Abstract
Metabotropic glutamate receptor 4 (mGlu4) is emerging as a potential therapeutic target for numerous central nervous system indications, including Parkinson’s disease (PD). As the glutamate binding sites among the eight mGlu receptors are highly conserved, modulation of receptor activity via allosteric sites within the receptor transmembrane domains using positive and negative allosteric modulators (PAMs and NAMs, respectively) has become a common strategy. We and others have used PAMs targeting mGlu4 to show that potentiation of receptor signaling induces antiparkinsonian activity in a variety of PD animal models, including haloperidol-induced catalepsy and 6-hydroxydopamine-induced lesion. Recently, mGlu4 has been reported to form heteromeric complexes with other mGlu receptor subtypes, such as mGlu2, and the resulting heteromer exhibits a distinct pharmacological profile in response to allosteric modulators. For example, some mGlu4 PAMs do not appear to potentiate glutamate activity when mGlu2 and mGlu4 are coexpressed, whereas other compounds potentiate mGlu4 responses regardless of mGlu2 coexpression. We report here the discovery and characterization of VU0418506, a novel mGlu4 PAM with activity in rodent PD models. Using pharmacological approaches and Complemented Donor–Acceptor resonance energy transfer (CODA-RET) technology, we find that VU0418506 does not potentiate agonist-induced activity when mGlu2 and mGlu4 are heterodimerized, suggesting that the antiparkinsonian action of mGlu4 PAMs can be induced by compounds without activity at mGlu2/4 heteromers.
Keywords: Allosteric modulator, Parkinson’s disease, metabotropic glutamate receptor
Graphical Abstract
The primary pathological change underlying the motor symptoms associated with Parkinson’s disease (PD) is death of dopaminergic neurons in the substantia nigra pars compacta (SNc) of the basal ganglia (BG).1 Traditionally, therapeutic strategies for improving motor function in PD patients have relied on replacement of lost dopamine using L-DOPA or dopamine receptor agonists. However, increasing efforts have focused on developing nondopaminergic drug treatments for PD that normalize pathological changes in activity of BG output nuclei (the internal segment of the globus pallidus (GPi) and the substantia nigra pars reticulata (SNr)) that occur after loss of dopaminergic neurons. We and others have used this underlying biology as rationale for the development of novel pharmacological agents with the potential to mimic the dramatic clinical efficacy observed using surgical ablation or deep brain stimulation of specific BG nuclei to reverse pathological changes in activity in the GPi (reviewed in refs 2 and 3).
In recent years, the mGlu4 subtype of metabotropic glutamate (mGlu) receptor has emerged as a promising new therapeutic target for PD, and activation or potentiation of mGlu4 has been proposed to normalize activity in the BG motor circuit in PD patients.4,5 The mGlu4 receptor is highly expressed on presynaptic GABAergic terminals projecting from the striatum to the GPe,6,7 the first synapse of the BG “indirect pathway”, where activation of this receptor reduces GABAergic transmission.8 Activity at the striato-GPe synapse increases excitatory drive from the subthalamic nucleus (STN) to the GPi/SNr. Excitation of the GPi/SNr through the indirect pathway balances the inhibitory input of striatal direct pathway neurons onto these output nuclei. Agonists and highly selective positive allosteric modulators (PAMs) of mGlu4 reduce transmission at the striato-GPe synapse and have robust antiparkinsonian effects in rodent models.9–23 In addition, increasing evidence suggests that activation of mGlu4 may have neuroprotective effects by reducing excessive excitatory drive from the STN to dopaminergic neurons,24 as well as other neuroprotective actions in the SNc.
Unlike direct acting agonists, mGlu4 positive allosteric modulators (PAMs) act at allosteric binding sites in the transmembrane domain to potentiate the response to glutamate; some mGlu4 PAMs can also directly activate the receptor via an allosteric site.23,25 Multiple, structurally diverse mGlu4 PAMs have been reported that provide high selectivity for mGlu4, excellent pharmacokinetic properties, and robust efficacy in a range of rodent models of parkinsonian motor disability, as well as neuroprotective effects in models of toxin-induced dopaminergic cell loss.9–12,14–16,19,21,23 While the antiparkinsonian effects of mGlu4 PAMs have been hypothesized to be mediated primarily by actions at the striato-GPe synapse, mGlu4 is also localized in the striatum and recent studies raise the possibility that mGlu4-mediated inhibition of transmission at corticostriatal synapses or other sites within the striatum could also contribute to the antiparkinsonian activity of mGlu4 modulators.10,22,26,27
Recent studies suggest that mGlu4 can exist either as a constitutive homodimer or as a heterodimer with other mGlu receptor subtypes.28–30 We and others recently reported that mGlu2 and mGlu4 form a distinct complex when coexpressed in vitro and have provided evidence that the prototypical mGlu4 PAM, N-phenyl-7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxamide (PHCCC), does not result in observable potentiation of the activity of glutamate when mGlu2 is coexpressed with mGlu4.29,30 In contrast, two structurally distinct mGlu4 PAMs that are thought to bind to an alternate allosteric site on mGlu4,25 VU0155041 and Lu AF21934, induce potentiation regardless of mGlu2 expression, indicating a differential interaction of these two classes of compounds when mGlu2 and mGlu4 are coexpressed.30
We have performed and reported a series of pharmacological, biochemical, and electrophysiology studies suggesting that mGlu4 functions primarily as a heteromer at corticostriatal synapses.30 For example, the compounds VU0155041 and Lu AF21934, ligands that potentiate responses when mGlu2 and mGlu4 are expressed together, activate and potentiate mGlu receptor-mediated inhibition of transmission at corticostriatal synapses, whereas PHCCC is without effect.10,22,30 In contrast, in the presence of agonist, PHCCC has robust effects on mGlu4 modulation of transmission at multiple other synapses, including the striato-GPe synapse,21 suggesting that mGlu4 is likely to function as an mGlu4 homomer at this key synapse in the BG indirect pathway. These studies highlight the need to determine whether mGlu4 PAMs that may only potentiate the activity of mGlu4 when expressed in homomeric form will induce full efficacy in reducing parkinsonian motor disability or whether it is important to optimize mGlu4 PAMs that are also active at mGlu2/4 heteromers as PD therapeutics. PHCCC does not possess an ideal pharmacokinetic profile to test this hypothesis in vivo. We now report optimization of VU041850631 as a novel mGlu4 PAM with excellent pharmacokinetic properties, good brain penetration, and a profile that is consistent with activity only at homomeric mGlu4 when assessed using a Complemented Donor-Acceptor resonance energy transfer (CODA-RET) approach.32 This technique relies upon measuring the signal induced by activating a defined receptor heteromer (in this case, mGlu2/4); using this assay, we show that, in contrast to Lu AF21934, VU0418506 is not active at mGlu2/4 heteromers. Nonetheless, VU0418506 exhibits robust efficacy in rodent models of parkinsonian motor disability, suggesting that activity at mGlu2/4 heterodimers is not required for the antiparkinsonian effect of mGlu4 PAMs.
RESULTS
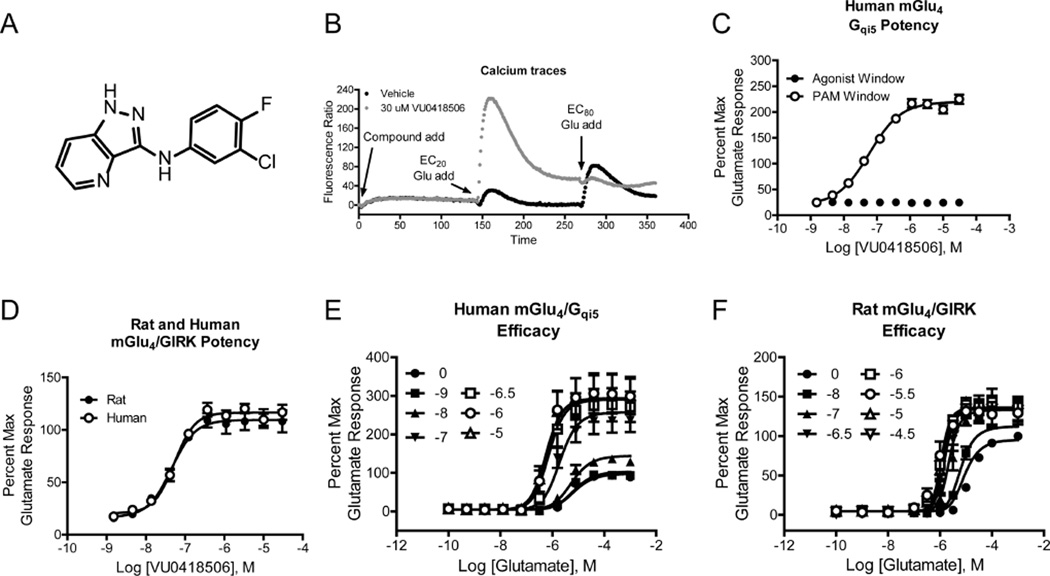
Development of VU0418506, a potent and selective mGlu4 PAM. VU0418506 (Figure 1A31) is a novel mGlu4 PAM with robust efficacy in cells expressing mGlu4. The compound is highly selective for mGlu4 relative to other mGlu receptor subtypes (with the exception of activity at mGlu6, a receptor which is primarily expressed in the retina), has an excellent pharmacokinetic profile in multiple species, and achieves high brain exposure after oral administration. Further characterization reveals that VU0418506 is a potent PAM of human mGlu4 activity assessed using two distinct assays (pEC50 = 7.17 ± 0.07, mean ± SEM, 68 nM, calcium assay, Figure 1B, C, pEC50 = 7.27 ± 0.08, EC50 = 55.7 nM, GIRK/thallium flux assay, Figure 1D). Additionally, VU0418506 potentiates the activity of glutamate at the rat mGlu4 receptor (pEC50 = 7.34 ± 0.04, 46 nM, Figure 1D). The compound also does not exhibit direct allosteric agonist activity in these assays as assessed by the lack of response when compound is added alone (Figure 1B, “compound add” and Figure 1C, black circles). Increasing concentrations of VU0418506 progressively left-shifted the concentration-response curve (CRC) for glutamate at both the human and rat receptors, with maximal efficacy achieved at approximately 1 µM (Figure 1E, F); again, no increase in the baseline was observed, suggesting a lack of allosteric agonist activity in this assay.
