Abstract
Rationale
The reinforcing effects of most abused drugs have been consistently demonstrated and studied in animal models, although those of marijuana were not, until the demonstration fifteen years ago that THC could serve as a reinforcer in self-administration (SA) procedures in squirrel monkeys. Until then, those effects were inferred using indirect assessments.
Objectives
The aim of this manuscript is to review the primary preclinical procedures used to indirectly and directly infer reinforcing effects of cannabinoid drugs.
Methods
Results will be reviewed from studies of cannabinoid-discrimination, intracranial-self-stimulation (ICSS), conditioned place preference (CPP), as well as change in levels of dopamine assessed in brain areas related to reinforcement, and finally from self-administration procedures. For each procedure, an evaluation will be made of the predictive validity in detecting the potential abuse liability of cannabinoids based on seminal papers, with the addition of selected reports from more recent years especially those from Dr. Goldberg’s research group.
Results and Conclusions
ICSS and CPP do not provide consistent results for the assessment of potential for abuse of cannabinoids. However, drug-discrimination and neurochemistry procedures appear to detect potential for abuse of cannabinoids, as well as several novel “designer cannabinoid drugs.” Though after 15 years it remains somewhat problematic transfer the self-administration model of marijuana abuse from squirrel monkeys to other species, studies with the former species have substantially advanced the field, and several reports have been published with consistent self-administration of cannabinoid agonists in rodents.
Keywords: Cannabis, Delta-9-THC, Dopamine neurochemistry, Marijuana Substance use disorders, addiction, self-administration, drug discrimination, place conditioning, spice, Designer drugs
Introduction
The plant cannabis, along with its “therapeutic” and psychotropic properties, was described in several ancient books. However, in contrast to the psychoactive ingredients in other plants, such as cocaine from erythroxylum coca or morphine from papaver somniferum, delta-9-tetrahydrocannabinol (THC) from cannabis, was only discovered recently (Gaoni and Mechoulam 1964). The discovery of the chemical structure of THC has greatly accelerated cannabinoid research. Indeed, until the late 1980s the observable effects of THC administration were thought to be the result of a cellular/neuronal interaction not mediated by receptors, but rather through the high lipophilicity of the THC molecule, rendering it effective in modifying the lipid structure of the cells’ membranes (Leuschner et al. 1984). CB1 receptors were identified in 1989 (Devane et al. 1988), and cloned in the early 1990s (Matsuda et al. 1990). Surprisingly, CB1 receptors are the most abundant receptors in the mammalian brains, and researchers have discovered not only one, but several endogenous circulating ligands for these receptors (Devane et al. 1992; Di Marzo and De Petrocellis 2012). However, a reliable animal model of THC self-administration was only established at the start of the new millennium. Behavioral pharmacologists struggled for about 30 years (1970–2000) to understand why the self-administration procedure was able to capture the reinforcing effects of most drugs of abuse but not of the psychoactive principle ingredient of marijuana (Tanda and Goldberg 2003), one of the most abused drugs world-wide. Inability to demonstrate THC self-administration in animal models was used to support claims for legalization of recreational use of marijuana.
In addition to the establishment of a model of reinforcing effects of THC, the last 15 years has seen a transformation of knowledge about THC in terms of physiology, pharmacology and therapeutics, and several reviews are available to cover all of these topics (De Petrocellis and Di Marzo 2010; Di Marzo 2009; Di Marzo and De Petrocellis 2012; Elkashef et al. 2008; Micale et al. 2013; Pertwee 2008; Petrosino and Di Marzo 2010; Piomelli 2003; Tanda and Goldberg 2003; Toth et al. 2009; Vandrey and Haney 2009; Vemuri and Makriyannis 2015). The present paper is not intended to be a comprehensive review of the scientific literature on cannabinoids, but instead, to serve as a review of results from the primary behavioral procedures employed to indirectly infer reinforcing effects of THC before they were directly demonstrated in squirrel monkeys (Tanda et al. 2000). Indeed, while we had scientific clinical reports on subjective effects of cannabis derivatives and their potential for abuse in humans (Block et al. 1998; Chait and Zacny 1992; Fant et al. 1998), the lack of preclinical models of reinforcing effects of THC brought several other indirect procedures to the forefront of the research about abuse liability of cannabis derivatives (Balster and Prescott 1992; Gardner and Vorel 1998).
Subjective effects of cannabinoid agonists
Administration of drugs of abuse can produce specific subjective effects that human and animal subjects can be trained to discriminate. In a typical drug-discrimination procedure, animals are trained to emit one response after administration of drug, and another response after administration of vehicle. Appropriate responses, typically pressing different levers, are intermittently reinforced with food presentation. Thus, the subjective effect of the drug (or its absence) is the stimulus that controls on which lever presses produce food reinforcement.
As has been reported for other abused substances (Kamien et al. 1993; Schuster and Johanson 1988), both animals and human subjects can discriminate THC from vehicle (Chait et al. 1988; Wiley et al. 1993), and such effects likely play a role in the abuse liability of marijuana and other cannabis derivatives (Balster and Prescott 1992; Wiley 1999). Thus, drugs that produce in animals subjective effects similar to those produced by THC potentially induce similar subjective effects in human subjects.
The initial studies on THC drug discrimination established the relevance and specificity of the THC stimulus. Several studies tested whether natural components of marijuana smoke would substitute for THC similarly to those components when synthesized (See Table 1, Natural and Synthetic Cannabinoids)(Browne and Weissman 1981; Hiltunen and Jarbe 1986a; b; Jarbe and Henriksson 1974; Jarbe et al. 1977), and other studies aimed to test stereo-isomeric requirements as well (Browne and Weissman 1981; Jarbe et al. 1986; Jarbe et al. 1993; Jarbe et al. 1989; Jarbe et al. 1994; Mechoulam et al. 1987). It was also reported that the discriminative effects of CB1 agonists were centrally mediated (Perio et al., (1996).
Table 1.
Specificity of the subjective effects of cannabinoids under Drug Discrimination procedures.
| References | Training drug, dose- range, (mg/kg) & route # |
Test Drug, dose- range (mg/kg), route # |
Species, Procedure ($), schedule |
Result, % DLR |
|---|---|---|---|---|
| NON CANNABINOID DRUGS OF ABUSE | ||||
| (Solinas et al. 2010) | THC, 3.0 | Amphetamine 0.3– 1.8 |
Male, SD rats, TL, FR10 | <20 |
| (Browne and Weissman 1981) | THC, 3.2 | Amphetamine 0.32 | Male, SD rats, TL, FR10, CS– reinforced |
0 |
| (Jarbe and Henriksson 1974) | THC 5.0 | Amphetamine 2.5– 5.0 |
Male, SD rats, T-shaped water maze, escape reinforced |
No substitutio n |
| (Solinas et al. 2010) | THC, 3.0 | Cocaine 1–10 | Male, SD rats, TL, FR10 | <20 |
| (Jarbe and Henriksson 1974) | THC 5.0 | Cocaine 5–20 | Male, SD rats, T-shaped water maze, escape reinforced |
No substitutio n |
| (McMahon et al. 2008) | THC, 10.0 | Cocaine, 10–56 | Male C57BL/6J mice, TH, FR30, CM-reinforced |
<25 |
| (Wiley et al. 1995b) | THC, individual doses, 0.04–0.17 IM |
Diazepam 0.025–1.2 | Rhesus monkeys, TL, FR50, | 31 |
| (Mokler et al. 1986) | THC, 3.0 | Diazepam 0.1–10 | Male, SD rats, TL, FR10, SM– reinforced |
60 |
| (Jarbe and Henriksson 1974) | THC 5.0 | Ethanol, 1000–2000 | Male, SD rats, T-shaped water maze, escape reinforced |
No substitutio n |
| (Jarbe et al. 2010) | THC, 1.8 or Methanandamide 10.0 |
Ethanol, 300–1000 | Male, SD rats, TL, FR10 | <48 and <40 |
| (McMahon et al. 2008) | THC, 10.0 | Ethanol, 320–1000 | Male C57BL/6J mice, TNP, FR30, CM-reinforced |
<25 |
| (McMahon et al. 2008) | THC, 10.0 | Ketamine, 3.2–32 | Male C57BL/6J mice, TH, FR30, CM-reinforced |
<25 |
| (Browne and Weissman 1981) | THC, 3.2 | LSD, 0.1 | Male, SD rats, TL, FR10, CS– reinforced |
8 |
| (Jarbe and Henriksson 1974) | THC, 5.0 or Hashish smoke |
Morphine 1.25–10.0 | Male, SD rats, T-shaped water maze, escape reinforced |
No substitution |
| (Wiley et al. 1995b) | THC, individual doses, 0.04–0.17 IM |
Morphine, 0.1–1.0 | Rhesus monkeys, TL, FR50, | 0 |
| (Browne and Weissman 1981) | THC, 3.2 | Morphine, 3.2 | Male, SD rats, TL, FR10, CS– reinforced |
20 |
| (Wiley et al. 1995b) | THC, individual doses, 0.04–0.17 IM |
Phencyclidine 0.03– 0.3 |
Rhesus monkeys, TL, FR50, | 0 |
| NATURAL AND SYNTHETIC CANNBINOIDS | ||||
| (Wiley et al. 1995a) | THC, 3.0 | Anandamide, 30 | Male, SD rats, TL, FR10 | 80 |
| (Wiley et al. 1997) | THC, individual doses, 0.08–0.16 IM |
Anandamide, 0.1– 10.0 |
Rhesus monkeys, TL, FR50–100, | Not consistent |
| (Burkey and Nation 1997) | THC, 2.0 | Anandamide, 0.5– 16.0 |
Male, SD rats, TL, FR10, SW | <40 |
| (Jarbe et al. 2001) | THC 1.8–5.6 | Anandamide, 10–18 | Male, SD rats, TL, FR10 | 0–21 |
| (Jarbe et al. 2001) | Methanandamide, 10.0 | Anandamide, 10–18 | Male, SD rats, TL, FR10 | 85 |
| (Browne and Weissman 1981) | THC, 3.2 | Cannabidiol, 10– 32.0 |
Male, SD rats, TL, FR10, CS– reinforced |
25 |
| (Browne and Weissman 1981) | THC, 3.2 | Cannabinol, ED50 6.77 |
Male, SD rats, TL, FR10, CS– reinforced |
100 |
| (Burkey and Nation 1997) | THC, 2.0 | CP 55,940, 0.05–0.8 | Male, SD rats, TL, FR10, SW | 90 |
| (Jarbe and Henriksson 1974) | THC 5.0 or Hashish smoke |
Hashish smoke | Male, SD rats, T-shaped water maze, escape reinforced |
100 |
| (Burkey and Nation 1997) | THC, 2.0 | Methanandamide, 0.5–8.0 |
Male, SD rats, TL, FR10, SW | 90 |
| (Jarbe et al. 2001) | THC 3.0 | Methanandamide, 10–18 |
Male, SD rats, TL, FR10 | 80–100 |
| (Wiley et al. 1995c) | THC, 3.0 | Rimonabant, 0.3– 5.6 |
Male, SD rats, TL, FR10 | 0 |
| (Jarbe et al. 2001) | Methanandamide, 10.0 | THC, 0.1–3.0 | Male, SD rats, TL, FR10 | 100 |
| (Jarbe 1982) | Amphetamine, 1.6 IM | THC, 0.125–0.5 | Male, mixed strains, pigeons | <20 |
| (Jarbe 1984) | Cocaine, 3.0 IM | THC, 0.3 IM | Male, White Carneaux pigeons, two keys, FR15 |
<16 |
| (Wiley et al. 1995c) | THC, 3.0 | WIN55,212–2, 0.1– 3.0 |
Male, SD rats, TL, FR10 | 100 |
| SYNTHETIC ABUSED CANNABINOIDS FOUND IN SEIZED SPICE-LIKE BLENDS | ||||
| (Gatch and Forster 2014) | THC, 3.0 | JWH018 (AM678), 0.03-1 |
Male, SD rats, TL, FR10 | |
| (Jarbe et al. 2010) | THC, 1.8 or methanandamide 10 |
JWH018 (AM678), 0.03-1 |
Male, SD rats, TL, FR10 | 100 |
| (Gatch and Forster 2014) | THC, 3.0 | JWH073, 0.1–10 | Male, SD rats, TL, FR10 | 100 |
| (Gatch and Forster 2014) | THC, 3.0 | JWH200, 0.1–10 | Male, SD rats, TL, FR10 | 100 |
| (Gatch and Forster 2014) | THC, 3.0 | AM2201, 0.03-1 | Male, SD rats, TL, FR10 | 100 |
intraperitoneal route of administration, unless indicated otherwise
unless differently indicated, food was delivered as a reinforcer
IM=intramuscular route of administration
DLR=drug-lever responding
TL=two lever choice
TNP=Two nose-poke holes available
SD=Sprague Dawley rats
FR=fixed ratio
CM=condensed milk (50%) in tap water
CS=carnation slender diluted 1:1 to water
SM=sweetened milk
SW=saccharin (0.4%) in water
ED50=dose at which 50 of subjects responded to the drug lever
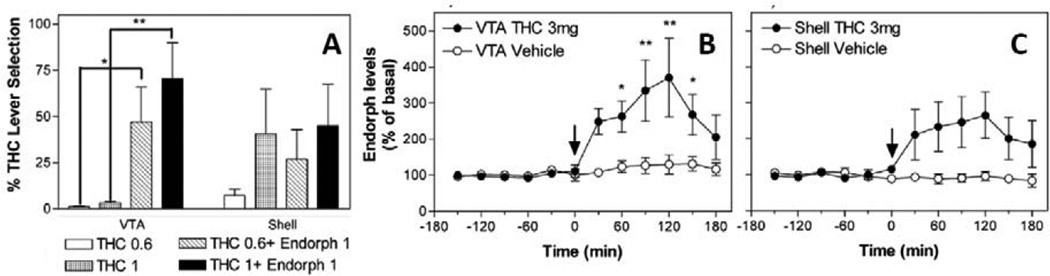
Recent experiments from Dr. Goldberg’s laboratory (Solinas et al. 2004) showed that, although morphine did not substitute for the discriminative stimulus effects of THC, it potentiated those effects. Indeed, β-endorphins and opioid agonists such as morphine, locally injected in the ventral tegmental area (VTA), or given systemically, potentiated the discriminative effects of small inactive doses of THC, an effect blocked by naloxone. Further, THC administration increased the concentration of endogenous opioid peptides in the VTA (see Figure 1)(Solinas et al. 2004). This effect is in agreement with both modulation of the subjective effects of THC by intra-VTA injections of β-endorphins (Solinas et al. 2004), and modulation of THC-induced increase in accumbens shell DA (Tanda et al. 1997), which was blocked by local intra-VTA µ-opioid antagonists or by systemic naloxone (Tanda et al. 1997). Although these experiments indicated that subjective effects of THC were centrally mediated, and that the opioid system is involved centrally in the behavioral effects of cannabinoids, contrasting results have been reported in human subjects (Haney 2007; Haney et al. 2003) and in non-human primates (Gerak et al. 2015; Justinova et al. 2004; Li et al. 2008; Maguire et al. 2013).
Figure 1. β-endorphins in the VTA mediate central THC actions.
Panel A shows that local intra VTA, but not intra-accumbens shell, injections of β-endorphins potentiate the discriminative effects of small doses of THC, measured by % increase in THC appropriate lever selection, in rats trained to discriminate the subjective effects of THC 3 mg/kg i.p. from THC-Vehicle i.p. Panels B and C show that administration of THC, 3 mg/kg i.p., but not its vehicle, stimulates β-endorphin levels in dialysates from the VTA but not significantly from the accumbens shell. Modified from Solinas et al 2004. See text and the original article for more details about experimental procedures and results.
The pharmacologic specificity of the discriminative stimulus effects of THC was documented by the large number of classes of compounds that have failed to substitute for THC in drug discrimination procedures in a variety of animal species (Table 1, Non-Cannabinoid Drugs of Abuse). Full substitution for THC has been demonstrated only with drugs that are agonists at CB1 receptors (Balster and Prescott 1992; Jarbe and Henriksson 1974; Wiley et al. 1995b). However, limited substitution with some cannabinoid CB1 agonists, such as anandamide, or methanadamide has been reported. These results may be due to partial agonist effects exerted by THC, or by differences in degrees of intrinsic cannabinoid activity among different drugs (De Vry and Jentzsch 2003; Jarbe et al. 1998). Alternatively, doses that significantly decrease response rates with these compounds may be lower than those necessary for full efficacy/generalization. Intrinsic efficacy and degree of tolerance to repeated treatment with THC have been studied and reported (Hruba et al. 2012). In that report, repeated THC injections produced extensive tolerance to its own discriminative effects. Such tolerance, however, only slightly affected the subjective effects produced by designer cannabinoid drugs with higher efficacy than THC. On the other hand, some cannabinoid CB1 agonists might have pharmacologic affinity and also bind to cannabinoid CB2, vanilloid TRPV1, GPR55, PPAR-alpha or PPAR-gamma receptors (Baskfield et al. 2004; McMahon et al. 2008). Functional activation of these receptors has been reported (Le Foll et al. 2013; Mascia et al. 2011; Mazzola et al. 2009), which may mask or interfere with the discriminative-stimulus effects mediated by CB1 receptors.
