Abstract
Dysregulated metabolism is one of the key characteristics of cancer cells. The most prominent alterations are present during regulation of cell respiration, which leads to a switch from oxidative phosphorylation to aerobic glycolysis. This metabolic shift results in activation of numerous signaling and metabolic pathways supporting cell proliferation and survival. Recent progress in genetics and metabolomics allowed us to take a closer look at the metabolic changes present in pheochromocytomas and paragangliomas (PHEOs/PGLs). These neuroendocrine tumors often exhibit dysregulation of mitochondrial metabolism, which is driven by mutations in genes encoding Krebs cycle enzymes or by activation of hypoxia signaling. Present metabolic changes are involved in processes associated with tumorigenesis, invasiveness, metastasis, and resistance to various cancer therapies. In this review, we discuss the metabolic nature of PHEOs/PGLs and how unveiling the metabolic disturbances present in tumors could lead to identification of new biomarkers and personalized cancer therapies.
Introduction
Recently, substantial progress in the understanding of pathophysiological mechanisms involved in various cancers has developed. Advancements in cancer research and molecular biology (including genomics) identified genes encoding metabolic enzymes and alterations in multiple signaling pathways, which are involved in tumorigenesis. Several lines of evidence suggest that activation of oncogenic signaling pathways leads to reprogramming of cell metabolism to fuel extensive cell proliferation and support cell survival (1, 2). Moreover, some of these metabolic alterations seem to be required for malignant transformation and this makes metabolic alterations in the cell one of the key hallmarks of cancer (1, 3). Thus, cancer metabolism is becoming paramount in understanding cancer pathophysiology and, therefore, tumor development, progression, senescence, and metastasis.
Decades ago, during the early period of cancer research, the link between carcinogenesis and cell metabolism alterations was proposed. In 1924, the German biochemist Otto Warburg hypothesized that cancer is a result of damage to the mitochondrial respiratory function and therefore, the replacement of oxidative phosphorylation (OXPHOS) by aerobic glycolysis for adenosine triphosphate (ATP) production. This became known as the Warburg effect (4, 5). Compared to normal healthy cells, such a shift in cell metabolism causes cancer cells to present with increased bioenergetics and altered anaplerotic (intermediate replenishing) processes driven by activation of mechanisms supporting cell survival (6). However, the Warburg effect itself is not sufficient enough to sustain cell proliferation (7). First, a cancer cell has to increase its uptake of nutrients from the environment, especially glucose and glutamine, which are the major nutrients needed for cancer cell survival and proliferation. They provide the cancer cell, through catabolism, with sufficient pools of carbon intermediates used for synthesis of various macromolecules and for ATP production. Second, to satisfy energy needs and ensure accelerated growth and proliferation, cancer cells metabolic reprogramming also includes an increase in protein, lipid, and nucleic acid biosynthesis (1). For essential biosynthetic processes, cancer cells use precursors derived from intermediates of the Krebs (tricarboxylic acid) cycle, which serves as a hub for these processes (8). Based on this, the Krebs cycle is considered one of the key metabolic pathways, which, if dysregulated, its dysfunction may result in tumorigenesis of certain tumors, including pheochromocytomas (PHEOs) and paragangliomas (PGLs).
PHEOs and PGLs are rare neuroendocrine tumors arising from chromaffin cells in the adrenal medulla or from extra-adrenal sympathetic and parasympathetic paraganglia, respectively (9, 10). These tumors, especially those arising from the sympathetic nervous system, are usually characterized by catecholamine production, which is responsible for clinical symptoms associated with PHEO/PGL. On the other hand, parasympathetic PGLs (head and neck PGLs) are mostly non-functional (11, 12). The majority of PHEOs/PGLs present as benign tumors. Yet, metastasis can also occur, notably, in patients with a specific genetic background (13–16).
Previous and recent genetic discoveries in PHEO/PGL research have led to the identification of PHEO/PGL-related unique metabolic abnormalities or pathways involved in oxygen sensing, hypermethylation, DNA repair, up-regulation of specific transporters and/or receptors, and particularly, Krebs cycle enzymes (17–20). These changes are tightly linked to metabolic reprogramming in PHEO/PGL, which points out the metabolic nature of PHEO/PGL, defining this cancer as a metabolic disease.
Mitochondria, Krebs cycle, and cancer cell metabolism
Normal, as well as cancer cells, largely depend on mitochondrial function. Besides being an essential producer of energy (in the form of ATP), mitochondria serve other functions fundamental for cell proliferation and survival, including biosynthetis of intermediates, heme and iron-sulfur clusters, and reactive oxygen species (ROS) (21). The highly flexible mitochondrial network allows the cell to adjust to changing intra- and extra-cellular conditions like hypoxia, nutrient deprivation, or other forms of cellular stress (6). The Krebs cycle is a crucial part of this network; it unifies carbohydrate, lipid, and protein metabolism (Figure 1) (22) and links the majority of metabolic pathways in the cell either directly or indirectly to the mitochondria. Besides that, NADH and FADH2 produced in the Krebs cycle provide electrons for mitochondrial electron transport chain to generate ATP. Thus, the Krebs cycle is fueling both energy production and anabolic processes in the cell (23). Dysfunction of the Krebs cycle enzymes (or a depletion or abundance of its substrates) leads to cycle malfunction and activation of adaptive mechanisms supporting cell survival. Many of these adaptive mechanisms are related to processes linked with tumorigenesis.
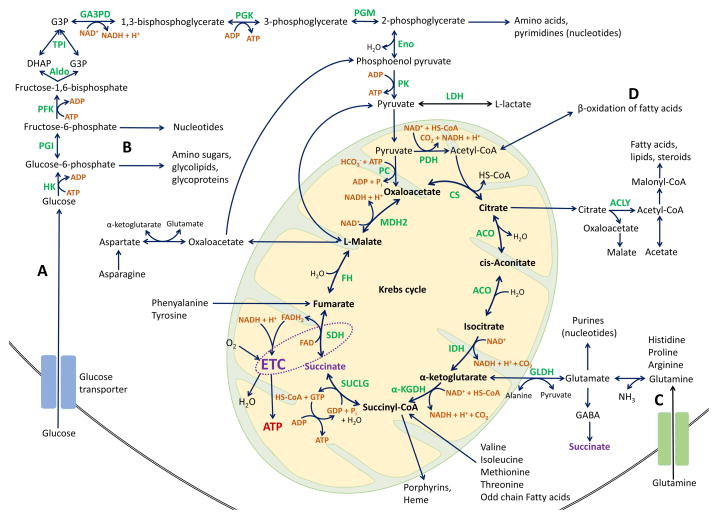
Figure 1. The Krebs (TCA) cycle and anaplerotic/cataplerotic pathways.
After entering the cell, glucose is phosphorylated by HK1 and then most of it is degraded via glycolysis (A) to pyruvate. Pyruvate enters the mitochondria, where it is decarboxylated and oxidized by PDH enzyme complex to acetyl-CoA, the main source of energy for Krebs cycle. After entering the Krebs cycle, acetyl-CoA condensates with oxaloacetate to produce citrate, catalyzed by CS. Citrate either stays in the mitochondria and is converted to isocitrate by ACO, or is exported to the cytoplasm to be used as a precursor for lipid biosynthesis (via conversion by ACLY). Isocitrate is subsequently decarboxylated to α-ketoglutarate by IDH. α-ketoglutarate is then either converted to succinyl-CoA by α-KGDH complex or exits the mitochondria and serves as a precursor for amino acid biosynthesis. Succinyl-CoA is either transformed to succinate in the reaction catalyzed by SUCLG or can be utilized for porphyrin biosynthesis. Succinate is then oxidized to fumarate by SDH, which also represents complex II of the ETC (dotted circle/ellipse). Fumarate is hydrated to malate by FH and, finally, malate is oxidized by MDH to restore oxaloacetate. In the Krebs cycle, hydrogen atoms reduce NAD+ and FAD to NADH + H+ and FADH2 respectively, which feed the ETC to produce ATP. The Krebs cycle as a biosynthetic pathway produces intermediates that leave the cycle (cataplerosis) to be converted primarily to glutamate, GABA, glutamine and aspartate, and also to glucose derivatives and fatty acids. A minor part of glycolytic glucose-6-phosphate is redirected to the pentose phosphate pathway (B) to produce ribose-5-phosphate and NADPH, which will be used to synthetize nucleotides. The triose phosphates can be used for lipids and phospholipids. In normal cells, amino acids follow the physiological turnover of the proteins and little part is used to synthetize the nucleotide bases. After deamination, the remainder of amino acids are used for energy production.
