Abstract
Background:
The objectives were to evaluate the effectiveness of an infection control bundle in controlling the meticillin resistant Staphylococcus aureus (MRSA) transmission in residential care homes for the elderly (RCHEs) in Hong Kong.
Methods:
This was a two-arm cluster randomised controlled trial. Infection control bundles focused on hand hygiene (HH), environmental hygiene, and modified contact precautions were applied to the intervention arm. Nasal swabs from residents; staff HH compliance and effectiveness; and environmental hygiene were assessed by microbiological sampling or observation at the baseline and quarterly after the intervention.
Results:
A total of 2776 residents from 36 RCHEs were recruited. The overall MRSA prevalence was 20.4% (95% confidence interval, 18.9%−21.9%). The intervention elicited an immediate effect of 2.4% absolute decrease in the prevalence and 3.7% in the intra-facility transmission, though the difference between the two arms was insignificant. Staff HH compliance increased substantially from 5.9% to 45.6% post-intervention (p < 0.001).
Conclusions:
We initiated the infection control culture into the RCHEs and gained their acceptance. However, this behavioural change takes time to emerge. Our study shows that relying on the bundle alone could not bring sustainable MRSA reduction. Administrative control for strengthening infection control infrastructure is important for continuous compliance and improvement.
Keywords: Care home, cluster randomised controlled trial, elderly, environmental hygiene, hand hygiene, infection control, long-term care, meticillin resistant Staphylococcus aureus, nursing home
Introduction
Meticillin resistant Staphylococcus aureus (MRSA) appeared in healthcare settings in the 1970s and is now an important nosocomial pathogen (Deurenberg and Stobberingh, 2008). In Europe, MRSA prevalence in care homes for the elderly varied from 1% to 23% across different countries (Dulon et al, 2011). Although there has been no major MRSA outbreak documented in elderly care homes in Hong Kong, local data show that around 48% of MRSA bacteraemia came from the residents there (unpublished data from the standardised web-based MRSA surveillance system in Hospital Authority, Hong Kong). Care homes for elderly people are potential amplifying pools for MRSA transmission. It is well established that institutionalisation, old age, and premorbid conditions are common risk factors for prolonged MRSA carriage among elderly people (Vovko et al, 2005; Daeschlein et al, 2006; Kerttula et al, 2006). Successful control of MRSA to a variable extent in hospitals and other tertiary healthcare settings has been demonstrated by implementing multi-pronged intervention bundles. However, at present, these bundles have not been fully promulgated and implemented in our elderly care homes, and their effectiveness in controlling MRSA or other multidrug resistant organisms (MDROs) remains unclear.
In Hong Kong, residential care homes for the elderly (RCHEs) provide residential care and facilities for elders aged 65 or above who, for personal, social, health and/or other reasons, cannot adequately be taken care of at home (Social Welfare Department, 2013). We hypothesised in our study that introducing a multifaceted intervention bundle targeted at the RCHEs would reduce MRSA transmission.
Methods
Setting and study design
This was a two-arm cluster randomised controlled trial. The intervention arm received an intervention bundle for MRSA control whereas the usual care arm received standard care. A baseline assessment was conducted in July and August 2009, followed by a preparatory phase of interventions in September to December. Thereafter, the intervention period started from January 2010 (phase 1, P1) with quarterly assessments until October 2010 (phase 4, P4). The study was approved by the Ethics Committee of the Department of Health, Hong Kong Special Administrative Region.
We targeted for 50-300-bed RCHEs located in three geographic districts with the same hospital catchment – Kowloon City, Yau Tsim Mong and Wong Tai Sin. These regions have the highest RCHE density in Hong Kong. Invitation letters were issued to eligible RCHEs, and agreed RCHEs were randomly allocated to one of the two arms, usual care or intervention. We applied stratified block randomisation where the stratum was the operation mode (run by non-governmental-organisations or run by the private sector) and the block size was two. To minimise the imbalance of the size of RCHEs across the two arms, the RCHE list was sorted by the bed capacity. The randomisation list was generated using the “rand” command in Microsoft Excel 2003. Afterwards, the research team sought written consent from all residents or their guardians of the recruited RCHEs. All participants were well-informed with an MRSA information sheet.
