Abstract
Background:
oral cancer is a result of disordered cellular behavior initiated by various stimuli which is characterized by the alteration of serum glycoproteins consisting of different monosaccharides. One of these is levo-fucose (L-fucose), a methyl pentose. Elevated levels of protein-bound fucose have been reported in various malignancies.
Aim:
The present study attempted to correlate levels of serum L-fucose as a biomarker with the various tumor node metastasis (TNM) stages of oral cancer.
Methodology:
The study was carried out on 90 subjects consisting of 30 healthy controls and 60 histopathologically proven oral squamous cell carcinoma (OSCC) cases. The serum fucose level estimation was done based on the method adopted by Winzler. Statistical analysis included independent sample's t-test, one-way ANOVA test, Karl–Pearson correlation test, and Tukey's HSD post hoc test to evaluate the significance and variability of values between groups.
Results:
Significant elevation in serum fucose levels was noticed among OSCC patients when compared with the controls and a progressive ascent of L-fucose levels were noted as the stage of severity increased. Serum fucose levels were independent of histopathological grading, age, and sex.
Conclusion:
Serum L-fucose levels were increased in OSCC patients, and a positive correlation was observed between serum L-fucose levels and TNM staging of OSCC. Thus, serum L-fucose can be used as an effective diagnostic and prognostic biomarker in OSCC patients.
KEY WORDS: Biomarkers, fucose, neoplasms
During the course of tumor development, a number of quantitative serological changes occur. These substances are called as tumor markers or biomarkers, to early detection and effective management leading to improved prognosis.[1]
Serum fucose levels are raised in different groups of malignancies including oral cancer. In association with clinical diagnostic procedures, serum levo-fucose (L-fucose) levels can be used as an effective biochemical indicator in oral cancer in monitoring recurrences and effectiveness or response to treatment.[2] With paucity of data on this biomarker in the Indian population, this study was done to determine the level of serum L-fucose in oral squamous cell carcinoma (OSCC) patients and correlate the same with the tumor node metastasis (TNM) stage.
Materials and Methods
Ethical approval was obtained from the “Institutional Human Ethics Committee” Ref. No: SMIMS/IHEC/2013/C/35. The study was conducted in the Department of Oral Medicine and Radiology and a Cancer Centre. The total sample consisting of 90 individuals was divided into a study group of 60 individuals and a control group of 30 individuals. The study group had 60 OSCC patients (both male and female) between the ages of 25 and 75 years with histologically proven oral cancer but before onset of any form of treatment. Individuals with other malignancies, liver disease, tuberculosis, diabetes mellitus, cardiovascular diseases, or any other chronic systemic diseases were excluded from the study. A group of 30 healthy volunteers aged 25–75 years without any history of debilitating systemic illness and no history of tobacco or alcohol consumption formed the control.
Details of the study are explained to the volunteers and written informed consent is obtained. Patient history was recorded, and TNM staging of the OSCC was done. Biopsy was taken in all suspected cancer patients for histopathological confirmation and grading. Venous blood was drawn from the antecubital vein and allowed to clot. The serum was separated by centrifugation and stored at 4°C. Standard L-fucose was procured from Megazyme Chemical Company, Ireland. Serum L-fucose level was estimated using clinical chemistry autoanalyzer based on Winzler method.
Statistical analysis
Statistical analysis was carried out using SPSS Inc. Released 2008. SPSS Statistics for Windows, Version 17.0. Chicago: SPSS Inc.. To compare the mean values between two groups (between genders), independent samples t-test was used. To compare the mean values between three or more groups (stages of cancer), one-way ANOVA was used.
Results
A comparison of the overall serum L-fucose levels between the OSCC patients and healthy controls showed the mean value of serum L-fucose levels of OSCC patients to be 10.85 mg/dl and the healthy controls had 3.47 mg/dl, thereby showing a difference of 7.38 mg/dl. Statistical analysis revealed a significant P value (P < 0.001) [Graph 1].
Graph 1.
