Abstract
Objective:
(1) To evaluate the corrosion resistance of four different orthodontic archwires and to determine the effect of 0.5% NaF (simulating high fluoride-containing toothpaste of about 2250 ppm) on corrosion resistance of these archwires. (2) To assess whether surface roughness (Ra) is the primary factor influencing the corrosion resistance of these archwires.
Materials and Methods:
Four different archwires (stainless steel [SS], nickel-titanium [NiTi], titanium molybdenum alloy [TMA], and ion-implanted TMA) were considered for this study. Surface characteristics were analyzed using scanning electron microscopy, atomic force microscopy (AFM), and energy dispersive spectroscopy. Linear polarization test, a fast electrochemical technique, was used to evaluate the corrosion resistance, in terms of polarization resistance of four different archwires in artificial saliva with NaF concentrations of 0% and 0.5%. Statistical analysis was performed by one-way analysis of variance.
Results:
The potentiostatic study reveals that the corrosion resistance of low-friction TMA (L-TMA) > TMA > NiTi > SS. AFM analysis showed the surface Ra of TMA > NiTi > L-TMA > SS. This indicates that the chemical composition of the wire is the primary influential factor to have high corrosion resistance and surface Ra is only secondary. The corrosion resistance of all wires had reduced significantly in 0.5% acidic fluoride-containing artificial saliva due to formation of fluoride complex compound.
Conclusion:
The presence of 0.5% NaF in artificial saliva was detrimental to the corrosion resistance of the orthodontic archwires. Therefore, complete removal of residual high-fluorinated toothpastes from the crevice between archwire and bracket during tooth brushing is mandatory.
KEY WORDS: Corrosion resistance, fluoride, polarization resistance
Orthodontic wires are formed into various configurations or appliances to apply forces to teeth and move them into desirable alignment. Various types of metallic orthodontic wires are used in the treatment of malocclusion based on their properties.
In the oral environment, orthodontic attachments are exposed to a number of potentially damaging physical and chemical agents. Corrosion, the graded degradation of materials by electrochemical attack, is of concern particularly when orthodontic appliances are placed in the hostile electrolytic environment provided by human mouth.[1] Electrolytic or electrochemical corrosion occurs in the oral cavity due, in part, to the wet environment. The surface of certain metals reacts with oxygen to form a surface oxide layer, which inhibits an attacking substance from reaching the metal surface. Corrosion of a metal that is covered by a protective film is dependent upon the properties of the film. Metallic materials are not susceptible to corrosion as long as the surface oxide layer is intact but when the breakdown potential of an alloy is reached, the oxide layer dissolves and the onset of surface corrosion and pitting begins.[2] Pitting corrosion is the most common type of corrosion seen in orthodontic wires and brackets because they are not perfectly smooth. At a microscopic level, they can exhibit many pits. This feature is thought to increase the susceptibility to corrosion because of their ability to harbor plaque forming microorganisms. These microorganisms cause localized reduction in pH and depletion of oxygen, which in turn affect the passivation process.[3,4]
The clinical significance of corrosion includes (1) corrosion increases orthodontic friction force between the archwire/bracket interface due to increase in surface roughness (Ra);[5] (2) corrosion products have been implicated in causing local pain or swelling in the region of orthodontic appliances in the absence of infection, which can lead to secondary infection;[6] (3) Cytotoxic and biological responses; and (4) Weakening of appliance.[7,8] Acidic conditions and chloride ions can accelerate corrosion process. Therefore, a diet rich in sodium chloride and acidic carbonated drinks provides a regular supply of corrosion agents. Another contribution to acidic oral conditions is fluoride-containing products. In dental applications, fluoride-containing, commercial mouthwashes, toothpastes, and prophylactic gels are generally used to avoid dental caries or to reduce dental sensitivity. The fluoride ions degrade the protective film permitting corrosion attack of the underlying alloy.[9]
The purpose of this study is to determine the corrosion resistance of various orthodontic wire alloys and to determine the effect of fluoride (0.5% NaF), simulating commercial fluoride-containing toothpastes on these archwires. This study also evaluates whether surface Ra is the primary influential factor for causing corrosion of these archwires.