Data collection
A bilateral nasal swab, a wound swab and, if the patient had a catheter, a catheterised urine sample were collected from each participant for MRSA culture by research nurses and doctors. Additionally, a questionnaire on epidemiological and medical information was completed by the RCHE staff. Specimens were directly inoculated onto the MRSA ID agar (BioMérieux, France) and incubated at 35°C in O2 overnight according to the laboratory standard operating protocol.
One trained researcher performed direct observation on the hand hygiene (HH) compliance of the RCHE staff according to the World Health Organization (2009) Five Moments, which are highlighted as: before and after touching the elderly person for any nursing caring procedure, before aseptic procedure, and before and after body fluid exposure risk. These observations were carried out in the intervention arm between every phase of the study as a part of the intervention. On the other hand, to avoid any interruption of usual practice and the Hawthorne effect in the usual care arm, we did HH observations in those RCHEs at the beginning and the end of the study only. To minimise the Hawthorne effect, the researcher visited the RCHE together with researchers for other purposes, such as training and taking environmental samples, to disguise his or her observation. Besides, HH effectiveness was evaluated by the glove juice method. We randomly invited six on-duty staff to perform HH before the sampling. They were instructed to insert their dominant hand into a sterile glove, and the glove was secured at the wrist and instilled with 75mL of sterile aqueous phosphate buffer solution (pH 7.8). The staff member was asked to massage the solution for 60 seconds. Aliquots of the “glove juice” were then extracted for MRSA culture.
To assess the environmental hygiene, an area of about 10x10cm of each pre-defined high touch spot (i.e. surfaces that are frequently touched by hands) was swabbed with pre-moistened sterile cotton swabs and these were subsequently sent for MRSA culture.
Sample size
A local study shows that the MRSA prevalence among RCHE residents was 5.1% (80/1563) in 2005 (Chow et al, 2006; Ho et al, 2008). Specifically, the pooled prevalence from RCHEs in our studied regions was 5.65% (unpublished data). Conversely, another rate of 32.5% (13/40) was found in RCHE residents newly admitted to a local hospital in August 2007 (unpublished data). These patients were screened for MRSA colonisation upon admission to the medical wards. As the figures are in stark contrast to each other, we took their mid-value, 19.075%, as our anticipated prevalence. We hypothesised that the colonisation rate would remain fairly constant in the usual care arm over the study period, while our intervention could reduce it by 40% relatively. This was supported by Raboud et al (2005) who demonstrated that a 10% increase in hand-washing compliance could improve nosocomial MRSA acquisition by 50%. As the RCHEs had a mean of 130 beds, we estimated an intra-cluster correlation coefficient of 0.025 (McCarthy, 2008) and a coefficient of variation of 0.4808 (Eldridge et al, 2006). With 80% power at 5% significance level and a 30% drop-out rate, the sample size was 1932 residents from 15 RCHEs per arm.
In the sample size calculations for HH compliance and environmental cleanliness, a conservative approach of taking 50% as the proportion was used due to unavailability of local data. The number of HH opportunities observed per staff (range, 2–17; mean, 8) and the number of staff observed (range, 3–26; mean, 11) were evaluated from our pilot visits. Following the same assumption of other parameters, we needed 490 HH opportunities per arm to detect a 10% increase after the intervention, and 306 environmental samples to identify a drop of 10%. Finally, the number of HH opportunities to be observed per RCHE (range, 13–47) was calculated pro rata to their bed capacity and an inflation of 25 environmental samples per RCHE was adopted.
Intervention
The multifaceted infection control intervention bundle comprised HH enhancement, environmental decontamination, and modified contact precautions. These were promulgated to the intervention arm through lectures, on-site demonstration, staff coaching, biweekly outreach visits and telephone consultancy services provided by infection control nurses. In order to facilitate the implementation of the bundle, we supplied the basic infection control consumables which were not readily available in the RCHEs. Staff compliance with the following bundle elements was assessed quarterly together with timely feedback to the stakeholders so as to drive performance improvement. Re-training and re-assessment were provided to the staff if necessary.
Hand hygiene enhancement
We introduced and installed alcohol-based hand rubs (AHR) at the nurses’ station, at each resident’s bedside, at napkin-round trollies, along the corridor and in the common room; together with eye-catching posters which demonstrated the Five Moments of correct HH indications and the Seven Steps of effective HH techniques (World Health Organization, 2009).