Figure 1.
In vitro pharmacological profile of VU0418506, a potent mGlu4 PAM. (A) Structure of VU0418506. (B) Calcium traces for a representative experiment performed in the presence and absence of 30 µM VU0418506. In this “triple addition” assay, a baseline read is taken for 2 s and compound or vehicle is added (designated with arrow and “compound add”). After approximately 2 min, a concentration of glutamate that elicits a 20% maximal response (“EC20 add”) is added. This addition detects the activity of potentiators. In the final addition (“EC80 add”), a concentration of glutamate that elicits an EC80 response is added. In this chimeric G protein assay, we anticipate that glutamate does not induce full coupling; inclusion of a PAM further stabilizes the active receptor state, resulting in a response in the presence of the PAM that is greater than the maximal response elicited by a saturating concentration of glutamate. As shown in the “compound add” section of the trace, VU0418506 does not elicit activity in this assay in the absence of glutamate. (C) Potency of VU0418506 in the absence (black symbols) and presence (white symbols) of an EC20 concentration of glutamate at human mGlu4 using the Gqi5-mediated calcium assay reflected in panel (B). The pEC50 is 7.24 ± 0.04 (mean ± SEM), EC50 = 59.6 nM, N = 10 determinations performed in triplicate. (D) Potency of rat and human mGlu4 using native coupling to GIRK channels as a readout. For rat mGlu4, the pEC50 is 7.34 ± 0.04 (mean ± SEM), EC50 = 46.6 nM, N = 3 determinations performed in triplicate. For human mGlu4, the pEC50 is 7.27 ± 0.08 (mean ± SEM), EC50 = 55.7 nM, N = 3 determinations performed in singlicate. (E, F) Increasing concentrations of VU0418506 were added to human mGlu4/Gqi5 cells or rat mGlu4/GIRK cells approximately 2 min prior to the addition of increasing concentrations of glutamate. Data represent three independent determinations performed in duplicate.
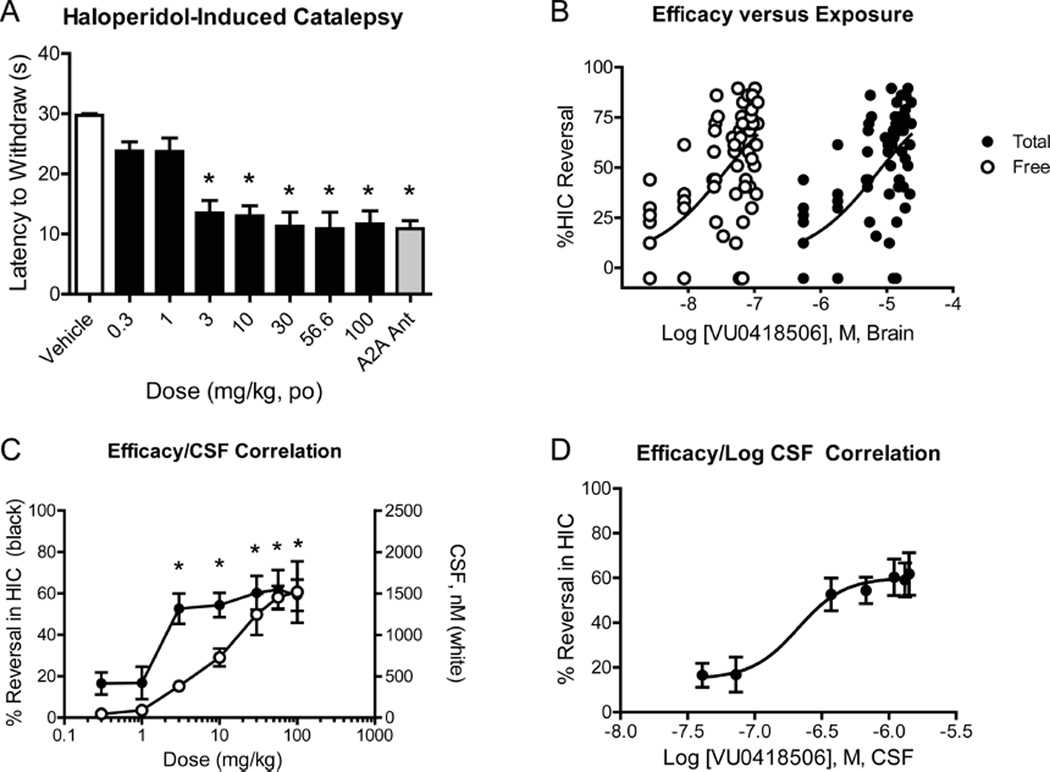
As mGlu4 has become a novel target that is being explored for the treatment of Parkinson’s disease, we next assessed the ability of VU0418506 to reverse haloperidol-induced catalepsy (HIC), a rodent model of parkinsonian motor disability. Animals were treated with a 1.5 mg/kg dose of haloperidol; 1 h later, animals were administered increasing doses of VU0418506 and catalepsy was assessed 30 min after VU0418506 dosing. VU0418506 showed robust activity in this model after oral administration, with significant activity in reversing catalepsy at doses of 3 mg/kg and higher (Figure 2A). The maximal efficacy achieved was similar to that observed with a 56.6 mg/kg oral dose of an adenosine A2A antagonist, which served as a positive control.33 In addition to assessing reversal of catalepsy, we also quantified plasma and brain levels of VU0418506 to establish a pharmacokinetic/pharmacodynamic (PK/PD) relationship for VU0418506 in the HIC model. Plasma and brain samples were taken immediately after catalepsy assessment (t = approximately 40 min after dosing) to measure total brain concentrations of VU0418506. Unbound brain levels were determined by multiplying total levels of drug by the unbound fraction assessed using a brain homogenate binding (BHB) assay (rat fu = 0.0045). As shown in Figure 2B, 50% reversal of HIC was observed with a total brain concentration of 7.1 µM and an estimated unbound concentration of 34 nM. This shows an excellent correlation with the in vitro EC50 of VU418506 in potentiating rat mGlu4 activity (46 nM).
Figure 2.
VU0418506 exhibits antiparkinsonian activity in reversing haloperidol-induced catalepsy at unbound brain and CSF concentrations at or above the in vitro EC50 at rat mGlu4. (A) A 1.5 mg/kg dose of haloperidol was given to rats, followed 1 h later by increasing doses of VU0418506 given orally; after 30 min, catalepsy was assessed. 56.6 mg/kg, dosed orally, of the adenosine A2A antagonist compound 2333 (gray bar) was used as a positive control. *p < 0.0001, one-way ANOVA with Bonferroni post-test. Data represent mean ± SEM, N = 9–10 animals. (B) Total (black circles) and predicted unbound/free (white circles) brain levels were determined. Calculated EC50 values were 7.1 µM (total) and 34 nM (unbound/free). Data represent mean ± SEM, N = 9–10 animals. (C) CSF levels of VU0418506 were determined after oral dosing and correlated with HIC reversal. Statistically significant reversals in HIC (data from panel (A)) were observed when CSF concentrations exceeded in the in vitro EC50 at rat mGlu4. (D) Concentration–response curve of CSF concentration versus response, with a calculated EC50 value of 210 nM.
To further establish a PK/PD relationship, we also assessed levels of VU0418506 in the cerebrospinal fluid (CSF), as this provides another assessment of “free” drug. In these studies, satellite animals were dosed and CSF samples were drawn at the same time points as plasma and brain samples were taken from the HIC studies (i.e., approximately 40 min postdose). As shown in Figure 2C, there was a good correlation between increasing CSF levels and in vivo activity in HIC; this is further elaborated in Figure 2D in which the log of the CSF concentration is plotted versus the % reversal in HIC, resulting in an in vivo EC50 value of 210 nM.
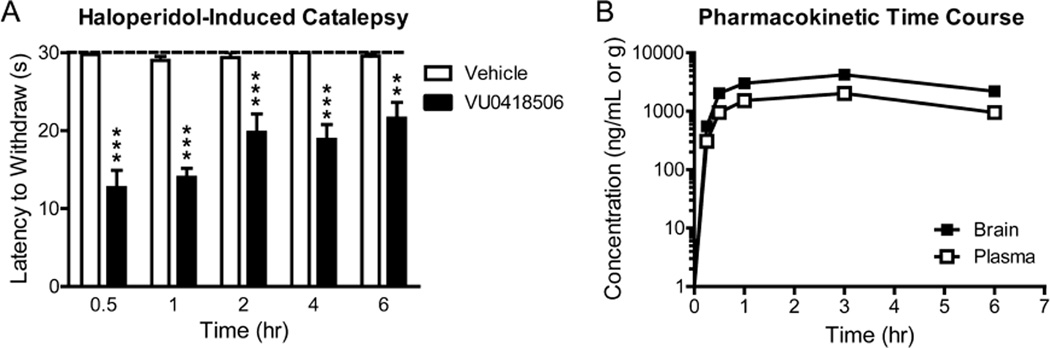
We also examined a time course of activity of VU0418506 in the reversal of HIC. In these studies, haloperidol was given at t = 0, a 30 mg/kg dose of VU0418506 was given at t = 1.5 h, and catalepsy was assessed 30, 60, 120, 240, and 360 min later (Figure 3A). Plasma and brain samples were taken from these same animals during this experiment, and this study revealed that sustained levels of VU0418506 were observed at least 6 h after dosing (Figure 3B), correlating with significant HIC reversals. As an initial assessment of effects on locomotor performance, we measured the effect of increasing doses of VU0418506 on rotorod performance (Supporting Information Figure 1). These studies showed that, at doses up to 100 mg/kg, we did not observe any impairment in the ability of rats to perform a rotorod challenge.