Materials and Methods
Commercial archwires of stainless steel (SS), nickel-titanium (NiTi), titanium molybdenum alloy (TMA), and low-friction TMA (L-TMA) were used (0.017 × 0.025 Ormco, Glendora, California, USA).
Scanning electron microscope (Hitachi, SU6600, Japan) was used to observe the surface morphology of the archwires. Elemental composition of the archwires was assessed using energy dispersive spectroscope (EDS) (Horiba-Emax). Atomic force microscope (AFM) (XE-100 Park, Korea) was used to evaluate the three-dimensional surface Ra of the archwires.
A potentiostat (Gill AC, ACM Instruments, England) was used to perform the linear polarization test, a fast and nearly nondestructive electrochemical technique. Archwires samples cut into 40 mm were used as working electrode. A saturated calomel electrode and platinum were used as the reference electrode and counter electrode, respectively. Modified Fusayama artificial saliva (Nacl [400 mg/L], KCl [400/mg/L], CaCl2 H2O [795 mg/L], Na2H2 PO4 H2O [690MG/L], KSCN [300 mg/L], Na2 S.9H2O [5 mg/L], and Urea [1000 mg/L]) with a pH of 6.5 at 37°C was used as the corrosion test electrolyte. To evaluate the effect of fluoride concentration on these archwires, 0.5% of NaF (simulating the fluoride concentration contained in commercial fluoridated toothpastes) was added to modified Fusayama artificial saliva.
Experimental groups
Eight groups were taken, and the sample size was 10 for each of these groups. The experimental groups are mentioned below:
Group 1: SS in artificial saliva
Group 2: NiTi in artificial saliva
Group 3: TMA in artificial saliva
Group 4: L-TMA in artificial saliva
Group 5: SS in artificial saliva with 0.5% NaF
Group 6: NiTi in artificial saliva with 0.5% NaF
Group 7: TMA in artificial saliva with 0.5% NaF
Group 8: L-TMA in artificial saliva with 0.5% NaF.
The linear polarization tests were carried out after dipping the archwire samples into the test electrolyte for 2 h. The linear polarization values were measured from −10 mV to +10 mV with a scan rate of 0.1 mV/S. From the present test, the polarization resistance (Rp [Ω cm2]) was obtained which is inversely proportional to the corrosion rate and directly proportional to corrosion resistance.
Results
Figure 1 shows the SEM observations of the different archwires. Figure 2 and Table 1 show the AFM observations and the corresponding surface (Ra, nm). TMA wires showed the greatest surface Ra. This was followed by NiTi, L-TMA, and SS, respectively. Statistical analysis using one-way analysis of variance (ANOVA) showed a significant influence on the Ra value (P < 0.003). EDS studies show the elemental composition of different archwires – SS (Fe - 73.2%, Cr - 18.8%, Ni - 8%), NiTi (Ni - 55.1%, Ti - 44.9%), TMA (Ti - 78%, Mo - 11.5%, Zr - 6%, Sn - 4.5%), and L-TMA (Ti - 78%, Mo - 10.5%, Zr - 5.5%, Sn - 4.5%, N - 1.5%). Table 2 shows Rp of different archwires in artificial saliva. The ranking of mean RP value was L-TMA > TMA > NiTi > SS. Table 3 reveals that RP value of all archwires decreased drastically in artificial saliva with 0.5% NaF. The collected data were subjected to statistical analysis using one-way ANOVA and was found that there was a statistically significant influence on the RP value (P < 0.001).
Figure 1.