High metabolic rates may also be among the reasons for the failure of anandamide to fully substitute for THC in some tests (Solinas et al. 2007b; Stewart and McMahon 2011). Further, methanandamide, a metabolic stable analog of anandamide, does not always fully substitute for THC in standard conditions. However, it does fully substitute under appropriate conditions, in which, for example lower doses of THC are employed to train the subjects, or (to increase selectivity) the THC stimulus is being discriminated from a different drug (morphine or PCP) rather than its vehicle (Alici and Appel 2004; Burkey and Nation 1997; Jarbe et al. 1998). Further, when FAAH (fatty-acid-amide-hydrolase), the enzyme that contributes to anandamide degradation is blocked, full generalization of intravenous anandamide to the discriminative effect of THC has been reported (Solinas et al. 2007b; Stewart and McMahon 2011). It has also been recently shown that simultaneous blockade of the enzymes FAAH and MGL (which blocks 2-AG metabolism) can produce THC-like subjective effects (Hruba et al. 2015). These latter results suggest that both endocannabinoid ligand levels have to be increased in the brain to obtain generalization with the THC stimulus. Indeed, selectively blocking each enzyme alone does not produce significant THC-like subjective effects (Hruba et al. 2015).
In summary, studies of THC as a discriminative stimulus have indicated that its effects are shared only by drugs from the cannabinoid pharmacologic class (Balster and Prescott 1992; Browne and Weissman 1981; Wiley 1999) and that THC and other CB1 agonists do not substitute for other drugs of abuse when the latter are used as training drugs (Doty et al. 1994; Jarbe 1982; Li et al. 2008; Maguire et al. 2013). Further, THC discrimination studies established the central mediation and stereospecificity of the subjective effects. Though this procedure does not directly assess the abuse liability of cannabinoids, it has been an important indicator of cannabinoid specific pharmacology, and can reasonably be used to predict whether new compounds have subjective effects like those of THC. Thus THC drug-discrimination procedures might have predictive validity about abuse liability of, for example, novel cannabinoid drugs. In this respect, it is interesting to note that new cannabinoids currently abused in both USA and Europe and available online, have been reported to fully substitute for the THC discriminative stimulus (Gatch and Forster 2014; Jarbe et al. 2010) (see table 1). Further the procedure identified antagonist effects of rimonabant, which has turned out to have inverse agonist effects (Landsman et al. 1997) and important psychiatric side effects in humans (E.M.A. 2008). However this procedure shows promise for identifying pure antagonists that can effectively block the subjective effects of THC, and possibly its abuse.
Intra-cranial Self-stimulation
The discovery by Olds and Milner (1954) that electrical stimulation of particular brain sites, intra-cranial self-stimulation (ICSS), could produce reinforcing effects in rats opened up new avenues in the field of psychopharmacology. For the present purposes, the potential of abused drugs to enhance the effects of brain stimulation suggested that the abuse liability of different drugs could be tested using this procedure (Kornetsky 1985; Kornetsky and Esposito 1979; Kornetsky et al. 1979). It is worth noting that drugs of abuse from different pharmacological classes produce a similar facilitation of ICSS whereas drugs lacking abuse liability do not (Olds and Fobes 1981; Wise 1996).
The direct behavioral effects of cannabinoids under this procedure were first studied by Stark and Dews (1980). In that report, THC, nabilone and canbisol decreased response rates of rats under ICSS procedures. However, only one, and a very high, comparison dose of THC was tested (10 mg/kg). Moreover, even with a 50 percent decrease in behavior, the effect of THC was not significant due to the high variability in the control response rates. Thus, Gardner and colleagues (Gardner et al. 1989; Gardner et al. 1988) were the first to show that i.p. administration of THC (1.5 mg/kg i.p.) in male Lewis rats lowered the brain stimulation threshold. Further, the facilitation of brain stimulation was antagonized by naloxone pretreatment, suggesting the involvement of opioid mechanisms in the behavioral effects of THC on brain stimulation (Gardner et al. 1989). The authors pointed out that their results strongly suggest that THC shares with other abused drugs the ability to facilitate reward mechanism/s under the ICSS procedure. Further, THC, like other drugs, might produce its euphorigenic effects through these brain mechanisms. The positive, “drug-abuse-like” results obtained with THC in this procedure have been replicated by the same and other research groups (Katsidoni et al. 2013; Lepore et al. 1996). Nonetheless, contrasting results have often been reported, even from the same authors. For example, a lack of effect of THC and other cannabinoid agonists has been reported (Arnold et al. 2001a; Gallo et al. 2014; Vlachou et al. 2003), as well as decreases in the effectiveness of brain stimulation (Fokos and Panagis 2010; Katsidoni et al. 2013; Mavrikaki et al. 2010; Vlachou et al. 2005; 2006; Vlachou et al. 2007; Wiebelhaus et al. 2015), an effect that was shown to be reversed by administration of very low doses, in the µg/kg range, of CB1 receptor antagonists (Vlachou et al. 2003; 2005; Vlachou et al. 2007).
Several factors might be taken into consideration to explain the different outcomes obtained with cannabinoids under this procedure. One of these is the strain of the rats used, as Lewis, but not Sprague-Dawley or Fisher rats showed a significant leftward shift of the number of brain stimulations obtained as a function of the current frequency (the rate-frequency curve)., obtained under an ICSS procedure (Lepore et al. 1996). However, even though genetic factors may be involved in the sensitivity to cannabinoid effects and to vulnerability to THC use and dependence (Arnold et al. 2001b; Cadoni et al. 2015; Gillespie et al. 2009; Kendler et al. 2008; Martin et al. 1999; Parker and Gillies 1995), only one dose of THC was tested in the report by Lepore et al (1996), thus there is lack of information about how different specific doses of THC might influence the rate-frequency curve. Indeed, a recent report explored again the contrasting results of cannabinoids in ICSS procedures, providing more emphasis on the range of THC doses employed (Katsidoni et al. 2013). Biphasic effects of THC on ICSS were found, with a low (0.1 mg/kg) dose decreasing and a moderate dose (1.0 mg/kg) increasing the ICSS threshold in Sprague Dawley rats. Both of these effects were blocked by rimonabant pretreatments (Katsidoni et al. 2013), confirming CB1 receptor involvement in the biphasic action of THC.
Taken together, the results obtained with cannabinoids in the ICSS procedure are widely mixed, and do not provide a level of confidence near that obtained with other drug classes to state that cannabinoid agonists would consistently produce a facilitation of brain stimulation. Thus, this methodology seems to be inadequate to understand the potential for abuse of cannabinoids or to screen either cannabinoid agonists or antagonists.
Place Conditioning
In place conditioning studies, subjects are confined inside one of the two distinguishable compartments during the conditioning session(s) with the drug, and inside the other compartment during conditioning session(s) with the drug vehicle. After typically several conditioning sessions, the allocation of time spent in the two compartments by the subjects is compared to that allocation before conditioning (Bardo and Bevins 2000; Tzschentke 1998; 2007). As shown by several research groups, this place conditioning increases the time allocation to the compartment associated with the injection of selected doses of abused drugs compared to little or no change with only vehicle injections. One advantage of the place conditioning procedure is that it is possible to detect both conditioned aversion and preference for the drug paired compartment.
Unfortunately, results for drugs belonging to the cannabinoid class (see Table 2) are not as straightforward as for other drug classes abused by humans (Tanda and Goldberg 2003). It is not uncommon for both conditioned preference and aversion to be reported for cannabinoids agonists (Tzschentke 1998). For example, the same doses of THC, injected at different time or pretreatment intervals, have been found to produce both preference and aversion in place-conditioning tests (Lepore et al. 1995; Valjent and Maldonado 2000). Moreover, failure to show conditioned preference with THC and other cannabinoid agonists has been repeatedly reported (Chaperon et al. 1998; Cheer et al. 2000; Mallet and Beninger 1998; McGregor et al. 1996; Sanudo-Pena et al. 1997). Most of these studies report a conditioned aversion produced by cannabinoid agonists, and two studies surprisingly show significant conditioned preference for a cannabinoid antagonist, rimonabant. This has been interpreted as evidence for the existence of a cannabinoid counter-rewarding system in the brain (Cheer et al. 2000; Sanudo-Pena et al. 1997), although other studies did not replicate those findings either with rimonabant (Chaperon et al 1998; Braida et al 2001, 2004), or with a different CB1 antagonist, AM251 (Scherma et al. 2008b).
Table 2.
Results from place conditioning tests with cannabinoid drugs.
| Reference | Test Drug | Dose (mg/kg) and route (#) |
Pretreatment time (min)/ session time (min)/number of exposures |
time (hours) between drug injections |
Species, strain/ Bias procedure? |
Result |
|---|---|---|---|---|---|---|
| (Lepore et al. 1995) | THC | 1.0. | 20/30/3 | 48 | Male, Long Evans rats/Unbiased |
A |
| 2.0, 4.0 | 48 | P | ||||
| 0.5 | 20/30/3 | 96 | Male, Long Evans rats/Unbiased |
NS | ||
| 1.0 | 96 | P | ||||
| 2.0, 4.0 | 96 | A | ||||
| (Parker and Gillies 1995) | THC | 0.2–1.5 | 5/30/3 | 72 | Male, Spraue Dawley rats/Unbiased |
NS |
| 0.2–1.5 | 5/30/3 | 72 | Male, Lewis rats/Unbiased |
NS | ||
| (McGregor et al. 1996) | CP 55,940 | 10 | 0/30/4 | 24 | Male, Wistar rats/Unbiased |
A |
| 100 | A | |||||
| (Sanudo-Pena et al. 1997) | THC | 1.5 | 0/30/2 | 24 | Male, Sprague Dawley rats |
NS |
| 15 | A | |||||
| Rimonabant | 0.5 | 0/30/2 | 24 | P | ||
| 5.0 | P | |||||
| (Mallet and Beninger 1998) | THC | 0.1, 0.5 | 30/30/4 | 48 | Male, Wistar rats/Unbiased |
NS |
| 1.0, 1.5 | A | |||||
| 2, 4, 8 | NS | |||||
| Anandamide + PMSF(*) |
0.031–16 | 5/30/4 | 48 | NS | ||
| (Chaperon et al. 1998) | WIN55,212–2 | 0.003–1 | 15/30/4 | 24 | Male, Wistar rats/Unbiased |
A |
| Rimonabant | 0.3–3 | 30/30/4 | 24 | NS | ||
| (Cheer et al. 2000) | THC | 1.5 | 10/10/3 | 24 | Male, Lister Hodded rats/Unbiased |
A |
| Rimonabant | 0.25–3 | 10/10/3 | 24 | P | ||
| HU210 | 0.02–0.1 | 10/10/3 | 24 | A | ||
| (Valjent and Maldonado 2000) | THC | 1.0 | 0/45/5 | 24 | Male, CD1 mice/Unbiased |
NS |
| 5.0 | A | |||||
| THC(***) | 1.0 | 0/45/5 | 24 | P | ||
| 5.0 | NS | |||||
| (Zimmer et al. 2001) | THC | 5.0 | 0/45/ 5 | 24 | wild type C57BL/6J mice; unbiased |
A |
| 5.0 | 0/45/5 | 24 | Dynorphin-deficient mice /Unbiased |
NS | ||
| (Ghozland et al. 2002) | THC | 1.0 | 0/45/5 | 24 | Hibrid mice, 1:1 129sv and C57BL6/Unbiased |
NS |
| 5.0 | A | |||||
| 1.0($) | 0/45/5 | 24 | Hibrid mice, 1:1 129sv and C57BL6/Unbiased |
P | ||
| 5.0($) | NS | |||||
| 1.0($) | 0/45/5 | 24 | µ-opiod-R KO mice/Unbiased |
NS | ||
| 5.0($) | NS | |||||
| 1.0 | 0/45/5 | 24 | κ-opiod-R KO mice/Unbiased |
P | ||
| 5.0 | NS | |||||
| 1.0($) | 0/45/5 | 24 | κ-opiod-R KO mice/Unbiased |
P | ||
| 5.0($) | NS | |||||
| 1.0($) | 0/45/5 | 24 | δ-opiod-R KO mice/Unbiased |
P | ||
| 5.0($) | A | |||||
| (Braida et al. 2001a) | CP55,940 | 0.02 | 10/30/4 | 48 | Male, Wistar rats/Biased |
P |
| Rimonabant | 0.5 | NS | ||||
| (Braida et al. 2004) | THC | 0.015 | 10/30/4 | 48 | Male, Wistar rats/Unbiased |
NS |
| 0.075–0.75 | P | |||||
| 1–3 | NS | |||||
| 6.0 | A | |||||
| Rimonabant | 0.25–1.0, | NS | ||||
| (Le Foll et al. 2006) | THC | 0.01 | 10/30/5 | 24 | Male, Sprague Dawley rats/Unbiased |
NS |
| 0.1 | P | |||||
| 1., 2.0 | NS | |||||
| (Scherma et al. 2008a) | Anandamide | 0.3–3 i.v. | 0/20/3 | 24 | Male, Sprague Dawley rats/Unbiased |
NS |
| Anandamide + URB597 (*) |
0.3–3.0 i.v. | A | ||||
| WIN55,212–2 | 0.05–3 i.v. | A | ||||
| AM251 | 3.0 | NS |
if not specified, the intraperitoneal route was used.
A=conditioned place aversion
P=conditioned place preference
PMSF and URB597 are inhibitors of the FAAH enzyme, and animals have been pretreated these drugs before anandamide to reduce a rapid anandamide metabolism.
Even if rats received saline or Rimonabant at the end of each THC session, there was no change in the results for THC effects at 1 or 5 mg/kg i.p.
Animals received a non-contingent injection of THC 24 hours before the first THC conditioning session. This treatment led to preference for the THC low dose and changed from aversion to NS the effects of the higher THC dose, independently form the Rimonabant pretreatment provided at the end of each THC session.
Animals received a non-contingent injection of THC 24 hours before the first THC conditioning session.
Several factors, in particular pharmacokinetics, have been suggested to explain the contrasting results from place conditioning tests obtained with THC. When administered i.p. or orally THC has a slow onset and offset of effects, which might require that the conditioning session be exquisitely timed. Previous studies indicated that the onset of effects can depend on the vehicle used to solubilize THC, a factor that has been shown to play a role in its bioavailability (Carney et al. 1977; Mantilla-Plata and Harbison 1974; 1975; Ohlsson et al. 1980; 1981). The duration of THC action is also influenced by its very high lipophilic profile which is likely prolonged as a result of slow dissociation from fat depots, prolonging its duration of action.