Figure 3.
VU0418506 exhibits antiparksonian activity out to 6 h after administration, paralleling exposure levels. (A) Animals were administered vehicle or a 30 mg/kg dose of VU0418506 and efficacy in reversing catalepsy was assessed 0.5, 1, 2, 4, or 6 h after dosing. Haloperidol was given 1.5 h before each catalepsy measurement as described in Methods. **p < 0.01, ***p < 0.0001. Data represent mean ± SEM, N = 8–10 animals. (B) Plasma (white squares) and brain (black squares) exposures were calculated up to 6 h after a 30 mg/kg dose of VU0418506. Data represent the average calculated from two animals.
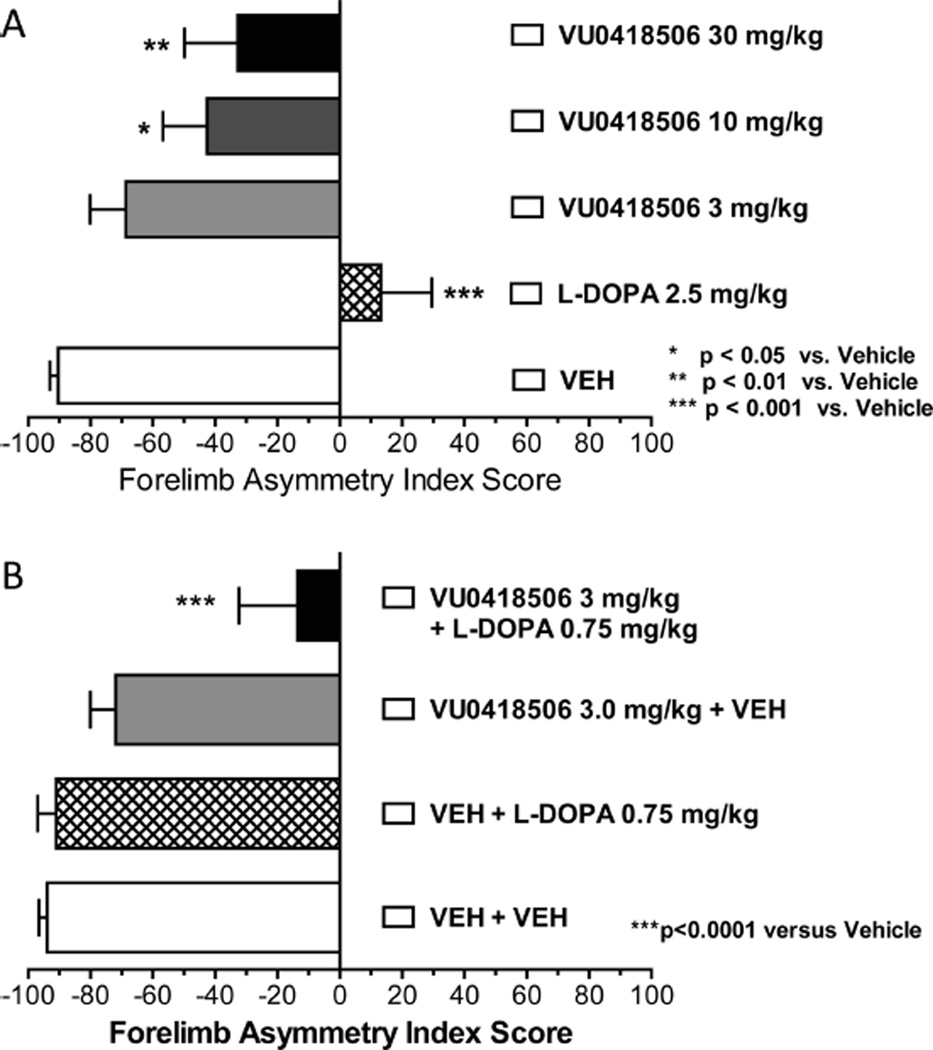
As an additional assessment of antiparkinsonian activity, we also performed studies in rats with unilateral 6-hydroxydopamine (6-OHDA) lesions and assessed the effects of VU0418506 on the reversal of forelimb asymmetry. Unilateral 6-OHDA injection into the medial forebrain bundle results in loss of use of the paw on the affected side, and an ability to restore equivalent use of both paws is considered a measure of antiparkinsonian activity. In these experiments, an acute dose of VU0418506 was administered and animals were assessed for asymmetry after 30 min. As can be seen in Figure 4A, a 2.5 mg/kg dose of L-DOPA completely reversed motor deficits assessed by measures of forelimb asymmetry. VU0418506 administered alone (10 and 30 mg/kg) also induced statistically significant reversals of forelimb asymmetry. Several mGlu4 PAMs, including ADX88178, VU0346770 and Lu AF21934, have also been shown to potentiate the effects of subthreshold doses of L-DOPA in various 6-OHDA lesion models.10,14,16 When combined, doses of L-DOPA and VU0418506 that were inactive alone (0.75 and 3 mg/kg, respectively) now exhibited statistically significant efficacy (Figure 4B). Overall, this profile indicates that VU0418506 is a potent mGlu4 PAM and exhibits a good pharmacokinetic profile with high brain penetration. Importantly, VU0418506 has robust antiparkinsonian activity in two rodent models and exhibits an excellent PK/PD relationship.
Figure 4.
VU0418506 reverses forelimb asymmetry deficits, both alone and with a subthreshold dose of L-DOPA, in a 6-OHDA model of Parkinson’s disease. (A) 2.5 mg/kg dose of L-DOPA (hatched bar), as well as 10 and 30 mg/kg VU0418506 (gray and black bars, respectively), significantly reverse forelimb asymmetry deficits (p-values shown in figure, one-way ANOVA with Dunnett’s multiple comparison test). (B) Doses of 0.75 mg/kg L-DOPA and 3 mg/kg VU0418506 are ineffective alone, but, when paired together, significant reversals of forelimb asymmetry phenotypes are observed (*p < 0.0001 versus vehicle, one-way ANOVA with Dunnett’s multiple comparison test). Dose groups for both studies represent mean ± SEM from 6 to 18 animals.
Finally, we compared the activity of VU0418506 and Lu AF21934 in cells coexpressing mGlu2 and mGlu4 using both traditional pharmacological approaches as well as CODA-RET. While all mGlu4 receptor PAMs shift the glutamate concentration response curve (CRC) in cells expressing mGlu4 alone, our previous studies revealed that mGlu4 PAMs appear to fall into two broad classes, one class that does not shift the glutamate CRC in cells coexpressing mGlu2 and mGlu4 (i.e., PHCCC), and a second class of compounds, represented by Lu AF21934, that can potentiate responses in these cells.30 We evaluated the effects of VU0418506 in cells expressing mGlu4 alone and mGlu4 in combination with mGlu2. While VU0418506 induced significant potentiation in cells only expressing mGlu4 (Figure 1 and Table 1), this compound had no effect on the glutamate CRC in cells expressing mGlu2 and mGlu4 (Figure 5A). In contrast, and as reported previously,30 Lu AF21934 induced a leftward shift in the glutamate CRC in cells expressing both mGlu2 and mGlu4 receptors (Figure 5B, Table 1). Overall, these data are consistent with the hypothesis that a distinct population of receptors is present in cells coexpressing mGlu2 and mGlu4 that is potentiated by Lu AF21934 but not by VU0418506; the simplest explanation for these findings is that this population is a heterodimer of mGlu2 and mGlu4, but this cannot be definitely established in cells coexpressing both receptors given the possibility of forming both homomers and heteromers.
Table 1.
Profiles of mGlu4 PAMs in Cells Expressing mGlu4 Alone versus Cells Expressing mGlu2 and mGlu4a
| VU0418506 |
Lu AF21934 |
|||
|---|---|---|---|---|
| mGlu4 | pEC50 | fold shift | pEC50 | fold shift |
| DMSO | 4.80 ± 0.06 | 4.81 ± 0.04 | ||
| −8 | 5.08 ± 0.07 | 1.9 ± 0.12c | 4.85 ± 0.03 | 1.1 ± 0.03 |
| −7 | 5.55 ± 0.17b | 6.0 ± 1.34 | 4.99 ± 0.07b | 1.5 ± 0.12 |
| −6.5 | 5.67 ± 0.14b | 7.7 ± 1.44c | 5.00 ± 0.04b | 1.6 ± 0.09c |
| −6 | 5.93 ± 0.12b | 14.0 ± 1.78c | 5.14 ± 0.07b | 2.1 ± 0.17 |
| −5.5 | 6.07 ± 0.21b | 20.8 ± 5.82 | 5.32 ± 0.07b | 3.2 ± 0.28c |
| −5 | 5.87 ± 0.13b | 12.2 ± 2.29c | 5.39 ± 0.06b | 3.8 ± 0.21c |
| −4.5 | 5.96 ± 0.15b | 15.1 ± 2.90c | 5.43 ± 0.08b | 4.2 ± 0.42 |
| mGlu2/4 | pEC50 | fold shift | pEC50 | fold shift |
| DMSO | 5.86 ± 0.07 | 5.91 ± 0.08 | ||
| −8 | 5.86 ± 0.08 | 1.0 ± 0.03c | 5.91 ± 0.11 | 1.0 ± 0.07 |
| −7 | 5.87 ± 0.05 | 1.0 ± 0.05 | 5.99 ± 0.06 | 1.2 ± 0.07 |
| −6.5 | 5.89 ± 0.07 | 1.1 ± 0.04c | 6.00 ± 0.09b | 1.2 ± 0.05c |
| −6 | 5.94 ± 0.06 | 1.2 ± 0.06c | 6.13 ± 0.09b | 1.7 ± 0.04 |
| −5.5 | 6.02 ± 0.02 | 1.5 ± 0.18 | 6.29 ± 0.06b | 2.4 ± 0.13c |
| −5 | 5.93 ± 0.07 | 1.2 ± 0.04c | 6.36 ± 0.08b | 2.8 ± 0.07c |
| −4.5 | 5.94 ± 0.06 | 1.2 ± 0.04c | 6.41 ± 0.08b | 3.2 ± 0.07 |
N = 3 independent experiments performed in duplicate.
p < 0.05. For pEC50 comparison, the potency in the presence of compound was compared to the respective DMSO control for either mGlu4 or mGlu2/4 using repeated measures ANOVA with a Bonferroni post-test to compare the potency of glutamate in the presence of increasing concentrations of the PAM with DMSO vehicle control.
p < 0.05. For the fold shift of the glutamate concentration-response curve, responses were compared for a given concentration of PAM between mGlu4 and mGlu2/4 using a paired, two-tailed t test.