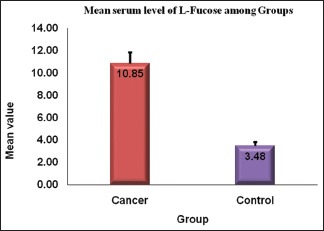
Serum level of levo-fucose between oral cancer and healthy individuals
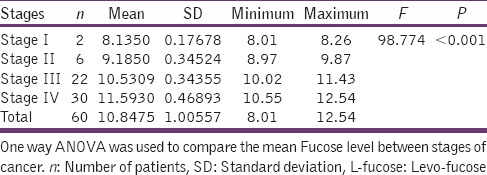
Among the 60 OSCC, 2 belong to Stage I with a mean serum L-fucose level of 8.13 mg/dl. The serum L-fucose level progressively increased with its level being 9.18 mg/dl in Stage II, 10.53 mg/dl in Stage III, and 11.59 mg/dl in Stage IV. Stage IV showed the highest level with a maximum value of 12.54 mg/dl and minimum of 10.55 mg/dl. Statistical analysis shows a significant P value (P < 0.001) [Table 1].
Table 1.
Serum level of L-fucose in various stages of oral cancer
An attempt to correlate the serum L-fucose levels with histopathological grading between genders and ages yielded no significant results thereby inferring that serum L-fucose levels are directly indicative of the degree of tissue destruction.
Discussion
Group of neoplasms affecting any region of the oral cavity is termed as oral cancer, among which OSCC represents >90% of all the neoplasm. Early diagnosis is important for the successful treatment of oral cancer.[3]
Recently, the knowledge about cancer biomarkers has increased, and numerous studies have been carried out regarding these. This has paved the way for improving the management of cancer patients by enhancing the accuracy of detection and efficacy of treatment. Understanding the relevance of biomarkers before using them is very important for diagnosis, treatment, and proper follow-up.[4]
Tumor markers are produced either by the tumor itself, as a tumor by product, by the body in response to the presence of cancer or certain benign (noncancerous) conditions. These can aid in the diagnosis of cancer and in the assessment of tumor burden.[5] One of them is L-fucose, which is a surface tumor marker.[6] It exists in both D forms as well as L form.[7] It is the only sugar which is present in L form. L-fucose is a monosaccharide, which is a common component of many N- and O-linked glycans and glycolipids produced by mammalian cells. Lack of a hydroxyl group on the carbon at the 6-position (C-6) and the L-configuration is the two structural features which help to distinguish fucose from other six-carbon sugars present in mammals.[8]
It is the most essential sugars that the body demands for normal cell-to-cell communication. Physiologically, the concentrations in serum are less but are increased in cancer and other diseases.[6]
Glycosylation is the most prevalent form of posttranslational modification of proteins. It is important in many of the signaling pathways which turns a healthy cell into a dysplastic cell. The protein diversity is achieved by varying sequence and structure of sugar moieties or glycan attachment.[9] Cellular glycosylation changes are associated with various types of neoplastic transformation. Mammalian cells either express or mediate many of their properties through the cell surface.[1] Change in glycosylation of cell surface proteins is essential in tumor progression, especially the terminal epitopes of glycoproteins, which have been proposed to play an essential role in cell–cell interactions, cell adhesion, malignant transformation, and metastasis.[10]
Fucosylation of glycoproteins (the addition of L-fucose at the terminal end of the oligosaccharide chain) is one of the most important features that mediate several specific biologic functions. Tumor cells alter their surface by increasing fucosylation levels to escape recognition; this contributes to numerous abnormal characteristics of tumor cells such as decreased adhesion and uncontrolled tumor growth. Several studies have suggested that estimating the serum/tissue fucose levels could be a promising approach for the early detection, diagnosis, and prognosis of various cancer types.[11]
Group of enzymes catalyze incorporation of fucose from activated nucleotide donor guanosine diphosphate-fucose to the reducing end of complex glycans in a linkage-specific manner. They are known as fucosyltransferases (FucT). These enzymes are expressed in many tissues and are elevated in serum and tumors of cancer patients. It has been mentioned in the article that increased fucosylation is associated with elevated FucT activity.[10]
Cancer cells which are shed or released into circulation from the primary tumor often overexpress fucosylated glycans on their surface.[1] The expression of fucosylated glycoproteins (i.e., fucoproteins) has been detected by means of specific lectins. Several lectin-based studies have indicated that fucoproteins are increased in various cancers. Profound fucosylation of the serum microenvironment may be a reason that interrupts adhesion and induces the formation of metastases (For example), several fucose-containing “natural ligands” reportedly are involved in the migration of tumor cells. Increased expression of fucosylated cell surface antigens, such as Lewis x/y (Lex/y) or sialyl Lex/α, and the upregulation of α1, 3/4-FucT have been associated with malignant transformation and increased metastatic potential of tumors, which leads to poor prognosis of patients with cancer. α-L-fucosidase is a lysosomal enzyme that catalyzes the hydrolytic cleavage of terminal fucose residue that is involved in maintaining the homeostasis of fucose metabolism.[12]
Extensive studies on the nature of metastasis have shown that only a small subpopulation of cells in tumors possess the characteristics necessary for their release from the primary tumor and transport to and establishment of tumor foci in distant organs. Carbohydrate moieties on cell surface glycoconjugates play an important role in this metastatic spread since it could be demonstrated that they are involved in adhesion processes.[10,12]
Several studies have mentioned that estimation of serum L-fucose levels could be a reassuring approach for the early detection, diagnosis, and prognosis of various types of cancer including Oral Carcinoma. Clinically, susceptible lesion can be analyzed with biomarker along with routine tests.[6] Thus, the present study was undertaken to estimate the serum level of L-fucose (tumor marker) among various TNM stages in oral cancer patients and compare them with healthy individuals.