Environmental decontamination
We provided disposable cleaning kits which included gloves, wipes, absorbent materials and waste bags. There were three different kinds of colour-coded kits dedicated to the environmental cleaning for MRSA carriers’ rooms – vomitus, blood and body fluid spillage respectively. An instruction sheet was supplemented to indicate the proper use of disinfectants. Environmental cleaning protocol, which elaborated the cleaning principles, such as special attention to high touch areas, cleaning sequence from clean to dirty zone and more frequent cleaning schedules for the MRSA cases, was formulated with inputs from RCHE staff to make the protocol workable and practicable as far as possible. In particular, we used the Wash and Glow Professional kit (Glowtec, UK) to evaluate the proficiency of cleaning skills of the staff by prior application of UV marks to the areas with a follow-up inspection of compliance. We aimed at a removal of at least 80% of the fluorescent marks during the assessment.
Modified contact precautions
Known MRSA carriers who had been diagnosed during hospital admission were recommended to be isolated in single rooms or in a cohort separated by partitioned barriers under the local prevailing guidelines. We helped put this into practice by giving advice on the isolation management. In brief, MRSA carriers should be physically isolated from vulnerable residents who had indwelling catheters or skin lesions. Contact precaution should be observed strictly when handling the device and wounds of the MRSA carriers. MRSA carriers without any wounds or indwelling catheters who managed to observe good personal hygiene were allowed to participate in social activities.
Device and wound care management
This was planned as one of the intervention elements in the original study design, but when the study commenced, we came to realize that these procedures were provided by the community nursing services (CNS), an outreach nursing team from public hospitals. Therefore, this topic was removed from the intervention.
Outcome measures
The primary outcome measure was the MRSA prevalence, which was the percentage of residents with an MRSA positive result in any of the specimens collected. The intra-facility transmission was determined by the overall percentage of MRSA-free residents who converted to MRSA carriers in the subsequent phase, excluding those who acquired MRSA during hospitalisation.
Statistical analysis
All analysis was undertaken on an intention-to-treat basis. Exploratory analysis was done at cluster level to evaluate the difference between the two arms. Chi-square test or Fisher’s exact test for categorical variables and Mann-Whitney test or Kruskal-Wallis test for continuous variables were used where appropriate. Regression models were applied to examine factors associated with the MRSA colonisation using Statistical Package for the Social Sciences Version 16.0 for Windows (SPSS Inc., Chicago, IL, USA). The generalised estimating equations (GEE) method was applied to account for a clustering effect using Stata Version 10 (StataCorp., College Station, TX, USA).
Results
Recruitment of RCHEs and residents
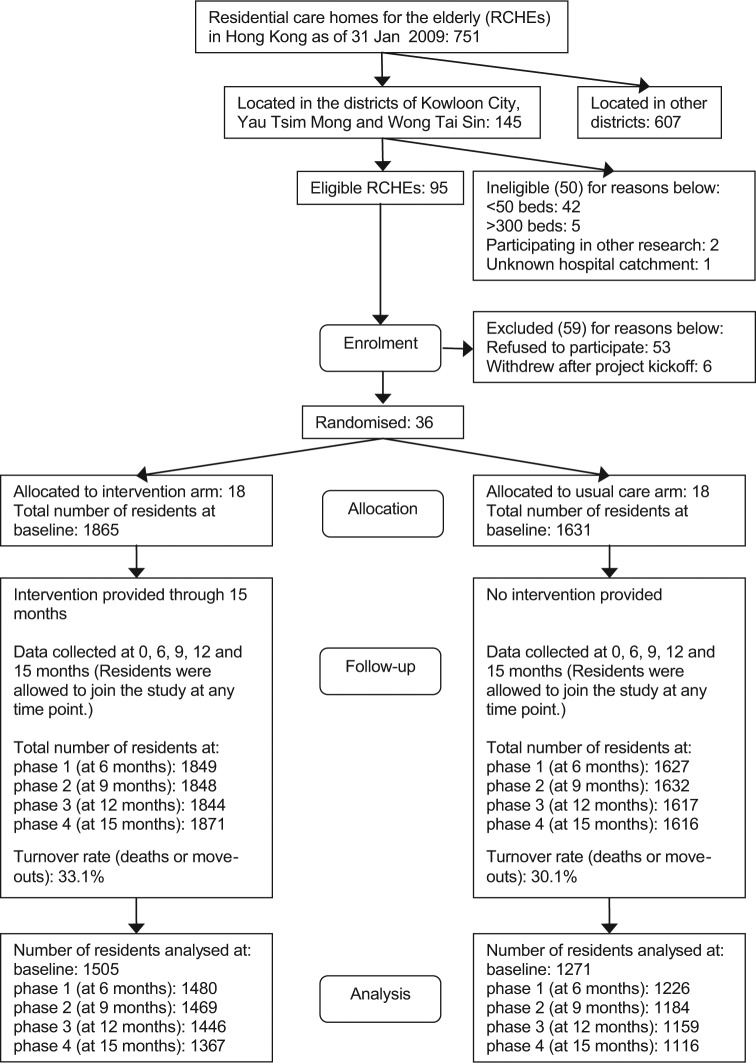