When Krebs cycle ketoacids are consumed or removed, they need to be replaced to permit the Krebs cycle sustained function. This process is called anaplerosis and is tightly coupled with cataplerosis (100). The anaplerotic reactions of Krebs cycle include the catabolism of essential amino acids (histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine) as well as odd chain fatty acids. Anaplerotic reactions provide the Krebs cycle with fumarate, oxaloacetate, α-ketoglutarate, malate, and succinyl-CoA. Oxaloacetate is formed via carboxylation of pyruvate by PC, from malate through oxidation by malate dehydrogenase, or by transamination of aspartate. Pyruvate can also be decarboxylated to malate. Glutaminolysis (C) serves as the source of the Krebs cycle intermediate α-ketoglutarate and oxidation of odd chain fatty acids or metabolism of methionine and isoleucine provide succinyl-CoA. Acetyl-CoA can be replenished from β-oxidation of fatty acids (D).
Abbreviations: α-KGDH, alpha-ketoglutarate dehydrogenase; ACLY, ATP-citrate lyase; ACO, aconitase; ADP, adnesoine diphosphate; Aldo, aldolase; ATP, adenosine triphosphate; CO2, carbon dioxide; CoA, coenzyme A; CS, citrate synthase; DHAP, dihydroxyacetone phosphate; Eno, enolase; ETC, electron transport chain; FAD, flavin adenine dinucleotide; FADH2, reduced FAD; FH, fumarate hydratase; G3P, glycerol-3-phosphate; GA3PD, glyceraldehyde-3-phosphate dehydrogenase; GABA, gamma-aminobutyric acid; GDP, guanosine diphosphate; GLDH, glutamate dehydrogenase; GTP, guanosine triphosphate; H2O, water; HK, hexokinase; HS-CoA, Coenzyme A; IDH, isocitrate dehydrogenase; LDH, lactate dehydrogenase; MDH, malate dehydrogenase; NAD+, nicotinamide adenine dinucleotide, oxidized; NADH, reduced form of NAD; PC, pyruvate carboxylase; PDH, pyruvate dehydrogenase; PFK, phosphofructokinase; PGI, glucose-6-phosphate isomerase; PGK, phosphoglycerate kinase; PGM, phosphoglycerate mutase; Pi, inorganic phosphate; PK, pyruvate kinase; SDH, succinate dehydrogenase; SUCLG, succinyl-CoA synthetase; TCA, tricarboxylic acid; TPI, triosephosphate isomerase
Mechanisms regulating increased metabolism in cancer cells are usually driven by mutations that chronically enhance specific metabolic pathways or alter levels of their substrates or end products, allowing cancer cells to maintain their biosynthetic metabolic phenotype independently of normal physiological regulations (8). Mutations in genes encoding proteins with enzymatic functions, including Krebs cycle enzymes, lead to dysregulation of cell metabolism and eventually, tumorigenesis. For instance, mutations in genes encoding the succinate dehydrogenase (SDH) complex subunits (SDHx: SDHA, SDHB, SDHC, SDHD), fumarate hydratase (FH), isocitrate dehydrogenase (IDH), and malate dehydrogenase (MDH) are known to cause accumulation of Krebs cycle metabolites/substrates, such as succinate, pyruvate, fumarate, citrate, or glutamine, which can promote tumorigenesis via generation of pseudohypoxic state (i.e. hypoxia-inducible factors (HIFs) signaling activation), epigenetic alterations, and dysregulation of other metabolic processes; reviewed in (24).
Warburg effect (aerobic glycolysis)
In a normal cell, glucose is metabolized to pyruvate during glycolysis. Pyruvate enters the mitochondria and is used in the Krebs cycle, which generates NADH and FADH2 to supply the mitochondrial electron transport chain with electrons for energy production (Figure 1). In this process, one molecule of glucose produces 36 molecules of ATP (5). When oxygen supply is insufficient (hypoxia), normal cells can switch to anaerobic glycolysis – the transformation of glucose to lactate with much lower ATP production (2 molecules of ATP per one molecule of glucose).
In contrast, a majority of cancer cells exhibit an altered glucose metabolism – they switch to glycolysis even in the abundance of oxygen (8, 25, 26, 27). This is, as previously mentioned, the Warburg effect or, ‘aerobic glycolysis,’ mediated mostly by changes in the fate of the end-product of glycolysis – pyruvate, as discussed below. Although aerobic glycolysis is much less efficient in ATP production compared to OXPHOS (2 vs. 36 molecules of ATP) and may seem to be a disadvantage for the cancer cell, the opposite is true. Cancer cells, to compensate for the lower generation of ATP during glycolysis, display substantially increased glucose uptake mediated by glucose transporters and hexokinase 2 upregulation (28–30). A high glucose influx and glycolytic rate can secure a higher production of ATP than that produced by OXPHOS (8, 25). Moreover, most of the pyruvate, instead of being oxidized through mitochondrial metabolism, is converted to lactate due to suppression of pyruvate dehydrogenase activity and overexpression of lactate dehydrogenase A (LDHA). This reaction allows tumor cells to regenerate NADH and therefore not only sustain, but accelerate the glycolysis rate (6, 8, 26, 28, 31). Conversion of excess glycolytic flux to lactate also helps avoid saturating the mitochondria with a supply of NADH that would suppress the Krebs cycle (32). Furthermore, lactate is released into the tumor microenvironment and nurtures cancer cells, which do not have a sufficient supply of nutrients (27, 33). In addition, conversion of pyruvate to lactate reduces production of ROS and lowers the pH of the extracellular microenvironment resulting in enabling the activity of metalloproteases for breaking down extracellular matrix. Therefore, lactate serves as an inducer of tumor invasion and metastasis (34, 35).
Besides energy production, glycolysis generates metabolic intermediates, which serve as substrates for other metabolic pathways, especially for synthesis of amino acids and macromolecules, which are essential for cell proliferation and survival (32) (Figure 1). Glucose-6-phosphate (oxidative pathway) or fructose-6-phosphate (non-oxidative pathway) are redirected to the pentose phosphate pathway where they are utilized for synthesis of nucleotides and NADPH. A substantial increase in lipid production allows for the synthesis of DNA and proteins, formation of lipid bilayers, modification of membrane-targeted proteins, and adaptation of membrane composition to oxidative stress (32, 36, 37). Fatty acid synthesis influences cell signaling and growth and lipid metabolism has been accepted as one of the major metabolic pathways involved in cancer development and progression (36). The Krebs cycle-derived citrate is cleaved to acetyl-CoA and oxaloacetate by Akt (protein kinase B) activated ATP-citrate lyase in cytosol. Acetyl-CoA is then used as a substrate for de novo biosynthesis of fatty acids (38, 39). Dihydroxyacetone phosphate, the metabolic intermediate of glycolysis, is also used for lipid synthesis and malonyl-CoA can be utilized for de novo biosynthesis of cholesterol (32, 40). Moreover, pyruvate can be imported into the mitochondria and converted into substrates for the production of additional amino or fatty acids, or can be used to sustain mitochondrial membrane potential (41).
Metabolic adaptation of proliferating/cancer cells can be affected by various oncogenes, such as c-Myc, RAS, or HIF-1α, involved in the regulation of genes involved in aerobic glycolysis, including glucose transporters, glycolytic enzymes, and LDHA. Activation of the PI3K/Akt/mTOR pathway (one of the RAS downstream signaling pathways) promotes cell biosynthesis through multiple actions, including increasing the surface expression of nutrient transporters, increasing glycolysis and lactate production, and enhancing the biosynthesis of macromolecules, as discussed in (8, 32, 42). These effects are, in part, mediated through oxygen independent HIF-α stabilization and activation of HIF signaling. Decreased oxygen availability (hypoxia) or conditions leading to stabilization of HIF-α, even in the presence of sufficient amount of oxygen (pseudohypoxia), trigger the switch of metabolism from OXPHOS to glycolysis (18, 24, 43, 44).
