Abstract
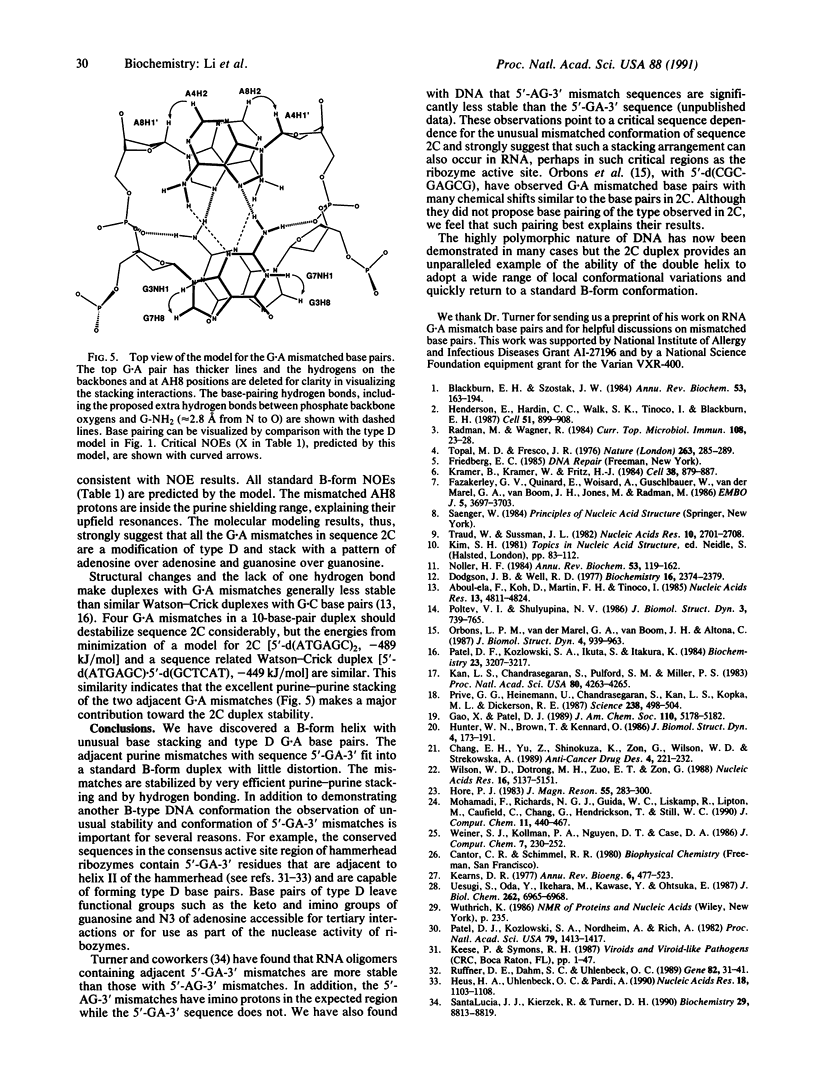
1H NMR experiments indicate that the oligomer 5'-d(ATGAGCGAATA) forms an unusual 10-base-pair duplex with 4 G.A base pairs (underlined) and a 3' unpaired adenosine. NMR results indicate that guanosine imino protons of the G.A mismatches are not hydrogen bonded but are stacked in the helix. A G----I substitution in either G.A base pair causes a dramatic decrease in duplex stability and indicates that hydrogen bonding of the guanosine amino group is critical. Nuclear Overhauser effect spectroscopy (NOESY) and two-dimensional correlated spectroscopy (COSY) results indicate that the overall duplex conformation is in the B-family. Cross-strand NOEs in two-dimensional NOESY spectra between a mismatched AH2 and an AH1' of the other mismatched base pair and between a mismatched GH8 and GNH1 of the other mismatch establish a purine-purine stacking pattern, adenosine over adenosine and guanosine over guanosine, which strongly stabilizes the duplex. A computer graphics molecular model of the unusual duplex was constructed with G.A base pairs containing A-NH2 to GN3 and G-NH2 to AN7 hydrogen bonds and B-form base pairs on both sides of the G.A pairs [5'-d(ATGAGC)]. The energy-minimized duplex satisfies all experimental constraints from NOESY and COSY results. A hydrogen bond from G-NH2 of the mismatch to a phosphate oxygen is predicted.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboul-ela F., Koh D., Tinoco I., Jr, Martin F. H. Base-base mismatches. Thermodynamics of double helix formation for dCA3XA3G + dCT3YT3G (X, Y = A,C,G,T). Nucleic Acids Res. 1985 Jul 11;13(13):4811–4824. doi: 10.1093/nar/13.13.4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E. H. The molecular structure of centromeres and telomeres. Annu Rev Biochem. 1984;53:163–194. doi: 10.1146/annurev.bi.53.070184.001115. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Yu Z., Shinozuka K., Zon G., Wilson W. D., Strekowska A. Comparative inhibition of ras p21 protein synthesis with phosphorus-modified antisense oligonucleotides. Anticancer Drug Des. 1989 Oct;4(3):221–232. [PubMed] [Google Scholar]
- Dodgson J. B., Wells R. D. Action of single-strand specific nucleases on model DNA heteroduplexes of defined size and sequence. Biochemistry. 1977 May 31;16(11):2374–2379. doi: 10.1021/bi00630a010. [DOI] [PubMed] [Google Scholar]
- Fazakerley G. V., Quignard E., Woisard A., Guschlbauer W., van der Marel G. A., van Boom J. H., Jones M., Radman M. Structures of mismatched base pairs in DNA and their recognition by the Escherichia coli mismatch repair system. EMBO J. 1986 Dec 20;5(13):3697–3703. doi: 10.1002/j.1460-2075.1986.tb04702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson E., Hardin C. C., Walk S. K., Tinoco I., Jr, Blackburn E. H. Telomeric DNA oligonucleotides form novel intramolecular structures containing guanine-guanine base pairs. Cell. 1987 Dec 24;51(6):899–908. doi: 10.1016/0092-8674(87)90577-0. [DOI] [PubMed] [Google Scholar]
- Heus H. A., Uhlenbeck O. C., Pardi A. Sequence-dependent structural variations of hammerhead RNA enzymes. Nucleic Acids Res. 1990 Mar 11;18(5):1103–1108. doi: 10.1093/nar/18.5.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter W. N., Brown T., Kennard O. Structural features and hydration of d(C-G-C-G-A-A-T-T-A-G-C-G); a double helix containing two G.A mispairs. J Biomol Struct Dyn. 1986 Oct;4(2):173–191. doi: 10.1080/07391102.1986.10506338. [DOI] [PubMed] [Google Scholar]