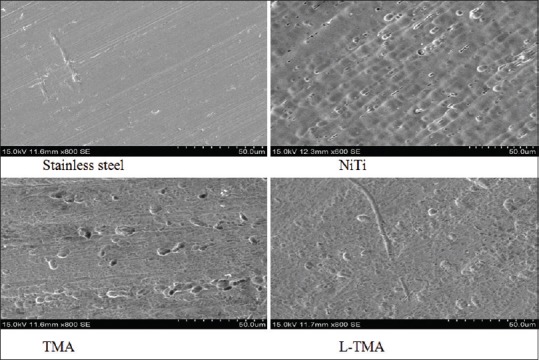
Scanning electron microscopic images. NiTi: Nickel titanium, TMA: Titanium molybdenum, L-TMA: Low-friction titanium molybdenum alloy
Figure 2.
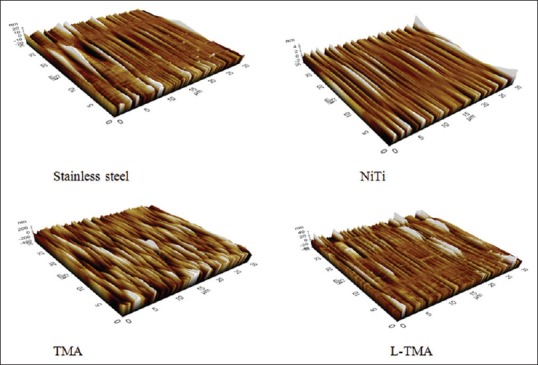
Atomic force microscopic images. NiTi: Nickel titanium, TMA: Titanium molybdenum, L-TMA: Low-friction titanium molybdenum alloy
Table 1.
Surface roughness (Ra, nm) for different wires

Table 2.
Polarization resistances (Ω cm2) for various groups in artificial saliva without sodium fluoride
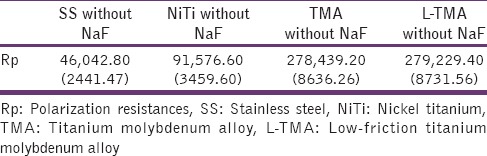
Table 3.
Polarization resistances (Ω cm2) for various groups in artificial saliva with sodium fluoride

Discussion
The SS and titanium alloys used in orthodontic appliances rely on the formation of a passive surface oxide film to resist corrosion. The protective layer is not infallible; it is susceptible to both mechanical and chemical disruption.[3] Potentiodynamic polarization experiments and scanning electron microscopic observations of archwires composed of SS, NiTi, and TMA exposed to electrochemical corrosion in artificial saliva have shown evidence of pitting corrosion formed on the wire surface.
As for SS archwires, it is well known that chromium element in the SS alloy can form a thin and adherent Cr2O3-based protective film which provides the corrosion resistance of a substrate alloy. A minimum chromium content of around 11% is required to form a protective passive film on the SS wire.[10] In case of NiTi archwires, the TiO2-based (also small traces of NiO) passive film can provide a good measure of NiTi alloy biocompatibility.[11,12] TMA wires form a passive film of TiO2 and traces of MO3, ZrO2, SnO, and L-TMA form the same protective layer as TMA wire together with the formation of traces of NO due to ion bombardment of nitrogen.[13] Edie et al.[14] in their study stated that the corrosion potential of SS and NiTi is not different. However, the present study [Table 3] suggests that more surface pitting and corrosion occurred in SS than in NiTi, TMA, and L-TMA. These results were almost similar to the results reported by Suarez et al. and Kim and Johnson.[2,15] They concluded that the titanium wires appear to be the most inert wire of those tested and are unlikely to release metal ions when used intraorally. They also stated that nitride coating did not affect the corrosion of the alloy. However, in this study, L-TMA was found to have a slightly higher corrosion resistance than TMA wires. This result was consistent to the results obtained earlier.[16] Ion implantation decreased friction and improved the corrosion resistance decreasing the corrosion rate. The improvement in corrosion resistance of L-TMA was believed due to the presence of NO in the outermost surface of the alloys.[13] It was found that TiO2-based passive film formed on titanium metal has better corrosion resistance in acidic artificial saliva than the Cr2O3-based passive film on SS. This result was also consistent with a previous study which has also concluded that TiO2 passive film is more corrosion resistant than Cr2O3.[10] The better corrosion resistance of TMA wires than NiTi might be due to the increased titanium content in them (78% in TMA and 45% in NiTi).[13] This is clearly seen in the present study in which the composition of the archwire is determined by energy dispersive spectroscope (EDS).