Insufficient activity of tumor suppressors, such as p53, also alleviates activation of tumorigenic metabolic pathways (32, 37, 40, 41). Actually, p53, c-Myc, and HIFs represent master regulators of cancer glycolysis and glycolytic enzymes play an active role in promoting cancer cell survival, invasion, metastasis, regulation of gene expression, and many other key cellular processes (40).
Glutamine metabolism (glutaminolysis)
The second most abundant nutrient in cancer cells is glutamine, which serves as the carbon and nitrogen shuttle between organs and is a major source of nitrogen for nonessential amino acids, nucleotides, and hexosamines (45). In mitochondria, glutamine is converted to glutamate by glutaminase and glutamate is, in turn, deaminated to α-ketoglutarate. Glutaminolysis products, particularly α-ketoglutarate, fuel the Krebs cycle as anaplerotic substrates for biosynthesis of lipids, cholesterol, amino acids and other vital metabolites when glucose derived citrate is re-routed to the cytoplasm (37, 46).
Glucose and glutamine are versatile, and in some cases, can compensate each other to maintain Krebs cycle function (40, 47). Glutaminolysis upregulation in cancer cells is mediated by c-Myc, which promotes uptake of glutamine as well as glutamine catabolism, especially by upregulation of glutaminase 1 expression (32).
Metabolic alterations in PHEOs/PGLs
Today, it is clear that many more PHEOs/PGLs are caused by germline mutations than previously anticipated – around 40% of these tumors are genetically inherited (48, 49). Germline and/or somatic mutations in at least eighteen different genes were described in PHEOs/PGLs (50–52) (Table 1). Each of these genes is involved in regulating key biological processes, including cell development, proliferation, growth, the cell’s ability to respond to changes in nutrients, oxygen, iron or energy, and cell transformation, including tumorigenesis and ultimately, metastasis (53–55).
Table 1.
| Molecular cluster | Gene | Location | Syndrome | Germline mutation frequency* | Somatic mutation frequency* |
|---|---|---|---|---|---|
|
| |||||
| Krebs cycle (Cluster 1a) | SDHA | 5p15 | PGL5 | <5% | 0% |
| SDHB | 1p36.13 | PGL4 | 10% | 0% | |
| SDHC | 1q23.3 | PGL3 | <5% | 0% | |
| SDHD | 11q23 | PGL1 | 10% | 0% | |
| SDHAF2 | 11q12 | PGL2 | <1% | 0% | |
| FH | 1q42.1 | <5% | 0% | ||
| MDH2 | unknown | unknown | |||
| IDH | unknown | unknown | |||
|
| |||||
| Pseudohypoxic (Cluster 1b) | VHL | 3p25.3 | Von Hippel-Lindau | 10% | 10% |
| HIF2A | 2p21 | Pacak-Zhuang Syndrome | <5% | 5–7% | |
| PHD1/EGLN2 | 19q13.2 | unknown | unknown | ||
| PHD2/EGLN1 | 1q42.2 | unknown | unknown | ||
|
| |||||
| Kinase signaling (Cluster 2) | NF1 | 17q11.2 | Neurofibromatosis type 1 | <5% | 20–40% |
| RET | 10q11.21 | MEN2 | 10% | 10% | |
| TMEM127 | 2q11.2 | <5% | 0% | ||
| MAX | 14q23.3 | <5% | <5% | ||
| KIF1Bβ | 1p36.22 | 0% | NA | ||
| HRAS | 11p15.5 | 0% | 10% | ||
| ATRX | Xq21.1 | unknown | unknown | ||
Germline mutation/somatic mutation frequency among all PHEOs/PGLs
Abbreviations: ATRX, alpha thalassemia/mental retardation syndrome X-linked; EGLN1/2, Egl-9 family hypoxia inducible factor 1/2FH, fumarate hydratase; HIF2A, hypoxia-inducible factor 2α; HRAS, Harvey rat sarcoma viral oncogene homolog; IDH, isocitrate dehydrogenase; KIF1Bβ; Inesin family member 1B; MAX, myc associated factor X; MDH2, malate dehydrogenase 2; NF1, neurofibromin 1; PHD1/2, prolyl hydroxylase domain-containing protein 1/2; RET, rearranged during transfection protooncogene; SDHA, -B, -C, -D, succinate dehydrogenase complex, subunit A, B, C, D; SDHAF2, SDH complex assembly factor 2; TMEM127, transmembrane protein 127; VHL, von Hippel-Lindau.
Current data suggests that most of the PHEO/PGL susceptibility gene mutations are associated with dysregulation of several metabolic pathways, which subsequently leads to defects in hypoxia signaling pathways and adaptive responses (18, 56). Pseudohypoxia and mitochondrial enzymes disruption may have a direct oncogenic or tumor suppressive effect by regulating and controlling diverse cellular processes (57–59). Activation of the hypoxia signaling pathway in PHEOs/PGLs can occur directly, driven by mutations in genes encoding proteins crucial for HIF-α hydroxylation and degradation, such as SDHx, PHD1/2, HIF2A, FH, or malate dehydrogenase 2 (MDH2). HIF-α can also be stabilized indirectly and it occurs in tumors with mutations in neurofibromin 1 (NF1), RET protooncogene, transmembrane protein 127 (TMEM127), H-RAS, and Myc-associated factor X (MAX), which are linked with the previous group through the mTOR and PI3K signaling pathways (18) (Figure 2).
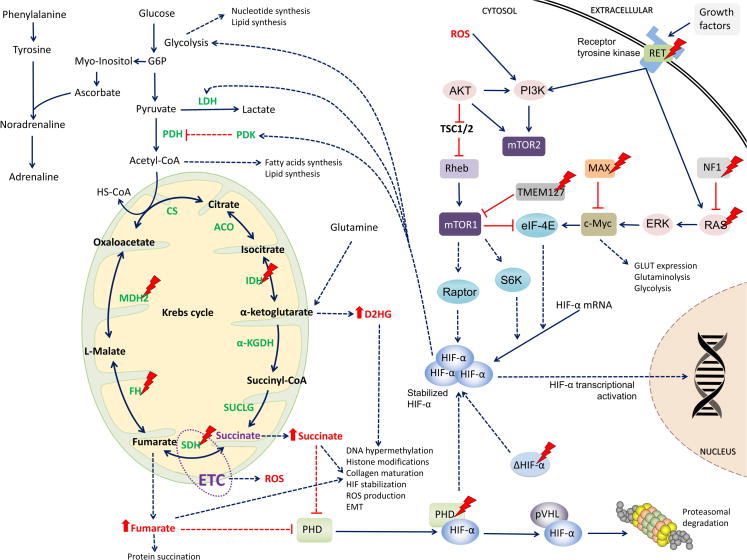
Figure 2. Metabolic changes in PHEO/PGL.
Schematic representation of mitochondrial genes as well as others involved in PHEO/PGL development with emphasis to the Krebs cycle enzymes, as explained in the text. Dotted arrows represent changes resulting from mutations in certain proteins. Actual treatment targets are explained in Table 2 and Supplementary Table S1.