- Kan L. S., Chandrasegaran S., Pulford S. M., Miller P. S. Detection of a guanine X adenine base pair in a decadeoxyribonucleotide by proton magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4263–4265. doi: 10.1073/pnas.80.14.4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns D. R. High-resolution nuclear magnetic resonance studies of double helical polynucleotides. Annu Rev Biophys Bioeng. 1977;6:477–523. doi: 10.1146/annurev.bb.06.060177.002401. [DOI] [PubMed] [Google Scholar]
- Kramer B., Kramer W., Fritz H. J. Different base/base mismatches are corrected with different efficiencies by the methyl-directed DNA mismatch-repair system of E. coli. Cell. 1984 Oct;38(3):879–887. doi: 10.1016/0092-8674(84)90283-6. [DOI] [PubMed] [Google Scholar]
- Noller H. F. Structure of ribosomal RNA. Annu Rev Biochem. 1984;53:119–162. doi: 10.1146/annurev.bi.53.070184.001003. [DOI] [PubMed] [Google Scholar]
- Orbons L. P., van der Marel G. A., van Boom J. H., Altona C. An NMR study of the polymorphous behavior of the mismatched DNA octamer d(m5C-G-m5C-G-T-G-m5C-G) in solution. The B, Z, and hairpin forms. J Biomol Struct Dyn. 1987 Jun;4(6):939–963. doi: 10.1080/07391102.1987.10507691. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Kozlowski S. A., Ikuta S., Itakura K. Deoxyguanosine-deoxyadenosine pairing in the d(C-G-A-G-A-A-T-T-C-G-C-G) duplex: conformation and dynamics at and adjacent to the dG X dA mismatch site. Biochemistry. 1984 Jul 3;23(14):3207–3217. doi: 10.1021/bi00309a015. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Kozlowski S. A., Nordheim A., Rich A. Right-handed and left-handed DNA: studies of B- and Z-DNA by using proton nuclear Overhauser effect and P NMR. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1413–1417. doi: 10.1073/pnas.79.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltev V. I., Shulyupina N. V. Simulation of interactions between nucleic acid bases by refined atom-atom potential functions. J Biomol Struct Dyn. 1986 Feb;3(4):739–765. doi: 10.1080/07391102.1986.10508459. [DOI] [PubMed] [Google Scholar]
- Privé G. G., Heinemann U., Chandrasegaran S., Kan L. S., Kopka M. L., Dickerson R. E. Helix geometry, hydration, and G.A mismatch in a B-DNA decamer. Science. 1987 Oct 23;238(4826):498–504. doi: 10.1126/science.3310237. [DOI] [PubMed] [Google Scholar]
- Radman M., Wagner R. Effects of DNA methylation on mismatch repair, mutagenesis, and recombination in Escherichia coli. Curr Top Microbiol Immunol. 1984;108:23–28. doi: 10.1007/978-3-642-69370-0_3. [DOI] [PubMed] [Google Scholar]
- Ruffner D. E., Dahm S. C., Uhlenbeck O. C. Studies on the hammerhead RNA self-cleaving domain. Gene. 1989 Oct 15;82(1):31–41. doi: 10.1016/0378-1119(89)90027-9. [DOI] [PubMed] [Google Scholar]
- SantaLucia J., Jr, Kierzek R., Turner D. H. Effects of GA mismatches on the structure and thermodynamics of RNA internal loops. Biochemistry. 1990 Sep 18;29(37):8813–8819. doi: 10.1021/bi00489a044. [DOI] [PubMed] [Google Scholar]
- Topal M. D., Fresco J. R. Complementary base pairing and the origin of substitution mutations. Nature. 1976 Sep 23;263(5575):285–289. doi: 10.1038/263285a0. [DOI] [PubMed] [Google Scholar]
- Traub W., Sussman J. L. Adenine-guanine base pairing ribosomal RNA. Nucleic Acids Res. 1982 Apr 24;10(8):2701–2708. doi: 10.1093/nar/10.8.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesugi S., Oda Y., Ikehara M., Kawase Y., Ohtsuka E. Identification of I:A mismatch base-pairing structure in DNA. J Biol Chem. 1987 May 25;262(15):6965–6968. [PubMed] [Google Scholar]
- Wilson W. D., Dotrong M. H., Zuo E. T., Zon G. Unusual duplex formation in purine rich oligodeoxyribonucleotides. Nucleic Acids Res. 1988 Jun 10;16(11):5137–5151. doi: 10.1093/nar/16.11.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]