Abstract
Background:
Percutaneous endoscopic gastrostomy (PEG) is used to provide enteral access in patients who are unable to swallow. Infection of the stoma is an important complication and there is little data from India on this problem, which can be used to inform infection prevention and prophylactic strategies.
Aim:
The objective was to assess the prevalence and the role of contributory factors in PEG site infections.
Methods:
A total of 173 patients underwent PEG insertion from January 2011 to May 2012. Clinical and microbiological data were collected for culture-positive cases. Insertion was performed using a standard sterile pull-through technique. Infections were defined as two of: peristomal erythema, induration, and purulent discharge.
Results:
A total of 54 PEG infections occurred in 43 patients (28.85%). Seventy-seven organisms were isolated. Pseudomonas aeruginosa was the most common (n=29) followed by coliforms (n=21) and meticillin resistant Staphylococcus aureus (MRSA) (n=6). Thirty-one (72%) received amoxicillin-clavulanic acid as prophylaxis and 12 (28%) were receiving concomitant antibiotics for their underlying conditions. The occurrence of PEG site infections was statistically independent of the administered prophylactic antibiotics (p=0.3).
Conclusions:
This study has demonstrated the importance of PEG sites as a cause of healthcare associated infections. Educating patients on wound care practices would play a significant role in prevention of PEG site infections.
Keywords: India, infection prevention, PEG site infections
Introduction
Percutaneous endoscopic gastrostomy (PEG) is used to provide enteral access in patients with a normally functioning gut who are unable to swallow The access site secured on the abdominal wall is the PEG site, and the procedure is usually performed malignancy, neurological conditions, and in bedridden patients. From 1980 it has been preferred to gastrostomy because it takes less time, carries less risk and costs less. One third of patients with a PEG develop various complications such as dislodgement, leakage and infection. Infection of the stoma is an important complication, with frequency ranging from 6%–34% in different settings (Gauderer et al, 1980; McClave and Chang, 2003; Janes Simon et al, 2005; Mahadeva et al, 2009). A 30-day mortality rate of 4.1%–26% has been reported for stomal infections (Abuksis et al, 2000 ). PEG stoma infection can compromise patients’ quality of life and impact on morbidity. Meticillin resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa, and Gram negative enteric bacteria have been implicated as a cause of these infection (Chaudhary et al, 2002; Gopal Rao et al, 2004; Maine et al, 2006; Duarte et al, 2012a,b). Single dose broad-spectrum antibiotic prophylaxis such as cephalosporins reduces the incidence with absolute risk reduction of 14%–17% (Allison et al, 2009). Generally diagnosed within 30 days post insertion, infections may occasionally be reported even months later. There is sparse microbiological data from India as the facility exists in only a few tertiary centres across the country. The objective of our study was to assess the prevalence, the aetiology of infections and the role of contributory factors (if any) in the prevention of PEG site infections.
Methodology
A total of 173 patients who had undergone PEG insertion in our institution from January 2011 to May 2012 (1.5 years) were enrolled into this cross-sectional study. Demographic (age, sex, risk factors, antibiotic prophylaxis administered) and microbiological data of culture-positive cases who presented with infection during their follow-up appointment during the first month were retrospectively collected. Microbiological processing included plating of samples on routine sheep blood agar and MacConkey agar with manual biochemical identification. Wound discharge and wound swabs were the principal samples. Insertion was performed using a standard sterile, pull-through technique. All patients received the routine postoperative betadine- dressings after the insertion and were recommended to keep the wound clean with soap and clean water after discharge. Infections were defined as having at least two of the following: peristomal erythema, induration, purulent discharge – with a positive culture report/microscopic finding as suggested by Jain and Larson (1987). Positive growth with organisms but no clinical signs were regarded as colonisation and were excluded in the study. Data on PEG site wound care practices could not be collected.
