 | 117 | 59 | 72 | 52 | 22% | - | Exp 1 + 2 |
| P05 | Cardiac arrest | 63 | 17 | 134 | 126 | 86 | 78 | 86 | N/A | N/A | Exp 1 + 2 |
| P06 | Stroke | 50 | 20 | 111 | 99 | 60 | 65 | 58 | 43% | - | Exp 1 |
| P07 | Cardiac arrest | 65 | 16 | 110 | 92 | 86 | 78 | 83 | N/A | N/A | Exp 1 + 2 |
| P08 | Anoxia/Ischemia | 47 | 12 | 103 | 95 | 59 | 68 | 55 | 46% | - | Exp 1 + 2 |
| P09 | Encephalitis | 73 | 13 | 99 | 104 | 49 | 56 | 58 | N/A | N/A | Exp 1 + 2 |
| P10 | Stroke | 62 | 18 | 117 | 88 | 67 | 75 | 55 | 62% | - | Exp 2 |
Note: Age, age in years; Edu, education in years; WAIS-III, Wechsler Adult Intelligence Scale-III (Wechsler, 1997a); WMS-III, Wechsler Memory Scale-III (Wechsler, 1997b); VIQ, verbal IQ; WMI, working memory index; GM, general memory; VD, visual delayed; AD, auditory delayed; CO, carbon monoxide; Hippocampal, bilateral hippocampal volume loss; Subhippocampal, bilateral parahippocampal gyrus volume loss; N/A, not available.
Volume loss in left anterior parahippocampal gyrus (i.e., entorhinal cortex, medial portion of the temporal pole, and the medial portion of perirhinal cortex; (see Kan, et al., 2007 for methodology).
Figure 1.
Structural CT and MRI scans, which depict medial temporal lobe (MTL) lesions for eight of the amnesic participants (see Method). The left side of the brain is displayed on the right side of the image. CT slices show lesion location for P01 in the axial plane. T1-weighted MRI images depict lesions for P02, P03, P04, P06, P08, and P10 in the coronal and axial plane. T2-Flair MRI images depict lesion locations for P09 in the axial plane.
Twelve healthy control participants (5 women) were matched to the amnesic group in age (60.2 ± 6.3 years), education (15.1 ± 2.6 years) and verbal IQ (110.4 ± 10.1), which was assessed with the Wechsler Adult Intelligence Scale-III (WAIS-III; Wechsler, 1997a). All participants provided informed consent in accordance with the procedures of the VA Boston Healthcare System Institutional Review Board.
2.1.2. Materials and Procedure
Participants considered hypothetical monetary rewards in a novel intertemporal choice task that was modified from Palombo et al. (2015c) and Benoit et al. (2011). The task included two experimental conditions: baseline and semantic. In the baseline condition, participants were asked to indicate their preference for a hypothetical sum of money available in the future (e.g., $42 in 4 months) or a smaller, hypothetical sum of money available immediately (held constant at $30 dollars). As in Palombo et al. (2015c), to avoid the use of a strictly economic strategy (i.e., decisions made on the basis of a consideration of inflation and/or interest rates), participants were told that the money needed to be spent at the time of receipt (i.e., it could not be saved or invested). The magnitude of the future reward and the delay of delivery varied across 6 rewards ($34, $38, $42, $48, $54 and $58) and 6 delays (2 mo, 4 mo, 6 mo, 9 mo, 1 yr, and 2 yr), which were crossed completely to yield a total of 36 trials that were presented in a randomized order.
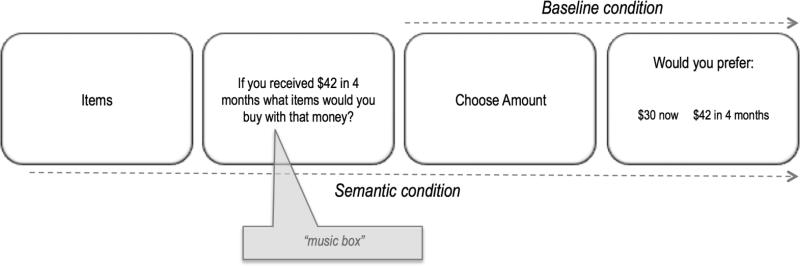
All stimuli were presented on a computer screen (using E-prime 2.0 software). Trials initiated with a self-paced cue (i.e., “Choose Amount”), which was provided to prepare participants for the task ahead and to provide reminders of instructions when necessary (see Figure 2). Participants were then cued to indicate their preference either for the future reward (e.g., $42 in 4 months) or the immediate $30 reward. Participants were given as much time as they needed to make their choice. Choices were read aloud by the experimenter and responses were self-paced and keyed in by the experimenter. Participants were first given practice trials to familiarize themselves with the materials and procedure.