We approached 95 eligible RCHEs and 42 of them accepted our invitation at the beginning. Six RCHEs withdrew from the study before the baseline assessment. Consequently, 36 RCHEs were included, with 18 RCHEs per arm (Figure 1). At the baseline, a total of 2776 out of 3496 residents participated, making a response rate of 79.4%.
Figure 1.
Flow diagram of the participants through the phases.
The turnover rate of the consented residents was 31.8% (either the person died or left the RCHEs) in both arms. Specifically, the mortality rate was 22.0%. During the study period, new residents admitted to participating RCHEs were also welcomed to join in at any time points. As a result, the overall response rate was maintained from a range of 71.2% to 77.8% in other phases, and still 66.1% of the baseline participants remained at the end.
Baseline characteristics of RCHEs and residents
The majority (83%) of the recruited RCHEs were operated by the private sector and 17% by non-governmental organisations (NGOs). The staff to resident ratio was on average 1:5 (range, 1:3–1:8). Personal care workers (51.7%) and health workers (20.3%) who had low education level predominated the staff composition. Registered or enrolled nurses accounted for only 5%. The baseline characteristics of the participants in the two arms are illustrated in Table 1.
Table 1.
Baseline characteristics of participating residents.
| Variables | Overall | Intervention arm | Usual care arm |
|---|---|---|---|
| Antibiotic use in the past 3 months | 22.13% | 342/1500 (22.80) | 270/1266 (21.33) |
| Barthel Index | |||
| Total dependence | 45.24% | 642/1496 (42.91) | 607/1265 (47.98) |
| Severe dependence | 18.83% | 296/1496 (19.79) | 224/1265 (17.71) |
| Moderate dependence | 17.57% | 283/1496 (18.92) | 202/1265 (15.97) |
| Slight dependence | 10.18% | 152/1496 (10.16) | 129/1265 (10.20) |
| Independent | 8.19% | 123/1496 (8.22) | 103/1265 (8.14) |
| Medical devices in the past 1 month (any of the following) | 17.82% | 276/1500 (18.40) | 217/1266 (17.14) |
| IV central | 0.00% | 0/1500 (0.00) | 0/1266 (0.00) |
| Intravenous peripheral line | 5.75% | 83/1500 (5.53) | 76/1266 (6.00) |
| Urinary catheter | 5.68% | 91/1500 (6.07) | 66/1266 (5.21) |
| Tenckhoff catheter | 0.61% | 11/1500 (0.73) | 6/1266 (0.47) |
| Feeding tube | 8.71% | 147/1500 (9.80) | 94/1266 (7.42) |
| Percutaneous gastrostomy tube | 0.22% | 2/1500 (0.13) | 4/1266 (0.32) |
| Prosthetic implant | 0.14% | 0/1500 (0.00) | 4/1266 (0.32) |
| Haemodialysis vascular access | 0.00% | 0/1500 (0.00) | 0/1266 (0.00) |
| Drain | 0.07% | 0/1500 (0.00) | 2/1266 (0.16) |
| Use of inhaled medication | 8.28% | 127/1500 (8.47) | 102/1266 (8.06) |
| Male | 36.74% | 564/1505 (37.48) | 456/1271 (35.88) |
| MRSA | 20.35% | 316/1505 (21.00) | 249/1271 (19.59) |
| Age (Year) | |||
| Median (min – max) | 84 (32–105) | 83 (32–104) | 84 (40–105) |
| Mean (SD) | 82.56 (9.50) | 82.42 (9.45) | 82.72 (9.55) |
| Charlson score | |||
| Median (min–max) | 0 (0–12) | 0 (0–12) | 0 (0–12) |
| Mean (SD) | 0.6204 (1.30) | 0.6180 (1.27) | 0.6232 (1.34) |
| Charlson score > 0 | 28.52% | 431/1500 (28.73) | 358/1266 (28.28) |
Data are expressed as number of participants exposed to the variable/total number of participants (%), unless otherwise indicated.