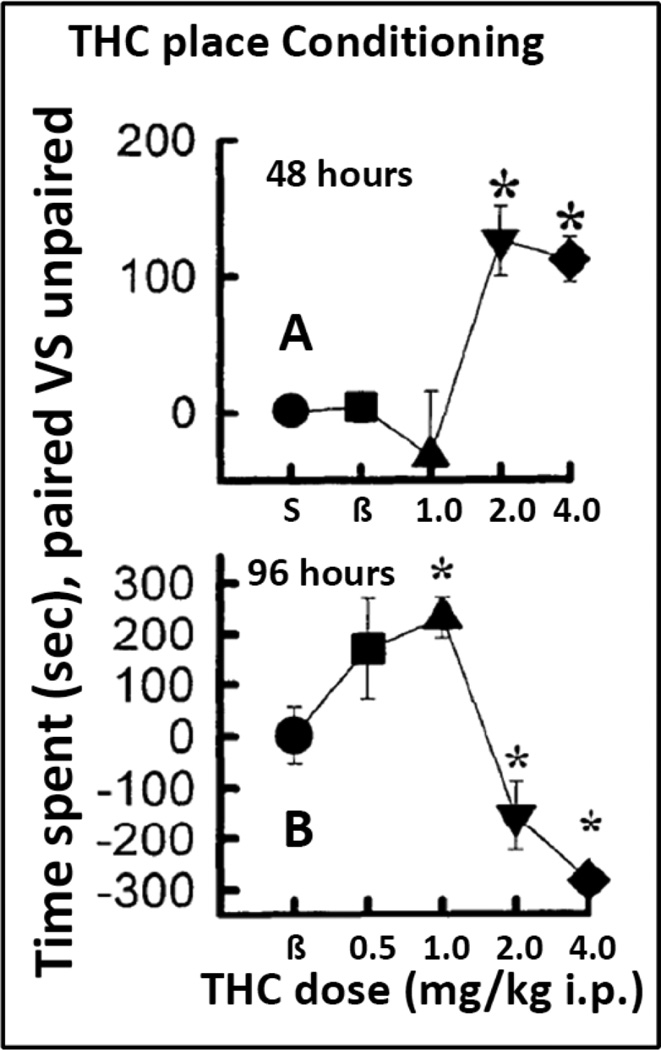
Lepore et al (1995) showed that when the THC conditioning sessions were conducted at intervals of 48 hours, lower doses of THC, 1 mg/kg, produced aversion, while higher doses, 2–4 mg/kg, produced place preference. However, when the experiments were conducted with THC conditioning sessions spaced 96 hours apart, lower doses induced place preference and higher doses produced aversion (see figure 2). The explanation offered for the different effects was that when time between THC conditioning injections does not allow the possible dysphoric actions to dissipate, the subsequent injections occurred during a dysphoric, non-hedonic state. Indeed, as suggested by the authors, low doses of THC might produce a dysphoric rebound measured by brain stimulation procedures that lasts for more than 48 hours (Lepore et al. 1996). A dose-dependent, biphasic and inverted U-shaped effect was obtained when doses of THC were administered every 96 hours (Lepore et al. 1995). Alternatively, we could explain the difference among these THC experiments as a leftward shift of the dose-response effects of THC when delaying the interval of THC injections from 48 to 96 hours. As can be seen in figure 2, the longer intervals between THC injections potentiate the preference- producing effects of smaller doses of THC, while increasing the aversive effects of larger doses. In order to minimize dysphoric effects that could interfere with the conditioning procedure, Valjent and Maldonado (2000) studied a conditioning procedure that revealed a place preference in THC conditioned mice. Mice were injected or primed non-contingently with a dose of THC the day before the start of the conditioning sessions. Under those conditions the authors observed place preference with low THC doses and neither preference nor aversion with higher doses that in non-primed mice produced place aversion (Valjent and Maldonado 2000).
Figure 2. Effects of changing the time between THC sessions on place conditioning.
Panel A shows effects of THC when conditioning sessions with THC were conducted 48 hours apart. Potential dysphoric effects of THC were hypothesized to reduce the likelihood of place preference at low THC doses (1 mg/kg i.p.). In Panel B with 96 hours between THC injections, there was a significant place preference at 1 mg/kg i.p., but also place aversion at higher doses. Modified from Lepore et al 1995. See text and the original article for more details about experimental procedures and results.
Mechanisms for the place preference or aversion to cannabinoids have been studied in genetically engineered mice. In contrast to wild-type mice, dynorphin-deficient mice did not show THC conditioned aversion (Zimmer et al. 2001). Moreover, using the previously detailed THC-priming procedure (Valjent and Maldonado 2000), Gohzland et al (2002) showed that while THC-induced preference is under control of µ-opioid receptors, THC-induced aversion depends on κ-opioid receptors (see Table 2). THC has been shown to induce stimulation of extracellular levels of opioid peptides, for example, β-endorphins in the VTA (Solinas et al. 2004) and dynorphin-B in the spinal cord (Houser et al. 2000). Thus, genetic deletion of specific opioid receptors blunts the effectiveness of THC in producing specific effects mediated by those receptors. It is interesting to note that local injections of THC or cannabinoid agonists, given i.c.v. or directly into specific brain areas, VTA and accumbens shell, would produce place preference in rats (Braida et al. 2004; Zangen et al. 2006).
In addition, environmental factors can contribute to differences in the induction of preference or aversion, in place-conditioning studies. For example, WIN55212-2 has been shown to produce place preference in rats raised in an enriched environment, but not in a standard one (Gobbi et al. 2005).
In summary, as was seen with the ICSS procedures, cannabinoids do not produce consistent results in place-conditioning procedures. Both pharmacological and environmental factors may influence outcome. As with ICSS procedures, predicting abuse liability of novel cannabinoid drugs with results obtained through this procedure should be considered cautiously until we better understand all of the factors involved in the contrasting outcomes obtained with cannabinoids.
Neurochemistry
THC has been suggested to interact with several neurotransmitter systems (Acquas et al. 2000; Egashira et al. 2002; Gessa et al. 1998; Mishima et al. 2002; Pisanu et al. 2006), but in this section the focus will be mainly on the interactions of cannabinoids with dopamine (DA) neurochemistry. Indeed, the neurochemical, DAergic effects of THC have been another example of how to infer reinforcing effects of marijuana by comparing the effects of its main psychoactive ingredient, THC, with those produced by standard drugs of abuse (Pontieri et al. 1995; Pontieri et al. 1996; Tanda et al. 1997).
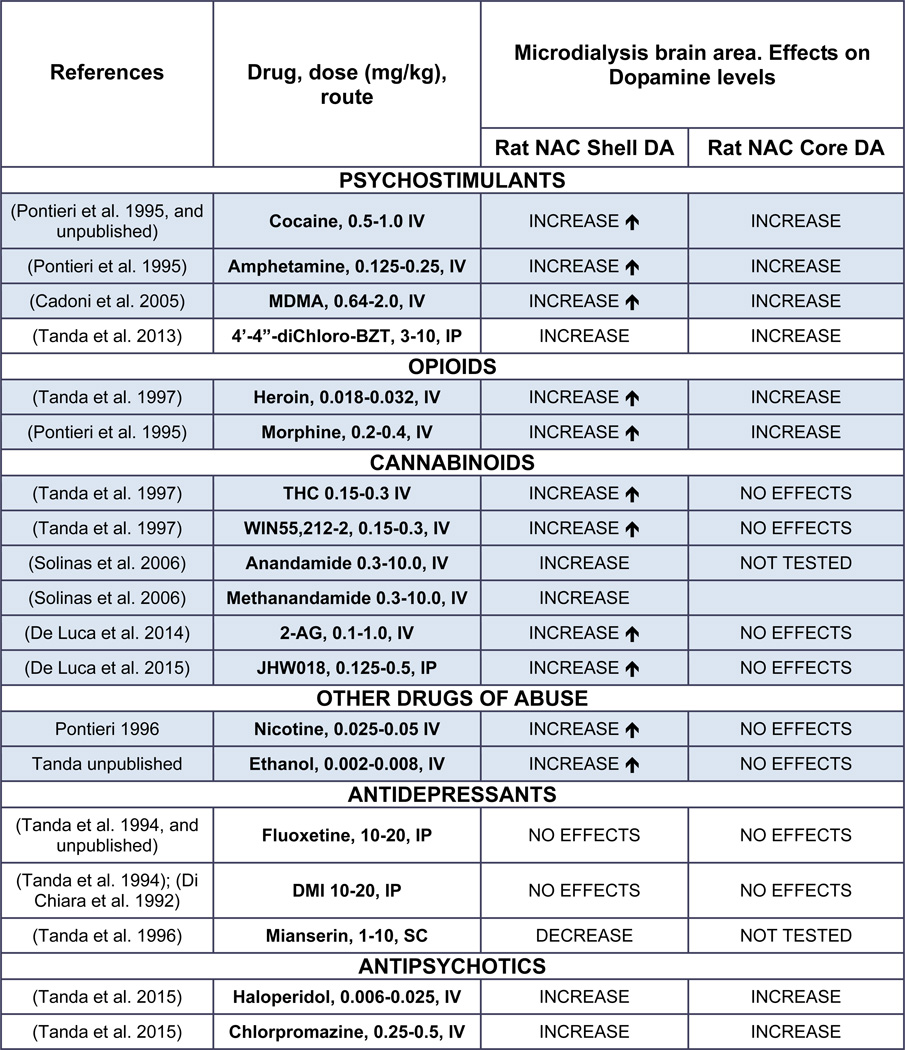
Most drugs of abuse produce a larger increase in extracellular DA levels in the nucleus accumbens as compared to that obtained in the dorsal striatum (Di Chiara et al. 1993a; Di Chiara et al. 1999; Koob 2000). Neuropharmacological and behavioral studies have also suggested that DA transmission in the nucleus accumbens plays a critical role in mediating the rewarding/reinforcing effects of drugs of abuse, and their abuse liability (Di Chiara et al. 1993a; Di Chiara and Imperato 1988), a relationship that we know today is more complicated (Di Chiara et al. 1998; Koob 1999). Detailed anatomical and histological studies of the shell and core sub-regions of the accumbens demonstrated regional differences in their input/output connectivity and in their basic functions, with the shell related to “limbic” functions and the core related to somato-motor functions (de Olmos and Heimer 1999; Di Chiara et al. 1993b; Heimer et al. 1991; Pontieri et al. 1995; Pontieri et al. 1996; Zahm and Heimer 1990; 1993). Table 3 shows the effects of several drug classes on stimulation of DA in the shell/core subdivisions of the accumbens in rodents, and the relation to self-administration behavior.
Table 3.
Drug-induced changes in DA levels in brain dopaminergic terminal areas and relationship with self-administration behavior.
DA= dopamine; ↑=significantly different from NAC core DA; IV=intravenous administration; IP= intraperitoneal administration; SC = subcutaneous administration.
Shadowed cells indicate that self-administration behavior is maintained by the drug in experimental animals.
Early evidence that THC stimulates ventral striatal DA neurotransmission (Chen et al. 1993; Chen et al. 1990a; Chen et al. 1990b; Chen et al. 1991; Ng Cheong Ton et al. 1988) was accompanied by other reports in which no significant changes in DA levels were obtained in striatal areas (Castaneda et al. 1991; Chen et al. 1991). These contrasting results on THC-induced stimulation of DA transmission were mostly obtained in early studies when DA synthesis or levels of DA were determined ex vivo in brain slices or tissue that included striatal areas (Bloom and Dewey 1978; Bloom et al. 1978; Lew and Richardson 1981; Navarro et al. 1993; Rodriguez De Fonseca et al. 1992; Sakurai-Yamashita et al. 1989). Two factors seemed to play a role in whether or not THC stimulated DA in striatal areas: 1) the animal strain, as was also suggested for place preference and ICSS procedures; 2) brain region. For instance, significantly greater THC-induced increase in extracellular DA in the accumbens of Lewis compared to Sprague Dawley rats was reported (Chen et al. 1990b), and confirmed several years later (Cadoni and Di Chiara 2007; Tanda and Dichiara, Unpublished observations). However, after careful review of the available literature, it appears that the stimulating effects of THC on DA transmission depend more on specific regional requirements, i.e. accumbens shell versus core or dorsal caudate, than on strain, as has been shown for other drugs of abuse (see Table 3). The assessment of pharmacological specificity of brain areas to THC effects on DA levels was reported (Tanda et al. 1997). In that study, the selective effects of THC on accumbens shell VS core DA levels were also mimicked by the CB1 agonist, WIN55,212-2, and blunted by pretreatments with the cannabinoid CB1 receptor antagonist, rimonabant. Further, using the same brain coordinates as in Tanda et al (1997), large increases in DA levels in the accumbens shell have been obtained with doses of THC up to 3 mg/kg i.p. (Justinova et al. 2013; Solinas et al. 2007a). Thus, lack of THC effects on DA levels in striatal areas reported in several studies might result from implanting the microdialysis probe in areas less responsive to THC effects, i.e. the caudate putamen or the accumbens core (Castaneda et al. 1991; Chen et al. 1990b; Chen et al. 1991; Tanda et al. 1997). In agreement with this regional specificity, Melis et al. (2000) showed THC-induced stimulation of firing in VTA DAergic neurons, which project to limbic DAergic terminal areas such as the accumbens shell. A similar neuronal firing enhancement by THC was not found in the substantia nigra pars compacta, which projects primarily on DA terminal fields such as the caudate-putamen or accumbens core. These experiments revealed a difference in regional brain sensitivity to THC effects in subpopulations of DA neurons, in agreement with the different change in DA levels among DA terminal areas (de Olmos and Heimer 1999; Tanda et al. 1997).
The studies by Melis et al (2000) confirmed the CB1 pharmacologic specificity of THC effects on DA by antagonism with rimonabant pretreatments, confirming also that rimonabant pretreatments did not affect heroin-induced stimulation of DA levels (Caille and Parsons 2006; Tanda et al. 1997). However, the nonselective opioid antagonist, naloxone, and the µ1-selective antagonist, naloxonazine that blocked both heroin and THC enhancement of DA levels (Chen et al. 1990b; Tanda et al. 1997), did not block the electrophysiological effects of THC on DA cell firing (French 1997; Melis et al. 2000). Several reasons may explain these different effects of opioid antagonists. It is possible that the different experimental conditions for freely-moving animals in microdialysis versus anesthetized or paralyzed animals in the electrophysiology experiments interacted with THC or opioids to produce different effects on neuronal firing. Alternatively the microdialysis experiment was performed with local VTA injections of naloxonazine, which may produce a more robust, area-selective effect than systemic injections. The antagonistic effects of naloxone and naloxonazine on THC-induced stimulation of DA would suggest blockade of effects exerted by endogenous VTA opioid peptides released by THC actions. As shown above, Dr. Goldberg’s group reported THC-induced release of β-endorphins in the VTA (see Figure 1)(Solinas et al. 2004). Stimulation of opioid µ1 receptors in the VTA results in a reduction of GABA inhibitory effects on firing of DA neurons and, in turn, of DA release (Di Chiara and North 1992). This release of endogenous opioid peptides was functionally related to THC actions, since it was found capable to modulate THC subjective effects in rats trained to discriminate THC from vehicle (Solinas et al. 2004)(Figure 1).
Thus, endogenous opioids could play a role in the behavioral actions of THC and cannabinoids. Lately, endogenous ligands for cannabinoid receptors have been discovered. Researchers have explored the possibility that endocannabinoids that share with THC affinity for CB1 receptors might participate to brain functions and pathways potentially related to reward. Recently, indeed, changes in nucleus accumbens shell DA transmission produced by endogenous cannabinoids have been reported (De Luca et al. 2014; Solinas et al. 2006). The increases in DA levels produced by anandamide and 2 AG parallel the findings on potential reinforcing effects of these drugs assessed in self-administration procedures (De Luca et al. 2014; Justinova et al. 2005b). These papers suggest that the endogenous cannabinoids activate reward-related brain structures and pathways producing reinforcing effects similarly to plant cannabinoids like THC.
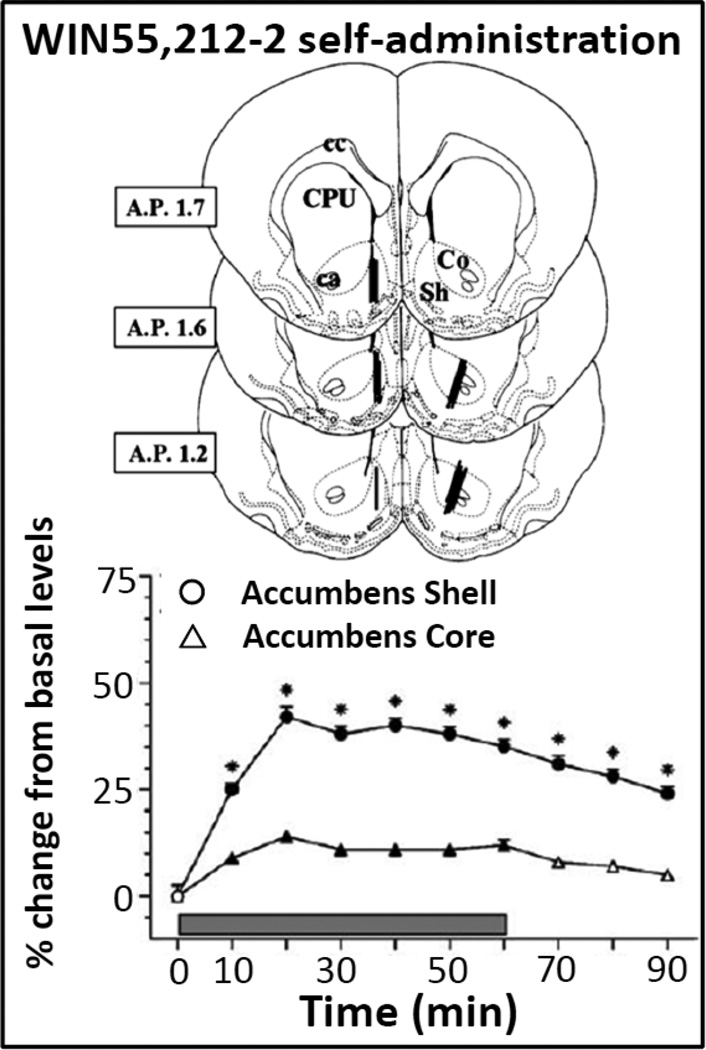
It is worth noting that even though the reinforcing effects of THC have not been demonstrated in rodents, intravenous self-administration of a cannabinoid agonist, WIN 55,212-2, has been shown in rats and mice (Fattore et al. 2001; Lecca et al. 2006; Ledent et al. 1999; Martellotta et al. 1998). Additionally, increases in DA levels related to WIN 55,212,2 intake have been reported to be greater in the shell than in the core of the accumbens (see Figure 3) during the behavioral sessions of self-administration (Lecca et al. 2006). Further, the increase in DA measured by microdialysis was related to self-administration of the cannabinoid agonist, because during extinction (when receiving saline instead of WIN55,212-2), DA levels did not change despite continued but decreasing rates of responding. In more recent reports an increase in DA levels related to systemic administration and self-administration of the synthetic cannabinoid JWH018 (De Luca et al. 2015), known as “spice,” has been demonstrated in rats. Again, larger increases in extracellular DA levels have been reported in the shell as compared to the core of the accumbens, as a confirmation of potential for abuse (Table 3).