Abbreviations: α-KGDH, alpha-ketoglutarate dehydrogenase; ACO, aconitase; Akt, RAC-alpha serine/threonine-protein kinase; CoA, coenzyme A; CS, citrate synthase; c-Myc, Myc proto oncogene; eIF-4E, eukaryotic translation initiation factor 4E; ERK, mitogen-activated protein kinase 2; ETC, electron transport chain; FH, fumarate hydratase; HIF-α, hypoxia-inducible factor alpha; HK, hexokinase; HS-CoA, Coenzyme A; IDH, isocitrate dehydrogenase; LDH, lactate dehydrogenase; MAX, myc-associated factor X; mTORC1, mammalian target of rapamycin complex 1; mTORC2, mammalian target of rapamycin complex 2; MDH2, malate dehydrogenase 2; NF1, neurofibromin 1; PDH, pyruvate dehydrogenase; PHD, prolyl hydroxylase domain protein; PI3K, phosphoinositide 3-kinase; pVHL, von Hippel-Lindau protein; Raptor, regulatory associated protein of mTOR; RAS, rat sarcoma oncogene; RET, rearranged during transfection proto-oncogene; Rheb, RAS homolog enriched in brain; ROS, reactive oxygen species; S6K, S6 kinase; SDH, succinate dehydrogenase; SUCLG, succinyl-CoA synthetase; TMEM127, transmembrane protein 127; TSC1/2, tuberous sclerosis complex 1/2
In SDHx- or FH- and MDH2-mutated PHEOs/PGLs, accumulation of Krebs cycle substrates/intermediates succinate and fumarate is paramount for tumorigenesis. In high levels both, succinate and fumarate act like oncometabolites. They function as competitive inhibitors of α-ketoglutarate-dependent dioxygenases, including HIF prolyl hydroxylases (PHDs), which are necessary for HIF-α hydroxylation, its further recognition by pVHL and subsequent proteasomal degradation (60–63); reviewed in (18) (Figure 2 and Figure 3). Thus, succinate and fumarate promote HIF-α signaling pathway activation and expression of HIF target genes, resulting in induction of adaptive changes in cell metabolism, activation of angiogenesis, cell migration and intra- and extravasation, and other protumorigenic mechanisms, including micrometastases (64). Activation of HIF signaling enhances the glycolytic pathway by increasing the expression of target genes involved in glycolysis and anabolic processes, such as glucose transporters 1, 2, 3, hexokinase 2, pyruvate kinase isoenzyme M2, or LDHA (56, 65, 66) and thus, switches cell metabolism to aerobic glycolysis.
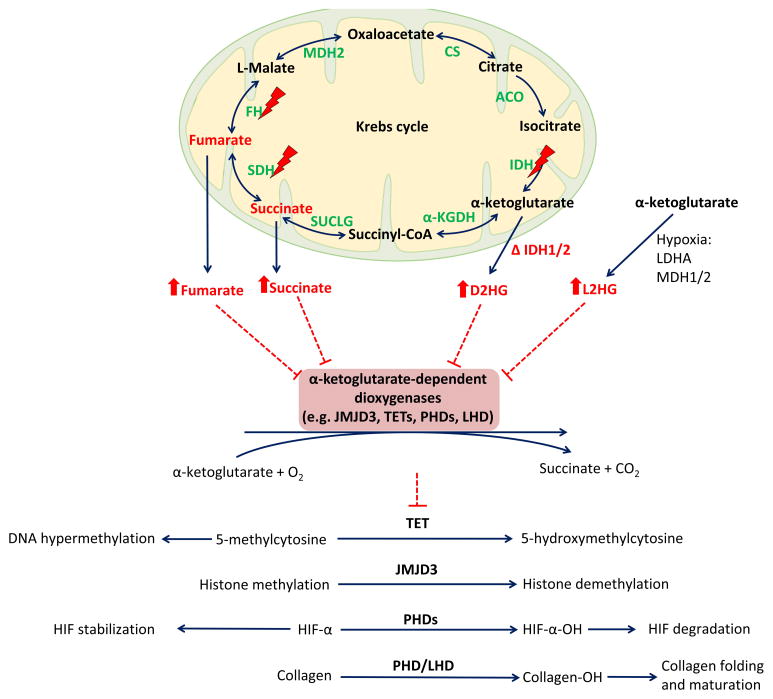
Figure 3. Inhibition of α-ketoglutarate-dependent dioxygenases by Krebs cycle intermediates and 2-HG.
Mutations in SDHx and FH genes lead to an accumulation of succinate and fumarate, mutated IDH1/2 exhibit neomorphic activity that results in conversion of α-ketoglutarate to oncometabolite, D2HG. Under hypoxic conditions, L2HD accumulation occurs, as described in the text. Succinate, fumarate, D2HG, and L2HG function as a competitive inhibitors of α-ketoglutarate-dependent dioxygenases. Reactions of α-ketoglutarate-dependent dioxygenases potentially inhibited by succinate, fumarate, D2HG, and L2HG are depicted in the lower part of the scheme. All four reactions convert α-ketoglutarate to succinate and CO2, incorporate O2, and require iron and ascorbate as cofactors. Inhibition of these reactions results in DNA and histone hypermethylation, activation of hypoxic responses, and inhibition of collagen maturation and folding.
Abbreviations: Δ, mutant; 2-HG, 2-hydroxyglutarate; α-KGDH, alpha-ketoglutarate dehydrogenase; ACO, aconitase; CO2, carbon dioxide; CS, citrate synthase; D2HG, D-2-hydroxyglutarate; FH, fumarate hydratase; HIF, hypoxia-inducible factor, IDH, isocitrate dehydrogenase; JMJD3, Jumoni C domain-containing histone lysine demethylases; L2HG, L-2-hydroxyglutarate; LDHA, lactate dehydrogenase A, LHD, lysyl hydroxylase, MDH, malate dehydrogenase; O2, oxygen; PHD, prolyl hydroxylases, SDH, succinate dehydrogenase; SUCLG, succinyl-CoA synthetase; TET, ten-eleven translocation family of 5-methylcytosine (5mC) hydroxylases
With regard to PHEOs/PGLs, HIF-2α was shown to be overexpressed in both SDHx- and VHL-mutated tumors (67) and is significantly more expressed in SDHx-mutated tumors compared to sporadic ones (68, 69). HIF-α stabilization in SDHx-mutated tumors can also be triggered by ROS signals (Figure 2). The mitochondrial electron transport chain is the major endogenous source of ROS, which can damage cells or various cellular components. SDHB mutations were reported to especially cause a significant increase in ROS production and mitochondrial DNA mutability (70, 71), although pseudohypoxia can be observed in SDH-suppressed cells even in the absence of oxidative stress (61, 72).
Moreover, decreased levels of several Krebs cycle intermediates, namely citrate, isocitrate, and cis-aconitate, were detected in SDHx- and VHL-mutated PHEOs/PGLs, which are associated with epigenetic and metabolic changes involved in tumorigenesis and confirmed decreased OXPHOS and presence of pseudohypoxia in those tumors (73). For instance, citrate, under physiological conditions, slows down/inhibits the Krebs cycle and glycolysis, and stimulates gluconeogenesis and lipid synthesis. Moreover, ATP-citrate lyase, the cytosolic enzyme amenable for citrate cleavage to oxaloacetate and acetyl-CoA, is involved in metabolic regulation of the PI3K/Akt/mTOR pathway (6, 24, 74). Loss of the citrate synthase enzyme, and thus, decrease of citrate levels, was also linked to the induction of epithelial-to-mesenchymal transition, which confers to cancer cell invasion and metastasis (75).
Fumarate also exhibits HIF-independent mechanisms of oncogenesis through succination of proteins, a non-enzymatic irreversible process, where fumarate covalently binds to cysteine residues of proteins. Some of the succinated proteins were identified to be associated with tumorigenesis (62, 76).
Mutations in IDH1 or 2, resulting in conversion of α-ketoglutarate to oncometabolite, D-2-hydroxyglutarate (Figure 3), were identified only in one case of PHEO/PGL so far (77). Thus, these mutations do not seem to play an important role in PHEO/PGL pathogenesis.
Recent studies have stressed the interconnection between the Krebs cycle and epigenomic changes. Succinate and fumarate have the ability to remodel the epigenome and alter gene expression. An accumulation of succinate/fumarate results in inhibition of ten-eleven-translocation methylcytosine dioxygenase and in DNA hypermethylation (17, 78) (Figure 3). Moreover, the L-2-hydroxyglutarate, a product of LDHA and MDH1/2 metabolism in hypoxic cells, has been shown to regulate histone methylation and response to hypoxia (79, 80) (Figure 3). These new findings strengthen the role of hypoxic signaling in cancer.