Figure 2.
Trial overview for experiment 1 (see Method).
The semantic condition was similar to the baseline condition (and used the same 36 trials presented in a different random order), except that, on each trial, participants were first given a semantic cue, which required them to think about what items they would purchase given the amount and delay provided (e.g., “If you received $42 in 4 months what items would you buy with that money?”). Participants were asked to generate the items and were told that they could use the money to purchase a single item (e.g., a silk blouse) or multiple items (e.g., a silk blouse and a pair of slacks). Participants were additionally told that the item(s) purchased could either be for themselves or for another person (e.g., a silk blouse for a loved one). As shown in Figure 2, trials began with a self-paced cue (i.e., “Items”). Next, the semantic cue was presented and participants provided aloud their response to the cue (e.g., “a silk blouse”), which was recorded by the experimenter. Immediately after providing their response, participants were cued to indicate their preference either for the future reward (e.g., $42 in 4 months) or the immediate $30 reward. Participants were additionally told that the decision was independent of the preceding situation, such that the amount chosen did not have to be spent on the item(s) just considered (Benoit, et al., 2011; Palombo, et al., 2015c). As described in the Supplementary Materials, piloting in a separate group of healthy participants (N = 12) as a manipulation check of the current paradigm, suggested that participants largely engaged in different cognitive processes while performing the semantic task used in the present study versus the episodic cueing procedure used in Palombo et al. (2015c). More specifically, whereas participants tended to more strongly imagine a scene or an unfolding scenario in the episodic as compared to the semantic cueing condition (t11=8.09, p<.0001), they tended to imagine single objects (but not a scene) to a greater extent in the semantic as compared to the episodic cueing condition (t11=7.85, p<.0001; See Supplementary Materials).
In the present study, immediately after completing all trials, for 12 (one third) of the cues, participants were asked to rate how much they would enjoy receiving the item(s) they selected, using a 6-point scale, with a higher score indicating greater enjoyment (e.g., “Earlier you mentioned getting a new silk blouse with $42 in 4 months. How much would you enjoy getting that?”). This rating provided a proxy of the affective value participants assigned to the future reward. The 12 trials used for the enjoyment ratings were quasi-randomly selected from the complete set of 36 trials, such that each of the 6 levels of reward and 6 levels of delay were represented equally often.
To avoid confusion with task instructions, the two conditions (baseline, semantic) were presented in blocked format, with the semantic block always presented second to avoid carryover effects that might contaminate the baseline condition. The semantic condition was preceded by a simple math task, in which participants had to answer a series of questions involving similar dollar amounts to those provided in the intertemporal choice task (e.g., “How many of these are divisible by two: $54, $38, $26?”). The purpose of this math “distracter task” was to shift participants’ cognitive set and interfere with their memory for the amounts used in the baseline condition and their corresponding selections. To keep the baseline and semantic conditions as analogous as possible, an additional set of math questions was also presented prior to the baseline task.
2.1.3. Quantifying Intertemporal Choice
Following Palombo et al (2015c), the intertemporal choice (i.e., temporal discounting) dependent measure was a “reward index,” which reflects the extent to which the accumulated reward exceeds the amount that would be obtained by always choosing the immediate reward (see Benoit et al. 2011 for similar methodology). It is calculated as the difference between a participant's actual accumulated reward and the minimum accumulated reward possible, divided by the difference between the maximum accumulated reward possible and the minimum accumulated reward possible (Palombo, et al., 2015c). Thus, the value of the reward index ranged from 0.0 to 1.0, with consistent selection of the smaller, immediate reward yielding a reward index of 0.0, and consistent selection of the larger, later reward yielding a reward index of 1.0.