Figure 5.
Differential interactions of (A) VU0418506 and (B) Lu AF21934 with mGlu4 in the presence and absence of mGlu2. Increasing concentrations of compound were applied prior to the application of increasing concentrations of glutamate and concentration-response curves were assessed. Data represent three independent experiments performed in duplicate.
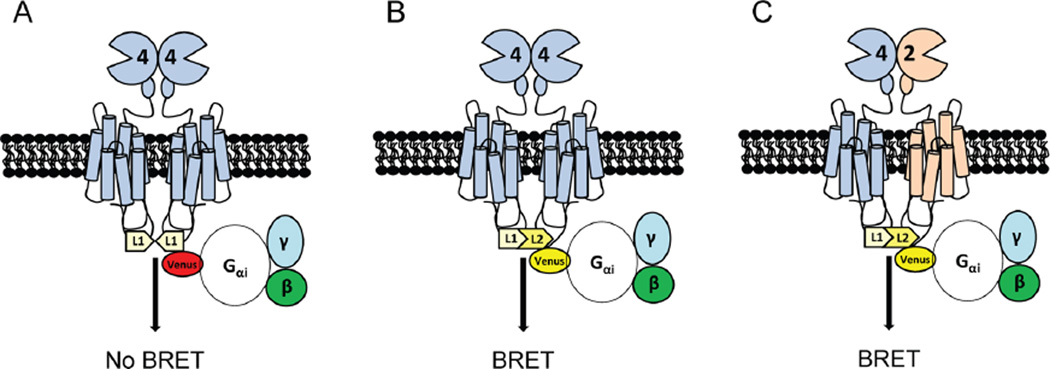
To verify that VU0418506 can activate mGlu4 homomers but not mGlu2/mGlu4 heterodimers, whereas Lu AF21934 can activate both homomers and heteromers, we turned to CODA-RET technology.32 This system relies on a luciferase complementation assay coupled with bioluminescence resonance energy transfer (BRET) to measure proximity. In these experiments, different subtypes of receptor (in this case mGlu2 and mGlu4) are labeled with N- and C-terminal fragments of a luminescent donor molecule (in Figure 6, termed L1 and L2). When two different receptor subunits form a heterodimer, these two halves complement, forming a functional luciferase molecule. In contrast, in a homodimer only a fragment of the donor molecule will be present and a luminescent signal will not be generated in response to substrate. In parallel, we express a defined G protein, in this case Gαi, labeled with the acceptor molecule, mVenus. Activation of the complemented luminescent heterodimeric receptor leads to recruitment of this acceptor-labeled Gαi protein; the resulting proximity of the complemented donor and the acceptor molecule leads to BRET.
Figure 6.
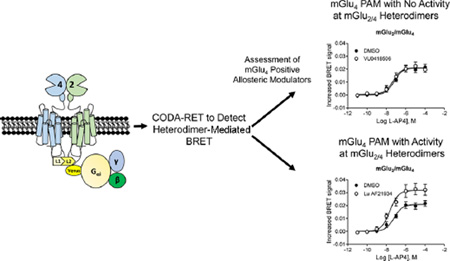
Schematic of CODA-RET technology to detect activity of heteromeric mGlu2/4. (A) When expressed alone, mGlu4 or mGlu2 (mGlu4 shown) form obligate homodimers due to disulfide linkages. If only mGlu4 receptors labeled with the L1 fragment of luciferase are expressed, no complementation between homomers occurs and no luminescence or BRET signal is detected. (B) If mGlu4 receptors tagged with L1 and L2 are coexpressed, luciferase is complemented and a BRET signal is produced upon G protein recruitment. (C) If mGlu4 tagged with L1 and mGlu2 tagged with L2 are expressed, complementation occurs, resulting in a BRET signal with G protein. This allows detection of a signal from heteromer activation without contamination of the signal by homomeric mGlu2 or mGlu4.
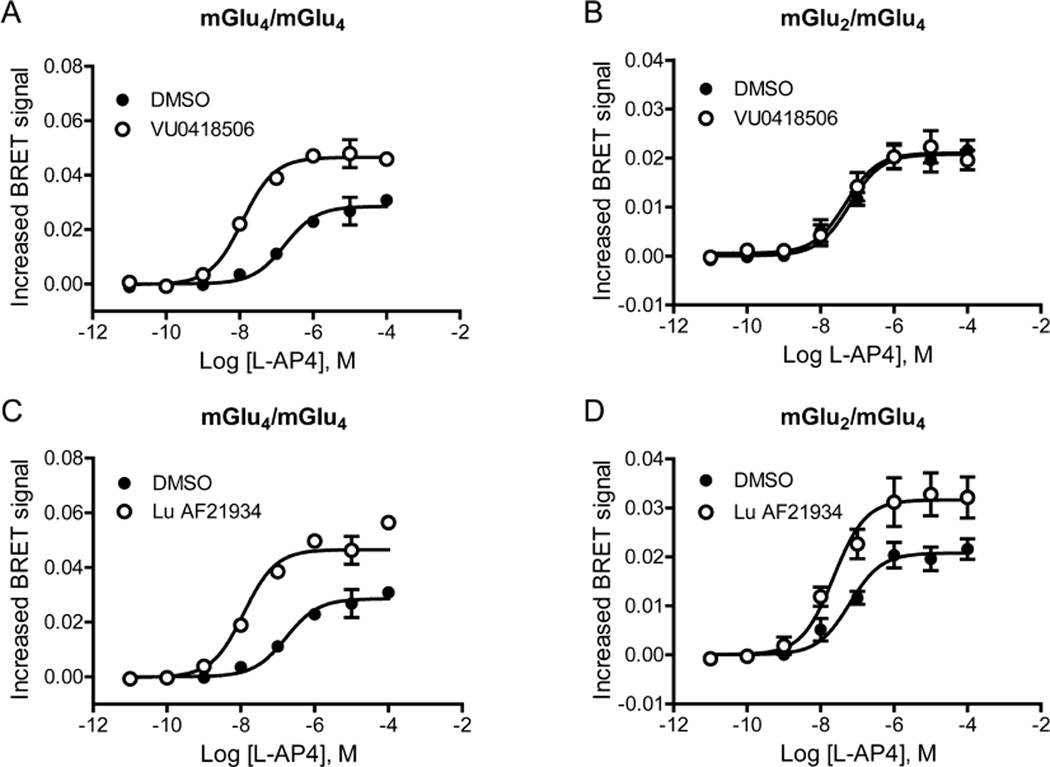
Using this system, we coexpressed either mGlu4_L1/mGlu4_L2 or mGlu4_L1/mGlu2_L2 and tested the effects of PAMs with the selective group III mGlu receptor agonist L-(+)-2-Amino-4-phosphonobutyric acid (L-AP4, Figure 7). In contrast to its activity when mGlu4_L1 and mGlu4_L2 are coexpressed (Figure 7A), VU0418506 is unable to potentiate L-AP4 responses when mGlu4_L1 and mGlu2_L2 are coexpressed (Figure 7B). Lu AF21934, however, is able to potentiate responses in cells expressing both mGlu4_L1/mGlu4_L2 (Figure 7C) as well as mGlu4_L1/mGlu2_L2 combinations (Figure 7D). In control studies (Supporting Information Figure 2), complementing two mGlu2 subunits generates a signal when the selective group II mGlu agonist, (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine (DCG-IV), is used for receptor activation. The signal induced by DCG-IV is unchanged in the presence of unlabeled mGlu4. If cells under these same conditions (i.e., labeled mGlu2 subunits and unlabeled mGlu4) are activated using the group III mGlu agonist L-(+)-2-amino-4-phosphonobutyric acid (L-AP4), no signal is generated (Supporting Information Figure 2). These important control experiments indicate that a BRET signal is only generated by activation of the complemented pair of receptors, and not by a higher order complex of receptors. Taken together, the CODA-RET data validate the hypothesis that mGlu2/mGlu4 heterodimers are potentiated by Lu AF21934 but not by VU0418506, whereas both compounds activate mGlu4 homomers.
Figure 7.
CODA-RET technology reveals that VU0418506 is ineffective in the mGlu2/4 heteromeric conformation while Lu AF21934 induces potentiation. (A, C) As illustrated in Figure 6, receptor constructs were generated in which mGlu4 was labeled with either L1 or L2 of Renilla luciferase as described in Methods and expressed in cells with mVenus-labeled Gαi G protein as well as unlabeled Gβ1 and Gγ2. Both VU0418506 and Lu AF21934 were able to potentiate responses by shifting the curve to the left ((A) pEC50 DMSO, 6.75 ± 0.07, mean ± SEM, pEC50 VU0418506, 7.91 ± , 0.06, *p = 0.0039; max response DMSO 0.029 ± 0.001, max response VU0418506, 0.047 ± 0.0004, *p = 0.0005; (C) pEC50 DMSO, 6.75 ± 0.07, pEC50 Lu AF21934, 7.70 ± 0.01, *p = 0.0026; max response DMSO, 0.029 ± 0.001, max response Lu AF21934, 0.050 ± 0.003, *p = 0.0043). (B, D) In contrast, in cells coexpressing labeled mGlu2 and mGlu4 with mVenus-labeled Gai G protein, VU0418506 was unable to potentiate responses, while Lu AF21934 induced potentiation ((B) pEC50 DMSO, 7.27 ± 0.19, mean ± SEM, pEC50 VU0418506, 7.31 ± , 0.17, *p = 0.274; max response DMSO, 0.021 ± 0.002, max response VU0418506, 0.021 ± 0.002, *p = 0.196; (D) pEC50 DMSO, 7.27 ± 0.19, pEC50 Lu AF21934, 7.66 ± 0.11, *p = 0.077; max response DMSO, 0.021 ± 0.002, max response Lu AF21934, 0.032 ± 0.004, *p = 0.0043). All t tests were one tailed, paired t tests. Data represent 3–4 experiments performed in triplicate.