Moreover, metabolomics studies in PHEO/PGL revealed a different metabolomics profile in SDHx-mutated tumors compared to sporadic or tumors with other mutations (30, 81). In SDHx-related PHEOs/PGLs, lower activity of SDH (mitochondrial electron transport chain complex II) enzyme and succinate accumulation has been observed compared to other PHEOs/PGLs (30). An interesting finding was increased activities of remaining complexes I, III, and IV of the mitochondrial electron transport chain and citrate synthase in SDHx-mutated tumors. However, the increased activities do not lead to full restoration of ATP/ADP/AMP. Imperiale et al. (81) also found increased glutamine levels in SDHx- mutated tumors, suggesting that glutamine metabolism is involved in pathogenesis of SDHx-related PHEOs/PGLs as well. PHEOs/PGLs exhibit differences in catecholamine synthesis and secretion. SDHx- and VHL-related tumors mostly produce norepinephrine and were found to be associated with lower catecholamine content compared to tumors with other mutations. PHEOs/PGLs associated with RET and NF1 mutations secrete both epinephrine and norepinephrine and exhibit low rate constants for catecholamine secretion (30, 81, 82). These differences in catecholamine synthesis and secretion may partially be explained by mutation-dependent changes in energy metabolism (30). In particular, ascorbate, which was found to be accumulated in PHEOs/PGLs, serves as a cofactor in the conversion of dopamine to norepinephrine and its levels were found to correlate with catecholamine concentrations (81). ATP production was found not to be impaired in SDH deficient PHEOs/PGLs (81), which suggests that the Krebs cycle and OXPHOS are not completely ‘turned off,’ and that they function together with glycolysis.
Thereby, in light of new research studies on PHEO/PGL, it is obvious that these tumors exhibit a variety of metabolic changes engaged in tumorigenesis. Thus, we can conclude that PHEOs/PGLs are in essence, metabolic endocrine tumors.
Perspectives and future therapeutic options
Our understanding of cancer pathophysiology and metabolism is a continuously evolving process. Recent progress in metabolomics and other ‘omics’-based research is providing us with new insights into the metabolic alterations of a cancer cell. Metabolic profiling is becoming an increasingly important tool in defining tumor phenotype and behavior, as well as in developing biomarkers for diagnosis and monitoring anticancer therapies. The latest studies on the PHEO/PGL metabolic profile revealed either an accumulation of certain proteins or a decrease in levels of other proteins. As described above, succinate levels in SDHx-mutated tumors were increased, suggesting succinate as a perspective biomarker for SDHx related PHEOs/PGLs. Moreover, these findings prove the usefulness of metabolic profiling using mass spectrometry analyses. Mass spectrometry should become an integral part of the routine diagnostic process preceding genetic testing, because based on the known genotype-metabolic phenotype relation, it could help to narrow the number of possible susceptibility gene mutations in a particular patient. Additionally, genotype-specific differences in tumor metabolite contents highlight the importance of metabolic imaging in tumor localization and patient follow-up (30). The metabolic profile of tumors will also serve as a basis for decision making in personalized and targeted anticancer therapy.
Our current understanding of the genetic, biochemical, and metabolic changes involved in tumorigenesis allows us to look for new, tumor specific therapeutic targets. Modifying or inhibiting the metabolic processes and enzymes that participate in metabolic reprogramming poses a promising therapeutic strategy (Table 2).
Table 2.
Potential metabolic therapeutic targets in PHEO/PGL
| Therapeutic target | Treatment effects |
|---|---|
|
| |
| HIF signaling (reviewed in 84, 93, 96) | |
|
| |
| HIF-α mRNA/protein expression | Inhibition of HIF-α mRNA or protein expression resulting in decreased HIF-α accumulation and activation |
| HIF-α/HIF-β dimerization | Inhibition of HIF-α/HIF-1β dimerization |
| HIF binding to DNA | Inhibition of HIF dimers binding to DNA |
| HIF transcriptional activity | Inhibition of transcription of HIF target genes |
| Hypoxia | Apoptosis of hypoxic cell |
| Angiogenesis | VEGF, VEGFR inhibition |
|
| |
| Glycolysis (reviewed in 86, 96) | |
|
| |
| Glucose uptake | Inhibition of glucose transport |
| HK 1/2 | Inhibition |
| PDK1 | Inhibition (to allow activity of PDH) |
| PFKB3 | Inhibition |
| LDHA | Inhibition |
| PKM2 | Induction of apoptosis |
| MCTs | Inhibition of lactate transport |
|
| |
| Glutaminolysis (reviewed in 87) | |
|
| |
| Glutamine uptake | Glutamine transporters inhibition |
| glutaminase | Inhibition |
| GOT2/GPT2 | Inhibition |
| GDH1 | Inhibition |
|
| |
| Fatty Acid and Lipid Synthesis (reviewed in 91, 97, 98) | |
|
| |
| ACLY | Inhibition |
| Acyl-CoA synthase | Inhibition |
| Acetyl-CoA carboxylase | Inhibition, induction of apoptosis/autophagy |
| Fatty acid synthase | Inhibition, induction of apoptosis |
| Choline kinase | Inhibition |
| Phospholipid metabolism | Inhibition |
|
| |
| Dysfunctional Krebs Cycle Enzymes And Metabolites (reviewed in 96, 99) | |
|
| |
| IDH1/2 | Inhibition of IDH mutants, inhibition of 2HG production |
| α-ketoglutarate-dependent dioxygenases | Restoring the function |
| Low citrate | Increase in citrate levels, inhibition of PFK, arrest of glycolysis, induction of apoptosis |
|
| |
| Proton Extrusion (reviewed in 96, 97) | |
|
| |
| Na+/H+ exchanger | Inhibition |
| Bicarbonate/Cl− exchanger | Inhibition |
| MCT1 lactate/H+ symporter | Inhibition |
| Carbonic anhydrases 9 and 12 | Inhibition |
| F1F0 ATP synthase | Inhibition |
| V-ATPase | Inhibition |
|
| |
| Other (reviewed in 97) | |
|
| |
| DNA methylation | Inhibition of methylation |
| AMPK | Activation |
| LAT1 | Inhibition of amino acid transport |
| SIRT1 | Stimulation of SIRT1-dependent deacetylation PGC1α |
| ROS | Neutralizing ROS by antioxidants to reduce HIF-α activation |
| ROS | Induction of ROS overproduction |
| Antioxidant systems (GSH) | Inhibition to achieve ROS accumulation |
Examples of drugs/compounds used or tested to achieve the desired treatment effect are provided in Supplementary Table S1.
Abbreviations: Acetyl-CoA, acetyl coenzyme A; ACLY, ATP citrate lyase; AMPK, AMP-activated protein kinase; ATP, adenosine triphosphate; GDH1, glutamate dehydrogenase 1; GOT2, glutamate oxaloacetate transaminase 2; GPT2, glutamate pyruvate transaminase 2; HIF, hypoxia-inducible factor; HK, hexokinase; IDH, isocitrate dehydrogenase; LAT1, L-type amino acid transporter 1; LDHA, lactate dehydrogenase A; MCT, monocarboxylase transporter; PDH, pyruvate dehydrogenase; PFK, phosphofructokinase; mRNA, messenger RNA; PGC1α, peroxisome proliferator-activated receptor-γ co-activator 1α; PKM2, pyruvate kinase, isoenzyme M2; ROS, reactive oxygen species; SIRT1, sirtuin 1.
In PHEO/PGL, the initial stages of tumor development are associated with hypoxia due to excessive growth and/or high metabolic activity and an insufficient oxygen supply. A subsequent switch to aerobic glycolysis provides cells with an increased chance of survival under hypoxic conditions (83). Thus, modifying/interrupting the HIF signaling pathway seems to be a promising therapeutic target. Several different approaches for HIF signaling pathway inhibition are undergoing testing. For instance, drugs inhibiting HIF mRNA or protein expression (such as antiangiogenic agents or heat shock protein 90 activity inhibitors). Activation of the HIF signaling pathway can also be compromised by inhibition of HIF dimerization, which is the step involved in HIF-α activation, or by inhibition of HIF binding to DNA and HIF transcriptional activity (24, 84) (Table 2 and Supplementary Table S1).