Results
A total of 54 episodes of PEG infections occurred in 43 patients (28.85%). A single episode was noted in 34 patients, two episodes in seven patients and three episodes in two patients. In the first three months, 44 infections were reported and in the next three months, nine were reported. Thirty-six were males and seven were females. Most (36) were >50 years (15 in the 60–70 years range, 13 in the 50–60 years range and 8 in the 70–80 years range). Indications for PEG were malignancy (35 patients), stroke from neurosurgery (seven patients) and one patient with ventilator associated pneumonia and encephalopathy. A total of 77 organisms were isolated. Pseudomonas aeruginosa was the most common (n=29) followed by Coliforms (n=21), MRSA (n=6) and others (Table 1). Eight Gram negative Multi-drug resistant organisms (MDROs), seven ESBL producing Enterobacteriaceae and three Col-S Acinetobacter baumanni were isolated. Polymicrobial infection (>/=2 pathogens) was noted in 19 patients. Gram stain microscopy with pus cells of the positive samples complemented the culture in 27 (62%) of the patients. All the infections were local (cellulits, abscess, and ulcers). No concomitant bacteraemia or attributable mortality was noted during the episodes. Of the 43 infected patients, 31 (72%) received amoxicillin-clavulanic acid (hospital policy) as prophylaxis and 12 (28%) were receiving concomitant antibiotics for other pre-existing infections. However, PEG site infections were, statistically independent of the administered prophylactic antibiotics (p=0.3, chi-square test). Of the 43 infected patients, 35 were diabetic and on medications (p=0.001). All received culture sensitive antibiotics, levofloxacin (n=18) being the most commonly used. Figure 1 shows the monthly distribution of PEG insertions and infections during the study period.
Table 1.
Organisms causing PEG site infections during the study period.
| Organism (n=77) | Number (%) |
|---|---|
| Pseudomonas aeruginosa | 29 (37.6) |
| Klebsiella pneumoniae | 15 (19.4) |
| Escherichia coli | 3 (3.8) |
| Enterobacter Spp | 3 (3.8) |
| Meticillin resistant Staphylococcus aureus | 6 (7.7) |
| Meticillin susceptible Staphylococcus aureus | 2 (2.5) |
| Acinetobacter baumanni | 4 (5.1) |
| Candida albicans | 6 (7.7) |
| Candida tropicalis | 6 (7.7) |
| Enterococcus faecalis | 2 (2.5) |
| Proteus mirabilis | 1 (1.2) |
Figure 1.
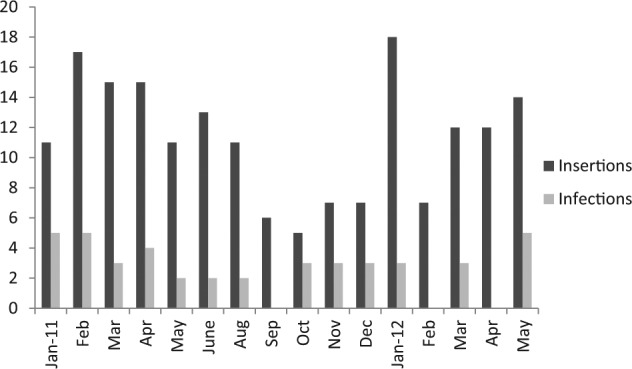
Monthly comparison of number of PEG-insertions and infections in the study.
Discussion
There is considerable data on the prevalence of PEG site infections from across the world, as in Brisbane, Australia (32%), 17% from Kansas, USA, and Pakistan (12%) with a variation in the number of patients and the duration studied (Karman et al, 2002; Davis et al, 2004; Khokhar et al, 2005). The prevalence rate from our study was found to be 28.8%. To the best of our knowledge, this study is the first descriptive hospital epidemiological study in India on PEG site infections. The only other study on PEG at Pune looked at patients with ventilator associated pneumonia and did not document any PEG site infection (Date et al, 2007). The most common indications for PEG were malignancy (oral and gastrointestinal) as in other studies (Dormann et al, 2001). Diabetes mellitus (p=0.001) was found to be common in most of the patients in our study (Lee et al, 2002). However, we did not assess other risk factors such as obesity, lower nutritional status and steroid therapy. The common organisms in our study were P. aeruginosa (37%), Klebsiella pneumoniae (19.4%) and Candida Spp (15.4%) and others (Table 1).