Present study suggested that serum level of L-fucose cannot be correlated with age and gender. This was similar to other studies which were published in the literatures.[13,14]
In our study, out of 60 cancer patients, 31 were well differentiated, 27 - moderately differentiated, and 2 poorly differentiated and anaplastic - nil. However, there was a negative correlation between serum fucose levels and histopathological tumor differentiation in our study as well as in the literatures.[15]
In our study, the mean serum value of L-fucose in cancer group (10.85 mg/dl) and control group (3.47 mg/dl) shows a wide margin of difference (7.38 mg/dl). Moreover, all the 60 patients in the cancer group were categorized according to TNM staging. Comparison of all the four TNM stages in cancer group showed increase in fucose levels as the stages progressed.
Similarly, Wilma Delphine Silvia et al. in 2001 conducted a study on thirty untreated oral cancer patients and thirty healthy control subjects. They assessed the levels of glycoprotein-associated carbohydrates such as hexose; hexosamine, fucose, and sialic acid. They found a significant rise in the level in cases when compared to control subjects. Furthermore, there was a progressive rise in these markers as the stages of oral cancer advances.[16]
In cancer patients, elevated levels have been observed more so in advanced stages and in cases with metastasis than in subjects without metastasis.[8] Serum fucose is considered one of the better biochemical markers in OSCC. Some studies have concluded that it is the most effective of the essential sugars when it comes to slowing the growth of cancer cells.[17] Fall in serum fucose levels after treatment has been reported in many studies although it is emphasized that the follow-up period has to be long enough for significant serological alteration.[18]
Unfortunately, rise in serum fucose level is not specific for cancers, as elevated serum fucose levels have also been reported in other pathological states such as cirrhosis liver, meningitis, rickets, osteomalacia, tuberculosis, cardiovascular disorders as well as in depressive disorders.[19] Furthermore, it has been observed that the serum fucose level is raised in different groups of malignancies such as breast cancer, ovarian cancer, colorectal adenocarcinomas, and leukemia as well as brain tumors.[20] Thus, it becomes important to exclude other degenerative and proliferative diseases, while estimating the serum fucose levels in oral cancer. The size of the lesion and secondary inflammation could alter these levels furthermore.[18]
Conclusion
Literature is replete with studies on fucose levels in various malignancies including a few on oral cancer. Based on the analysis of the results of our study, we can conclude that there was a positive correlation between the serum L-fucose levels and TNM stages of OSCC. Serum L-fucose levels remained constant among healthy individuals and was raised proportionately in oral cancer patients, commensurate with the stage of cancer. In conjunction with clinic-diagnostic procedures, serum L-fucose levels can be used as an easy, noninvasive, cost-effective, biochemical indicator of cancer detection, staging, therapeutic success, prognosis, and as a posttreatment evaluation tool. Further investigation on a large scale would in all probability prove serum L-fucose as an effective tumor marker in the diagnosis and management of oral cancer.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kadam CY, Katkam RV, Suryakar AN, Kumbar KM, Kadam DP. Biochemical markers in oral cancer. Biomed Res. 2011;22:76–80. [Google Scholar]
- 2.Rathan Shetty KS, Kali A. Prognostic significance of serum L-fucose level in head and neck malignancies. Int J Pharm Bio Sci. 2014;5:210–6. [Google Scholar]