2.2. Results
Like controls, amnesic participants were able to generate a response to the semantic cues on every trial. Examples are provided in the Supplementary Materials (Table S1). Intertemporal choice data (i.e., reward index) were analyzed using a 2×2 mixed-design ANOVA, with factors of group (amnesic vs. control) and condition (baseline vs. semantic). There was no main effect of condition (F1, 19 = 2.19, p = .16, ηp2 = 0.10) or group (F1, 19 = 0.05, p = .83, ηp2 = 0.003); however, there was a significant interaction between group and condition (F1, 19 = 15.82, p = .001, ηp2 = 0.45). Post-hoc analyses revealed that the reward index was higher for the semantic condition (mean = .70) relative to the baseline condition (M = .61) only in the control group (t11 = −2.92, p = .01); in amnesics the reward index was significantly lower in the semantic condition (M = .52) relative to the baseline condition (mean = .72; t9 = 2.70, p = .027). In other words, whereas controls made more patient choices following semantic cueing, amnesic participants made less patient choices such that the mean difference score (semantic minus baseline) was positive in the control group (indicating reduced temporal discounting in the semantic condition), but negative in the amnesic group (indicating increased temporal discounting in the semantic condition)2 (Figure 3). One amnesic participant (P05) completed only approximately one third of the semantic condition of the intertemporal choice paradigm due to fatigue (and did not complete the enjoyment ratings). Exclusion of this amnesic participant's data did not change the overall pattern of results (Group × Condition: F1, 18 = 18.04, p < .001, ηp2 = 0.50). The pattern of results was similar when we examined four amnesic participants with lesions restricted to the hippocampus (Group × Condition: F1, 14 = 13.22, p = .003, ηp2 = 0.49).
Figure 3.
Mean temporal discounting difference (semantic – baseline) scores for the reward index for healthy controls and amnesic participants for experiment 1. Error bars indicate SEM.
Groups did not significantly differ in the perceived enjoyment of the items they generated in the semantic cueing portion of the task (t18 = .33, p = .75, Cohen's d = .16; controls: M = 4.70, SD = .58; amnesics: M = 4.59, SD = .91), suggesting that amnesics and controls did not differ in the affective value assigned to the items they selected. This null group difference was also observed when we examined amnesic participants with lesions restricted to the hippocampus (t14 = 1.15, p = .27, Cohen's d = .61). No association was observed between the magnitude of change in discounting following cueing and perceived enjoyment in either amnesics (p = .91) or controls (p = .32).
3. Discussion
In experiment 1 we investigated whether semantic future thinking could modulate intertemporal choices in amnesic participants with damage to the MTL. We found that, relative to a baseline task involving standard intertemporal choice, healthy individuals were more inclined to select a larger, later reward when they first engaged in semantic future thinking. In other words, semantic cueing of the future choices attenuated the typical human tendency to discount the future. Contrary to our hypothesis, however, semantic cueing did not have a similar effect in amnesic participants, and in fact, paradoxically augmented their temporal discounting; semantic cueing significantly increased the tendency for amnesics to choose the smaller, sooner reward.
What is the nature of the impairment in amnesic participants that prevents them from experiencing the semantic-cueing-induced shift toward more optimal intertemporal choices? One way to answer this question is to consider the mechanisms that give rise to that shift in control participants. Theorists have proposed that future thinking can affect intertemporal choices by allowing individuals to vicariously sample future outcomes, which in turn, helps one establish an appropriate subjective value (Kurth-Nelson, Bickel, & Redish, 2012)3. For example, the subjective value of $42, available in 4 months, can more readily be assigned if one considers using the money to buy a much-needed shovel for the impending winter. Amnesic participants did not appear to have difficulty reasoning an appropriate application for the future reward: Much like controls, the majority of amnesics, while spontaneously thinking out loud about what item(s) they would purchase, considered various future circumstances that they might face (e.g., “it will be the middle of the summer”; “it would be Christmastime”; “my wife's birthday is in October”). Additionally, amnesic participants derived the same level of hypothetical “enjoyment” as healthy controls when considering their future purchases, suggesting that they are as capable as controls of assigning subjective value to a future reward (see Benoit, et al., 2011; Benoit, Szpunar, & Schacter, 2014). Thus, a breakdown in the ability to assign subjective value is unlikely to account for their failure to show attenuated temporal discounting with semantic cues. Moreover, “enjoyment” ratings did not predict the shift in discounting behavior in either group. Thus, it appears that the cuing-induced shift in temporal discounting (and the absence of this shift in amnesic participants) is not linked to the effect that semantic future thinking has on the perceived value of the future, at least with respect to perceived future enjoyment.