DISCUSSION
In recent years, multiple structurally diverse compounds, acting via a variety of mechanisms, have been reported as selective activators or potentiators of mGlu4 and have demonstrated promise in the reversal of motor deficits and induction of neuroprotective effects in PD models.9–16,34,35 We now present a novel mGlu4 PAM, VU0418506, with suitable properties as a brain penetrant, orally bioavailable, mGlu4 tool compound. VU0418506 exhibits robust antiparkinsonian activity in two PD models, and we show that its effects correlate with compound exposure in the HIC model, generating a pharmacokinetic/pharmacodynamic relationship. As reported by Engers et al.,31 VU0418506 exhibits a desirable in vitro and in vivo PK profile across three species, good brain penetration, and is highly selective for mGlu4.
Previous studies suggest that mGlu4 can function as homomeric receptors or in heteromeric form with mGlu2.28 Furthermore, recent studies suggest that mGlu2/4 heterodimers are present at the corticostriatal synapse,30 a key synapse involved in regulating motor function. Finally, two previously reported mGlu4 PAMs, VU0155041 and Lu AF21934, potentiate activity in cells expressing mGlu2 and mGlu4 and also have robust efficacy in models of parkinsonian motor disability.10,23,30 Based on this latter finding, it is possible that mGlu4 PAM action specifically at mGlu2/4 complexes could contribute to the antiparkinsonian effects of mGlu4 PAMs. However, VU0418506 had no effect on agonist responses in cells in which mGlu2 and mGlu4 were coexpressed. Using a CODA-RET approach that specifically monitors activity of the heterodimeric receptor configuration, we show here that VU0418506 is inactive at mGlu2/4, despite its robust antiparkinsonian activity in both HIC and 6-OHDA lesion models. These data suggest that activity at mGlu2/4 heteromers is not required to achieve antiparkinsonian activity of mGlu4 PAMs. In addition to potential implications for the roles of mGlu4 in regulating motor function, these data have important practical implications and suggest that it is not necessary to intentionally optimize mGlu4 PAMs that have activity at mGlu2/4 heteromers to achieve antiparkinsonian efficacy; activity at only mGlu4 homomers may also provide a selectivity advantage if heteromers of mGlu4 with other mGlus are widespread in the CNS or highly expressed in peripheral tissues.
Our current studies also shed new light on the activity of PAMs when mGlu2 and mGlu4 are coexpressed. Our original hypothesis was that there was a preferential interaction between mGlu2 and mGlu4 compared to each homomeric combination as we had shown that transfection ratios of 1:10 (mGlu2:mGlu4) resulted in a total loss of potentiation induced by certain mGlu4 PAMs.30 However, theoretically, we would predict that there should be three populations of receptors in our cell line: mGlu2/2, mGlu4/4 and mGlu2/4. As glutamate is approximately 10-fold more potent at mGlu2 compared to mGlu4, we would be unable to detect potentiation of mGlu4 homomers with weaker PAMs that do not shift the glutamate response at mGlu4 by at least 10-fold. However, compounds with higher efficacies at mGlu4 might induce measurable potentiation if the efficacy is sufficient to increase the potency of glutamate to a point that is left-shifted compared to the potency of glutamate at mGlu2 or mGlu2/4. To overcome these complexities of receptor coexpression, we turned to CODA-RET technology. This assay relies upon complementation and bioluminescence resonance energy transfer (BRET) and allows the detection of a signal induced only by a defined mGlu2/4 heterodimer without any contribution from homodimers that may be present. These data show that the heterodimer can be activated by the mGlu4 selective agonist, L-AP4, and responds by recruiting and coupling with the cognate Gαi protein. These studies clearly show that VU0418506 does not potentiate agonist responses when mGlu2 and mGlu4 are heterodimerized (Figure 7B). In contrast, as predicted from our previous pharmacology studies, Lu AF21934 potentiates agonist-induced responses at an mGlu2/4 heterodimer using a CODA-RET readout.
The finding that VU0418506 does not potentiate mGlu2/4 responses could suggest that this mGlu4 PAM fails to bind to the receptor when it is heterodimerized with mGlu2. Alternatively, VU0418506 might act as a silent allosteric ligand (SALs) at this receptor complex; SALs are compounds that bind to allosteric sites on GPCRs without inhibiting or potentiating responses.36,37 While additional studies will be needed to address these mechanisms, the differentiation of Lu AF21934 and VU0418506 presented here is interesting in light of studies showing that Lu AF21934 engages an allosteric site that is distinct from the binding site of other mGlu4 PAMs.25 Further studies using a combination of CODA-RET, muta-genesis and/or radioligand binding will be required to differentiate between these intriguing possibilities.
In summary, we report here the discovery and characterization of VU0418506, a compound with activity in two antiparkinsonian rodent models. VU0418506 exhibits an excellent PK:PD relationship in an antiparkinsonian model. Based on our in vitro CODA-RET studies with defined homomers and heteromers, we show that VU0418506, while active at mGlu4 homomers, fails to potentiate the activity of an mGlu4 agonist at mGlu2/4 heterodimers. These findings are consistent with the hypothesis that the antiparkinsonian activity of mGlu4 PAMs is mediated by actions at mGlu4 homomeric receptors.
METHODS
Synthesis
Lu AF21934 was synthesized as described in ref 30. The A2A antagonist (N-(6-(3,5-dimethyl-1H–pyrazol-1-yl)-2-(furan-2-yl)-pyrimidin-4-yl)-2-(4-methylpiperazin-1-yl)acetamide, compound 23), previously disclosed by Neurocrine Biosciences, was used as a positive control and was synthesized as described.38 VU0418506 was synthesized as described in ref 31.
Drugs
Haloperidol, L-DOPA methyl ester, 6-hydroxydopamine hydrobromide, apomorphine, and clozapine were obtained from Sigma (St. Louis, MO). Buprenorphine was obtained from Patterson Veterinary (Devens, MA). l-Glutamate was obtained from Tocris Biosciences (Ellisville, MO). VU0418506 and LuAF21934 were suspended in an aqueous solution of 10% Tween 80. Benserazide and L-DOPA methyl ester were dissolved in 0.9% saline, while haloperidol was dissolved in 0.85% lactic acid (Sigma) and 0.5% Cremophor EL (Sigma), respectively. Except for L-DOPA, which is unstable at neutral pH, all drug formulations were adjusted to a pH of approximately 7 using 1 N sodium hydroxide. Drugs were administered in a volume of 1–2 mL/kg (i.p. and s.c.) or 10 mL/kg (p.o.).
Cell Line Establishment and Cell Culture
Cell lines for all non-CODA-RET experiments were established and cultured as described in refs 14 and 30. Briefly, human mGlu4/Gqi5/CHO cells were grown in 90% Dulbecco’s modified Eagle’s medium (DMEM), 10% dialyzed fetal bovine serum (FBS), 100 units/mL penicillin/streptomycin, 20 mM HEPES (pH 7.3), 1 mM sodium pyruvate, 20 µg/mL proline, 2 mM glutamine, 400 µg/mL G418 sulfate (Mediatech, Inc., Herndon, VA), and 5 nM methotrexate (Calbiochem, EMD Chemicals, Gibbstown, NJ). Cell culture reagents were purchased from Life Technologies (Carlsbad, CA) unless otherwise noted. Rat mGlu2 or rat mGlu4 coding sequences were cloned into the pIRESpuro3 vector, transfected into HEK/GIRK cells, and selected with puromycin. Polyclonal rat mGlu2/HEK/GIRK and rat mGlu4/HEK/GIRK cells were cultured in growth media as previously described in ref 30, supplemented with Non-Essential Amino Acids. Rat mGlu4 was also subcloned into the pIREShyg3 vector, and the resulting plasmid was transfected into rat mGlu2/HEK/GIRK cells; cells were then selected with 200 µg/mL hygromycin B. Polyclonal cells were cultured in growth media supplemented with 100 µg/mL hygromycin B.