Figure 3. Neuroanatomical probe placements and neurochemical effects of WIN55,212-2 self-administration in Sprague-Dawley rats.
Top Panel. Schematic drawings showing the location of microdialysis probes in the nucleus accumbens shell or core in rats self-administering WIN55,212-2 (12.5 µg/kg/infusion). Bottom panel shows the time course of changes from basal DA levels in dialysates from the shell and core of the nucleus accumbens in rats during 4 weeks (12 sessions) of WIN55,212-2 self-administration. Modified from Lecca et al 2006. See text and the original article for more details about experimental procedures and results.
Preclinical studies on cannabinoids self-administration behavior in rodents and monkeys
With only a few exceptions, drugs of abuse are also self-administered by experimental animals, intravenously or orally (Katz and Goldberg 1988). In its simplest configuration, this behavioral procedure allows the animal to freely move inside a cage, to inject a drug or drink a solution of the substance, after specific behavioral requirements have been met (for details of the procedure see Spealman and Goldberg 1978). The most frequently employed intravenous self-administration procedure is one in which each response or a fixed number of responses (fixed-ratio or FR schedule) produces a drug injection. With the addition of a time-out, a signaled time during which responses have no consequences, following each injection, the pattern of responding maintained by drug injection is similar to patterns of responding maintained by more conventional reinforcers, such as food presentation (Goldberg 1973).
In contrast to other drugs of abuse, THC is not self-administered in rodents or rhesus monkeys, and has to date only been demonstrated in squirrel monkeys. The reasons why THC self-administration is not obtained in rodents and in rhesus monkeys is not definitively known. Several research groups have recently tested THC as a reinforcer in self-administration studies in rhesus monkeys or rats (Li et al. 2012; Scherma, Tanda and Goldberg, unpublished observation). Several factors have been taken into consideration to provide a reasonable explanation for the unsuccessful attempts to obtain THC self-administration behavior in rodents and rhesus monkeys (Justinova et al. 2005a; Tanda and Goldberg 2003). In the next paragraphs, and in the Table 4, the procedures of unsuccessful attempts at THC self-administration in experimental animals are compared with those that were successful in squirrel monkeys. Three main factors will be compared and discussed: 1) the schedule of self-administration; 2) the doses and bioavailability of the drug; 3) the animal species
Table 4.
Self-Administration behavior, tests with cannabinoid drugs
| Reference | Drug reinforcer. Dose and vehicle |
Species | Procedure/schedule | Main Results |
|---|---|---|---|---|
| T H C | ||||
|
Deneau and kaymakcalan 1971; Harris et al. 1974 |
100–400 µg/kg Tween20/salineine or 25–300 µg/kg PVP/saline |
Rhesus monkeys |
IVSA, FR1, naïve subjects | No significant acquisition |
|
Mansbach et al. 1994; Pickens et al. 1973 |
17–100 µg/kg in PVP/saline or EL620/EtOH/saline |
IVSA, FR1, Substitution for phencyclidine | No significant substitution | |
|
Carney et al. 1977; Harris et al. 1974; Kaymakcalan 1973 |
3–300 µg/kg EL620; 25–200 µg/kg PVP/saline;100–400 µg/kg Tween20/saline |
IVSA, substitution for cocaine | No significant substitution | |
| Harris et al. 1974 | 25–300 µg/kg PVP/saline | IVSA, cocaine/THC combinations. Automated programmed delivery |
No significant acquisition/substitution |
|
| Kaymakcalan 1973 | 100–400 µg/kg Tween20/saline | IVSA, Monkeys made dependent on THC | No significant acquisition (2 out of 6) |
|
| Pickens et al. 1973 | 25 mg burned hashish | Inhalation and food presentation/FR3 and 4 sec. mouth contact to smoke tube, 24 hours per day, 4 days each week |
No significant difference from vehicle responding |
|
| Li et al. 2012 | 3.2–32 µg/kg EL620/EtOH/saline | IVSA, history of heroin SA or naive | No significant acquisition | |
| Corcoran and Amit 1974 | 0.25 mg (hashish)/ml | Rats | Forced drinking | No significant acquisition |
| van Ree et al. 1978 | 7.5–300 µg/kg tween20/saline | IVSA, naïve | 40% positive | |
| Takahashi and Singer 1979; 1980 | 6.25–50 µg/kg tween80/saline | IVSA, naïve, food deprived, with automatic food pellet delivery |
Positive results maintained only with food deprivation and pellet delivery |
|
| Braida et al. 2004 | 0.01–1 µg/inf cerebrospinal fluid, EtOH, cremophor |
ICVSA, trained with water | Positive results, max responding at 0.02 µg/inf |
|
|
Tanda et al. 2000; Justinova et al. 2008; Justinova et al. 2003 |
1–16 µg/kg EtOH/Tween80/saline | Squirrel monkeys |
IVSA, naïve or cocaine trained with saline extinction/FR or second order |
Positive results, max responding at 4 µg/kg |
| Anandamide and Methanandamide | ||||
| Justinova et al. 2005 | 2.5–160 µg/kg tween80/saline/eth | Squirrel monkeys |
FR10, IVSA, drug naïve | Positive results, max responding at 40 µg/kg |
| 2-AG | ||||
| De Luca et al. 2014 | 12.5–50 µg/kg tween80/saline/eth | Sprague Dawley Rats |
IVSA, naïve | 90% positive, max responding at 25 µg/kg |
| Justinova et al. 2011 | 0.1–100 µg/kg EtOH/Tween80/saline | Squirrel monkeys |
IVSA, nicotine substitution or history IVSA of anandamide, anandamide reuptake inhibitors or nicotine |
100% positive, max responding at 3 µg/kg |
| CP55,940 | ||||
| Mansbach et al. 1994 | 0.3–3 µg/kg emulphor/ EtOH/saline | Rhesus monkey |
IVSA,PCP substitution | No acquisition |
| Braida et al. 2001 | 0.1–1.6 µg cerebrospinal fluid, EtOH, cremophor |
Wistar Rats |
IVSA | Positive, max responding at 0.4 µg/kg |
| WIN55,212-2 | ||||
| Martellotta et al. 1998 | 10–500 µg/kg ? | CD1 Mice | IVSA, naïve | Positive, max responding at 100 µg/kg |
| Fattore et al. 2001 | 6.25–50 µg/kg Tween 80/heparin/saline |
Wistar Rats |
IVSA, naïve | 87% positive, max responding at 12.5 µg/kg |
| Solinas et al. 2007 | 12.5–25 µg/kg Tween 80/saline | Long– Evans Rats |
Positive, max responding at 12.5 µg/kg |
|
| Justinova et al 2013 | 12.5 µg/kg Tween- 80/heparin/saline | Long– Evans Rats/Spra gue Dawley |
IVSA, naïve | Positive |
| Lecca et al. 2006 | 12.5 µg/kg Tween 80/saline | Sprague Dawley Rats |
IVSA, naïve | Positive |
| JWH 018 | ||||
| De Luca et al. 2015 | 10–20 µg/kg EtOH/Tween80/saline | Sprague– Dawley Rats |
IVSA, naïve | 90% positive, max responding at 20 µg/kg |
| De Luca et al. 2015 | 15–30 µg/kg EtOH/Tween80/saline | C57BL/6 mice |
IVSA, naïve | 90% positive, at 30 µg/kg |
IVSA = intravenous self-administration
1. Schedule of self-administration
The self-administration behavior maintained by THC in squirrel monkeys was obtained using an FR 10 schedule (ten lever presses on the active lever produced an intravenous injection) of drug-delivery (Tanda et al. 2000), or using a second-order schedule of drug-delivery (Justinova et al. 2008) that will be discussed later in this section.
As shown in Table 4, different, and sometimes very complicated, schedules of drug injection or delivery have been tested in rhesus monkey. In early studies inhalation, oral or intravenous routes were unsuccessfully used to test THC or cannabis-related compounds (Amit et al. 1973; Corcoran and Amit 1974; Leite and Carlini 1974; Pickens et al. 1973). For example, in a report by Harris and coworkers (Harris et al. 1974), naïve and cocaine-experienced rhesus monkey were tested with THC under an FR schedule. Some tests were performed with delivery of intravenous mixtures of cocaine and THC, or after a month of exposure to response-independent programmed injections of 2 mg/kg of THC daily. None of those studies demonstrated reliable THC self-administration above levels maintained by vehicle injections.
Longer-term exposures to response-independent THC in monkeys have also been examined (Kaymakcalan 1973; Kaymakçalan 1972). The logic of this “pretreatment” evolved from earlier studies with other psychoactive agents (Deneau et al. 1969) in which self-administration of certain drugs in some naïve monkeys was only obtained after automatically programmed response-independent injections. After programmed injections two out of six monkeys self-administered THC at low rates (Kaymakcalan 1973; Kaymakçalan 1972).
In other reports monkeys were trained to self-administer drugs of abuse such as phencyclidine or cocaine. However THC failed to maintain behavior when substituted for these training drugs (Mansbach et al. 1994; Pickens et al. 1973). For example, the potent cannabinoid agonist, CP55,940, was substituted in rhesus monkeys trained to reliably self-administer phencyclidine by Mansbach and colleagues, (1994). Despite reliable phencyclidine self-administration, response rates above vehicle levels were not maintained by either THC or CP55,940. Similarly, THC failed to substitute in rhesus monkeys self-administering heroin (Li et al. 2012).
In the report by Tanda et al (2000) monkeys were trained with cocaine and tested with various drugs in cocaine experiments for longer than 1 year; these monkeys had at least one month of washout and 2 weeks of saline availability before they were assigned to the THC self-administration group. Cocaine training was thought to be a key factor in THC self-administration in squirrel monkeys (Maldonado 2002). However, a subsequent demonstration of THC self-administration in drug-naïve squirrel monkeys, and the successful demonstrations of anandamide and methanandamide self-administration, again, in drug-naïve squirrel monkey, made it clear that cocaine training is not a necessary condition for acquisition of cannabinoid self-administration. Although self-administration of THC has not been found in other species, except for local brain injections of THC (Braida et al. 2004; Zangen et al. 2006), intravenous self-administration of other cannabinoid agonists, like WIN55212, 2-AG, JWH018 has been reported in rodents (De Luca et al. 2015; De Luca et al. 2014; Fattore et al. 2001; Lecca et al. 2006; Martellotta et al. 1998). This is of particular importance since the widespread use of new designer cannabinoids. Indeed, samples of “spice” blends, which is a new format of drugs of abuse on the street, have shown that several high affinity cannabinoid agonist are present in this abused substance (Debruyne and Le Boisselier 2015).
As shown in the Table 4, intravenous injections of THC also served as a reinforcer under second-order schedules of self-administration. Under these procedures the completion of one schedule requirement is treated as a unit of behavior that is in turn reinforced according to another schedule of reinforcement (Kelleher 1966). Often an exteroceptive stimulus is briefly presented at the completion of each of the “unit” schedules as well as at completion of the unit schedule that produces the reinforcer. The briefly presented stimulus can facilitate the maintenance of behavior such that an extended sequence of behavior is maintained before delivery of the reinforcer. For example, Goldberg et al. (1976) maintained responding of rhesus monkeys under an FI 60-min (FR 10:S) second-order schedule in which each completion of the FR 10 unit schedule produced a brief light illumination and the first completion of the FR 10 produced the brief visual stimulus and an injection of morphine, ending the experimental session for the day. Under this schedule, large numbers of responses were emitted, though concentrated towards the end of the hour, and importantly these responses occurred before drug was injected and therefore were not influenced by consequent intoxication. As indicated in the previous section, intoxication from large doses of THC may have interfered with a clear demonstration of reinforcing effects in the earliest studies (see also Carriero et al. 1998). Thus, a second-order schedule seemed a natural initial approach to studying reinforcing effects of THC, and was used in initial studies of THC self-administration, though those results were ultimately published later (Justinova et al. 2008)
Responding of squirrel monkeys under the second-order schedule was well maintained throughout the session and was characteristic of those performances previously maintained with other drugs or food reinforcement. In addition, rates of responding were related to dose with an inverted U-shaped function characteristic of dose-effects with other abused drugs. When the consequent THC injections were replaced with vehicle injections response rates decreased to low levels. Because all THC injections were delivered at the end of the experimental session the decreases in response rates were observed in the second session after THC was replaced with vehicle. When rimonabant (0.3 mg/kg) was administered before sessions, response rates immediately decreased to low levels before THC administration. Removal of the brief stimulus that followed FR unit schedule completions also immediately decreased rates of responding to very low levels. When the brief stimulus and THC were discontinued, reinstatement of responding was reliably obtained with reintroduction of the brief stimulus only. Interestingly, this reinstatement was blocked by rimonabant injection. Further, after responding had been reduced by elimination of the brief stimulus and removal of the consequent THC injection it was reinstated with injections of THC, the endogenous cannabinoid anandamide (or its metabolic stable congener, methanandamide), the endocannabinoid uptake blocker AM404, morphine, and to a lesser degree by relatively high doses of cocaine.
Rimonabant also blocked THC-induced reinstatement but not morphine induced reinstatement, whereas naltrexone blocked morphine- but not THC-induced reinstatement, indicating that each antagonist specifically blocked the reinstatement of a behavior induced by the corresponding receptor specific agonist. The blockade by rimonabant was of considerable interest as it suggested CB1 receptor antagonism would not only block the reinforcing effects of THC but also the effects of stimuli associated with THC administration. This more global blockade suggests the potential of CB1 antagonism as a strategy to treat cannabis use disorder as well as all of the phenomena surrounding the conditioned effects of stimuli associated with THC abuse (Justinova et al. 2008). Unfortunately rimonabant for human use has been discontinued and suspended due to severe psychiatric side effects (E.M.A. 2008). However, the study of neutral antagonists of CB1 receptors, as opposed to the antagonist/inverse-agonist rimonabant, is an important area for future research and suggests it may be possible to reduce THC reinforcing effects without unwanted psychiatric side-effects.
2. Doses and bioavailability of the drug
In review of the unsuccessful tests of THC self-administration in experimental animals, the majority of papers used i.v. THC doses as high as 0.2-0.4 mg/kg/inj. Several studies report that subjects were intoxicated after the session, even with the delivery of only a few THC injections at a frequency not greater than that for vehicle (Harris et al. 1974; Mansbach et al. 1994; Pickens et al. 1973). In contrast, the first successful studies with THC self-administration used THC doses that ranged from 1 to 16 µg/kg/injection (Justinova et al. 2003; Tanda et al. 2000). With those doses the squirrel monkeys when observed post-session did not show signs of intoxication. Further, in the few “almost successful” earlier attempts at establishing THC self-administration, doses of THC were in the low microgram range (Takahashi and Singer 1979; 1980; 1981). Thus, most of the unsuccessful attempts to obtain self-administration of THC used doses of THC much higher than those subsequently shown to be effective in squirrel monkeys.