The three major determinants of PEG infections are antibiotic prophylaxis, insertion techniques and wound care practices. Several studies have shown a positive effect of antibiotic prophylaxis in reducing the risk of infection after PEG insertions (Preclick et al, 1999; Sharma and Howden, 2000; Adnan et al, 2011; Durate et al, 2012). Although, a single intravenous dose of cefuroxime one hour prior to the procedure is recommended by the British Society of Gastroenterology (Allison et al, 2009), the current hospital policy recommends the prophylaxis of amoxicillin-clavulanic acid one hour before PEG insertions (Preclik et al, 1999) as S. aureus was the most common causative agent from 2005. This prophylaxis is no longer appropriate since the study has indicated the current predominance of P. aeruginosa. The current prophylaxis guidelines are now under review in the light of the results of this study. Also, due to the increasing incidence of MRSA noted in many hospitals, cephalosporins may no longer be appropriate. It is therefore suggested that the antibiotic prophylaxis should be adjusted according to periodic reviews of the local organisms.
The study results clearly indicate that it is time for PEG site infections to be given serious consideration at specialty centers, where the procedures are carried out in developing countries. The patients were followed up only during the first month after insertion and the documented number of infections could have been an under-estimate due to a lack of self-reporting or reporting elsewhere after this period. Awareness about the significance of this infection is important among surgeons, clinical microbiologists and infection control specialists and more data needs to be reported across the country. A proposed multi-strategy preventive approach (being studied further) may be employed to reduce the high rates. vigilant surveillance focused on PEG site inflections in addition to the main four HCAI may be necessary. Extended post discharge surveillance, which is not currently conducted, especially at outpatient follow-up clinics, would help to estimate the true burden of this infection. A second strategy would be to strengthen the antimicrobial stewardship practices by periodically reviewing and updating institutional guidelines in this era of antibiotic resistance. Patient education is a third strategy, with discharge instructions to PEG site recipients about wound care and hygiene. Reporting of potential infections would help to improve PEG site management and reduce rates of infection.
Conclusion
More attention should be paid to PEG site infections, especially in developing countries where most hospitals are in their infancy in relation to infection prevention. This study provides distinct descriptive data regarding PEG site infections from India for the first time. The current microbiological data should be seen as a guide to infection prevention and treatment strategies in hospitals. Hospital infection control teams must extend surveillance to this important healthcare associated infection as a part of surgical site infections. Strengthening the antimicrobial stewardship, and tailoring antibiotic prophylaxis to local organisms will reduce PEG site infection. Hospitals should have an antibiotic prophylaxis policy in place for insertions, and guidelines specifying the type of dressings, frequency of dressing and cleansing agent usage. Discharge education to patients on wound care practices such as sterile dressings, hand hygiene and topical antiseptics would play a significant role in the prevention of PEG site infections.
Acknowledgments
Thanks go to Sister Fini, Infection Control Nurse, Amrita Institute of Medical Sciences & Research Center, Cochin, Kerala, India for facilitating the study.
Footnotes
Authors’ note: Oral presentation at National Conference, Microbiology (MICROCON), New Delhi, November 2012
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Peer review statement: Not commissioned; blind peer-reviewed.
References
- Abuksis G, Mor M, Segal N, Shemesh I, Plout S, Sulkes J, et al. (2000) Percutaneous endoscopic gastrostomy: high mortality rates in hospitalized patients. American Journal of Gastroenterology 95: 128–132. [DOI] [PubMed] [Google Scholar]