Still, it has been argued that future thinking does more than just facilitate value assignment; when we can represent the subjective value of a future reward in the present, we are better equipped to bypass current goals (Boyer, 2008). That is, future thinking may affect intertemporal choices by bringing the future outcome into our present “mind's eye” so that the subjective value of the future can be appreciated “right now.” That amnesic participants were unable to use semantic future thinking to elicit more patient choices may, at first blush, suggest that they simply have a deficit in semantic future thinking. Still, that amnesics could semantically reason what items they could plausibly purchase at a given time frame (and are able to assign the item a normal subjective value as described above) argues against a semantic future thinking deficit as accounting for the present results. We suggest instead that the generation of such semantic information was devoid of future “tagging,” a term we use here to refer to an inability to bind the generated semantic item to the future time frame. That is, amnesic participants cannot maintain a representation of the future value as subjectively being in the future. In the absence of this subjective future tag, an item that is generated from the future-oriented cue is conceptualized as “belonging” to the present. The notion that MTL damage produces deficits in this type of future tagging, albeit post hoc, provides an explanation for the finding that temporal discounting was augmented by semantic cueing in amnesics in the present study: Because the generated item is conceptualized as being part of the present, the immediate reward becomes more appealing than it was in the absence of semantic cueing because the cueing process makes more apparent what that immediate reward could be used for. Note that this tagging deficit need not be specific to a future time orientation and may involve binding two pieces of information together more broadly (i.e., the generated item needs to be bound to the subsequently presented future time frame at the decision phase).
In light of this interpretation, it remains an open question what other factors may have been responsible for the cueing effect in some of the amnesic participants in Kwan et al. (2015), and why such a tagging deficit did not preclude some amnesics from making farsighted choices in their study. One procedural feature of their study that warrants consideration is the fact that the cues used to elicit future thinking remained present on the screen during the decision phase. For example, when participants saw e.g., “$50 now versus $100 in 3 years” in Kwan et al. (2015), the cue “Imagine 40th wedding anniversary in 3 years” was simultaneously displayed on the screen. On the one hand, a bias towards selecting the future option could occur even in the absence of future thinking, simply by linking the cued event with the future dollar amount by virtue of the shared time frame (i.e., a cueing bias). On the other hand, simultaneous presentation of the event and the decision options may have helped some amnesic participants better maintain the future tag associated with the reward, the process we hypothesize is deficient in amnesic participants in experiment 1. In other words, the presentation of the cued event during the decision phase in Kwan et al. (2015) served as a scaffold to help amnesics maintain the experienced reward value as “belonging” to the future.
The goal of experiment 2 was to explore the aforementioned “tagging” hypothesis by presenting the generated semantic items on the screen during the decision phase, similar to Kwan et al. (2015). To eliminate the potential contribution of the abovementioned cueing bias, the semantic item(s) generated were paired both with the present and with the future reward.
4. Experiment 2
4.1. Method
4.1.1. Participants
Eight patients with amnesia (3 women) participated in experiment 2 (see Table 1 for demographic and neuropsychological data), which included the amnesic participants who had participated in experiment 1 who were available as well as one additional amnesic participant (P10; see Table 1 for etiology, lesion, and demographic information) who had not participated in experiment 1. A new group of 12 healthy control participants (6 women) were recruited, and were matched to the amnesic group in age (58.8 ± 10.8 years), education (15.3 ± 2.4 years) and verbal IQ (108.6 ± 12.7). All participants provided informed consent in accordance with the procedures of the VA Boston Healthcare System Institutional Review Board.
4.1.2. Materials and Procedure
The procedures used in experiment 2 were similar to those of experiment 1 with the baseline followed by the semantic condition. By contrast to experiment 1, in experiment 2, in the semantic condition, the item(s) generated from the cue was presented at the time of choice (e.g., would you prefer $30 now to spend on a silk blouse or $42 in 4 months to spend on a silk blouse). By necessity, these items were harvested in a pre-session (completed after the baseline and prior to the semantic condition) to be fed into the intertemporal choice task so that the items could be presented simultaneously on the screen (see Figure 4). (This is in contrast to experiment 1, in which an item was generated just prior to each intertemporal choice decision.) A short break of a few minutes was implemented after the pre-session to allow the experimenter to enter the generated items into the computer for the intertemporal choice task. In the pre-session, participants generated for each time delay an item that was “high” in value (around $48-$58) and one that was “low” in value (around $34-42), which were then paired with the high ($48, $54, and $58) and low value ($34, $38, $42) dollar amounts, respectively, in the choice phase. Thus, each generated item appeared in 3 different trials. Notably, this was in contrast to experiment 1, in which an item was generated in response to each value—a methodological difference implemented in experiment 2 for practical reasons to ensure that this experiment was not unduly long for amnesic participants. (Cueing the participants with the value range instead of each specific value reduced the number of items that had to be generated, thus expediting the cue generation portion of the task so that the entire task was not significantly longer than in experiment 1.). The order in which participants generated items in response to cues with respect to time period and dollar amount was random for each subject. The remaining methods were identical to experiment 1.