Calcium Mobilization Assays
Potency experiments were performed as described in ref 14. Human mGlu4/Gqi5/CHO cells (30 000 cells/20 µL/well) were plated in black-walled, clear-bottomed, TC treated, 384-well plates (Greiner Bio-One, Monroe, North Carolina) in DMEM containing 10% dialyzed FBS, 20 mM HEPES, 100 units/mL penicillin/streptomycin, and 1 mM sodium pyruvate (Plating Medium). The cells were grown overnight at 37 °C in the presence of 5% CO2. The next day, the medium was removed and replaced with 20 µL of 1 µM Fluo-4, AM (Invitrogen, Carlsbad, CA) prepared as a 2.3 mM stock in DMSO and mixed in a 1:1 ratio with 10% (w/v) pluronic acid F-127 and diluted in Assay Buffer (Hank’s Balanced Salt Solution (HBSS), 20 mM HEPES and 2.5 mM Probenecid (Sigma-Aldrich, St. Louis, MO)) for 45 min at 37 °C. Dye was removed and replaced with 20 µL of Assay Buffer. For concentration-response curve experiments, compounds were serially diluted 1:3 into 10 point concentration response curves in DMSO, transferred to daughter plates using an Echo acoustic plate reformatter (Labcyte, Sunnyvale, CA) and diluted in Assay Buffer to a 2× final concentration. Ca2+ flux was measured using the Functional Drug Screening System 6000 (FDSS6000, Hamamatsu, Japan). After establishment of a fluorescence baseline for 2 s (2 images at 1 Hz; excitation, 470 ± 20 nm; emission, 540 ± 30 nm), 20 µL of test compounds were added to the cells, and the response was measured. After 142 s later, 10 µL (5×) of an EC20 concentration of glutamate was added to the cells, and the response of the cells was measured. 147 s after this add, an EC80 concentration of glutamate was added (assay is schematically shown in Figure 1B). Calcium fluorescence was recorded as fold over basal fluorescence and raw data were normalized to the maximal response to glutamate. Potency (EC50) and maximum response (% Glu Max) for compounds were determined using a four parameter logistical equation in GraphPad Prism (La Jolla, CA). For efficacy experiments, a constant amount of compound was applied 142 s prior to the addition of a full glutamate concentration-response curve and the left shift of the EC50 of the curves was calculated as “fold shift”, which represents compound efficacy.
Thallium Flux Assays
Thallium flux assays were performed according to methods described in ref 23 with minor modifications. For dye loading, media was exchanged with Assay Buffer (HBSS containing 20 mM HEPES, pH 7.4) using an ELX405 microplate washer (BioTek), leaving 20 µL/well, followed by addition of 20 µL/well 2× FluoZin-2 AM (330 nM final) indicator dye (Life Technologies, prepared as a DMSO stock and mixed in a 1:1 ratio with pluronic acid F-127) in Assay Buffer. After a 1 h incubation at room temperature, dye was exchanged with Assay Buffer, leaving 20 µL/well. Thallium flux was measured at room temperature using a Functional Drug Screening System 7000 (FDSS 7000, Hamamatsu). Baseline readings were taken (2 images at 1 Hz; excitation, 470 ± 20 nm; emission, 540 ± 30 nm), and test compounds (2×) were added in a 20 µL volume and incubated for 140 s before the addition of 10 µL of Thallium Buffer with or without agonist (5×). Data were collected for an additional 2.5 min and analyzed using Excel (Microsoft Corp, Redmond, WA) as previously described,23 and the concentration-response curves were fitted to a four-parameter logistic equation to determine potency estimates using GraphPad Prism:
where A is the molar concentration of the compound; bottom and top denote the lower and upper plateaus of the concentration-response curve; HillSlope is the Hill coefficient that describes the steepness of the curve; and EC50 is the molar concentration of compound required to generate a response halfway between the top and bottom.
CODA-RET Assays: Constructs for Expression Vectors and Transfection
cDNAs for rat mGlu2 and mGlu4 were N-terminally tagged with a hemagglutinin (HA) epitope tag using standard molecular biology procedures. cDNAs encoding the L1 (residues 1-229) or L2 (residues 230-311) fragments of Renilla Luciferase 8 (RLuc8, a gift from Sam Gambhir, Stanford) were fused in frame to the C terminus of mGlu4 and mGlu2 following the linker “GSPP-ARAT” in the pcDNA3.1 vector. The following G protein constructs were also used: Gαi-mVenus with the mVenus inserted at position 60, untagged Gβ1, and untagged Gγ2. The integrity of all the constructs was confirmed with sequencing analysis. Cultured HEK-293T cells were transfected with a constant amount of plasmid cDNA using polyethylenimine (Polysciences Inc.) in a 1:1 ratio in 10 cm dishes. The ratio of transfected plasmids was optimized to maximize the luminescence of the complemented donor as well as the dynamic range of the BRET response to L-AP4. In the case of mGlu4 homomers, the ratio of mGlu4-L1, mGlu4-L2, Gαi-mVenus, Gβ1 and Gγ2 was 4:4:1:1:1 (for a 10 cm dish, 4, 4, 1, 1, and 1 µg respectively). For the mGlu2 and mGlu4 heteromer, the ratio of mGlu4-L1, mGlu2-L2, Gαi-mVenus, Gβ1, and Gγ2 was 4:8:1:1:1 (for a 10 cm dish, 4, 8, 1, 1, and 1 µg respectively). Cells were maintained in culture with DMEM supplemented with 10% FBS. Experiments were performed 48 h after transfection.
CODA-RET Assay
Cells were harvested, washed twice, and resuspended in PBS. Approximately 200 000 cells per well were distributed in 96-well plates and stimulated by indicated drugs dissolved in prewarmed PBS for 5 min at 37 °C. A concentration of 5 µM coelenterazine H (the substrate for luciferase) was added to each well (Dalton Pharma Services). Two minutes after the addition of coelenterazine H, the fluorescence and luminescence was quantified (Pherastar, BMG Labtech) and the BRET signal was determined by calculating the ratio of the emission of mVenus (510–540 nm) over the emission of Rluc8 (485 nm).
Haloperidol-Induced Catalepsy
Catalepsy was assessed in Sprague–Dawley rats using a horizontal bar placed 6 cm from the testing surface as described in and performed.14 The forepaws of each rat were placed on the bar with the body positioned at an angle of ∼45° to the testing surface. The latency in seconds required for the rat to remove one or both forepaws from the bar was manually measured with a cutoff time of 30 s. Rats were randomly assigned to treatment groups and injected with haloperidol (1.5 mg/kg, i.p.) and returned to home cages for 60 min. Vehicle, VU0418506 (1–100 mg/kg, p.o.), or the adenosine A2A antagonist (N-(6-(3,5-dimethyl-1H-pyrazol-1-yl)-2-(furan-2-yl)pyrimidin-4-yl)-2-(4-methylpiperazin-1-yl)acetamide, 56.6 mg/kg, p.o.) was administered and catalepsy was assessed 30 min later. We have previously demonstrated that this dose of adenosine A2A antagonist referred to as compound 2333 produces a robust reversal of haloperidol-induced catalepsy that serves as positive control for this assay. For the time course study, haloperidol was always administered 1.5 h prior to catalepsy assessment. For the 30 min group, haloperidol was administered 1 h prior to compound; for the 60 min group, haloperidol was given 30 min before compound; for the 120 min group, compound was given 30 min before haloperidol, for the 240 min group, compound was given 2.5 h before haloperidol, and for the 360 min group, compound was given 4.5 h before haloperidol.
Rotarod Test
The effects of VU0418506 on motor performance were evaluated using a rotarod as described in ref 14 (Med Associates, Inc., St. Albans, VT). All rats were given an initial training trial of 120 s, followed by two additional training trials of 85 s, approximately 10 min apart, using a rotarod (7.5 cm in diameter) rotating at a constant speed of 20 rpm After initial training trials, a baseline trial of 120 s was conducted, and any rats that did not reach the 120 s criterion were excluded from the study. Rats were then treated with vehicle or VU0418506 (10, 30, 56.6, or 100 mg/kg, p.o.) and tested 30 min later. The time each animal remained on the rotarod was recorded and animals that did not fall off of the rotarod were given a maximal score of 120 s.
6-OHDA Lesions-Induced Forelimb Asymmetry
Unilateral 6-OHDA Lesions. In these studies, rats were first assessed in a baseline forelimb asymmetry assay to ensure that there was no inherent bias in paw use prior to lesions. Animals were then injected with buprenorphine (0.03 mg/kg, s.c.) and anesthetized with isoflurane before being placed in a stereotaxic frame. Unilateral lesions of the nigrostriatal dopamine projections were made by injecting 6-hydroxydopamine (6-OHDA) into the left medial forebrain bundle (AP −3.6 mm, ML 1.8 mm, DV −8.6 mm).39 Briefly, a 5 µL microsyringe with an outer diameter of 0.47 mm (Fisher Scientific, Pittsburgh, PA) was lowered to the injection site where 4 µL of 6-OHDA hydrobromide (3.6 µg/µL in ice-cold saline containing 0.015% ascorbic acid) were injected over a period of 4 min. The sham lesion procedure involved lowering the injection cannula 2 mm dorsal to the lesion site without delivering an intracerebral injection. After suturing the scalp rats were injected with 5% dextrose in Ringer’s solution (3 mL s.c.) to prevent postsurgical dehydration. In order to minimize weight loss encountered in some 6-OHDA-lesioned rats, the regular chow was supplemented with Bacon Softies (Bio-Serv, Frenchtown, NJ).
Apomorphine-Induced Rotation
Three weeks after surgery rats were tested for apomorphine-induced rotation.40 Briefly, rats were injected with apomorphine (0.05 mg/kg, s.c.), connected to a tether, and immediately placed into a cylinder-style rotometer (AccuScan, Columbus, OH). In this assay, animals were administered apomorphine at a dose that only activates supersensitive dopamine receptors (on the lesioned side), which induced turning behavior that was used as a functional assessment of dopamine depletion. The number and direction of full 360° turns was automatically recorded over a period of 30 min. The total number of turns (contralateral + ipsilateral) and the net number of contralateral turns (contralateral-ipsilateral) were calculated. Animals passing criteria were then assessed to ensure forelimb asymmetry had been achieved; animals with >50 apomorphine-induced rotations/30 min and an asymmetry score of < −60 were progressed to drug studies.
Forelimb Asymmetry Assay
6-OHDA lesion-induced forelimb asymmetry was assessed using the cylinder test.41 This assay measures the rats’ forelimb use during spontaneous exploration of a Plexiglas cone. Briefly, rats were gently placed into a clear Plexiglas cone (bottom diameter 21 cm, top diameter 37 cm [CleverSys, Reston, VA]) and their behavior was recorded over a period of 10 min by a video camera placed underneath the Plexiglas cone using Pinnacle Studio software (Pinnacle Systems, Mountain View, CA). Video recordings were analyzed offline by an observer blinded to both lesion status and drug treatment. The VLC media player (http://www.videolan.org/vlc/download-windows.html) was used to replay videos at various playback speeds (0.125–1.0× real-time speed) in order to facilitate detailed analysis of forepaw placement during rearing and landing.