That the dose-range examined is a critical factor for THC self-administration is not unexpected as it is important for all pharmacological actions. However, an important question is how the doses used relate to those used by humans. In the study by Tanda et al (2000) the total i.v. intake of THC obtained by monkeys ranged from 50 to 120 µg/kg over a one-hour session at doses of 2 and 4 µg/kg/injection. In a clinical study (Ohlsson et al. 1981), i.v. injection of 5 mg of THC (~70 µg/kg) produced the same degree of "high" as that reported by humans after smoking marijuana or hashish. That ~70 µg/kg dose is similar to the total intake of THC self-administered by squirrel monkeys.. Further, as THC bioavailability from smoking marijuana is on average about 20% (Agurell et al. 1986), the actual THC intake would be about 3 mg in human subjects smoking a cigarette containing 15 mg of THC. Distributed over 10 to 15 puffs, each puff would contain 200 to 300 µg of THC or approximately 2.9 to 4.3 µg/kg of THC delivered per puff. This dose per puff is in good agreement with the range of injection doses (2 to 4 µg/kg) that maintained responding in the squirrel monkey self-administration studies.
Bioavailability likely also plays a role in the reinforcing effects of THC. THC is usually commercially available as an ethanol solution, since it is practically insoluble in water. Several reports describe THC solutions for intravenous injections in vehicles that kept the compound in suspension, with obvious limits to bioavailability, even when injected i.v.. In the study by Tanda et al (2000) THC was dissolved in a tween 80/ethanol/saline vehicle, using a modification of the procedure described by Olsen et al (1973). This vehicle provides a rapid distribution of THC to different tissues, especially the brain, compared to other commonly employed vehicles (Mantilla-Plata and Harbison 1975). This vehicle was selected based on its previous use in a study in which THC was injected intravenously and significantly elevated DA levels in the shell of the nucleus accumbens of the rat during the first 10 to 20 minutes after injection (Tanda et al. 1997). This effect on DA, which resembles that produced by heroin, cocaine, or amphetamine, appears critical for addictive behaviors (Di Chiara et al. 1999; Koob et al. 1998).
3. Animal species
We know from previously cited studies in rats that genetic background can have a profound impact in the effects of THC and other cannabinoid agonists in place preference, brain self-stimulation and DA microdialysis procedures. What we do not know, yet, is whether squirrel monkeys, like human subjects, have the ability to self-administer THC because of specific genetic/physiologic determinants or differences in metabolism/pharmacokinetics from other species. It remains possible that the best THC self-administration conditions for other species simply have not yet been found. Interestingly, the same squirrel monkeys used to establish THC self-administration by Tanda et al. (2000) had been used previously without success using a similar THC i.v. dose range but with a different vehicle (Poly-Vinyl-Pyrrolidone) (S. Yasar and S. Goldberg, unpublished observations). It is worth noting that nicotine self-administration has similarly been found to be effective in the squirrel monkeys (Goldberg et al. 1981), when its reinforcing effects in other species were inconsistently obtained (Stolerman and Jarvis 1995).
Thus, it is likely that several factors play a role in the difficulty to find the best experimental conditions for studying the potential reinforcing effects of cannabinoids in different animal species. Even trying to “reverse translate” human behaviors, related to abuse of substances, to animals does not seem to help. For instance, most of the self-administration studies using FR 1 schedules (one injection for each response) show a pattern with multiple drug injections, which might seem at variance with the pattern of drug intake shown by human subjects abusing drugs like heroin or cocaine, usually taken in limited amounts, often once per day, thus with drug use not really distributed over time. So, it is worth noting that drugs like nicotine or marijuana, often smoked and with a pattern of intake distributed more broadly over time, as in self administration sessions, have been among the most refractory to the establishment of robust self-administration in animals. Nicotine self-administration has ultimately been documented in squirrel monkeys, baboons, rats and mice, though only under specific procedural conditions (Ator and Griffiths 1983; Caggiula et al. 2002; Caggiula et al. 2001; Corrigall 1999; Goldberg and Henningfield 1988; Henningfield and Goldberg 1983). Thus, as with nicotine (Stolerman and Jarvis 1995), it is possible that the necessary conditions for more widespread success in the study of reinforcing effects of THC are soon to be better documented.
Conclusions
Several behavioral procedures have been reviewed here in an attempt to understand how the potential for abuse of cannabis derivatives could be studied using indirect assessments of their reinforcing actions. Inconsistency of results obtained with place conditioning and ICSS procedures suggest that drug-discrimination and neurochemistry are the best indirect predictors of cannabinoid-like pharmacology and abuse liability of new cannabinoid compounds, respectively.
Since the first paper reliably demonstrating reinforcing effects of THC was published in the year 2000, the THC self-administration model of marijuana abuse has provided the possibility of directly studying reinforcing effects and abuse liability of THC as well as other cannabinoid agonists. That study and those that followed showed that like THC, the endogenous brain cannabinoids, anandamide and 2AG, are self-administered. These results suggest an important involvement of brain endocannabinoid systems in the central mechanisms of reinforcement. Self-administration procedures have also been of paramount importance for evaluating pharmacological treatments as therapies for cannabis use disorders (Justinova et al. 2013; Justinova et al. 2004). Reliable THC self-administration allows the testing of potential medications for THC abuse to be realized in a manner that has substantial experimental utility. With new research from pharmaceutical companies on cannabinoids to treat pain (Kirkpatrick et al. 2015; Maione et al. 2013; Vemuri and Makriyannis 2015) their testing for abuse liability will be facilitated by self-administration models. New pain therapies are particularly important today with prescription opioid abuse reaching epidemic proportions; however there are a host of other potential therapeutic applications for cannabinoids. The availability of the squirrel monkey model of THC self-administration behavior will allow the development of new compounds with confidence in safety testing regarding their potential for abuse.
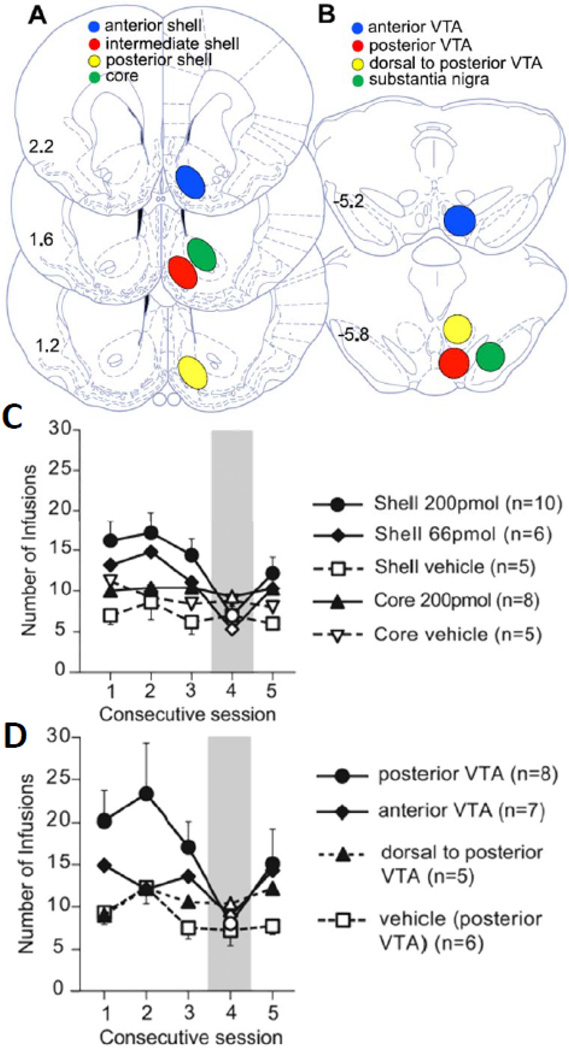
Figure 4. Neuroanatomical location of THC injections and number of self-infusion of THC in the shell or VTA as a function of daily sessions.
Top panels (A and B) show the location of THC site infusions in the anterior, intermediate or posterior shell, or in the core, and in the anterior, posterior, or dorsal to posterior VTA, or in the substantia nigra. Panels C and D show the number of self-infusions of THC into different accumbens (C) or midbrain (D) sites, earned by rats during consecutive sessions. Session number 4 was characterized by substitution of THC by its vehicle. Modified from Zangen et al, 2006. Please see text or the original article for more details about experimental procedures and results..
Acknowledgments
This work was funded by the Medication Development Program, NIDA-IRP, NIH/DHHS.
I would like to thank Claudio Zanettini and Mark Coggiano for discussion of previous version of this review, and Jonathan Katz for his extensive reading of the current version of this manuscript, and his comments and suggestions.
Finally, I can never be thankful enough for crossing the scientific path of Dr. Steve Goldberg. There are not enough words I know (even in Italian) to express my gratitude for the many things I learned from Steve. We were a great team for about 16 years, even though I was a guest researcher in Steve’s Preclinical Pharmacology Section for only two of those years. Perhaps to make my feelings about our collaboration more understandable, Steve’s lab was for me like the Eagles’ Hotel California, “you can check out any time you like, but you can never leave.”
Footnotes
The author declares no conflict of interests.
List of References
- Acquas E, Pisanu A, Marrocu P, Di Chiara G. Cannabinoid CB(1) receptor agonists increase rat cortical and hippocampal acetylcholine release in vivo. European journal of pharmacology. 2000;401:179–185. doi: 10.1016/s0014-2999(00)00403-9. [DOI] [PubMed] [Google Scholar]
- Acquas E, Tanda G, Di Chiara G. Differential effects of caffeine on dopamine and acetylcholine transmission in brain areas of drug-naive and caffeine-pretreated rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2002;27:182–193. doi: 10.1016/S0893-133X(02)00290-7. [DOI] [PubMed] [Google Scholar]
- Agurell S, Halldin M, Lindgren JE, Ohlsson A, Widman M, Gillespie H, Hollister L. Pharmacokinetics and metabolism of delta 1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacol Rev. 1986;38:21–43. [PubMed] [Google Scholar]
- Alici T, Appel JB. Increasing the selectivity of the discriminative stimulus effects of delta 9-tetrahydrocannabinol: complete substitution with methanandamide. Pharmacol Biochem Behav. 2004;79:431–437. doi: 10.1016/j.pbb.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Amit Z, Corcoran ME, Charness ME, Shizgal P. Intake of diazepam and hashish by alcohol preferring rats deprived of alcohol. Physiology & behavior. 1973;10:523–527. doi: 10.1016/0031-9384(73)90215-1. [DOI] [PubMed] [Google Scholar]
- Arnold JC, Hunt GE, McGregor IS. Effects of the cannabinoid receptor agonist CP 55,940 and the cannabinoid receptor antagonist SR 141716 on intracranial self-stimulation in Lewis rats. Life sciences. 2001a;70:97–108. doi: 10.1016/s0024-3205(01)01366-2. [DOI] [PubMed] [Google Scholar]
- Arnold JC, Topple AN, Mallet PE, Hunt GE, McGregor IS. The distribution of cannabinoid-induced Fos expression in rat brain: differences between the Lewis and Wistar strain. Brain research. 2001b;921:240–255. doi: 10.1016/s0006-8993(01)03127-4. [DOI] [PubMed] [Google Scholar]
- Ator NA, Griffiths RR. Nicotine self-administration in baboons. Pharmacology, biochemistry, and behavior. 1983;19:993–1003. doi: 10.1016/0091-3057(83)90406-9. [DOI] [PubMed] [Google Scholar]
- Balster RL, Prescott WR. Delta 9-tetrahydrocannabinol discrimination in rats as a model for cannabis intoxication. Neuroscience and biobehavioral reviews. 1992;16:55–62. doi: 10.1016/s0149-7634(05)80051-x. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Baskfield CY, Martin BR, Wiley JL. Differential effects of delta9-tetrahydrocannabinol and methanandamide in CB1 knockout and wild-type mice. The Journal of pharmacology and experimental therapeutics. 2004;309:86–91. doi: 10.1124/jpet.103.055376. [DOI] [PubMed] [Google Scholar]
- Bassareo V, Tanda G, Petromilli P, Giua C, Di Chiara G. Non-psychostimulant drugs of abuse and anxiogenic drugs activate with differential selectivity dopamine transmission in the nucleus accumbens and in the medial prefrontal cortex of the rat. Psychopharmacology. 1996;124:293–299. doi: 10.1007/BF02247433. [DOI] [PubMed] [Google Scholar]
- Block RI, Erwin WJ, Farinpour R, Braverman K. Sedative, stimulant, and other subjective effects of marijuana: relationships to smoking techniques. Pharmacology, biochemistry, and behavior. 1998;59:405–412. doi: 10.1016/s0091-3057(97)00453-x. [DOI] [PubMed] [Google Scholar]
- Bloom AS, Dewey WL. A comparison of some pharmacological actions of morphine and delta9-tetrahydrocannabinol in the mouse. Psychopharmacology. 1978;57:243–248. doi: 10.1007/BF00426745. [DOI] [PubMed] [Google Scholar]
- Bloom AS, Johnson KM, Dewey WL. The effects of cannabinoids on body temperature and brain catecholamine synthesis. Res Commun Chem Pathol Pharmacol. 1978;20:51–57. [PubMed] [Google Scholar]
- Braida D, Iosue S, Pegorini S, Sala M. Delta9-tetrahydrocannabinol-induced conditioned place preference and intracerebroventricular self-administration in rats. Eur J Pharmacol. 2004;506:63–69. doi: 10.1016/j.ejphar.2004.10.043. [DOI] [PubMed] [Google Scholar]
- Braida D, Pozzi M, Cavallini R, Sala M. Conditioned place preference induced by the cannabinoid agonist CP 55,940: interaction with the opioid system. Neuroscience. 2001a;104:923–926. doi: 10.1016/s0306-4522(01)00210-x. [DOI] [PubMed] [Google Scholar]
- Braida D, Pozzi M, Parolaro D, Sala M. Intracerebral self-administration of the cannabinoid receptor agonist CP 55,940 in the rat: interaction with the opioid system. Eur J Pharmacol. 2001b;413:227–234. doi: 10.1016/s0014-2999(01)00766-x. [DOI] [PubMed] [Google Scholar]
- Browne RG, Weissman A. Discriminative stimulus properties of delta 9-tetrahydrocannabinol: mechanistic studies. J Clin Pharmacol. 1981;21:227S–234S. doi: 10.1002/j.1552-4604.1981.tb02599.x. [DOI] [PubMed] [Google Scholar]
- Burkey RT, Nation JR. (R)-methanandamide, but not anandamide, substitutes for delta 9-THC in a drug-discrimination procedure. Experimental and clinical psychopharmacology. 1997;5:195–202. doi: 10.1037//1064-1297.5.3.195. [DOI] [PubMed] [Google Scholar]
- Cadoni C, Di Chiara G. Differences in dopamine responsiveness to drugs of abuse in the nucleus accumbens shell and core of Lewis and Fischer 344 rats. Journal of neurochemistry. 2007;103:487–499. doi: 10.1111/j.1471-4159.2007.04795.x. [DOI] [PubMed] [Google Scholar]
- Cadoni C, Simola N, Espa E, Fenu S, Di Chiara G. Strain dependence of adolescent Cannabis influence on heroin reward and mesolimbic dopamine transmission in adult Lewis and Fischer 344 rats. Addiction biology. 2015;20:132–142. doi: 10.1111/adb.12085. [DOI] [PubMed] [Google Scholar]
- Cadoni C, Solinas M, Pisanu A, Zernig G, Acquas E, Di Chiara G. Effect of 3,4-methylendioxymethamphetamine (MDMA, "ecstasy") on dopamine transmission in the nucleus accumbens shell and core. Brain research. 2005;1055:143–148. doi: 10.1016/j.brainres.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Chaudhri N, Perkins KA, Evans-Martin FF, Sved AF. Importance of nonpharmacological factors in nicotine self-administration. Physiology & behavior. 2002;77:683–687. doi: 10.1016/s0031-9384(02)00918-6. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacology, biochemistry, and behavior. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Caille S, Parsons LH. Cannabinoid modulation of opiate reinforcement through the ventral striatopallidal pathway. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31:804–813. doi: 10.1038/sj.npp.1300848. [DOI] [PubMed] [Google Scholar]
- Carney JM, Uwaydah IM, Balster RL. Evaluation of a suspension system for intravenous self-administration studies of water-insoluble compounds in the rhesus monkey. Pharmacology, biochemistry, and behavior. 1977;7:357–364. doi: 10.1016/0091-3057(77)90232-5. [DOI] [PubMed] [Google Scholar]