SD, standard deviation.
Outcome measures
MRSA prevalence and intra-facility MRSA transmission within RCHE
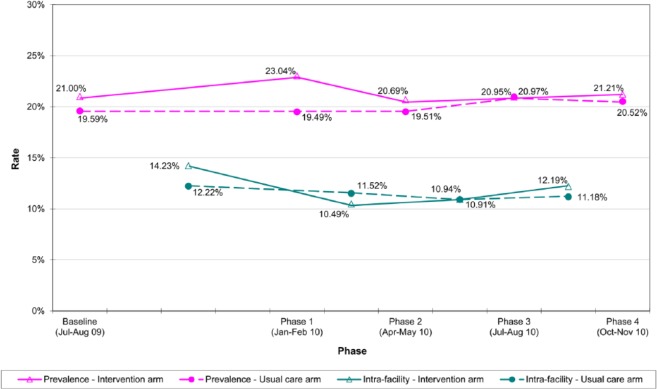
The overall MRSA prevalence in the recruited RCHEs was 20.4% (95% confidence interval, 18.9% to 21.9%) at the baseline and it was comparable in both arms. The MRSA prevalence in the intervention arm dropped in the initial phases and became steady afterwards. It decreased 1.8% from P1 to P4, whereas for the usual care arm, it increased 1.0%. Yet the difference between the two arms was not statistically significant (Table 2). The aggregate intra-cluster correlation coefficient (ICC) was 0.0212.
Table 2.
Meticillin-resistant Staphylococcus aureus (MRSA) prevalence at residential care homes for the elderly across different phases.
| Phase | MRSA positive/total (%) |
Odds ratioa (95% CI) | pa | |
|---|---|---|---|---|
| Intervention arm | Usual care arm | |||
| Baseline | 316/1505 (21.0) | 249/1271 (19.6) | 1.1125 (0.8569,1.4445) | 0.423 |
| Phase 1 | 341/1480 (23.0) | 239/1226 (19.5) | 1.1774 (0.8659,1.6011) | 0.298 |
| Phase 2 | 304/1469 (20.7) | 231/1184 (19.5) | 1.0562 (0.8062,1.3837) | 0.692 |
| Phase 3 | 303/1446 (21.0) | 243/1159 (21.0) | 0.9913 (0.7357,1.3357) | 0.954 |
| Phase 4 | 290/1367 (21.2) | 229/1116 (20.5) | 1.0369 (0.7206,1.4920) | 0.845 |
Adjusted by generalised estimating equations (GEE) method.
CI, confidence interval.
The transmission rate of the intervention arm dropped substantially in the initial phases (14.2% to 10.5%) and slightly bounced back afterwards (12.2%) whereas the rate of the usual care arm stayed static at around 11–12% (Figure 2). No statistically significant difference was found between the two arms.
Figure 2.
Meticillin-resistant Staphylococcus aureus (MRSA) prevalence and intra-facility transmission rate of the intervention arm and the usual care arm across different phases.