Forelimb usage was analyzed as outlined by Lundblad et al.42 In order to avoid scoring incidental contact with the cylinder wall only weight-bearing contacts as evidenced by splayed digits were scored. Subsequent wall contacts were only counted after a rat completely withdrew its paw(s) from the wall resulting in their weight being exclusively supported by their hindlimbs. All floor landings from a rearing or grooming position onto either an individual forelimb or onto both forelimbs simultaneously were scored. The following parameters were used to calculate a composite measure of forelimb usage termed Forelimb Asymmetry (FLA) Index Score according to the formula below:
where Wunaffected is number of wall contacts of the unaffected (ipsilateral) forelimb, Wimpaired is number of wall contacts of the impaired (contralateral) forelimb, Lunaffected is number of landings on the unaffected (ipsilateral) forelimb, and Limpaired is number of landings on the impaired (contralateral) forelimb.
The FLA Index Score provides continuous data ranging between −100 (100% preference of the unaffected limb) and +100 (100% preference of the impaired limb), with a score of zero indicating a lack of forepaw preference.
Lesion Verification
After completion of the behavioral studies, lesioned animals were sacrificed and dopamine content in the lesioned and intact striatal side were determined by HPLC with electrochemical detection (as done in ref 14). Animals with less than 95% striatal dopamine depletion were excluded from statistical analysis. Rats were decapitated under deep isoflurane anesthesia, and the brains extracted. Tissue punches of the dorsolateral striatum (ipsi- and contralateral to the lesion) were frozen on dry ice and tissue concentrations of dopamine, 3,4-dihypdroxyphenylacetic acid (DOPAC), and homo-vanillic acid (HVA) were analyzed by HPLC with electrochemical detection.
Supplementary Material
Acknowledgments
We would like to thank Ms. Rocio Zamorano for excellent technical assistance.
Funding
This work was supported by grants from the NIH (NS078262 to C.M.N., NS048334 to P.J.C./C.M.N., MH108498 to C.M.N./C.W.L., NS031373 to P.J.C., U54MH084659 to C.W.L., DA22413 and MH054137 to J.A.J.), funding from the Michael J. Fox Foundation, and Bristol Myers-Squibb.
ABBREVIATIONS
- ACSF
artificial cerebrospinal fluid
- CNS
central nervous system
- DOI
2,5-dimethyloxy-4-iodoamphetamine
- GIRK
G protein inwardly rectifying potassium
- GPCR
G protein-coupled receptor
- HTS
high-throughput screening
- PHCCC
N-phenyl-7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxamide
- L-AP4
l-2-amino-4-phosphonobutyric acid
- DCG-IV
(2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine
- mGlu
metabotropic glutamate receptor
- NAM
negative allosteric modulator
- PAM
positive allosteric modulator
- SAR
structure-activity relationship
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acschemneur-o.6b00036.
Effect of increasing doses of VU0418506 on rotorod performance; signal strength when selective agonist is used for receptor activation (PDF)
Author Contributions
C.M.N., C.K.J., X.L., M.B., A.T.G., A.L.B., A.L.R., M.T.L., J.S.D., J.A.J., P.J.C. conducted, analyzed, or provided input into interpretation of data from biological experiments. D.W.E., C.W.L., and C.R.H. performed the synthetic chemistry work. All authors contributed to the writing of the manuscript.
The authors declare the following competing financial interest(s): C.M.N., C.K.J., A.L.B., D.W.E., J.S.D., C.W.L., C.R.H., and P.J.C. have received funding and royalties from Bristol-Myers Squibb.
REFERENCES
- 1.Schapira AH. Neurobiology and treatment of Parkinson’s disease. Trends Pharmacol. Sci. 2009;30:41–47. doi: 10.1016/j.tips.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Giugni JC, Okun MS. Treatment of advanced Parkinson’s disease. Curr. Opin. Neurol. 2014;27:450–460. doi: 10.1097/WCO.0000000000000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moldovan AS, Groiss SJ, Elben S, Sudmeyer M, Schnitzler A, Wojtecki L. The treatment of Parkinson’s disease with deep brain stimulation: current issues. Neural Regener. Res. 2015;10:1018–1022. doi: 10.4103/1673-5374.160094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conn PJ, Battaglia G, Marino MJ, Nicoletti F. Metabotropic glutamate receptors in the basal ganglia motor circuit. Nat. Rev. Neurosci. 2005;6:787–798. doi: 10.1038/nrn1763. [DOI] [PubMed] [Google Scholar]
- 5.Marino MJ, Awad H, Poisik O, Wittmann M, Conn PJ. Localization and physiological roles of metabotropic glutamate receptors in the direct and indirect pathways of the basal ganglia. Amino Acids. 2002;23:185–191. doi: 10.1007/s00726-001-0127-1. [DOI] [PubMed] [Google Scholar]
- 6.Bradley SR, Standaert DG, Rhodes KJ, Rees HD, Testa CM, Levey AI, Conn PJ. Immunohistochemical localization of subtype 4a metabotropic glutamate receptors in the rat and mouse basal ganglia. J. Comp. Neurol. 1999;407:33–46. [PubMed] [Google Scholar]
- 7.Corti C, Aldegheri L, Somogyi P, Ferraguti F. Distribution and synaptic localisation of the metabotropic glutamate receptor 4 (mGluR4) in the rodent CNS. Neuroscience. 2002;110:403–420. doi: 10.1016/s0306-4522(01)00591-7. [DOI] [PubMed] [Google Scholar]
- 8.Valenti O, Marino MJ, Wittmann M, Lis E, DiLella AG, Kinney GG, Conn PJ. Group III metabotropic glutamate receptor-mediated modulation of the striatopallidal synapse. J. Neurosci. 2003;23:7218–7226. doi: 10.1523/JNEUROSCI.23-18-07218.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Battaglia G, Busceti CL, Molinaro G, Biagioni F, Traficante A, Nicoletti F, Bruno V. Pharmacological activation of mGlu4 metabotropic glutamate receptors reduces nigrostriatal degeneration in mice treated with 1-methyl-4-phenyl-1,2,3,6-tetrahy-dropyridine. J. Neurosci. 2006;26:7222–7229. doi: 10.1523/JNEUROSCI.1595-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennouar KE, Uberti MA, Melon C, Bacolod MD, Jimenez HN, Cajina M, Kerkerian-Le Goff L, Doller D, Gubellini P. Synergy between L-DOPA and a novel positive allosteric modulator of metabotropic glutamate receptor 4: implications for Parkinson’s disease treatment and dyskinesia. Neuropharmacology. 2013;66:158–169. doi: 10.1016/j.neuropharm.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 11.Betts MJ, O’Neill MJ, Duty S. Allosteric modulation of the group III mGlu4 receptor provides functional neuroprotection in the 6-hydroxydopamine rat model of Parkinson’s disease. British journal of pharmacology. 2012;166:2317–2330. doi: 10.1111/j.1476-5381.2012.01943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beurrier C, Lopez S, Revy D, Selvam C, Goudet C, Lherondel M, Gubellini P, Kerkerian-LeGoff L, Acher F, Pin JP, Amalric M. Electrophysiological and behavioral evidence that modulation of metabotropic glutamate receptor 4 with a new agonist reverses experimental parkinsonism. FASEB J. 2009;23:3619–3628. doi: 10.1096/fj.09-131789. [DOI] [PubMed] [Google Scholar]
- 13.Engers DW, Field JR, Le U, Zhou Y, Bolinger JD, Zamorano R, Blobaum AL, Jones CK, Jadhav S, Weaver CD, Conn PJ, Lindsley CW, Niswender CM, Hopkins CR. Discovery, synthesis, and structure-activity relationship development of a series of N-(4-acetamido)phenylpicolinamides as positive allosteric modulators of metabotropic glutamate receptor 4 (mGlu(4)) with CNS exposure in rats. J. Med. Chem. 2011;54:1106–1110. doi: 10.1021/jm101271s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones CK, Bubser M, Thompson AD, Dickerson JW, Turle-Lorenzo N, Amalric M, Blobaum AL, Bridges TM, Morrison RD, Jadhav S, Engers DW, Italiano K, Bode J, Daniels JS, Lindsley CW, Hopkins CR, Conn PJ, Niswender CM. The metabotropic glutamate receptor 4-positive allosteric modulator VU0364770 produces efficacy alone and in combination with L-DOPA or an adenosine 2A antagonist in preclinical rodent models of Parkinson’s disease. J. Pharmacol. Exp. Ther. 2012;340:404–421. doi: 10.1124/jpet.111.187443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones CK, Engers DW, Thompson AD, Field JR, Blobaum AL, Lindsley SR, Zhou Y, Gogliotti RD, Jadhav S, Zamorano R, Bogenpohl J, Smith Y, Morrison R, Daniels JS, Weaver CD, Conn PJ, Lindsley CW, Niswender CM, Hopkins CR. Discovery, synthesis, and structure-activity relationship development of a series of N-4-(2,5-dioxopyrrolidin-1-yl)phenylpicolinamides (VU0400195, ML182): characterization of a novel positive allosteric modulator of the metabotropic glutamate receptor 4 (mGlu(4)) with oral efficacy in an antiparkinsonian animal model. J. Med. Chem. 2011;54:7639–7647. doi: 10.1021/jm200956q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Poul E, Bolea C, Girard F, Poli S, Charvin D, Campo B, Bortoli J, Bessif A, Luo B, Koser AJ, Hodge LM, Smith KM, DiLella AG, Liverton N, Hess F, Browne SE, Reynolds IJ. A potent and selective metabotropic glutamate receptor 4 positive allosteric modulator improves movement in rodent models of Parkinson’s disease. J. Pharmacol. Exp. Ther. 2012;343:167–177. doi: 10.1124/jpet.112.196063. [DOI] [PubMed] [Google Scholar]