Figure 4.
Trial overview for experiment 2 (see Method).
4.2. Results
As in experiment 1, amnesic participants did not have difficulty generating items to purchase. Intertemporal choice data (i.e., reward index) were analyzed using a 2×2 mixed-design ANOVA, with factors of group (amnesic vs. control) and condition (baseline vs. semantic). There was a main effect of condition (F1, 18= 7.35, p = .01, ηp2 = 0.29), with reduced temporal discounting observed in the semantic relative to baseline condition, but no significant effect of group (F1, 18 = .29, p = .60, ηp2 = 0.02), or interaction between group and condition (F1, 18 = .41, p = .53, ηp2 = 0.02; see Figure 5); in other words, both controls and amnesic participants became significantly more “patient” in their decision making following the semantic cueing.4 The pattern of results was similar when we examined four amnesics with lesions restricted to the hippocampus with a main effect of condition, albeit at trend level (F1, 14 = 3.78 p = .07, ηp2 = 0.21), with no significant effect of group (F1, 14 = .10, p = .76, ηp2 = 0.07), or interaction between group and condition (F1, 14 = .61, p = .45, ηp2 = 0.04).
Figure 5.
Mean temporal discounting difference (semantic – baseline) scores for the reward index for healthy controls and amnesic participants for experiment 2. Error bars indicate SEM.
Groups did not significantly differ in the perceived enjoyment of the items they selected in the semantic cueing portion of the task (t18 = .32, p = .76, Cohen's d = .14; controls: M = 4.79, SD = .81; amnesics: M = 4.67, SD = .85), as in experiment 1. This null group difference was also observed when we examined amnesic participants with lesions restricted to the hippocampus (t14 = .58, p = .57, Cohen's d = .32). No association was observed between the magnitude of change in discounting following cueing and perceived enjoyment either in amnesic participants (p = .56) or in controls (p = .47).
A direct comparison of the 7 amnesics who participated both in experiment 1 and experiment 2 demonstrated a significantly larger change score (semantic – baseline) in experiment 2 in comparison to experiment 1 (t6 = −3.00, p = .02; Mexperiment1 = −.21, SD = .25, Mexperiment2 = .14, SD = .24).
4.3. Discussion
Experiment 2 was designed to explore the hypothesis that the increased temporal discounting observed in experiment 1 in amnesic participants was due to a deficit in future “tagging.” In support of this hypothesis, we found that when the items generated from the cueing procedure were presented on the screen during the decision phase, amnesic individuals, like controls, shifted their decision making towards more patient choices. These findings suggest that the MTL is not needed for making optimal intertemporal decisions that draw on semantic future thinking provided that scaffolding is given to support accurate time-tagging.
5. General Discussion
The aim of the present investigation was to determine whether cueing the future semantically would drive amnesic participants with MTL lesions towards more patient choices. In doing so, our goal was to address the results of Kwan et al. (2015) in comparison to our prior work (Palombo, et al., 2015c), both involving episodic cueing in intertemporal choice. As noted in the introduction, Kwan et al. (2015) suggested that the highly personal nature of the episodic cues used in their study involving amnesic participants with MTL (and associated) lesions might have enabled some amnesics to leverage personal semantic information about the future to inform their intertemporal choices, whereas the episodic cues in our prior study (Palombo, et al., 2015c) were less personally salient, and thus less likely to elicit personally-relevant semantic future thinking (Kwan, et al., 2015; also see Palombo et al., 2015a for discussion). In the current study (experiment 1), the use of personally relevant semantic cues alone did not induce more future-oriented choices in amnesics. In fact, it had the opposite effect, resulting in less patient choices in amnesic participants. As noted, we interpreted this result as a future “tagging” (binding) deficit, in that amnesics were unable to maintain that the semantic item generated from the cue was related to the future. In experiment 2, the fact that the item(s) generated in response to the larger, future reward was present on the screen during the decision phase, helped to keep the generated item tied to the future. Accordingly, this resulted in more patient choices in amnesic participants as well as in controls. These findings suggest that the ability to bind the item to the future is a critical process underlying the shift towards more patient choices, but when such binding is not necessary for task performance (as in experiment 2), amnesic participants can make use of semantic future thinking in the service of optimal decision making.