Hand hygiene compliance and effectiveness
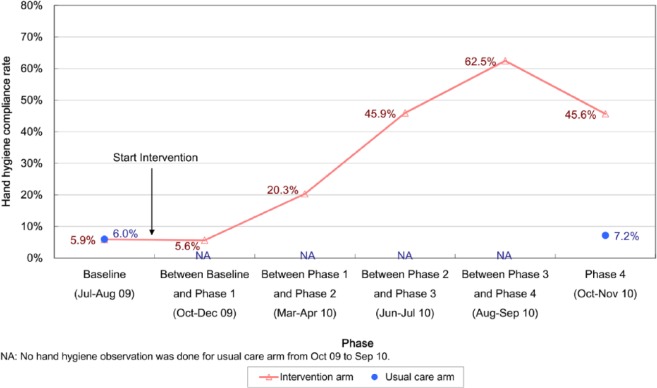
A range of 546–823 HH opportunities in each arm was observed at different time-points. The HH compliance among RCHE staff was 5.9% at the baseline in both arms (Figure 3). After the implementation of the bundle, we found a significant increase in the compliance rate in the intervention arm (p < 0.001). It was boosted to the highest before the final phase. At the end of the study, the HH compliance was significantly higher as compared to the usual care arm (45.6% vs 7.2%, p < 0.001). The aggregate ICC was 0.0899.
Figure 3.
Hand hygiene compliance rate of staff in residential care homes for the elderly (RCHEs).
For the preference between AHR and hand washing, staff were not in the habit of using AHR at the baseline, and this accounted for only 7.1% of the HH performance. However, AHR outnumbered hand washing in the subsequent phases in the intervention arm, with a proportion ranging from 60% to 80%.
A range of 95–108 glove juice samples per arm was collected at each phase. At the baseline, the post-HH MRSA contamination rate in both arms was 9.9%. After implementation of the intervention, the rates dropped in both arms but the intervention arm achieved a greater rate reduction than the usual care arm, and the hand contamination rate at the post-intervention phase was 5.6% as compared to 8.7% of the usual care arm (p = 0.43).The contamination reduction in the intervention arm was 4.7% (10.3% vs 5.6%, p = 0.23) since its baseline.
Environmental hygiene
A range of 425–440 environmental samples was obtained from each arm in various phases. MRSA was commonly found from bedside table tops (19.4%), hand covers (15.6%), and commodes (11.4%), and others included sofas (8.6%), handrails of arm chairs (8.3%), soap dispensers (5.7%), and wheelchairs (5.6%). The MRSA rate of environmental samples remained steady at around 4% throughout the study period in the intervention arm, while there was a significant increase in the usual care arm (2.3% at P1 to 6.6% at P4, p < 0.001). However, no statistically significant difference was found between the two arms at the post-intervention phase.
Discussion
This is the first cluster randomised controlled trial to assess the effectiveness of the multi-pronged infection control intervention bundle to control and prevent MRSA transmission in RCHEs. The intervention programme successfully drew a reduction of 3.7% in MRSA intra-facility transmission and 2.4% in MRSA prevalence in the intervention arm right after the intervention implementation, whereas small changes were observed in the usual care arm (0.7% decrease in intra-facility transmission and 0.02% increase in prevalence). However, the difference in the MRSA rate reduction between the two arms was not significant and the effectiveness of the intervention arm was not sustained as the MRSA rate reached a plateau in the subsequent phases. The initial reduction was probably due to the novelty effect, the tendency for better performance when facing a new project. Every staff member in both arms was well-informed of the institution of this study except the disclosure of the intervention content to the usual care arm. This might increase their awareness towards the infection control issue. The rebounds in the MRSA prevalence and the intra-facility transmission demonstrated that the intervention programme alone was not effective for significant MRSA reduction. Rigorous personal and environmental hygiene as advocated in our study were not warranted on eliminating the MRSA carriage. Although we put the clinically identified MRSA carriers with known skin lesions and indwelling catheters under modified contact precautions so as to prevent the spread of MRSA to other vulnerable residents, this proportion was only the tip of the iceberg and in stark contrast to the massive occult MRSA reservoir picked up by the surveillance cultures during the study. The reservoir that was 20% of all residents continued to shed the organism within the RCHEs. Hence, active screening and decolonisation regimes, which have been shown to be successful in the eradication of MRSA in several studies (Kotilainen et al, 2001; Lona et al, 2003), may be considered as an additional strategic measure to identify the occult shedders and control this ongoing transmission in elderly care homes in future research.