Since cancer cells metabolism is predominantly fueled by glucose and glutamine, altering the glucose and/or glutamine uptake and metabolism in cancer cells represents a promising treatment option. Glucose metabolism can be therapeutically altered at two levels: 1) glucose uptake (inhibition of glucose transporters) and 2) glycolytic enzymes (e.g. hexokinase 2, LDH, or PDK inhibitors) (85, 86). Another option is inhibitors of glutaminolysis or SLC1A5 glutamine transporter (87). Glucose and glutamine utilization is finely balanced and both nutrients function interdependently of tumor metabolism. Thus, simultaneous inhibition of both glycolysis and glutaminolysis may be also beneficial (88) (Table 2).
Restoring the enzymatic activity of nonfunctioning Krebs cycle enzymes, replenishing depleted substrates for the cycle, or inhibiting activity of overexpressed enzymes are the other options for targeted PHEO/PGL therapy. Therapeutic agents under development include small molecule inhibitors of certain proteins or drugs restoring the functionality of mitochondrial enzymes (24). For instance, in SDHB-deficient cells, stability and total amount of mitochondrial SDHB protein can be increased by proteostasis regulators, such as histone deacetylase inhibitors (89). An inhibition of PDKs reverses the Warburg effect, increases OXPHOS, and thus, inhibits cancer cell proliferation (90). Another therapeutic possibility is the small-molecule inhibition of key enzymes involved in metabolic pathways such as lipids and fatty acid synthesis. Several inhibitors of lipid biosynthesis are under investigation. For example, fatty acid synthase inhibitors were shown to selectively target cancer cells for apoptosis (91). Metabolic changes in PHEOs/PGLs also affect DNA methylation. Tumors driven by VHL mutations display promoter hypermethylation of a few targets and prevalent hypomethylation outside CpG islands. Although hypomethylation has been associated with genomic instability, its significance in VHL-mutated PHEOs/PGLs is not clear, since the increase in chromosome instability in these tumors has not been observed (92). SDH- and FH- mutated tumors are characterized by a hypermethylator phenotype (17), suggesting that DNA demethylating agents can also be utilized in treatment of some PHEOs/PGLs (93).
Conclusions
PHEOs/PGLs are tumors resulting from various genetic, epigenetic, and metabolic changes. Treatment of PHEOs/PGLs (especially when they present as metastatic disease) is challenging, and still in early stages. This review provides novel insight regarding the metabolic disturbances we believe will soon be utilized in the treatment of metastatic disease. Many of these disturbances are related to recent discoveries of new cell metabolism-related genes, many of them in the Krebs cycle, well-proven to be linked to PHEO/PGL pathogenesis. Continuous search for additional metabolic changes in these tumors will undoubtedly result in the identification of new diagnostic methods, strategies, and therapeutic targets.
Supplementary Material
Acknowledgments
The authors thank Katherine I Wolf, BS for her technical assistance. This research was supported, in part, by the Intramural Research Program of the National Institutes of Health, NICHD.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of potential conflicts of interest: No potential conflicts of interest were disclosed.
References
- 1.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 2.Metallo CM, Vander Heiden MG. Understanding metabolic regulation and its influence on cell physiology. Mol Cell. 2013;49:388–98. doi: 10.1016/j.molcel.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8:519–30. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu J, Tan M, Cai Q. The Warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett. 2015;356:156–64. doi: 10.1016/j.canlet.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kruspig B, Zhivotovsky B, Gogvadze V. Mitochondrial substrates in cancer: drivers or passengers? Mitochondrion. 2014;19(Pt A):8–19. doi: 10.1016/j.mito.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Dang CV. Links between metabolism and cancer. Genes & development. 2012;26:877–90. doi: 10.1101/gad.189365.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 9.DeLellis RA, Lloyd RV, Heitz PU, Eng C, editors. WHO classification of tumours of endocrine organs. Lyon (France): IARC Press; 2004. [Google Scholar]
- 10.Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366:665–75. doi: 10.1016/S0140-6736(05)67139-5. [DOI] [PubMed] [Google Scholar]
- 11.Favier J, Gimenez-Roqueplo AP. Pheochromocytomas: The (pseudo)-hypoxia hypothesis. Best Pract Res Clin Endocrinol Metab. 2010;24:957–68. doi: 10.1016/j.beem.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Kantorovich V, Pacak K. Pheochromocytoma and paraganglioma. Prog Brain Res. 2010;182:343–73. doi: 10.1016/S0079-6123(10)82015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benn DE, Gimenez-Roqueplo AP, Reilly JR, Bertherat J, Burgess J, Byth K, et al. Clinical presentation and penetrance of pheochromocytoma/paraganglioma syndromes. J Clin Endocrinol Metab. 2006;91:827–36. doi: 10.1210/jc.2005-1862. [DOI] [PubMed] [Google Scholar]
- 14.Ayala-Ramirez M, Feng L, Johnson MM, Ejaz S, Habra MA, Rich T, et al. Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: primary tumor size and primary tumor location as prognostic indicators. J Clin Endocrinol Metab. 2011;96:717–25. doi: 10.1210/jc.2010-1946. [DOI] [PubMed] [Google Scholar]
- 15.Brouwers FM, Eisenhofer G, Tao JJ, Kant JA, Adams KT, Linehan WM, et al. High frequency of SDHB germline mutations in patients with malignant catecholamine-producing paragangliomas: implications for genetic testing. J Clin Endocrinol Metab. 2006;91:4505–9. doi: 10.1210/jc.2006-0423. [DOI] [PubMed] [Google Scholar]
- 16.Eisenhofer G, Lenders JW, Siegert G, Bornstein SR, Friberg P, Milosevic D, et al. Plasma methoxytyramine: a novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. Eur J Cancer. 2012;48:1739–49. doi: 10.1016/j.ejca.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Letouze E, Martinelli C, Loriot C, Burnichon N, Abermil N, Ottolenghi C, et al. SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell. 2013;23:739–52. doi: 10.1016/j.ccr.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 18.Jochmanova I, Yang C, Zhuang Z, Pacak K. Hypoxia-inducible factor signaling in pheochromocytoma: turning the rudder in the right direction. J Natl Cancer Inst. 2013;105:1270–83. doi: 10.1093/jnci/djt201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chae YC, Angelin A, Lisanti S, Kossenkov AV, Speicher KD, Wang H, et al. Landscape of the mitochondrial Hsp90 metabolome in tumours. Nat Commun. 2013;4:2139. doi: 10.1038/ncomms3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Cubas AA, Korpershoek E, Inglada-Perez L, Letouze E, Curras-Freixes M, Fernandez AF, et al. DNA methylation profiling in pheochromocytoma and paraganglioma reveals diagnostic and prognostic markers. Clin Cancer Res. 2015;21:3020–30. doi: 10.1158/1078-0432.CCR-14-2804. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan LB, Chandel NS. Mitochondrial metabolism in TCA cycle mutant cancer cells. Cell Cycle. 2014;13:347–8. doi: 10.4161/cc.27513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheffler IE. Mitochondria. 2. Hoboken (NJ): John Wiley & Sons; 2008. [Google Scholar]
- 23.Desideri E, Vegliante R, Ciriolo MR. Mitochondrial dysfunctions in cancer: genetic defects and oncogenic signaling impinging on TCA cycle activity. Cancer Lett. 2015;356:217–23. doi: 10.1016/j.canlet.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 24.Jochmanova I, Zhuang Z, Pacak K. Pheochromocytoma: gasping for air. Horm Cancer. 2015;6:191–205. doi: 10.1007/s12672-015-0231-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 26.Yeung SJ, Pan J, Lee MH. Roles of p53, MYC and HIF-1 in regulating glycolysis - the seventh hallmark of cancer. Cell Mol Life Sci. 2008;65:3981–99. doi: 10.1007/s00018-008-8224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dang CV, Lewis BC, Dolde C, Dang G, Shim H. Oncogenes in tumor metabolism, tumorigenesis, and apoptosis. J Bioenerg Biomembr. 1997;29:345–54. doi: 10.1023/a:1022446730452. [DOI] [PubMed] [Google Scholar]