A previous study[17] had suggested that surface Ra of orthodontic archwires ought to be taken as an important indicator of the trend toward archwire corrosion resistance. In our study [Table 1], TMA showed the highest surface Ra value and SS the least surface Ra. From the potentiostatic tests, it was confirmed that TMA and L-TMA exhibited the highest corrosion resistance. Contrary to this, earlier studies were reported[7,18] but our study was consistent with the study reported by D’Anto et al.[19] From this, it can be concluded that chemical composition of the wire is the primary factor influencing the corrosion resistance and surface Ra is only secondary.
From [Table 3], it is very clear that the corrosion resistance of all wires had reduced drastically in the presence of 0.5% NaF (although L-TMA showed the highest RP value). The Cr2O3 passive film of SS reacts with NaF and the following reaction takes place.[20,21]
Cr2O3 + 2NaF → CrF2 + Na2O + CrO2
In case of NiTi and TMA wires, TiO2 reacts with NaF to form titanium-fluoride complex compound.[11]
TiO2 + NaF → Na2 TiF6
However, TMA and L-TMA archwires showed a better corrosion resistance than NiTi. This may be due to the presence of some other oxides in the surfaces passive film (MOO3, ZrO, and SnO).[13]
Conclusion
From the AFM analysis, TMA showed the highest surface Ra. This was followed by NiTi, L-TMA, and SS in decreasing order. From the potentiostatic study, it was found that TMA and L-TMA had the highest corrosion resistance. This indicates that the chemical composition of the wire is the primary influential factor to have high corrosion resistance and surface Ra is only secondary.
In the present study, the RP values were measured after exposing the archwires to artificial saliva and artificial saliva-containing sodium fluoride (0.5%) for 2 h. This simulated the in vivo corrosion resistance of the archwires in the oral cavity. It also simulated the in vivo corrosion resistance of archwires when residual fluorinated toothpastes interacted with the archwires 2 h after brushing. It was seen that the in vivo corrosion resistance of the archwires in the oral environment might decrease if the highly fluorinated toothpastes were used and/or the exposure time of archwires to residual fluorinated toothpastes was prolonged. Therefore, complete removal of residual high-fluorinated toothpastes from the crevice between archwire and bracket during tooth brushing is required. Furthermore, the repair of the protectiveness of the surface oxide film on the archwires might occur after full removal of residual fluorinated toothpastes by mechanical tooth brushing.
However, the effect of a lesser concentration of fluoride and variation in the exposure time to this particular concentration of fluoride on the archwires is still to be investigated.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Maijer R, Smith DC. Biodegradation of the orthodontic bracket system. Am J Orthod Dentofacial Orthop. 1986;90:195–8. doi: 10.1016/0889-5406(86)90065-x. [DOI] [PubMed] [Google Scholar]
- 2.Kim H, Johnson JW. Corrosion of stainless steel, nickel-titanium, coated nickel-titanium, and titanium orthodontic wires. Angle Orthod. 1999;69:39–44. doi: 10.1043/0003-3219(1999)069<0039:COSSNT>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.House K, Sernetz F, Dymock D, Sandy JR, Ireland AJ. Corrosion of orthodontic appliances – Should we care? Am J Orthod Dentofacial Orthop. 2008;133:584–92. doi: 10.1016/j.ajodo.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 4.Eliades T, Athanasiou AE. In vivo aging of orthodontic alloys: Implications for corrosion potential, nickel release, and biocompatibility. Angle Orthod. 2002;72:222–37. doi: 10.1043/0003-3219(2002)072<0222:IVAOOA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 5.Matasa CG. Attachment corrosion and its testing. J Clin Orthod. 1995;29:16–23. [PubMed] [Google Scholar]