On the other hand, our study provided an opportunity to grasp the picture of the infrastructure of local RCHEs, in terms of administration, finance, manpower sustainability, staff mix, vulnerability of the residents and infection control culture. This gave us some clues to the ineffectiveness of the intervention. For instance, high staff turnover without immediate replacement, or employment of non-experienced or low-educated staff, disrupts the quality of service and thus the continuity of the intervention. This also requires additional resources for re-training. Besides, provision of infection control resources demands extra expenditure which is hindered by the profit margins of most private RCHEs. This results in poor infection control culture and lack of knowledge. Moreover, a constant flow of vulnerable residents who have multiple chronic diseases and require frequent hospital admission provides a potential channel for bringing forward the MRSA burden into the RCHEs. Inevitably, all these fundamental factors, which are echoed by a previous study (Baldwin et al, 2010), are great challenges in translating infection control interventions into a reduced MRSA burden.
Nevertheless, our study served as a pioneer strategic programme to successfully initiate the infection control culture into local RCHEs. The audit scores indicated poor or substandard infection control knowledge and compliance among the majority of RCHE staff. After the intervention, a seven-fold increase in the HH compliance rate was achieved, although to a lesser extent, improvement in environmental hygiene and HH technique were also reflected by the microbiological findings. Apart from the infection control knowledge given in lectures and regular on-site coaching, the free provision of the infection control consumables also facilitated the realistic implementation of the intervention bundle. A recent study shows that adherence to a simple bundle of infection prevention and control strategies was found to be associated with a nationwide reduction of healthcare associated MRSA infections over the 42-month study period in Veterans Affairs long-term care facilities, despite an increase in MRSA admission prevalence (Evans et al, 2014). The bundle featured the culture change to ensure that infection control was everyone’s responsibility. An MRSA Prevention Coordinator was designated at each centre to coordinate all aspects of the MRSA Prevention Initiative. Similarly, we believe that ongoing intensive support is crucial. However, in the middle of our study, we were compelled to change the frequency of on-site visits from bi-weekly to monthly due to unforeseeable nursing staff deployment. This might hamper the resulting efficacy of our intervention. All in all, the introduction of infection control programmes requires cultural and behavioural change which take time to emerge. Engagement from senior management and corresponding administrative control for strengthening infection control infrastructure is important for continuous compliance and improvement.
There are other limitations to this study. Firstly, the MRSA carriage status of RCHE staff was not examined; the MRSA positive staff might serve as a potential mysterious reservoir for the continuous shedding and spread of MRSA. Secondly, other studies suggest that additional sampling sites of perineal, groin or throat regions may increase the sensitivity of MRSA detection (Nilsson and Ripa, 2006; Mertz et al, 2007; Eveillard et al, 2008), and therefore, we might have underestimated the true prevalence and transmission rate.
In summary, we showed that MRSA reduction was not guaranteed by relying only on the frontline staff to implement infection control measures; administrative controls to address the poor infection control infrastructure should be considered as equally important. The future direction in policy formulation should encompass a strategic plan which addresses both administrative and infection control originating from hospitals and institutions, and this might be the sole way to successfully control MRSA.
Acknowledgments
Special thanks to Dr Dominic NC Tsang’s for his invaluable advice and to his microbiology laboratory team members for providing surveillance culture services. We thank all residents and staff of the participating RCHEs for their kind support and also all the staff of the Infection Control Branch, Centre for Health Protection who contributed to the study.
Footnotes
Declaration of conflicting interest: None declared.
Funding: This work was supported by the Research Fund for the Control of Infectious Diseases (RFCID) of the Food and Health Bureau of Hong Kong SAR Government (grant number CHP-NS-04).
Peer review statement: Not commissioned, blind peer-reviewed.