- 17.Lopez S, Turle-Lorenzo N, Acher F, De Leonibus E, Mele A, Amalric M. Targeting group III metabotropic glutamate receptors produces complex behavioral effects in rodent models of Parkinson’s disease. J. Neurosci. 2007;27:6701–6711. doi: 10.1523/JNEUROSCI.0299-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacInnes N, Messenger MJ, Duty S. Activation of group III metabotropic glutamate receptors in selected regions of the basal ganglia alleviates akinesia in the reserpine-treated rat. Br. J. Pharmacol. 2004;141:15–22. doi: 10.1038/sj.bjp.0705566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maj M, Bruno V, Dragic Z, Yamamoto R, Battaglia G, Inderbitzin W, Stoehr N, Stein T, Gasparini F, Vranesic I, Kuhn R, Nicoletti F, Flor PJ. (-)-PHCCC, a positive allosteric modulator of mGluR4: characterization, mechanism of action, and neuroprotection. Neuropharmacology. 2003;45:895–906. doi: 10.1016/s0028-3908(03)00271-5. [DOI] [PubMed] [Google Scholar]
- 20.Marino M, Valenti O, Conn PJ. Group III metabotropic glutamate receptor mediated modulation of excitatory transmission on to midbrain dopaminergic neurons of the rat. J. Neurosci. 2002;26:359–357. doi: 10.1196/annals.1300.058. [DOI] [PubMed] [Google Scholar]
- 21.Marino MJ, Williams DL, Jr, O’Brien JA, Valenti O, McDonald TP, Clements MK, Wang R, DiLella AG, Hess JF, Kinney GG, Conn PJ. Allosteric modulation of group III metabotropic glutamate receptor 4: a potential approach to Parkinson’s disease treatment. Proc. Natl. Acad. Sci. U. S. A. 2003;100:13668–13673. doi: 10.1073/pnas.1835724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gubellini P, Melon C, Dale E, Doller D, Kerkerian-Le Goff L. Distinct effects of mGlu4 receptor positive allosteric modulators at corticostriatal vs. striatopallidal synapses may differentially contribute to their antiparkinsonian action. Neuropharmacology. 2014;85:166–177. doi: 10.1016/j.neuropharm.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 23.Niswender CM, Johnson KA, Weaver CD, Jones CK, Xiang Z, Luo Q, Rodriguez AL, Marlo JE, de Paulis T, Thompson AD, Days EL, Nalywajko T, Austin CA, Williams MB, Ayala JE, Williams R, Lindsley CW, Conn PJ. Discovery, characterization, and antiparkinsonian effect of novel positive allosteric modulators of metabotropic glutamate receptor 4. Mol. Pharmacol. 2008;74:1345–1358. doi: 10.1124/mol.108.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valenti O, Mannaioni G, Seabrook GR, Conn PJ, Marino MJ. Group III metabotropic glutamate-receptor-mediated modulation of excitatory transmission in rodent substantia nigra pars compacta dopamine neurons. J. Pharmacol. Exp. Ther. 2005;313:1296–1304. doi: 10.1124/jpet.104.080481. [DOI] [PubMed] [Google Scholar]
- 25.Rovira X, Malhaire F, Scholler P, Rodrigo J, Gonzalez-Bulnes P, Llebaria A, Pin JP, Giraldo J, Goudet C. Overlapping binding sites drive allosteric agonism and positive cooperativity in type 4 metabotropic glutamate receptors. FASEB J. 2015;29:116–130. doi: 10.1096/fj.14-257287. [DOI] [PubMed] [Google Scholar]
- 26.Bogenpohl J, Galvan A, Hu X, Wichmann T, Smith Y. Metabotropic glutamate receptor 4 in the basal ganglia of parkinsonian monkeys: ultrastructural localization and electrophysiological effects of activation in the striatopallidal complex. Neuropharmacology. 2013;66:242–252. doi: 10.1016/j.neuropharm.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pisani A, Calabresi P, Centonze D, Bernardi G. Activation of group III metabotropic glutamate receptors depresses glutamatergic transmission at corticostriatal synapse. Neuropharmacology. 1997;36:845–851. doi: 10.1016/s0028-3908(96)00177-3. [DOI] [PubMed] [Google Scholar]
- 28.Doumazane E, Scholler P, Zwier JM, Trinquet E, Rondard P, Pin JP. A new approach to analyze cell surface protein complexes reveals specific heterodimeric metabotropic glutamate receptors. FASEB J. 2011;25:66–77. doi: 10.1096/fj.10-163147. [DOI] [PubMed] [Google Scholar]
- 29.Kammermeier PJ. Functional and pharmacological characteristics of metabotropic glutamate receptors 2/4 heterodimers. Mol. Pharmacol. 2012;82:438–447. doi: 10.1124/mol.112.078501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin S, Noetzel MJ, Johnson KA, Zamorano R, Jalan-Sakrikar N, Gregory KJ, Conn PJ, Niswender CM. Selective actions of novel allosteric modulators reveal functional heteromers of metabotropic glutamate receptors in the CNS. J. Neurosci. 2014;34:79–94. doi: 10.1523/JNEUROSCI.1129-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engers DW, Blobaum AL, Gogliotti RD, Cheung YY, Salovich JM, Garcia-Barrantes PM, Daniels JS, Morrison R, Jones CK, Soars MG, Zhuo X, Hurley J, Macor JE, Bronson JJ, Conn PJ, Lindsley CW, Niswender CM, Hopkins CR. Discovery, Synthesis and Pre-Clinical Characterization of N-(3-chloro-4-fluorophenyl)-1H-pyrazolo[4,3-b]pyridin-3-amine (VU0418506), a novel positive allosteric modulator of the metabotropic glutamate receptor 4 (mGlu4) ACS Chem. Neurosci. 2016 doi: 10.1021/acschemneuro.6b00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urizar E, Yano H, Kolster R, Gales C, Lambert N, Javitch JA. CODA-RET reveals functional selectivity as a result of GPCR heteromerization. Nat. Chem. Biol. 2011;7:624–630. doi: 10.1038/nchembio.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slee DH, Zhang X, Moorjani M, Lin E, Lanier MC, Chen Y, Rueter JK, Lechner SM, Markison S, Malany S, Joswig T, Santos M, Gross RS, Williams JP, Castro-Palomino JC, Saunders J. Identification of novel, water-soluble, 2-amino-N-pyrimidin-4-yl acetamides as A2A receptor antagonists with in vivo efficacy. J. Med. Chem. 2008;51:400–406. doi: 10.1021/jm070623o. [DOI] [PubMed] [Google Scholar]
- 34.Goudet C, Vilar B, Courtiol T, Deltheil T, Bessiron T, Brabet I, Oueslati N, Rigault D, Bertrand HO, McLean H, Daniel H, Amalric M, Acher F, Pin JP. A novel selective metabotropic glutamate receptor 4 agonist reveals new possibilities for developing subtype selective ligands with therapeutic potential. FASEB J. 2012;26:1682–1693. doi: 10.1096/fj.11-195941. [DOI] [PubMed] [Google Scholar]
- 35.East SP, Bamford S, Dietz MG, Eickmeier C, Flegg A, Ferger B, Gemkow MJ, Heilker R, Hengerer B, Kotey A, Loke P, Schanzle G, Schubert HD, Scott J, Whittaker M, Williams M, Zawadzki P, Gerlach K. An orally bioavailable positive allosteric modulator of the mGlu4 receptor with efficacy in an animal model of motor dysfunction. Bioorg. Med. Chem. Lett. 2010;20:4901–4905. doi: 10.1016/j.bmcl.2010.06.078. [DOI] [PubMed] [Google Scholar]
- 36.Burford NT, Clark MJ, Wehrman TS, Gerritz SW, Banks M, O’Connell J, Traynor JR, Alt A. Discovery of positive allosteric modulators and silent allosteric modulators of the mu-opioid receptor. Proc. Natl. Acad. Sci. U. S. A. 2013;110:10830–10835. doi: 10.1073/pnas.1300393110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gregory KJ, Malosh C, Turlington M, Morrison R, Vinson P, Daniels JS, Jones C, Niswender CM, Conn PJ, Lindsley CW, Stauffer SR. Identification of a high affinity MPEP-site silent allosteric modulator (SAM) for the metabotropic glutamate subtype 5 receptor (mGlu5) Bethesda, MD: In Probe Reports from the NIH Molecular Libraries Program, National Institutes of Health; 2010. [PubMed] [Google Scholar]
- 38.Slee DH, Zhang X, Moorjani M, Lin E, Lanier MC, Chen Y, Rueter JK, Lechner SM, Markison S, Malany S, Joswig T, Santos M, Gross RS, Williams JP, Castro-Palomino JC, Crespo MI, Prat M, Gual S, Diaz JL, Wen J, O’Brien Z, Saunders J. Identification of novel, water-soluble, 2-amino-N-pyrimidin-4-yl acetamides as A2A receptor antagonists with in vivo efficacy. J. Med. Chem. 2008;51:400–406. doi: 10.1021/jm070623o. [DOI] [PubMed] [Google Scholar]
- 39.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th. Amsterdam: Elsevier; 2007. [DOI] [PubMed] [Google Scholar]
- 40.Marshall JF, Ungerstedt U. Supersensitivity to apomorphine following destruction of the ascending dopamine neurons: quantification using the rotational model. Eur. J. Pharmacol. 1977;41:361–367. doi: 10.1016/0014-2999(77)90256-4. [DOI] [PubMed] [Google Scholar]
- 41.Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 42.Lundblad M, Picconi B, Lindgren H, Cenci MA. A model of L-DOPA-induced dyskinesia in 6-hydroxydopamine lesioned mice: relation to motor and cellular parameters of nigrostriatal function. Neurobiol. Dis. 2004;16:110–123. doi: 10.1016/j.nbd.2004.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.