- 29.Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer. 2008;8:705–13. doi: 10.1038/nrc2468. [DOI] [PubMed] [Google Scholar]
- 30.Rao JU, Engelke UF, Rodenburg RJ, Wevers RA, Pacak K, Eisenhofer G, et al. Genotype-specific abnormalities in mitochondrial function associate with distinct profiles of energy metabolism and catecholamine content in pheochromocytoma and paraganglioma. Clin Cancer Res. 2013;19:3787–95. doi: 10.1158/1078-0432.CCR-12-3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325–37. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 32.Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Semenza GL. Tumor metabolism: cancer cells give and take lactate. J Clin Invest. 2008;118:3835–7. doi: 10.1172/JCI37373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonuccelli G, Tsirigos A, Whitaker-Menezes D, Pavlides S, Pestell RG, Chiavarina B, et al. Ketones and lactate “fuel” tumor growth and metastasis: evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle. 2010;9:3506–14. doi: 10.4161/cc.9.17.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez-Outschoorn UE, Prisco M, Ertel A, Tsirigos A, Lin Z, Pavlides S, et al. Ketones and lactate increase cancer cell “stemness,” driving recurrence, metastasis and poor clinical outcome in breast cancer: achieving personalized medicine via metabolo-genomics. Cell Cycle. 2011;10:1271–86. doi: 10.4161/cc.10.8.15330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baenke F, Peck B, Miess H, Schulze A. Hooked on fat: the role of lipid synthesis in cancer metabolism and tumour development. Dis Model Mech. 2013;6:1353–63. doi: 10.1242/dmm.011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayers JR, Vander Heiden MG. Famine versus feast: understanding the metabolism of tumors in vivo. Trends Biochem Sci. 2015;40:130–40. doi: 10.1016/j.tibs.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berwick DC, Hers I, Heesom KJ, Moule SK, Tavare JM. The identification of ATP-citrate lyase as a protein kinase B (Akt) substrate in primary adipocytes. J Biol Chem. 2002;277:33895–900. doi: 10.1074/jbc.M204681200. [DOI] [PubMed] [Google Scholar]
- 39.Bauer DE, Hatzivassiliou G, Zhao F, Andreadis C, Thompson CB. ATP citrate lyase is an important component of cell growth and transformation. Oncogene. 2005;24:6314–22. doi: 10.1038/sj.onc.1208773. [DOI] [PubMed] [Google Scholar]
- 40.Phan LM, Yeung SC, Lee MH. Cancer metabolic reprogramming: importance, main features, and potentials for precise targeted anti-cancer therapies. Cancer Biol Med. 2014;11:1–19. doi: 10.7497/j.issn.2095-3941.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones RG, Thompson CB. Tumor suppressors and cell metabolism: a recipe for cancer growth. Genes Dev. 2009;23:537–48. doi: 10.1101/gad.1756509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maxwell JE, KSS, Howe JR. Translational diagnostics and therapeutics in pancreatic neuroendocrine tumors. Clin Cancer Res. 2016;22 doi: 10.1158/1078-0432.CCR-16-0435. xxx-xxx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gordan JD, Simon MC. Hypoxia-inducible factors: central regulators of the tumor phenotype. Curr Opin Genet Dev. 2007;17:71–7. doi: 10.1016/j.gde.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012;33:207–14. doi: 10.1016/j.tips.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hensley CT, Wasti AT, DeBerardinis RJ. Glutamine and cancer: cell biology, physiology, and clinical opportunities. J Clin Invest. 2013;123:3678–84. doi: 10.1172/JCI69600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anastasiou D, Cantley LC. Breathless cancer cells get fat on glutamine. Cell Res. 2012;22:443–6. doi: 10.1038/cr.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang C, Ko B, Hensley CT, Jiang L, Wasti AT, Kim J, et al. Glutamine oxidation maintains the TCA cycle and cell survival during impaired mitochondrial pyruvate transport. Mol Cell. 2014;56:414–24. doi: 10.1016/j.molcel.2014.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neumann HP, Bausch B, McWhinney SR, Bender BU, Gimm O, Franke G, et al. Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med. 2002;346:1459–66. doi: 10.1056/NEJMoa020152. [DOI] [PubMed] [Google Scholar]
- 49.Gimenez-Roqueplo AP, Dahia PL, Robledo M. An Update on the genetics of paraganglioma, pheochromocytoma, and associated hereditary syndromes. Horm Metab Res. 2012;44:328–33. doi: 10.1055/s-0031-1301302. [DOI] [PubMed] [Google Scholar]
- 50.Jochmanova I, Zelinka T, Widimsky J, Jr, Pacak K. HIF signaling pathway in pheochromocytoma and other neuroendocrine tumors. Physiol Res. 2014;63(Suppl 2):S251–62. doi: 10.33549/physiolres.932789. [DOI] [PubMed] [Google Scholar]
- 51.Burnichon N, Buffet A, Gimenez-Roqueplo AP. Pheochromocytoma and paraganglioma: molecular testing and personalized medicine. Curr Opin Oncol. 2016;28:5–10. doi: 10.1097/CCO.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 52.Pillai S, Gopalan V, Smith RA, Lam AK. Updates on the genetics and the clinical impacts on phaeochromocytoma and paraganglioma in the new era. Crit Rev Oncol Hematol. 2016;100:190–208. doi: 10.1016/j.critrevonc.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 53.Linehan WM, Ricketts CJ. The metabolic basis of kidney cancer. Semin Cancer Biol. 2013;23:46–55. doi: 10.1016/j.semcancer.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaelin WG., Jr The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer. 2008;8:865–73. doi: 10.1038/nrc2502. [DOI] [PubMed] [Google Scholar]
- 55.Semenza GL. Oxygen Sensing, Homeostasis, and Disease. New Engl J Med. 2011;365:537–47. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- 56.Vicha A, Taieb D, Pacak K. Current views on cell metabolism in SDHx-related pheochromocytoma and paraganglioma. Endocr Relat Cancer. 2014;21:R261–77. doi: 10.1530/ERC-13-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8:967–75. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wise DR, Ward PS, Shay JE, Cross JR, Gruber JJ, Sachdeva UM, et al. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc Natl Acad Sci U S A. 2011;108:19611–6. doi: 10.1073/pnas.1117773108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–34. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pollard PJ, Briere JJ, Alam NA, Barwell J, Barclay E, Wortham NC, et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum Mol Genet. 2005;14:2231–9. doi: 10.1093/hmg/ddi227. [DOI] [PubMed] [Google Scholar]
- 61.Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 62.Yang M, Ternette N, Su H, Dabiri R, Kessler BM, Adam J, et al. The succinated proteome of FH-mutant tumours. Metabolites. 2014;4:640–54. doi: 10.3390/metabo4030640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cascon A, Comino-Mendez I, Curras-Freixes M, de Cubas AA, Contreras L, Richter S, et al. Whole-exome sequencing identifies MDH2 as a new familial paraganglioma gene. J Natl Cancer Inst. 2015:107. doi: 10.1093/jnci/djv053. [DOI] [PubMed] [Google Scholar]
- 64.Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest. 2013;123:3664–71. doi: 10.1172/JCI67230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fliedner SM, Kaludercic N, Jiang XS, Hansikova H, Hajkova Z, Sladkova J, et al. Warburg effect’s manifestation in aggressive pheochromocytomas and paragangliomas: insights from a mouse cell model applied to human tumor tissue. PLoS One. 2012;7:e40949. doi: 10.1371/journal.pone.0040949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soga T. Cancer metabolism: key players in metabolic reprogramming. Cancer Sci. 2013;104:275–81. doi: 10.1111/cas.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Favier J, Briere JJ, Burnichon N, Riviere J, Vescovo L, Benit P, et al. The Warburg effect is genetically determined in inherited pheochromocytomas. PLoS One. 2009;4:e7094. doi: 10.1371/journal.pone.0007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gimenez-Roqueplo AP, Favier J, Rustin P, Mourad JJ, Plouin PF, Corvol P, et al. The R22X mutation of the SDHD gene in hereditary paraganglioma abolishes the enzymatic activity of complex II in the mitochondrial respiratory chain and activates the hypoxia pathway. Am J Hum Genet. 2001;69:1186–97. doi: 10.1086/324413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gimenez-Roqueplo AP, Favier J, Rustin P, Rieubland C, Kerlan V, Plouin PF, et al. Functional consequences of a SDHB gene mutation in an apparently sporadic pheochromocytoma. J Clin Endocrinol Metab. 2002;87:4771–4. doi: 10.1210/jc.2002-020525. [DOI] [PubMed] [Google Scholar]