References
- Baldwin NS, Gilpin DF, Tunney MM, et al. (2010) Cluster randomised controlled trial of an infection control education and training intervention programme focusing on methicillin-resistant Staphylococcus aureus in nursing homes for older people. Journal of Hospital Infection 76(1): 36–41. [DOI] [PubMed] [Google Scholar]
- Chow SM, Yung WHR, Tsang HLI, et al. (2006) Prevalence study on MRSA colonization, associated risk factors, assessment of infection control knowledge and institutional characteristics among RCHEs of Hong Kong SAR. In: Fourteenth Annual Congress of Gerontology of Hong Kong Association of Gerontology; 25 November, Hong Kong. [Google Scholar]
- Daeschlein G, Assadian O, Rangous I, Kramer A. (2006) Risk factors for Staphylococcus aureus nasal carriage in residents of three nursing homes in Germany. Journal of Hospital Infection 63(2): 216–220. [DOI] [PubMed] [Google Scholar]
- Deurenberg RH, Stobberingh EE. (2008) The evolution of Staphylococcus aureus. Infection, Genetics and Evolution 8(6): 747–763. [DOI] [PubMed] [Google Scholar]
- Dulon M, Haamann F, Peters C, Schablon A, Nienhaus A. (2011) MRSA prevalence in European healthcare settings: a review. BMC Infectious Diseases 11: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge SM, Ashby D, Kerry S. (2006) Sample size for cluster randomized trials: effect of coefficient of variation of cluster size and analysis method. International Journal of Epidemiology 35: 1292–1300. [DOI] [PubMed] [Google Scholar]
- Evans ME, Kralovic SM, Simbartl LA, et al. (2014) Nationwide reduction of health care-associated methicillin-resistant Staphylococcus aureus infections in Veterans Affairs long-term care facilities. American Journal of Infection Control 42(1): 60–62. [DOI] [PubMed] [Google Scholar]
- Eveillard M, Charru P, Rufat P, et al. (2008) Methicillin-resistant Staphylococcus aureus carriage in a long-term care facility: hypothesis about selection and transmission. Age and Ageing 37(3): 294–299. [DOI] [PubMed] [Google Scholar]
- Ho PL, Lai EL, Chow KH, Chow LS, Yuen KY, Yung RW. (2008) Molecular epidemiology of methicillin-resistant Staphylococcus aureus in residential care homes for the elderly in Hong Kong. Diagnostic Microbiology and Infectious Disease 61(2): 135–142. [DOI] [PubMed] [Google Scholar]
- Kerttula AM, Lyytilkäinen O, Vuopio-Varkila J, et al. (2005) Molecular epidemiology of an outbreak caused by methicillin-resistant Staphylococcus aureus in a health care ward and associated nursing home. Journal of Clinical Microbiology 43(12): 6161–6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotilainen P, Routamaa M, Peltonen R, et al. (2001) Eradication of methicillin-resistant Staphylococcus aureus from a health center ward and associated nursing home. Archives of Internal Medicine 161(6): 859–863. [DOI] [PubMed] [Google Scholar]
- Lona M, Kauffman CA, McNeil SA, Galecki AT, Bradley SF. (2003) Mupirocin-based decolonization of Staphylococcus aureus carriers in residents of 2 long-term care facilities: a randomized, double-blind, placebo-controlled trial. Clinical Infectious Diseases 37(11): 1467–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy WF. (2008) The design and sample size requirement for a cluster randomized non-inferiority trial with two binary co-primary outcomes. COBRA Preprint Series 2008. July; Working Paper 36. [Google Scholar]
- Mertz D, Frei R, Jaussi B, et al. (2007) Throat swabs are necessary to reliably detect carriers of Staphylococcus aureus. Clinical Infectious Diseases 45(4): 475–477. [DOI] [PubMed] [Google Scholar]
- Nilsson P, Ripa T. (2006) Staphylococcus aureus throat colonization is more frequent than colonization in the anterior nares. Journal of Clinical Microbiology 44(9): 3334–3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raboud J, Saskin R, Simor A, et al. (2005) Modeling transmission of methicillin-resistant Staphylococcus aureus among patients admitted to a hospital. Infection Control and Hospital Epidemiology 26(7): 607–615. [DOI] [PubMed] [Google Scholar]
- Social Welfare Department. Residential care services for the elderly. Available at: www.swd.gov.hk/en/index/site_pubsvc/page_elderly/sub_residentia/id_introducti/ (accessed 1 August 2013).
- Vovko P, Retelj M, Cretnik TZ, et al. (2005) Risk factors for colonization with methicillin-resistant Staphylococcus aureus in a long-term-care facility in Slovenia. Infection Control and Hospital Epidemiology 26(2): 191–195. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2009) WHO Guidelines on Hand Hygiene in Health Care. Available at: http://whqlibdoc.who.int/publications/2009/9789241597906_eng.pdf?ua=1. (accessed January 2014).