- 3.Markopoulos AK. Current aspects on oral squamous cell carcinoma. Open Dent J. 2012;6:126–30. doi: 10.2174/1874210601206010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatt AN, Mathur R, Farooque A, Verma A, Dwarakanath BS. Cancer biomarkers – Current perspectives. Indian J Med Res. 2010;132:129–49. [PubMed] [Google Scholar]
- 5.Neena PD, Shah SA, Patel KB, Jhabuawala Munira F. Histological grading of oral cancer: A comparison of different systems and their relation to lymph node metastasis. Natl J Community Med. 2011;2:136–42. [Google Scholar]
- 6.Miyoshi E, Moriwaki K, Terao N, Tan CC, Terao M, Nakagawa T, et al. Fucosylation is a promising target for cancer diagnosis and therapy. Biomolecules. 2012;2:34–45. doi: 10.3390/biom2010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moldoveanu I, Stefan-van Staden RI, Kapnissi-Cristodoulou CP, van Staden JF, Aboul-Enein HY. Challenges in the enantioanalysis of fucose using stochastic and potentiometric microsensors. Sens Biosensing Res. 2014;1:1–7. [Google Scholar]
- 8.Sawke NG, Sawke GK. Serum fucose level in malignant diseases. Indian J Cancer. 2010;47:452–7. doi: 10.4103/0019-509X.73549. [DOI] [PubMed] [Google Scholar]
- 9.Vajaria BN, Patel KR, Begum R, Shah FD, Patel JB, Shukla SN, et al. Evaluation of serum and salivary total sialic acid and a-l-fucosidase in patients with oral precancerous conditions and oral cancer. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:764–71. doi: 10.1016/j.oooo.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Vanhooren PT, Vandamme EJ. L-fucose: Occurrence, physiological role, chemical, enzymatic and microbial synthesis. J Chem Technol Biotechnol. 1999;74:479–97. [Google Scholar]
- 11.Bose KS, Gokhale PV, Dwivedi S, Singh M. Quantitative evaluation and correlation of serum glycoconjugates: Protein bound hexoses, sialic acid and fucose in leukoplakia, oral sub mucous fibrosis and oral cancer. J Nat Sci Biol Med. 2013;4:122–5. doi: 10.4103/0976-9668.107275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah M, Telang S, Raval G, Shah P, Patel PS. Serum fucosylation changes in oral cancer and oral precancerous conditions: Alpha-L-fucosidase as a marker. Cancer. 2008;113:336–46. doi: 10.1002/cncr.23556. [DOI] [PubMed] [Google Scholar]
- 13.Wilma Delphine Silvia CR, Vasudevan DM, Prabhu KS. Evaluation of serum glycoproteins in oral carcinoma. Indian J Clin Biochem. 2001;16:113–5. doi: 10.1007/BF02867579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parwani RN, Parwani SR. Quantitative evaluation of serum fucose in oral squamous cell carcinoma patients. J Cancer Res Ther. 2011;7:143–7. doi: 10.4103/0973-1482.82928. [DOI] [PubMed] [Google Scholar]
- 15.Shetty RK, Bhandary SK, Kali A. Significance of serum L-fucose glycoprotein as cancer biomarker in head and neck malignancies without distant metastasis. J Clin Diagn Res. 2013;7:2818–20. doi: 10.7860/JCDR/2013/6681.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sultana NS, Ehtaih S, Kaul R, Shastry S, Bhat S. Tumor markers: A short overview. Int J Oral Maxillofac Pathol. 2013;4:7–15. [Google Scholar]
- 17.Elkins Rita MH. Miracle Sugars: The Glyconutrient link to Better Health. Pleasant Grove, Utah, USA: Woodland Publishing; 2003. p. 220. [Google Scholar]
- 18.Mesquita JA, Cavalvanti AL, Weege Nonaka CF, Godoy GP, Alves AP. Clinical and histopathological evidence of oral squamous cell carcinoma in young patients: Systematized review. J Bras Patol Med Lab. 2014;50:67–74. [Google Scholar]
- 19.Sharma NC, Sur BK. Serum fucose and sialic acid in rickets and osteomalacia. Clin Sci. 1969;36:317–21. [PubMed] [Google Scholar]
- 20.Arya DB, Bhatnagar KK. Evaluation of serum fucose level. Indian J Surg. 1974;36:224–8. [Google Scholar]