Importantly, that semantic cueing influenced amnesic participants’ intertemporal choices both in experiment 1 and 2 (albeit in opposite directions), is in contrast to our prior work with an episodic cueing paradigm (Palombo, et al., 2015c), in which the cueing procedure had no effect on amnesic participants’ intertemporal choices (i.e., the average change in discounting following the episodic cueing manipulation was essentially zero). That is, unlike in the case of semantic cueing, episodic cueing was unequivocally unsuccessful in influencing amnesic participants’ intertemporal choices in either direction. It remains an open question as to what critical feature of the semantic cueing procedure was important for influencing amnesic participants’ choices; was it the personal nature of the cues or their semantic content that was important? Relevant to this topic, it is noteworthy that a recent fMRI study of healthy individuals compared temporal discounting following future imagining of personally familiar events (e.g., meeting a close friend at a café) versus unfamiliar events (e.g., meeting Bill Clinton at a café). While there was greater involvement of the hippocampus for unfamiliar events relative to familiar ones (likely due to greater construction demands), the magnitude of hippocampal activation predicted the subjective value of the delayed reward in both conditions (Sasse, et al., 2015). In other words, this study suggests that the involvement of the hippocampus in modulating intertemporal choices does not depend on the degree of personal familiarity of the cues, at least with respect to episodic future thinking. To address this issue further, it would be necessary to compare the effects of personally familiar to that of personally unfamiliar semantic future thinking cues on intertemporal choices, particularly in the context of amnesia.
A possible alternative explanation for the observed shift to more patient choices in amnesic participants in experiment 2 is that amnesics opted for the larger, later reward to simply cover the cost of the generated item without reference to the timeframe information. Ruling out this possibility, an ancillary analysis showed that, like controls, amnesic participants were significantly less patient for rewards situated in the distant (9 months-2 years) relative to the near (2-6 months) future (controls: p < .001; amnesics: p = .003) and this distant versus near difference in amnesics was of the same magnitude to that observed in controls (Timeframe × Group: p = .80; amnesic difference = 33%; controls difference = 36%). This strongly affirms that amnesics considered the timeframe in their choices.
Our findings, in conjunction with previous work on this topic, provide evidence that amnesic participants with MTL damage cannot draw either on episodic (Palombo, et al., 2015c) or on semantic representations normally to make optimal intertemporal choices. They can, however, make “normal” decisions when intertemporal choices are not explicitly baited with either episodic or semantic cues, as in standard intertemporal choice paradigms (Kwan, et al., 2012; Kwan, et al., 2013) or when semantic cues are tagged to the future, as in experiment 2 in the present study. Real life decisions likely vary widely in the extent to which future thinking is critical and, when required, in the extent to which semantic versus episodic processes are most relevant (e.g., deciding to skip your yearly vacation may be based on a consideration of the fact that your child starts college soon [semantic future thinking]; deciding to wait in line to upgrade your seats at a baseball game may be based on envisioning how much clearer the field would be if seated closer [episodic future thinking]). A failure to engage fully in future thinking likely has important implications for amnesic patients’ abilities to make at least some types of decisions about aspects of daily functioning, as suggested by their limited functional independence. Nonetheless, our results suggest that amnesics can benefit from semantic future thinking under some circumstances—namely when adequate scaffolding is provided. Continuing to elucidate the consequences of MTL damage on decision making is a critical avenue for future work. Moreover, a better understanding of the ways in which such deficits can be ameliorated has important clinical relevance.
Supplementary Material
Highlights.