- Castaneda E, Moss DE, Oddie SD, Whishaw IQ. THC does not affect striatal dopamine release: microdialysis in freely moving rats. Pharmacology, biochemistry, and behavior. 1991;40:587–591. doi: 10.1016/0091-3057(91)90367-b. [DOI] [PubMed] [Google Scholar]
- Chait LD, Evans SM, Grant KA, Kamien JB, Johanson CE, Schuster CR. Discriminative stimulus and subjective effects of smoked marijuana in humans. Psychopharmacology (Berl) 1988;94:206–212. doi: 10.1007/BF00176846. [DOI] [PubMed] [Google Scholar]
- Chait LD, Zacny JP. Reinforcing and subjective effects of oral delta 9-THC and smoked marijuana in humans. Psychopharmacology (Berl) 1992;107:255–262. doi: 10.1007/BF02245145. [DOI] [PubMed] [Google Scholar]
- Chaperon F, Soubrie P, Puech AJ, Thiebot MH. Involvement of central cannabinoid (CB1) receptors in the establishment of place conditioning in rats. Psychopharmacology (Berl) 1998;135:324–332. doi: 10.1007/s002130050518. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Kendall DA, Marsden CA. Cannabinoid receptors and reward in the rat: a conditioned place preference study. Psychopharmacology (Berl) 2000;151:25–30. doi: 10.1007/s002130000481. [DOI] [PubMed] [Google Scholar]
- Chen J, Marmur R, Pulles A, Paredes W, Gardner EL. Ventral tegmental microinjection of delta 9-tetrahydrocannabinol enhances ventral tegmental somatodendritic dopamine levels but not forebrain dopamine levels: evidence for local neural action by marijuana's psychoactive ingredient. Brain research. 1993;621:65–70. doi: 10.1016/0006-8993(93)90298-2. [DOI] [PubMed] [Google Scholar]
- Chen J, Paredes W, Lowinson JH, Gardner EL. Delta 9-tetrahydrocannabinol enhances presynaptic dopamine efflux in medial prefrontal cortex. European journal of pharmacology. 1990a;190:259–262. doi: 10.1016/0014-2999(90)94136-l. [DOI] [PubMed] [Google Scholar]
- Chen JP, Paredes W, Li J, Smith D, Lowinson J, Gardner EL. Delta 9-tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology (Berl) 1990b;102:156–162. doi: 10.1007/BF02245916. [DOI] [PubMed] [Google Scholar]
- Chen JP, Paredes W, Lowinson JH, Gardner EL. Strain-specific facilitation of dopamine efflux by delta 9-tetrahydrocannabinol in the nucleus accumbens of rat: an in vivo microdialysis study. Neuroscience letters. 1991;129:136–180. doi: 10.1016/0304-3940(91)90739-g. [DOI] [PubMed] [Google Scholar]
- Chen YW, Fiscella KA, Bacharach SZ, Tanda G, Shaham Y, Calu DJ. Effect of yohimbine on reinstatement of operant responding in rats is dependent on cue contingency but not food reward history. Addiction biology. 2015;20:690–700. doi: 10.1111/adb.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran ME, Amit Z. Reluctance of rats to drink hashish suspensions: free-choice and forced consumption, and the effects of hypothalamic stimulation. Psychopharmacologia. 1974;35:129–147. doi: 10.1007/BF00429580. [DOI] [PubMed] [Google Scholar]
- Corrigall WA. Nicotine self-administration in animals as a dependence model. Nicotine Tob Res. 1999;1:11–20. doi: 10.1080/14622299050011121. [DOI] [PubMed] [Google Scholar]
- De Luca MA, Bimpisidis Z, Melis M, Marti M, Caboni P, Valentini V, Margiani G, Pintori N, Polis I, Marsicano G, Parsons LH, Di Chiara G. Stimulation OF IN VIVO dopamine transmission and intravenous self-administration in rats and mice by JWH-018, a Spice cannabinoid. Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2015.08.041. [DOI] [PubMed] [Google Scholar]
- De Luca MA, Valentini V, Bimpisidis Z, Cacciapaglia F, Caboni P, Di Chiara G. Endocannabinoid 2-Arachidonoylglycerol Self-Administration by Sprague-Dawley Rats and Stimulation of in vivo Dopamine Transmission in the Nucleus Accumbens Shell. Front Psychiatry. 2014;5:140. doi: 10.3389/fpsyt.2014.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Olmos JS, Heimer L. The concepts of the ventral striatopallidal system and extended amygdala. Annals of the New York Academy of Sciences. 1999;877:1–32. doi: 10.1111/j.1749-6632.1999.tb09258.x. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Di Marzo V. Non-CB1, non-CB2 receptors for endocannabinoids, plant cannabinoids, and synthetic cannabimimetics: focus on G-protein-coupled receptors and transient receptor potential channels. J Neuroimmune Pharmacol. 2010;5:103–121. doi: 10.1007/s11481-009-9177-z. [DOI] [PubMed] [Google Scholar]
- De Vry J, Jentzsch KR. Intrinsic activity estimation of cannabinoid CB1 receptor ligands in a drug discrimination paradigm. Behavioural pharmacology. 2003;14:471–476. doi: 10.1097/01.fbp.0000087739.21047.d8. [DOI] [PubMed] [Google Scholar]
- Debruyne D, Le Boisselier R. Emerging drugs of abuse: current perspectives on synthetic cannabinoids. Subst Abuse Rehabil. 2015;6:113–129. doi: 10.2147/SAR.S73586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneau G, Yanagita T, Seevers MH. Self-administration of psychoactive substances by the monkey. Psychopharmacologia. 1969;16:30–48. doi: 10.1007/BF00405254. [DOI] [PubMed] [Google Scholar]
- Deneau GA, kaymakcalan S. Physiological and Psychological dependence to synthetic delta-9-tetrahydrocannabinol (THC) in rhesus monkeys. The Pharmacologist. 1971:246. [Google Scholar]
- Devane WA, Dysarz FA, 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Acquas E, Tanda G, Cadoni C. Drugs of abuse: biochemical surrogates of specific aspects of natural reward? Biochem Soc Symp. 1993a;59:65–81. [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, North RA. Neurobiology of opiate abuse. Trends in pharmacological sciences. 1992;13:185–193. doi: 10.1016/0165-6147(92)90062-b. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Tanda G, Bassareo V, Pontieri F, Acquas E, Fenu S, Cadoni C, Carboni E. Drug addiction as a disorder of associative learning. Role of nucleus accumbens shell/extended amygdala dopamine. Annals of the New York Academy of Sciences. 1999;877:461–485. doi: 10.1111/j.1749-6632.1999.tb09283.x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Tanda G, Cadoni C, Acquas E, Bassareo V, Carboni E. Homologies and differences in the action of drugs of abuse and a conventional reinforcer (food) on dopamine transmission: an interpretative framework of the mechanism of drug dependence. Adv Pharmacol. 1998;42:983–987. doi: 10.1016/s1054-3589(08)60911-4. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Tanda G, Frau R, Carboni E. On the preferential release of dopamine in the nucleus accumbens by amphetamine: further evidence obtained by vertically implanted concentric dialysis probes. Psychopharmacology. 1993b;112:398–402. doi: 10.1007/BF02244939. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Tanda GL, Frau R, Carboni E. Heterologous monoamine reuptake: lack of transmitter specificity of neuron-specific carriers. Neurochem Int. 1992;20(Suppl):231S–235S. [PubMed] [Google Scholar]
- Di Marzo V. The endocannabinoid system: its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacological research. 2009;60:77–84. doi: 10.1016/j.phrs.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L. Why do cannabinoid receptors have more than one endogenous ligand? Philos Trans R Soc Lond B Biol Sci. 2012;367:3216–3228. doi: 10.1098/rstb.2011.0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty P, Dykstra LA, Picker MJ. Discriminative stimulus effects of phencyclidine: pharmacologically specific interactions with delta 9- and delta 8-tetrahydrocannabinol. Drug and alcohol dependence. 1994;35:151–158. doi: 10.1016/0376-8716(94)90122-8. [DOI] [PubMed] [Google Scholar]
- E.M.A. The European Medicines Agency recommends suspension of the marketing authorisation of Acomplia. Lodon UK: Press Office of the European Medicines Agency; 2008. Volume: [Google Scholar]
- Egashira N, Mishima K, Katsurabayashi S, Yoshitake T, Matsumoto Y, Ishida J, Yamaguchi M, Iwasaki K, Fujiwara M. Involvement of 5-hydroxytryptamine neuronal system in Delta(9)-tetrahydrocannabinol-induced impairment of spatial memory. European journal of pharmacology. 2002;445:221–229. doi: 10.1016/s0014-2999(02)01755-7. [DOI] [PubMed] [Google Scholar]
- Elkashef A, Vocci F, Huestis M, Haney M, Budney A, Gruber A, el-Guebaly N. Marijuana neurobiology and treatment. Subst Abus. 2008;29:17–29. doi: 10.1080/08897070802218166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fant RV, Heishman SJ, Bunker EB, Pickworth WB. Acute and residual effects of marijuana in humans. Pharmacology, biochemistry, and behavior. 1998;60:777–784. doi: 10.1016/s0091-3057(97)00386-9. [DOI] [PubMed] [Google Scholar]
- Fattore L, Cossu G, Martellotta CM, Fratta W. Intravenous self-administration of the cannabinoid CB1 receptor agonist WIN 55,212-2 in rats. Psychopharmacology (Berl) 2001;156:410–416. doi: 10.1007/s002130100734. [DOI] [PubMed] [Google Scholar]
- Finlay JM, Damsma G, Fibiger HC. Benzodiazepine-induced decreases in extracellular concentrations of dopamine in the nucleus accumbens after acute and repeated administration. Psychopharmacology. 1992;106:202–208. doi: 10.1007/BF02801973. [DOI] [PubMed] [Google Scholar]
- Fokos S, Panagis G. Effects of delta9-tetrahydrocannabinol on reward and anxiety in rats exposed to chronic unpredictable stress. Journal of psychopharmacology. 2010;24:767–777. doi: 10.1177/0269881109104904. [DOI] [PubMed] [Google Scholar]
- French delta9-Tetrahydrocannabinol excites rat VTA dopamine neurons through activation of cannabinoid CB1 but not opioid receptors. Neurosci Lett. 1997;226:159–162. doi: 10.1016/s0304-3940(97)00278-4. [DOI] [PubMed] [Google Scholar]
- Gallo A, Bouchard C, Fortier E, Ducrot C, Rompre PP. Cannabinoids reward sensitivity in a neurodevelopmental animal model of schizophrenia: a brain stimulation reward study. Eur Neuropsychopharmacol. 2014;24:1534–1545. doi: 10.1016/j.euroneuro.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of Hashish. Journal of American Chemistry Society. 1964;86:1646–1647. [Google Scholar]
- Gardner EL, Parades W, Smith D, Zukin RS. Facilitation of brain stimulation reward by delta-9-tetrahydrocannabinol is mediated by an endogenous opioid mechanism. Advances in Biosciences. 1989;75:671–674. [Google Scholar]
- Gardner EL, Paredes W, Smith D, Donner A, Milling C, Cohen D, Morrison D. Facilitation of brain stimulation reward by delta 9-tetrahydrocannabinol. Psychopharmacology. 1988;96:142–144. doi: 10.1007/BF02431546. [DOI] [PubMed] [Google Scholar]
- Gardner EL, Vorel SR. Cannabinoid transmission and reward-related events. Neurobiol Dis. 1998;5:502–533. doi: 10.1006/nbdi.1998.0219. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Forster MJ. Delta9-Tetrahydrocannabinol-like discriminative stimulus effects of compounds commonly found in K2/Spice. Behavioural pharmacology. 2014;25:750–757. doi: 10.1097/FBP.0000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerak LR, Zanettini C, Koek W, France CP. Cross-tolerance to cannabinoids in morphine-tolerant rhesus monkeys. Psychopharmacology. 2015;232:3637–3647. doi: 10.1007/s00213-015-4023-x. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Casu MA, Carta G, Mascia MS. Cannabinoids decrease acetylcholine release in the medial-prefrontal cortex and hippocampus, reversal by SR 141716A. European journal of pharmacology. 1998;355:119–124. doi: 10.1016/s0014-2999(98)00486-5. [DOI] [PubMed] [Google Scholar]
- Ghozland S, Matthes HW, Simonin F, Filliol D, Kieffer BL, Maldonado R. Motivational effects of cannabinoids are mediated by mu-opioid and kappa-opioid receptors. J Neurosci. 2002;22:1146–1154. doi: 10.1523/JNEUROSCI.22-03-01146.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie NA, Neale MC, Jacobson K, Kendler KS. Modeling the genetic and environmental association between peer group deviance and cannabis use in male twins. Addiction. 2009;104:420–429. doi: 10.1111/j.1360-0443.2008.02457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, Tontini A, Tarzia G, Mor M, Trezza V, Goldberg SR, Cuomo V, Piomelli D. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci U S A. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SR. Comparable behavior maintained under fixed-ratio and second-order schedules of food presentation, cocaine injection or d-amphetamine injection in the squirrel monkey. The Journal of pharmacology and experimental therapeutics. 1973;186:18–30. [PubMed] [Google Scholar]
- Goldberg SR, Henningfield JE. Reinforcing effects of nicotine in humans and experimental animals responding under intermittent schedules of i.v. drug injection. Pharmacology, biochemistry, and behavior. 1988;30:227–234. doi: 10.1016/0091-3057(88)90450-9. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Morse WH, Goldberg DM. Behavior maintained under a second-order schedule by intramuscular injection of morphine or cocaine in rhesus monkeys. The Journal of pharmacology and experimental therapeutics. 1976;199:278–286. [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD, Goldberg DM. Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Science. 1981;214:573–575. doi: 10.1126/science.7291998. [DOI] [PubMed] [Google Scholar]
- Haney M. Opioid antagonism of cannabinoid effects: differences between marijuana smokers and nonmarijuana smokers. Neuropsychopharmacology. 2007;32:1391–1403. doi: 10.1038/sj.npp.1301243. [DOI] [PubMed] [Google Scholar]
- Haney M, Bisaga A, Foltin RW. Interaction between naltrexone and oral THC in heavy marijuana smokers. Psychopharmacology (Berl) 2003;166:77–85. doi: 10.1007/s00213-002-1279-8. [DOI] [PubMed] [Google Scholar]
- Harris RT, Waters W, McLendon D. Evaluation of reinforcing capability of delta-9-tetrahydrocannabinol in rhesus monkeys. Psychopharmacologia. 1974;37:23–29. doi: 10.1007/BF00426679. [DOI] [PubMed] [Google Scholar]
- Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Goldberg SR. Nicotine as a reinforcer in human subjects and laboratory animals. Pharmacology, biochemistry, and behavior. 1983;19:989–992. doi: 10.1016/0091-3057(83)90405-7. [DOI] [PubMed] [Google Scholar]
- Hiltunen AJ, Jarbe TU. Cannabidiol attenuates delta 9-tetrahydrocannabinol-like discriminative stimulus effects of cannabinol. European journal of pharmacology. 1986a;125:301–304. doi: 10.1016/0014-2999(86)90042-7. [DOI] [PubMed] [Google Scholar]
- Hiltunen AJ, Jarbe TU. Interactions between delta 9-tetrahydrocannabinol and cannabidiol as evaluated by drug discrimination procedures in rats and pigeons. Neuropharmacology. 1986b;25:133–142. doi: 10.1016/0028-3908(86)90034-1. [DOI] [PubMed] [Google Scholar]
- Houser SJ, Eads M, Embrey JP, Welch SP. Dynorphin B and spinal analgesia: induction of antinociception by the cannabinoids CP55,940, Delta(9)-THC and anandamide. Brain research. 2000;857:337–342. doi: 10.1016/s0006-8993(00)01981-8. [DOI] [PubMed] [Google Scholar]
- Hruba L, Ginsburg BC, McMahon LR. Apparent inverse relationship between cannabinoid agonist efficacy and tolerance/cross-tolerance produced by Delta(9)-tetrahydrocannabinol treatment in rhesus monkeys. The Journal of pharmacology and experimental therapeutics. 2012;342:843–849. doi: 10.1124/jpet.112.196444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruba L, Seillier A, Zaki A, Cravatt BF, Lichtman AH, Giuffrida A, McMahon LR. Simultaneous inhibition of fatty acid amide hydrolase and monoacylglycerol lipase shares discriminative stimulus effects with Delta9-tetrahydrocannabinol in mice. The Journal of pharmacology and experimental therapeutics. 2015;353:261–268. doi: 10.1124/jpet.115.222836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarbe TU. Discriminative stimulus properties of d-amphetamine in pigeons. Pharmacology, biochemistry, and behavior. 1982;17:671–675. doi: 10.1016/0091-3057(82)90343-4. [DOI] [PubMed] [Google Scholar]