- 6.Chaturvedi TP. Corrosion behaviour of orthodontic alloys. Orthodontic Cyber J. 2008;18:6–16. [Google Scholar]
- 7.Hunt NP, Cunningham SJ, Golden CG, Sheriff M. An investigation into the effects of polishing on surface hardness and corrosion of orthodontic archwires. Angle Orthod. 1999;69:433–40. doi: 10.1043/0003-3219(1999)069<0433:AIITEO>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Platt JA, Guzman A, Zuccari A, Thornburg DW, Rhodes BF, Oshida Y, et al. Corrosion behavior of 2205 duplex stainless steel. Am J Orthod Dentofacial Orthop. 1997;112:69–79. doi: 10.1016/s0889-5406(97)70276-2. [DOI] [PubMed] [Google Scholar]
- 9.Schiff N, Dalard F, Lissac M, Morgon L, Grosgogeate B. Corrosion resistance of three orthodontic brackets: A comparative study of three fluoride mouth washes. Eur J Orthod. 2005;27:541–9. doi: 10.1093/ejo/cji050. [DOI] [PubMed] [Google Scholar]
- 10.Lin MC, Lin SC, Lee TH, Huang HH. Surface analysis and corrosion resistance of different stainless steel orthodontic brackets in artificial saliva. Angle Orthod. 2006;76:322–9. doi: 10.1043/0003-3219(2006)076[0322:SAACRO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.Huang HH, Lee H, Huang TK, Lin SY, Chen LK, Chou MY. Corrosion resistance of different nickel – Titanium archwires in acidic fluoride containing artificial saliva. Angle Orthod. 2010;80:547–53. doi: 10.2319/042909-235.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang HH. Variation in corrosion resistance of nickel-titanium wires from different manufacturers. Angle Orthod. 2005;75:661–5. doi: 10.1043/0003-3219(2005)75[661:VICRON]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.Huang HH, Wang CC, Chiu SM, Wang JF, Liaw YC, Lee TH, Chen FL. Corrosion behaviour of titanium containing orthodontic archwires in artificial saliva: Effects of fluoride ions and plasma immersion ion implantation treatment. China Dent J. 2005;24:134–40. [Google Scholar]
- 14.Edie JW, Andreasen GF, Zaytoun MP. Surface corrosion of nitinol and stainless steel under clinical conditions. Angle Orthod. 1981;51:319–24. doi: 10.1043/0003-3219(1981)051<0319:SCONAS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 15.Suarez C, Vilar T, Sevilla P, Gil J. In vitro corrosion behaviour of lingual orthodontic archwires. Int J Corros. 2011;132:1–9. [Google Scholar]
- 16.Kao CT, Ding SJ, He H, Chou MY, Huang TH. Cytotoxicity of orthodontic wire corroded in fluoride solution in vitro. Angle Orthod. 2007;77:349–54. doi: 10.2319/0003-3219(2007)077[0349:COOWCI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Widu F, Drescher D, Junker R, Bourauel C. Corrosion and biocompatibility of orthodontic wires. J Mater Sci Mater Med. 1999;10:275–81. doi: 10.1023/a:1008953412622. [DOI] [PubMed] [Google Scholar]
- 18.Yu JH, Wu LC, Hsu JT, Chang YY, Huang HH, Hung HL. Surface roughness and topography of four commonly used types of orthodontic archwires. J Med Biol Eng. 2011;31:367–70. [Google Scholar]
- 19.D’Anto V, Rongo R, Ametrano G, Spanuolo G, Manzo P, Martina R, et al. Evaluation of surface roughness of orthodontic wires by means of atomic force microscopy. Angle Orthod. 2012;82:922–8. doi: 10.2319/100211-620.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemand S, Puri BR. Text Book of Inorganic Chemistry. Trivandrum: New Jyothi Publication; 2007. [Google Scholar]
- 21.Walker MP, Ries D, Kula K, Ellis M, Fricke B. Mechanical properties and surface characterization of beta titanium and stainless steel orthodontic wire following topical fluoride treatment. Angle Orthod. 2007;77:342–8. doi: 10.2319/0003-3219(2007)077[0342:MPASCO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]