Participants made intertemporal choices cued by semantic future thinking
Healthy controls shifted to more patient choices following semantic future thinking
Amnesics shifted to less patient choices following semantic future thinking
When items were shown with intertemporal choices, amnesics also became more patient
Amnesics can make optimal decisions about the future when scaffolding is provided
Acknowledgements
D.J.P., M.M.K. and M.V. designed the research. D.J.P. conducted the research and analyses and wrote the manuscript with input from M.M.K. and M.V. This research was supported by the National Institutes of Mental Health (Grant number: MH093431) and the Clinical Science Research and Development Service of the Department of Veterans Affairs (Grant number: IO1 CX000925). The research was also supported by a Faculty Award from Wellesley College to M.M.K. and a Canadian Institutes of Health Research Postdoctoral Fellowship Award to D.J.P. The authors thank Rose Hopkins and Keely Burke for research assistance. The authors additionally thank the two anonymous reviewers for their insightful comments on the manuscript. The contents of this manuscript do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Another way to understand the difference in findings between the two studies is with reference to the fact that the future scenarios in Kwan et al. (2015) were more plausible than the generic events in Palombo et al. (2015c). Given that the hippocampus is more active when imagining implausible versus plausible events (Weiler, Suchan, & Daum, 2010), the impairment in Palombo et al. (2015c) might reflect greater demands on the hippocampus in that study. Indeed, other work shows that when amnesic participants are asked to select future events pertaining to their lives, they are more prone to select common events (i.e., events that are highly likely to occur in the population) relative to controls (Lenton-Brym, Kurczek, Rosenbaum, & Sheldon, 2016). Notably however, the reliance on personal semantic information would be easier for plausible events (as in Kwan et al., 2015) thus leading to the same proposed mechanism described above.
Although amnesics and controls did not significantly differ in the baseline condition (p = .46), the baseline was numerically higher in amnesics relative to controls. To ensure that the difference in baseline was not responsible for the differential effect of semantic future thinking in the two groups, we removed one control and one amnesic participant (with the lowest and highest baseline performance, respectively), to more closely match the baseline condition between groups [controls (M = .66); amnesics (M = .68)]. This yielded the same pattern of results (Group × Condition: p = .003).
Note that many proposed mechanisms were derived from theoretical accounts regarding the effect of episodic future thinking on intertemporal choice. Here we assume similar mechanisms may also account for the effect of semantic future thinking on intertemporal choice.
As in experiment 1, we observed a numerically higher baseline in amnesics (.57), relative to controls (.47) although amnesic participants and controls did not significantly differ in their baseline scores (p = .55). To ensure that the numeric difference in baseline was not contributing to the above results, we removed one control and one amnesic (with the lowest and highest baseline performance, respectively), to more closely match the baseline condition between groups [amnesics (M = .51); controls (M = .51)]. This analysis produced the same pattern of results (Condition effect: p = .009; Group effect: p = .96; Group × Condition: p = .93).
The authors declare no conflicts of interest. The contents of this manuscript do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
References
- Atance CM, O'Neill DK. Episodic future thinking. Trends Cogn Sci. 2001;5:533–539. doi: 10.1016/s1364-6613(00)01804-0. [DOI] [PubMed] [Google Scholar]
- Benoit RG, Gilbert SJ, Burgess PW. A neural mechanism mediating the impact of episodic prospection on farsighted decisions. J Neurosci. 2011;31:6771–6779. doi: 10.1523/JNEUROSCI.6559-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit RG, Szpunar KK, Schacter DL. Ventromedial prefrontal cortex supports affective future simulation by integrating distributed knowledge. Proc Natl Acad Sci USA. 2014;111:16550–16555. doi: 10.1073/pnas.1419274111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer P. Evolutionary economics of mental time travel? Trends Cogn Sci. 2008;12:219–224. doi: 10.1016/j.tics.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Gupta R, Duff MC, Denburg NL, Cohen NJ, Bechara A, Tranel D. Declarative memory is critical for sustained advantageous complex decision-making. Neuropsychologia. 2009;47:1686–1693. doi: 10.1016/j.neuropsychologia.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutbrod K, Krouzel C, Hofer H, Muri R, Perrig W, Ptak R. Decision-making in amnesia: do advantageous decisions require conscious knowledge of previous behavioural choices? Neuropsychologia. 2006;44:1315–1324. doi: 10.1016/j.neuropsychologia.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Kan IP, Giovanello KS, Schnyer DM, Makris N, Verfaellie M. Role of the medial temporal lobes in relational memory: neuropsychological evidence from a cued recognition paradigm. Neuropsychologia. 2007;45:2589–2597. doi: 10.1016/j.neuropsychologia.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SB. Future mental time travel: types of memory, types of selves, and types of temporality. Social Cognition. 2013;31:417–426. [Google Scholar]