- 70.Guzy RD, Sharma B, Bell E, Chandel NS, Schumacker PT. Loss of the SdhB, but Not the SdhA, subunit of complex II triggers reactive oxygen species-dependent hypoxia-inducible factor activation and tumorigenesis. Mol Cell Biol. 2008;28:718–31. doi: 10.1128/MCB.01338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goffrini P, Ercolino T, Panizza E, Giache V, Cavone L, Chiarugi A, et al. Functional study in a yeast model of a novel succinate dehydrogenase subunit B gene germline missense mutation (C191Y) diagnosed in a patient affected by a glomus tumor. Hum Mol Genet. 2009;18:1860–8. doi: 10.1093/hmg/ddp102. [DOI] [PubMed] [Google Scholar]
- 72.Mannelli M, Rapizzi E, Fucci R, Canu L, Ercolino T, Luconi M, et al. 15 YEARS OF PARAGANGLIOMA: Metabolism and pheochromocytoma/paraganglioma. Endocr Relat Cancer. 2015;22:T83–90. doi: 10.1530/ERC-15-0215. [DOI] [PubMed] [Google Scholar]
- 73.Richter S, Peitzsch M, Rapizzi E, Lenders JW, Qin N, de Cubas AA, et al. Krebs cycle metabolite profiling for identification and stratification of pheochromocytomas/paragangliomas due to succinate dehydrogenase deficiency. J Clin Endocrinol Metab. 2014;99:3903–11. doi: 10.1210/jc.2014-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iacobazzi V, Infantino V. Citrate--new functions for an old metabolite. Biol Chem. 2014;395:387–99. doi: 10.1515/hsz-2013-0271. [DOI] [PubMed] [Google Scholar]
- 75.Lin CC, Cheng TL, Tsai WH, Tsai HJ, Hu KH, Chang HC, et al. Loss of the respiratory enzyme citrate synthase directly links the Warburg effect to tumor malignancy. Sci Rep. 2012;2:785. doi: 10.1038/srep00785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Merkley ED, Metz TO, Smith RD, Baynes JW, Frizzell N. The succinated proteome. Mass Spectrom Rev. 2014;33:98–109. doi: 10.1002/mas.21382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gaal J, Burnichon N, Korpershoek E, Roncelin I, Bertherat J, Plouin PF, et al. Isocitrate dehydrogenase mutations are rare in pheochromocytomas and paragangliomas. J Clin Endocrinol Metab. 2010;95:1274–8. doi: 10.1210/jc.2009-2170. [DOI] [PubMed] [Google Scholar]
- 78.Castro-Vega LJ, Buffet A, De Cubas AA, Cascon A, Menara M, Khalifa E, et al. Germline mutations in FH confer predisposition to malignant pheochromocytomas and paragangliomas. Hum Mol Genet. 2014;23:2440–6. doi: 10.1093/hmg/ddt639. [DOI] [PubMed] [Google Scholar]
- 79.Intlekofer AM, Dematteo RG, Venneti S, Finley LW, Lu C, Judkins AR, et al. Hypoxia induces production of L-2-hydroxyglutarate. Cell Metab. 2015;22:304–11. doi: 10.1016/j.cmet.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oldham WM, Clish CB, Yang Y, Loscalzo J. Hypoxia-mediated increases in L-2-hydroxyglutarate coordinate the metabolic response to reductive stress. Cell Metab. 2015;22:291–303. doi: 10.1016/j.cmet.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Imperiale A, Moussallieh FM, Roche P, Battini S, Cicek AE, Sebag F, et al. Metabolome profiling by HRMAS NMR spectroscopy of pheochromocytomas and paragangliomas detects SDH deficiency: clinical and pathophysiological implications. Neoplasia. 2015;17:55–65. doi: 10.1016/j.neo.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Eisenhofer G, Pacak K, Huynh TT, Qin N, Bratslavsky G, Linehan WM, et al. Catecholamine metabolomic and secretory phenotypes in phaeochromocytoma. Endocr Relat Cancer. 2011;18:97–111. doi: 10.1677/ERC-10-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fang JS, Gillies RD, Gatenby RA. Adaptation to hypoxia and acidosis in carcinogenesis and tumor progression. Semin Cancer Biol. 2008;18:330–7. doi: 10.1016/j.semcancer.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wigerup C, Pahlman S, Bexell D. Review: Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer. Pharmacol Ther. 2016;164:152–69. doi: 10.1016/j.pharmthera.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 85.Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–46. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- 86.Granja S, Pinheiro C, Reis RM, Martinho O, Baltazar F. Glucose addiction in cancer therapy: advances and drawbacks. Curr Drug Metab. 2015;16:221–42. doi: 10.2174/1389200216666150602145145. [DOI] [PubMed] [Google Scholar]
- 87.Jin L, Alesi GN, Kang S. Glutaminolysis as a target for cancer therapy. Oncogene. 2016;35:3619–25. doi: 10.1038/onc.2015.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cervantes-Madrid D, Romero Y, Duenas-Gonzalez A. Reviving lonidamine and 6-diazo-5-oxo-L-norleucine to be used in combination for metabolic cancer therapy. Biomed Res Int. 2015;2015:690492. doi: 10.1155/2015/690492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang C, Matro JC, Huntoon KM, Ye DY, Huynh TT, Fliedner SM, et al. Missense mutations in the human SDHB gene increase protein degradation without altering intrinsic enzymatic function. FASEB J. 2012;26:4506–16. doi: 10.1096/fj.12-210146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang SL, Hu X, Zhang W, Tam KY. Unexpected discovery of dichloroacetate derived adenosine triphosphate competitors targeting pyruvate dehydrogenase kinase to inhibit cancer proliferation. J Med Chem. 2016;59:3562–8. doi: 10.1021/acs.jmedchem.5b01828. [DOI] [PubMed] [Google Scholar]
- 91.Mullen GE, Yet L. Progress in the development of fatty acid synthase inhibitors as anticancer targets. Bioorg Med Chem Lett. 2015;25:4363–9. doi: 10.1016/j.bmcl.2015.08.087. [DOI] [PubMed] [Google Scholar]
- 92.Castro-Vega LJ, Letouze E, Burnichon N, Buffet A, Disderot PH, Khalifa E, et al. Multi-omics analysis defines core genomic alterations in pheochromocytomas and paragangliomas. Nat Commun. 2015;6:6044. doi: 10.1038/ncomms7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Favier J, Amar L, Gimenez-Roqueplo AP. Paraganglioma and phaeochromocytoma: from genetics to personalized medicine. Nat Rev Endocrinol. 2015;11:101–11. doi: 10.1038/nrendo.2014.188. [DOI] [PubMed] [Google Scholar]
- 94.Bjorklund P, Pacak K, Crona J. Precision medicine in pheochromocytoma and paraganglioma: current and future concepts. J Intern Med. 2016 May 10; doi: 10.1111/joim.12507. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Luchetti A, Walsh D, Rodger F, Clark G, Martin T, Irving R, et al. Profiling of somatic mutations in phaeochromocytoma and paraganglioma by targeted next generation sequencing analysis. Int J Endocrinol. 2015;2015:138573. doi: 10.1155/2015/138573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Justus CR, Sanderlin EJ, Yang LV. Molecular Connections between Cancer Cell Metabolism and the Tumor Microenvironment. Int J Mol Sci. 2015;16:11055–86. doi: 10.3390/ijms160511055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13:472–82. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 98.Mashima T, Seimiya H, Tsuruo T. De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br J Cancer. 2009;100:1369–72. doi: 10.1038/sj.bjc.6605007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vander Heiden MG. Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov. 2011;10:671–84. doi: 10.1038/nrd3504. [DOI] [PubMed] [Google Scholar]
- 100.Sonnewald U. Glutamate synthesis has to be matched by its degradation - where do all the carbons go? J Neurochem. 2014;131:399–406. doi: 10.1111/jnc.12812. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.