- Jarbe TU. Discriminative stimulus properties of cocaine. Effects of apomorphine, haloperidol, procaine and other drugs. Neuropharmacology. 1984;23:899–907. doi: 10.1016/0028-3908(84)90003-0. [DOI] [PubMed] [Google Scholar]
- Jarbe TU, Henriksson BG. Discriminative response control produced with hashish, tetrahydrocannabinols (delta 8-THC and delta 9-THC), and other drugs. Psychopharmacologia. 1974;40:1–16. doi: 10.1007/BF00429443. [DOI] [PubMed] [Google Scholar]
- Jarbe TU, Henriksson BG, Ohlin GC. Delta9-THC as a discriminative cue in pigeons: effects of delta8-THC, CBD, and CBN. Arch Int Pharmacodyn Ther. 1977;228:68–72. [PubMed] [Google Scholar]
- Jarbe TU, Hiltunen AJ, Lander N, Mechoulam R. Cannabimimetic activity (delta 1-THC cue) of cannabidiol monomethyl ether and two stereoisomeric hexahydrocannabinols in rats and pigeons. Pharmacology, biochemistry, and behavior. 1986;25:393–399. doi: 10.1016/0091-3057(86)90015-8. [DOI] [PubMed] [Google Scholar]
- Jarbe TU, Hiltunen AJ, Mathis DA, Hanus L, Breuer A, Mechoulam R. Discriminative stimulus effects and receptor binding of enantiomeric pairs of cannabinoids in rats and pigeons; a comparison. The Journal of pharmacology and experimental therapeutics. 1993;264:561–569. [PubMed] [Google Scholar]
- Jarbe TU, Hiltunen AJ, Mechoulam R. Stereospecificity of the discriminative stimulus functions of the dimethylheptyl homologs of 11-hydroxy-delta 8-tetrahydrocannabinol in rats and pigeons. The Journal of pharmacology and experimental therapeutics. 1989;250:1000–1005. [PubMed] [Google Scholar]
- Jarbe TU, Lamb RJ, Lin S, Makriyannis A. (R)-methanandamide and Delta 9-THC as discriminative stimuli in rats: tests with the cannabinoid antagonist SR-141716 and the endogenous ligand anandamide. Psychopharmacology (Berl) 2001;156:369–380. doi: 10.1007/s002130100730. [DOI] [PubMed] [Google Scholar]
- Jarbe TU, Lamb RJ, Makriyannis A, Lin S, Goutopoulos A. Delta9-THC training dose as a determinant for (R)-methanandamide generalization in rats. Psychopharmacology (Berl) 1998;140:519–522. doi: 10.1007/s002130050797. [DOI] [PubMed] [Google Scholar]
- Jarbe TU, Li C, Vadivel SK, Makriyannis A. Discriminative stimulus functions of methanandamide and delta(9)-THC in rats: tests with aminoalkylindoles (WIN55,212-2 and AM678) and ethanol. Psychopharmacology. 2010;208:87–98. doi: 10.1007/s00213-009-1708-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarbe TU, Mechoulam R, Zahalka J. Discriminative stimulus- and open-field effects of the enantiomers of 11-hydroxy-delta-8-tetrahydrocannabinol in pigeons and gerbils. Pharmacology, biochemistry, and behavior. 1994;47:113–119. doi: 10.1016/0091-3057(94)90119-8. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Goldberg SR, Heishman SJ, Tanda G. Self-administration of cannabinoids by experimental animals and human marijuana smokers. Pharmacol Biochem Behav. 2005a;81:285–299. doi: 10.1016/j.pbb.2005.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Mascia P, Wu HQ, Secci ME, Redhi GH, Panlilio LV, Scherma M, Barnes C, Parashos A, Zara T, Fratta W, Solinas M, Pistis M, Bergman J, Kangas BD, Ferre S, Tanda G, Schwarcz R, Goldberg SR. Reducing cannabinoid abuse and preventing relapse by enhancing endogenous brain levels of kynurenic acid. Nature neuroscience. 2013;16:1652–1661. doi: 10.1038/nn.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Munzar P, Panlilio LV, Yasar S, Redhi GH, Tanda G, Goldberg SR. Blockade of THC-seeking behavior and relapse in monkeys by the cannabinoid CB(1)-receptor antagonist rimonabant. Neuropsychopharmacology. 2008;33:2870–2877. doi: 10.1038/npp.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Solinas M, Tanda G, Redhi GH, Goldberg SR. The endogenous cannabinoid anandamide and its synthetic analog R(+)-methanandamide are intravenously self-administered by squirrel monkeys. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005b;25:5645–5650. doi: 10.1523/JNEUROSCI.0951-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Munzar P, Goldberg SR. The opioid antagonist naltrexone reduces the reinforcing effects of Delta 9 tetrahydrocannabinol (THC) in squirrel monkeys. Psychopharmacology (Berl) 2004;173:186–194. doi: 10.1007/s00213-003-1693-6. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Redhi GH, Goldberg SR. Self-administration of delta9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology (Berl) 2003;169:135–140. doi: 10.1007/s00213-003-1484-0. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Yasar S, Redhi GH, Goldberg SR. The endogenous cannabinoid 2-arachidonoylglycerol is intravenously self-administered by squirrel monkeys. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:7043–7048. doi: 10.1523/JNEUROSCI.6058-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamien JB, Bickel WK, Hughes JR, Higgins ST, Smith BJ. Drug discrimination by humans compared to nonhumans: current status and future directions. Psychopharmacology. 1993;111:259–270. doi: 10.1007/BF02244940. [DOI] [PubMed] [Google Scholar]
- Katsidoni V, Kastellakis A, Panagis G. Biphasic effects of Delta9-tetrahydrocannabinol on brain stimulation reward and motor activity. Int J Neuropsychopharmacol. 2013;16:2273–2284. doi: 10.1017/S1461145713000709. [DOI] [PubMed] [Google Scholar]
- Katz JL, Goldberg SR. Preclinical assessment of abuse liability of drugs. Agents and actions. 1988;23:18–26. doi: 10.1007/BF01967174. [DOI] [PubMed] [Google Scholar]
- Kaymakcalan S. Tolerance to and dependence on cannabis. Bull Narc. 1973;25:39–47. [Google Scholar]
- Kaymakçalan S. Physiological and psychological dependence on THC in rhesus monkeys. In: Paton WDM, Crown J, editors. Cannabis and its derivatives. London: Oxford University Press; 1972. pp. 142–146. [Google Scholar]
- Kelleher RT. Conditioned reinforcement in second-order schedules. Journal of the experimental analysis of behavior. 1966;9:475–485. doi: 10.1901/jeab.1966.9-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Schmitt E, Aggen SH, Prescott CA. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Archives of general psychiatry. 2008;65:674–682. doi: 10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick DR, McEntire DM, Hambsch ZJ, Kerfeld MJ, Smith TA, Reisbig MD, Youngblood CF, Agrawal DK. Therapeutic Basis of Clinical Pain Modulation. Clin Transl Sci. 2015 doi: 10.1111/cts.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. The role of the striatopallidal and extended amygdala systems in drug addiction. Annals of the New York Academy of Sciences. 1999;877:445–460. doi: 10.1111/j.1749-6632.1999.tb09282.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neurobiology of addiction. Toward the development of new therapies. Ann N Y Acad Sci. 2000;909:170–185. doi: 10.1111/j.1749-6632.2000.tb06682.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- Kornetsky C. Brain-stimulation reward: a model for the neuronal bases for drug-induced euphoria. NIDA research monograph. 1985;62:30–50. [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU. Euphorigenic drugs: effects on the reward pathways of the brain. Fed Proc. 1979;38:2473–2476. [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU, McLean S, Jacobson JO. Intracranial self-stimulation thresholds: a model for the hedonic effects of drugs of abuse. Archives of general psychiatry. 1979;36:289–292. doi: 10.1001/archpsyc.1979.01780030055004. [DOI] [PubMed] [Google Scholar]
- Landsman RS, Burkey TH, Consroe P, Roeske WR, Yamamura HI. SR141716A is an inverse agonist at the human cannabinoid CB1 receptor. Eur J Pharmacol. 1997;334:R1–R2. doi: 10.1016/s0014-2999(97)01160-6. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Di Ciano P, Panlilio LV, Goldberg SR, Ciccocioppo R. Peroxisome proliferator-activated receptor (PPAR) agonists as promising new medications for drug addiction: preclinical evidence. Curr Drug Targets. 2013;14:768–776. doi: 10.2174/1389450111314070006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Wiggins M, Goldberg SR. Nicotine pre-exposure does not potentiate the locomotor or rewarding effects of Delta-9-tetrahydrocannabinol in rats. Behavioural pharmacology. 2006;17:195–199. doi: 10.1097/01.fbp.0000197460.16516.81. [DOI] [PubMed] [Google Scholar]
- Lecca D, Cacciapaglia F, Valentini V, Di Chiara G. Monitoring extracellular dopamine in the rat nucleus accumbens shell and core during acquisition and maintenance of intravenous WIN 55,212-2 self-administration. Psychopharmacology (Berl) 2006;188:63–74. doi: 10.1007/s00213-006-0475-3. [DOI] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Bohme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Leite JR, Carlini EA. Failure to obtain "cannabis-directed behavior" and abstinence syndrome in rats chronically treated with cannabis sativa extracts. Psychopharmacologia. 1974;36:133–145. doi: 10.1007/BF00421785. [DOI] [PubMed] [Google Scholar]
- Lepore M, Liu X, Savage V, Matalon D, Gardner EL. Genetic differences in delta 9-tetrahydrocannabinol-induced facilitation of brain stimulation reward as measured by a rate-frequency curve-shift electrical brain stimulation paradigm in three different rat strains. Life sciences. 1996;58:PL365–PL372. doi: 10.1016/0024-3205(96)00237-8. [DOI] [PubMed] [Google Scholar]
- Lepore M, Vorel SR, Lowinson J, Gardner EL. Conditioned place preference induced by delta 9-tetrahydrocannabinol: comparison with cocaine, morphine, and food reward. Life Sci. 1995;56:2073–2080. doi: 10.1016/0024-3205(95)00191-8. [DOI] [PubMed] [Google Scholar]
- Leuschner JT, Wing DR, Harvey DJ, Brent GA, Dempsey CE, Watts A, Paton WD. The partitioning of delta 1-tetrahydrocannabinol into erythrocyte membranes in vivo and its effect on membrane fluidity. Experientia. 1984;40:866–868. doi: 10.1007/BF01951999. [DOI] [PubMed] [Google Scholar]
- Lew EO, Richardson JS. Neurochemical and behavioural correlates of the interaction between amphetamine and delta 9-tetrahydrocannabinol in the rat. Drug and alcohol dependence. 1981;8:93–101. doi: 10.1016/0376-8716(81)90104-6. [DOI] [PubMed] [Google Scholar]
- Li JX, Koek W, France CP. Interactions between Delta(9)-tetrahydrocannabinol and heroin: self-administration in rhesus monkeys. Behavioural pharmacology. 2012;23:754–761. doi: 10.1097/FBP.0b013e32835a3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, McMahon LR, Gerak LR, Becker GL, France CP. Interactions between Delta(9)-tetrahydrocannabinol and mu opioid receptor agonists in rhesus monkeys: discrimination and antinociception. Psychopharmacology. 2008;199:199–208. doi: 10.1007/s00213-008-1157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Yang W, France CP. Interactions between mu-opioid receptor agonists and cannabinoid receptor agonists in rhesus monkeys: antinociception, drug discrimination, and drug self-administration. The Journal of pharmacology and experimental therapeutics. 2013;345:354–362. doi: 10.1124/jpet.113.204099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maione S, Costa B, Di Marzo V. Endocannabinoids: a unique opportunity to develop multitarget analgesics. Pain. 2013;154(Suppl 1):S87–S93. doi: 10.1016/j.pain.2013.03.023. [DOI] [PubMed] [Google Scholar]
- Maldonado R. Study of cannabinoid dependence in animals. Pharmacology & therapeutics. 2002;95:153–164. doi: 10.1016/s0163-7258(02)00254-1. [DOI] [PubMed] [Google Scholar]
- Mallet PE, Beninger RJ. Delta9-tetrahydrocannabinol, but not the endogenous cannabinoid receptor ligand anandamide, produces conditioned place avoidance. Life Sci. 1998;62:2431–2439. doi: 10.1016/s0024-3205(98)00226-4. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Nicholson KL, Martin BR, Balster RL. Failure of Delta(9)-tetrahydrocannabinol and CP 55,940 to maintain intravenous self-administration under a fixed-interval schedule in rhesus monkeys. Behav Pharmacol. 1994;5:219–225. doi: 10.1097/00008877-199404000-00014. [DOI] [PubMed] [Google Scholar]
- Mantilla-Plata B, Harbison RD. Effects of phenobarbital and SKF 525A pretreatment, sex, liver injury, and vehicle on delta9-tetrahydrocannabinol toxicity. Toxicol Appl Pharmacol. 1974;27:123–130. doi: 10.1016/0041-008x(74)90179-3. [DOI] [PubMed] [Google Scholar]
- Mantilla-Plata B, Harbison RD. Distribution studies of (14C)delta-9-tetrahydrocannabinol in mice: effect of vehicle, route of administration, and duration of treatment. Toxicol Appl Pharmacol. 1975;34:292–300. doi: 10.1016/0041-008x(75)90034-4. [DOI] [PubMed] [Google Scholar]
- Martellotta MC, Cossu G, Fattore L, Gessa GL, Fratta W. Self-administration of the cannabinoid receptor agonist WIN 55,212-2 in drug-naive mice. Neuroscience. 1998;85:327–330. doi: 10.1016/s0306-4522(98)00052-9. [DOI] [PubMed] [Google Scholar]
- Martin S, Manzanares J, Corchero J, Garcia-Lecumberri C, Crespo JA, Fuentes JA, Ambrosio E. Differential basal proenkephalin gene expression in dorsal striatum and nucleus accumbens, and vulnerability to morphine self-administration in Fischer 344 and Lewis rats. Brain Res. 1999;821:350–355. doi: 10.1016/s0006-8993(99)01122-1. [DOI] [PubMed] [Google Scholar]
- Mascia P, Pistis M, Justinova Z, Panlilio LV, Luchicchi A, Lecca S, Scherma M, Fratta W, Fadda P, Barnes C, Redhi GH, Yasar S, Le Foll B, Tanda G, Piomelli D, Goldberg SR. Blockade of nicotine reward and reinstatement by activation of alpha-type peroxisome proliferator-activated receptors. Biological psychiatry. 2011;69:633–641. doi: 10.1016/j.biopsych.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Mavrikaki M, Markaki E, Nomikos GG, Panagis G. Chronic WIN55,212-2 elicits sustained and conditioned increases in intracranial self-stimulation thresholds in the rat. Behavioural brain research. 2010;209:114–118. doi: 10.1016/j.bbr.2010.01.024. [DOI] [PubMed] [Google Scholar]
- Mazzola C, Medalie J, Scherma M, Panlilio LV, Solinas M, Tanda G, Drago F, Cadet JL, Goldberg SR, Yasar S. Fatty acid amide hydrolase (FAAH) inhibition enhances memory acquisition through activation of PPAR-alpha nuclear receptors. Learning & memory. 2009;16:332–337. doi: 10.1101/lm.1145209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor IS, Issakidis CN, Prior G. Aversive effects of the synthetic cannabinoid CP 55,940 in rats. Pharmacol Biochem Behav. 1996;53:657–664. doi: 10.1016/0091-3057(95)02066-7. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Ginsburg BC, Lamb RJ. Cannabinoid agonists differentially substitute for the discriminative stimulus effects of Delta(9)-tetrahydrocannabinol in C57BL/6J mice. Psychopharmacology. 2008;198:487–495. doi: 10.1007/s00213-007-0900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Lander N, Srebnik M, Breuer A, Segal M, Feigenbaum JJ, Jarbe TU, Consroe P. Stereochemical requirements for cannabimimetic activity. NIDA research monograph. 1987;79:15–30. [PubMed] [Google Scholar]
- Melis M, Gessa GL, Diana M. Different mechanisms for dopaminergic excitation induced by opiates and cannabinoids in the rat midbrain. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24:993–1006. doi: 10.1016/s0278-5846(00)00119-6. [DOI] [PubMed] [Google Scholar]
- Micale V, Di Marzo V, Sulcova A, Wotjak CT, Drago F. Endocannabinoid system and mood disorders: priming a target for new therapies. Pharmacology & therapeutics. 2013;138:18–37. doi: 10.1016/j.pharmthera.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Mishima K, Egashira N, Matsumoto Y, Iwasaki K, Fujiwara M. Involvement of reduced acetylcholine release in Delta9-tetrahydrocannabinol-induced impairment of spatial memory in the 8-arm radial maze. Life sciences. 2002;72:397–407. doi: 10.1016/s0024-3205(02)02274-9. [DOI] [PubMed] [Google Scholar]