- Klein SB, Loftus J, Kihlstrom JF. Memory and temporal experience: the effects of episodic memory loss on an amnesic patient's ability to remember the past and imagine the future. Social Cognition. 2002;20:353–379. [Google Scholar]
- Kurth-Nelson Z, Bickel W, Redish AD. A theoretical account of cognitive effects in delay discounting. Eur J Neurosci. 2012;35:1052–1064. doi: 10.1111/j.1460-9568.2012.08058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan D, Craver CF, Green L, Myerson J, Boyer P, Rosenbaum RS. Future decision-making without episodic mental time travel. Hippocampus. 2012;22:1215–1219. doi: 10.1002/hipo.20981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan D, Craver CF, Green L, Myerson J, Gao F, Black SE, Rosenbaum RS. Cueing the personal future to reduce discounting in intertemporal choice: Is episodic prospection necessary? Hippocampus. 2015;25:432–443. doi: 10.1002/hipo.22431. [DOI] [PubMed] [Google Scholar]
- Kwan D, Craver CF, Green L, Myerson J, Rosenbaum RS. Dissociations in future thinking following hippocampal damage: evidence from discounting and time perspective in episodic amnesia. J Exp Psychol Gen. 2013;142:1355–1369. doi: 10.1037/a0034001. [DOI] [PubMed] [Google Scholar]
- Lee H, Ghim JW, Kim H, Lee D, Jung M. Hippocampal neural correlates for values of experienced events. J Neurosci. 2012;32:15053–15065. doi: 10.1523/JNEUROSCI.2806-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenton-Brym A, Kurczek J, Rosenbaum RS, Sheldon S. A new method for assessing the impact of medial temporal lobe amnesia on the characteristics of generated autobiographical events. Neuropsychologia. 2016;85:35–43. doi: 10.1016/j.neuropsychologia.2016.02.023. [DOI] [PubMed] [Google Scholar]
- Lin H, Epstein LH. Living in the moment: Effects of time perspective and emotional valence of episodic thinking on delay discounting. Behav Neurosci. 2014;128:12–19. doi: 10.1037/a0035705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Feng T, Chen J, Li H. The value of emotion: how does episodic prospection modulate delay discounting? PLoS One. 2013;8:e81717. doi: 10.1371/journal.pone.0081717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Hassabis D. Role of the hippocampus in imagination and future thinking. Proc Natl Acad Sci U S A. 2011;108:E39. doi: 10.1073/pnas.1018876108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombo DJ, Keane MM, Verfaellie M. How do lesion studies elucidate the role of the hippocampus in intertemporal choice? Hippocampus. 2015a;25:407–408. doi: 10.1002/hipo.22433. [DOI] [PubMed] [Google Scholar]
- Palombo DJ, Keane MM, Verfaellie M. How does the hippocampus shape decisions? Neurobiol Learn Mem. 2015b;125:93–97. doi: 10.1016/j.nlm.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Palombo DJ, Keane MM, Verfaellie M. The medial temporal lobes are critical for reward-based decision making under conditions that promote episodic future thinking. Hippocampus. 2015c;25:345–353. doi: 10.1002/hipo.22376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Büchel C. Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron. 2010;66:138–148. doi: 10.1016/j.neuron.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Race E, Keane MM, Verfaellie M. Medial temporal lobe damage causes deficits in episodic memory and episodic future thinking not attributable to deficits in narrative construction. J Neurosci. 2011;31:10262–10269. doi: 10.1523/JNEUROSCI.1145-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race E, Keane MM, Verfaellie M. Losing sight of the future: Impaired semantic prospection following medial temporal lobe lesions. Hippocampus. 2013;23:268–277. doi: 10.1002/hipo.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasse LK, Peters J, Buchel C, Brassen S. Effects of prospective thinking on intertemporal choice: The role of familiarity. Hum Brain Mapp. 2015;36:4210–4221. doi: 10.1002/hbm.22912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, Szpunar KK. The future of memory: remembering, imagining, and the brain. Neuron. 2012;76:677–694. doi: 10.1016/j.neuron.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, van der Horst AS, McDuff SG, Frascino JC, Hopkins RO, Mauldin KN. Role of the hippocampus in remembering the past and imagining the future. Proc Natl Acad Sci U S A. 2010;107:19044–19048. doi: 10.1073/pnas.1014391107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Psychology. 1985;26:1–12. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale—Third Edition (WAIS-III) administration and scoring manual. Harcourt Assessment; San Antonio, TX: 1997a. [Google Scholar]
- Wechsler D. Wechsler Memory Scale—Third Edition (WMS–III) administration and scoring manual. The Psychological Corporation; San Antonio, TX: 1997b. [Google Scholar]
- Weiler JA, Suchan B, Daum I. Foreseeing the future: occurrence probability of imagined future events modulates hippocampal activation. Hippocampus. 2010;20:685–690. doi: 10.1002/hipo.20695. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.