- Agha A, Al Saudi D, Furnari M, Abdullhadi MM, Al-Majadah SSA, Savarino V, et al. (2011) Efficacy of 48-hr post-operative antibiotics prophylaxis for patients undergoing percutaneous endoscopic gastrostomy tube in preventing site infection. Journal of Gastrointestinal and Liver Diseases 20(2): 131–134. [PubMed] [Google Scholar]
- Allison MC, Sandoe JA, Tighe R, Simpson IA, Hall RJ, Elliot TS. (2009) Antibiotic prophylaxis in gastrointestinal endoscopy. Gut 58: 869–880. [DOI] [PubMed] [Google Scholar]
- Chaudhary KA, Smith OJ, Cuddy PG, Clarkston WK. (2002) PEG site infections: the emergence of methicillin resistant Staphylococcus aureus as a major pathogen. American Journal of Gastroenterology 97: 1713–1716. [DOI] [PubMed] [Google Scholar]
- Date SV, Pillai LV, Vaidya GV, Vaidya NV, Hussainy SMK. (2007) Feasibility, safety, efficacy of percutaneous endoscopic gastrostomy on ventilated patients in ICU. Indian Journal of Critical Care Medicine 11(3): 109–111. [Google Scholar]
- Davis JP, Entrop M, Read SJ. (2004) The incidence of percutaneous gastrostomy infection and variation in wound care practices. Primary Intention 12(2): 73–80. [Google Scholar]
- Dormann AJ, Huchzermeyer H, Lippert H. (2001) The relevance of systemic complications and the different outcomes of subgroups after percutaneous endoscopic gastrostomy (PEG). American Journal of Gastroenterology 96: 1951–1952. [DOI] [PubMed] [Google Scholar]
- Duarte H, Alcobia A, Fonseca J, Capelas ML. (2012a) Should peristomal infection after percutaneous endoscopic gastrostomy be considered a health-care associated infection? Role of antibiotic prophylaxis. European Journal of Hospital Pharmacy 19(2): 255–258. [Google Scholar]
- Duarte H, Santos C, Capelas ML, Fonseca J. (2012b) Peristomal infection after percutaneous endoscopic gastrostomy: 7-year surveillance of 297 patients. Arq Gastroenterol 49(4): 255–258. [DOI] [PubMed] [Google Scholar]
- Gauderer MW, Ponsky JL, Izant KJ., Jr. (1980) Gastrostomy without laprotomy: a percutaneous endoscopic technique. Journal of Pediatric Surgery 15: 872–875. [DOI] [PubMed] [Google Scholar]
- Rao GG, Osman M, Johnson L, Ramsey D, Jones S, Fidler H, et al. (2004) Prevention of percutaneous endoscopic gastrostomy site infections caused by methicillin-resistant Staphylococcus aureus. Journal of Hospital Infection 58(1): 81–83. [DOI] [PubMed] [Google Scholar]
- Jain NK, Larson DE. (1987) Antibiotic prophylaxis for percutaneous endoscopic gastrostomy. A prospective, randomized, double-blind clinical trial. Annals of Internal Medicine 107: 824–828. [DOI] [PubMed] [Google Scholar]
- Janes Simon EJ, Price CSG, Khan S. (2005) Percutaneous endoscopic gastrostomy: 30- day mortality trends and risk factors. Journal of Postgraduate Medicine 51(1): 23–29. [PubMed] [Google Scholar]
- Khokhar N, Gill ML. (2005) Percutaneous endoscopic gastrostomy: nine years’ experience in a tertiary care center in Pakistan. Journal of the Pakistan Medical Association 55(3): 108–110. [PubMed] [Google Scholar]
- Lee JH, Kim JJ, Kin YH, Jang JK, Son HJ, Paik KR, et al. (2002) Increased risk of peristomal wound infection after PEG in patients with diabetes mellitus. Digestive and Liver Disease 34: 857–861. [DOI] [PubMed] [Google Scholar]
- Mahadeva S, Sam IC, Khoo BL, et al. (2009) Antibiotic prophylaxis tailored to local organisms reduces percutaneous gastrostomy site infection. International Journal of Clinical Practice 63(5): 760–765. [DOI] [PubMed] [Google Scholar]
- Mainie I, Loughrey A, Watson J, Tham TC. (2006) Percutaneous endoscopic gastrostomy sites infected by methicillin-resistant Staphylococcus aureus: impact on outcome. Journal of Clinical Gastroenterology 40(4): 297–300. [DOI] [PubMed] [Google Scholar]
- McClave SA, Chang WK. (2003) Complications of enteral access. Gastrointestinal Endoscopy 58: 739–751. [DOI] [PubMed] [Google Scholar]
- Preclik G, Grüne S, Leser HG, Lebherz J, Heldwin W, Machka K, et al. (1999) Prospective, randomized, double blind trial of prophylaxis with single dose of co-amoxiclav before percutaneous endoscopic gastrostomy. BMJ 319: 881–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma VK, Howden CW. (2000) Meta-analysis of randomized, controlled trials of antibiotic prophylaxis before percutaneous endoscopic gastrostomy. American Journal of Gastroenterology 95: 3133–3136. [DOI] [PubMed] [Google Scholar]