- Mokler DJ, Nelson BD, Harris LS, Rosecrans JA. The role of benzodiazepine receptors in the discriminative stimulus properties of delta-9-tetrahydrocannabinol. Life sciences. 1986;38:1581–1589. doi: 10.1016/0024-3205(86)90497-2. [DOI] [PubMed] [Google Scholar]
- Navarro M, Fernandez-Ruiz JJ, de Miguel R, Hernandez ML, Cebeira M, Ramos JA. An acute dose of delta 9-tetrahydrocannabinol affects behavioral and neurochemical indices of mesolimbic dopaminergic activity. Behavioural brain research. 1993;57:37–46. doi: 10.1016/0166-4328(93)90059-y. [DOI] [PubMed] [Google Scholar]
- Ng Cheong Ton JM, Gerhardt GA, Friedemann M, Etgen AM, Rose GM, Sharpless NS, Gardner EL. The effects of delta 9-tetrahydrocannabinol on potassium-evoked release of dopamine in the rat caudate nucleus: an in vivo electrochemical and in vivo microdialysis study. Brain research. 1988;451:59–68. doi: 10.1016/0006-8993(88)90749-4. [DOI] [PubMed] [Google Scholar]
- Ohlsson A, Lindgren JE, Wahlen A, Agurell S, Hollister LE, Gillespie HK. Plasma delta-9 tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clinical pharmacology and therapeutics. 1980;28:409–416. doi: 10.1038/clpt.1980.181. [DOI] [PubMed] [Google Scholar]
- Ohlsson A, Lindgren JE, Wahlen A, Agurell S, Hollister LE, Gillespie HK. Plasma levels of delta 9-tetrahydrocannabinol after intravenous, oral, and smoke administration. NIDA research monograph. 1981;34:250–256. [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Olds ME, Fobes JL. The central basis of motivation: intracranial self-stimulation studies. Annu Rev Psychol. 1981;32:523–574. doi: 10.1146/annurev.ps.32.020181.002515. [DOI] [PubMed] [Google Scholar]
- Olsen JL, Makhani M, Davis KH, Wall ME. Preparation of 9-tetrahydrocannabinol for intravenous injection. J Pharm Pharmacol. 1973;25:344. doi: 10.1111/j.2042-7158.1973.tb10023.x. [DOI] [PubMed] [Google Scholar]
- Parker LA, Gillies T. THC-induced place and taste aversions in Lewis and Sprague-Dawley rats. Behav Neurosci. 1995;109:71–78. doi: 10.1037//0735-7044.109.1.71. [DOI] [PubMed] [Google Scholar]
- Perio A, Rinaldi-Carmona M, Maruani J, Barth F, Le Fur G, Soubrie P. Central mediation of the cannabinoid cue: activity of a selective CB1 antagonist, SR 141716A. Behav Pharmacol. 1996;7:65–71. [PubMed] [Google Scholar]
- Pertwee RG. Ligands that target cannabinoid receptors in the brain: from THC to anandamide and beyond. Addiction biology. 2008;13:147–159. doi: 10.1111/j.1369-1600.2008.00108.x. [DOI] [PubMed] [Google Scholar]
- Petrosino S, Di Marzo V. FAAH and MAGL inhibitors: therapeutic opportunities from regulating endocannabinoid levels. Current opinion in investigational drugs. 2010;11:51–62. [PubMed] [Google Scholar]
- Pickens R, Thompson T, Muchow DC. Cannabis and phencyclidine self-administered by animals. In: Goldfarb L, Hoffmeister F, editors. Psychic Dependence [Bayer-Symposium IV] Berlin: Springer-Verlag; 1973. pp. 78–86. [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Pisanu A, Acquas E, Fenu S, Di Chiara G. Modulation of Delta(9)-THC-induced increase of cortical and hippocampal acetylcholine release by micro opioid and D(1) dopamine receptors. Neuropharmacology. 2006;50:661–670. doi: 10.1016/j.neuropharm.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the "shell" as compared with the "core" of the rat nucleus accumbens. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Rodriguez De Fonseca F, Fernandez-Ruiz JJ, Murphy LL, Cebeira M, Steger RW, Bartke A, Ramos JA. Acute effects of delta-9-tetrahydrocannabinol on dopaminergic activity in several rat brain areas. Pharmacology, biochemistry, and behavior. 1992;42:269–275. doi: 10.1016/0091-3057(92)90526-l. [DOI] [PubMed] [Google Scholar]
- Sakurai-Yamashita Y, Kataoka Y, Fujiwara M, Mine K, Ueki S. Delta 9-tetrahydrocannabinol facilitates striatal dopaminergic transmission. Pharmacology, biochemistry, and behavior. 1989;33:397–400. doi: 10.1016/0091-3057(89)90521-2. [DOI] [PubMed] [Google Scholar]
- Sanudo-Pena MC, Tsou K, Delay ER, Hohman AG, Force M, Walker JM. Endogenous cannabinoids as an aversive or counter-rewarding system in the rat. Neurosci Lett. 1997;223:125–128. doi: 10.1016/s0304-3940(97)13424-3. [DOI] [PubMed] [Google Scholar]
- Scherma M, Fadda P, Le Foll B, Forget B, Fratta W, Goldberg SR, Tanda G. The endocannabinoid system: a new molecular target for the treatment of tobacco addiction. CNS & neurological disorders drug targets. 2008a;7:468–481. doi: 10.2174/187152708786927859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherma M, Medalie J, Fratta W, Vadivel SK, Makriyannis A, Piomelli D, Mikics E, Haller J, Yasar S, Tanda G, Goldberg SR. The endogenous cannabinoid anandamide has effects on motivation and anxiety that are revealed by fatty acid amide hydrolase (FAAH) inhibition. Neuropharmacology. 2008b;54:129–140. doi: 10.1016/j.neuropharm.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster CR, Johanson CE. Relationship between the discriminative stimulus properties and subjective effects of drugs. Psychopharmacol Ser. 1988;4:161–175. doi: 10.1007/978-3-642-73223-2_13. [DOI] [PubMed] [Google Scholar]
- Solinas M, Justinova Z, Goldberg SR, Tanda G. Anandamide administration alone and after inhibition of fatty acid amide hydrolase (FAAH) increases dopamine levels in the nucleus accumbens shell in rats. Journal of neurochemistry. 2006;98:408–419. doi: 10.1111/j.1471-4159.2006.03880.x. [DOI] [PubMed] [Google Scholar]
- Solinas M, Scherma M, Fattore L, Stroik J, Wertheim C, Tanda G, Fratta W, Goldberg SR. Nicotinic alpha 7 receptors as a new target for treatment of cannabis abuse. J Neurosci. 2007a;27:5615–5620. doi: 10.1523/JNEUROSCI.0027-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Tanda G, Justinova Z, Wertheim CE, Yasar S, Piomelli D, Vadivel SK, Makriyannis A, Goldberg SR. The endogenous cannabinoid anandamide produces delta-9-tetrahydrocannabinol-like discriminative and neurochemical effects that are enhanced by inhibition of fatty acid amide hydrolase but not by inhibition of anandamide transport. The Journal of pharmacology and experimental therapeutics. 2007b;321:370–380. doi: 10.1124/jpet.106.114124. [DOI] [PubMed] [Google Scholar]
- Solinas M, Tanda G, Wertheim CE, Goldberg SR. Dopaminergic augmentation of delta-9-tetrahydrocannabinol (THC) discrimination: possible involvement of D(2)-induced formation of anandamide. Psychopharmacology. 2010;209:191–202. doi: 10.1007/s00213-010-1789-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Zangen A, Thiriet N, Goldberg SR. Beta-endorphin elevations in the ventral tegmental area regulate the discriminative effects of Delta-9-tetrahydrocannabinol. Eur J Neurosci. 2004;19:3183–3192. doi: 10.1111/j.0953-816X.2004.03420.x. [DOI] [PubMed] [Google Scholar]
- Spealman RD, Goldberg SR. Drug self-administration by laboratory animals: control by schedules of reinforcement. Annu Rev Pharmacol Toxicol. 1978;18:313–339. doi: 10.1146/annurev.pa.18.040178.001525. [DOI] [PubMed] [Google Scholar]
- Stark P, Dews PB. Cannabinoids. I. Behavioral effects. The Journal of pharmacology and experimental therapeutics. 1980;214:124–130. [PubMed] [Google Scholar]
- Stewart JL, McMahon LR. The fatty acid amide hydrolase inhibitor URB 597: interactions with anandamide in rhesus monkeys. British journal of pharmacology. 2011;164:655–666. doi: 10.1111/j.1476-5381.2011.01388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology. 1995;117:2–10. doi: 10.1007/BF02245088. discussion 14–20. [DOI] [PubMed] [Google Scholar]
- Takahashi RN, Singer G. Self-administration of delta 9-tetrahydrocannabinol by rats. Pharmacol Biochem Behav. 1979;11:737–740. doi: 10.1016/0091-3057(79)90274-0. [DOI] [PubMed] [Google Scholar]
- Takahashi RN, Singer G. Effects of body weight levels on cannabis self-injection. Pharmacol Biochem Behav. 1980;13:877–881. doi: 10.1016/0091-3057(80)90222-1. [DOI] [PubMed] [Google Scholar]
- Takahashi RN, Singer G. Cross self-administration of delta 9-tetrahydrocannabinol and D-amphetamine in rats. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica [et al. 1981;14:395–400. [PubMed] [Google Scholar]
- Tanda G, Bassareo V, Di Chiara G. Mianserin markedly and selectively increases extracellular dopamine in the prefrontal cortex as compared to the nucleus accumbens of the rat. Psychopharmacology. 1996;123:127–130. doi: 10.1007/BF02246169. [DOI] [PubMed] [Google Scholar]
- Tanda G, Carboni E, Frau R, Di Chiara G. Increase of extracellular dopamine in the prefrontal cortex: a trait of drugs with antidepressant potential? Psychopharmacology. 1994;115:285–288. doi: 10.1007/BF02244785. [DOI] [PubMed] [Google Scholar]
- Tanda G, Goldberg SR. Cannabinoids: reward, dependence, and underlying neurochemical mechanisms--a review of recent preclinical data. Psychopharmacology (Berl) 2003;169:115–134. doi: 10.1007/s00213-003-1485-z. [DOI] [PubMed] [Google Scholar]
- Tanda G, Kopajtic TA, Katz JL. Cocaine-like neurochemical effects of antihistaminic medications. Journal of neurochemistry. 2008;106:147–157. doi: 10.1111/j.1471-4159.2008.05361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Li SM, Mereu M, Thomas AM, Ebbs AL, Chun LE, Tronci V, Green JL, Zou MF, Kopajtic TA, Newman AH, Katz JL. Relations between stimulation of mesolimbic dopamine and place conditioning in rats produced by cocaine or drugs that are tolerant to dopamine transporter conformational change. Psychopharmacology. 2013;229:307–321. doi: 10.1007/s00213-013-3109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Munzar P, Goldberg SR. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nature neuroscience. 2000;3:1073–1074. doi: 10.1038/80577. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- Tanda G, Valentini V, De Luca MA, Perra V, Serra GP, Di Chiara G. A systematic microdialysis study of dopamine transmission in the accumbens shell/core and prefrontal cortex after acute antipsychotics. Psychopharmacology. 2015;232:1427–1440. doi: 10.1007/s00213-014-3780-2. [DOI] [PubMed] [Google Scholar]
- Toth A, Blumberg PM, Boczan J. Anandamide and the vanilloid receptor (TRPV1) Vitam Horm. 2009;81:389–419. doi: 10.1016/S0083-6729(09)81015-7. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Progress in neurobiology. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addiction biology. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Maldonado R. A behavioural model to reveal place preference to delta 9-tetrahydrocannabinol in mice. Psychopharmacology (Berl) 2000;147:436–438. doi: 10.1007/s002130050013. [DOI] [PubMed] [Google Scholar]
- van Ree JM, Slangen JL, de Wied D. Intravenous self-administration of drugs in rats. The Journal of pharmacology and experimental therapeutics. 1978;204:547–557. [PubMed] [Google Scholar]
- Vandrey R, Haney M. Pharmacotherapy for cannabis dependence: how close are we? CNS drugs. 2009;23:543–553. doi: 10.2165/00023210-200923070-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri VK, Makriyannis A. Medicinal chemistry of cannabinoids. Clinical pharmacology and therapeutics. 2015;97:553–558. doi: 10.1002/cpt.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachou S, Nomikos GG, Panagis G. WIN 55,212-2 decreases the reinforcing actions of cocaine through CB1 cannabinoid receptor stimulation. Behavioural brain research. 2003;141:215–222. doi: 10.1016/s0166-4328(02)00370-4. [DOI] [PubMed] [Google Scholar]
- Vlachou S, Nomikos GG, Panagis G. CB1 cannabinoid receptor agonists increase intracranial self-stimulation thresholds in the rat. Psychopharmacology. 2005;179:498–508. doi: 10.1007/s00213-004-2050-0. [DOI] [PubMed] [Google Scholar]
- Vlachou S, Nomikos GG, Panagis G. Effects of endocannabinoid neurotransmission modulators on brain stimulation reward. Psychopharmacology. 2006;188:293–305. doi: 10.1007/s00213-006-0506-0. [DOI] [PubMed] [Google Scholar]
- Vlachou S, Nomikos GG, Stephens DN, Panagis G. Lack of evidence for appetitive effects of Delta 9-tetrahydrocannabinol in the intracranial self-stimulation and conditioned place preference procedures in rodents. Behav Pharmacol. 2007;18:311–319. doi: 10.1097/FBP.0b013e3282186cf2. [DOI] [PubMed] [Google Scholar]
- Wiebelhaus JM, Grim TW, Owens RA, Lazenka MF, Sim-Selley LJ, Abdullah RA, Niphakis MJ, Vann RE, Cravatt BF, Wiley JL, Negus SS, Lichtman AH. Delta9-tetrahydrocannabinol and endocannabinoid degradative enzyme inhibitors attenuate intracranial self-stimulation in mice. The Journal of pharmacology and experimental therapeutics. 2015;352:195–207. doi: 10.1124/jpet.114.218677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley J, Balster R, Martin B. Discriminative stimulus effects of anandamide in rats. Eur J Pharmacol. 1995a;276:49–54. doi: 10.1016/0014-2999(95)00010-i. [DOI] [PubMed] [Google Scholar]
- Wiley JL. Cannabis: discrimination of "internal bliss"? Pharmacology, biochemistry, and behavior. 1999;64:257–260. doi: 10.1016/s0091-3057(99)00059-3. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Barrett RL, Britt DT, Balster RL, Martin BR. Discriminative stimulus effects of delta 9-tetrahydrocannabinol and delta 9-11-tetrahydrocannabinol in rats and rhesus monkeys. Neuropharmacology. 1993;32:359–365. doi: 10.1016/0028-3908(93)90157-x. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Golden KM, Ryan WJ, Balster RL, Razdan RK, Martin BR. Evaluation of cannabimimetic discriminative stimulus effects of anandamide and methylated fluoroanandamide in rhesus monkeys. Pharmacol Biochem Behav. 1997;58:1139–1143. doi: 10.1016/s0091-3057(97)00327-4. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Huffman JW, Balster RL, Martin BR. Pharmacological specificity of the discriminative stimulus effects of delta 9-tetrahydrocannabinol in rhesus monkeys. Drug Alcohol Depend. 1995b;40:81–86. doi: 10.1016/0376-8716(95)01193-5. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Lowe JA, Balster RL, Martin BR. Antagonism of the discriminative stimulus effects of delta 9-tetrahydrocannabinol in rats and rhesus monkeys. J Pharmacol Exp Ther. 1995c;275:1–6. [PubMed] [Google Scholar]
- Wise RA. Neurobiology of addiction. Current opinion in neurobiology. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Heimer L. Two transpallidal pathways originating in the rat nucleus accumbens. J Comp Neurol. 1990;302:437–446. doi: 10.1002/cne.903020302. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Heimer L. Specificity in the efferent projections of the nucleus accumbens in the rat: comparison of the rostral pole projection patterns with those of the core and shell. J Comp Neurol. 1993;327:220–232. doi: 10.1002/cne.903270205. [DOI] [PubMed] [Google Scholar]
- Zangen A, Solinas M, Ikemoto S, Goldberg SR, Wise RA. Two brain sites for cannabinoid reward. J Neurosci. 2006;26:4901–4907. doi: 10.1523/JNEUROSCI.3554-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer A, Valjent E, Konig M, Zimmer AM, Robledo P, Hahn H, Valverde O, Maldonado R. Absence of delta −9-tetrahydrocannabinol dysphoric effects in dynorphin-deficient mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:9499–9505. doi: 10.1523/JNEUROSCI.21-23-09499.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]