Abstract
Background:
Contaminated blood cultures (BC) generate avoidable costs and prolong hospital stays. To measure our hospital’s performance against the recommended standard of <3% BC contamination, we performed a prospective study.
Methods:
We prospectively determined the frequency of contaminated and genuinely positive BC hospital-wide over seven months.
Results:
Overall, 73 of 1,829 blood cultures reviewed were contaminated (4.0%). However, distribution of contamination was not uniform. Finding a consistently higher incidence of contamination (11.7%) in our emergency department (ED) than elsewhere in the hospital (2.5%), we adopted a collaborative quality improvement strategy targeted to the ED. A combination of education, modified BC packs and regular feedback of BC results was associated with a significant reduction in contamination (7.4%, p=0.01) over the next six months. Our data suggests that contaminated BC were more likely to have been taken during regular day time hours (odds ratio (OR) 2.7, p=0.012), rather than overnight and were not associated with influxes of new junior medical staff. We found no evidence that the incidence of true bloodstream infection (12.8%) diagnosed by our ED was adversely affected by our intervention (10.7%, p=0.35).
Conclusions:
Using a simple and inexpensive collaborative intervention we reduced BC contamination without adversely affecting the detection of genuine BSI
Keywords: Blood culture, bloodstream infection, contamination, educational intervention, quality improvement
Introduction
Blood culture (BC) is the optimal test for diagnosing bloodstream infection (BSI). Both the Surviving Sepsis Campaign (Dellinger et al, 2012) and the Royal College of Emergency Medicine guidelines (Royal College of Emergency Medicine, 2013) recommend BC sampling prior to antibiotic administration when managing severe sepsis. However, clinically irrelevant bacteria from the patient’s skin, often termed BC contaminants, can also be cultured if a proper sampling technique is not observed. A contamination rate of <3% is considered acceptable, (Schifman et al, 1998; Department of Health, 2007) but contamination rates often exceed this, particularly in emergency departments (ED) (Madeo et al, 2005; Gander et al, 2009; Murillo et al, 2011; Self et al, 2013; Harding and Bollinger, 2013). Concern has been raised over the clinical and financial cost of BC contamination. A contaminated BC has been estimated to extend hospital stay by between 1 to 5.4 days and carry additional financial costs of £3,770 in the UK (Almahadi et al, 2011) and between $4,385 and $8,750 in the USA (Bates et al, 1991; Zwang and Albert, 2006; Gander et al, 2009). Minimising BC contamination would therefore be expected to provide cost savings and reduce clinical uncertainty.
Several studies have shown that reducing BC contamination is possible through simple, inexpensive interventions – typically a combination of education, feedback and use of BC packs (Madeo et al, 2005; Murillo et al, 2011; Marini and Truog, 2012; Youssef et al, 2012; Self et al, 2013; Harding and Bollinger, 2013). These studies have principally focused on reducing rates of contaminated cultures in order to reduce the costs associated with false positive cultures. While reducing contamination is important, it is possible that improvement drives – particularly if restrictive in nature – may unintentionally lead to fewer BC being taken. This may in turn impair the diagnosis of genuine BSI (Thomas et al, 2011). Our aims were threefold: to assess the hospital-wide quality of BC sampling; to reduce any excessive BC contamination; and to determine whether an effort to improve BC contamination rates prejudiced the diagnosis of genuine BSI.
Methods
We performed a prospective study in a 535 bed district general hospital (DGH) seeing around 68,000 ED attendances annually. BC were taken by medical and nursing staff, with no dedicated BC phlebotomy team. BC were sampled using a BC pack containing an aerobic and anaerobic BactALERT® culture bottle (Biomerieux), ChloraPrep® 0.67ml 2% chlorhexidine applicator (CareFusion), butterfly collecting set and adaptor. BC were incubated on the same site using a BactALERT®3D (Biomerieux), with positive cultures identified using Gram staining and conventional identification methods from sub-cultures. Contamination was defined pragmatically as the presence in BC of low pathogenicity skin commensals (such as coagulase-negative staphylococci, Micrococcus species and Corynebacteria species) in patients not known to have central venous access or indwelling prosthetic material. Other similar studies have used varying definitions of BC contamination, some microbiological (Madeo et al, 2005; Gander et al, 2009; Youssef et al, 2012; Self et al, 2013) and others supplemented with clinical correlation (Schifman et al, 1998; Halverson et al, 2013; Harding and Bollinger, 2013). Our definition was primarily microbiological and was chosen for its simplicity and reproducibility without the need for access to patient records, accepting that it may modestly overestimate true BC contamination.
We first determined the frequency of BC contamination and true positivity for all clinical areas in our institution by prospectively reviewing all BC request forms for the first 14 days of each month from May to November 2011. The means from this seven month period were used as baselines prior to intervention. Identifying the ED as an area with a high incidence of contamination, we reported these results to ED consultant staff, prompting a discussion around possible reasons for the high contamination rate. These reflections were used to inform the intervention phase.
We next generated data for all BC taken in the ED (total number of BC, number of contaminated BC and true positive BC, date and time of sampling) every 14 days and fed this back to the clinical staff in the ED through posters, safety briefs and, where necessary, individual feedback from an ED consultant. In addition, we introduced an augmented BC sampling pack specific for the ED containing warning labels about rates of contamination, instructions on how best to obtain a BC, and a second ChloraPrep® 0.67ml applicator. Data were collected over a six month period, from December 2011 until May 2012. Chi-squared tests were used to assess statistical significance of categorical variables. Ethical approval was not sought as this work was undertaken as part of normal quality improvement efforts.
Results
Baseline blood culture performance
We reviewed 1,829 BC from throughout the hospital, of which 73 were contaminated (4.0%) and 182 cultured genuine pathogens (10.0%). Based on informal observations we had suspected a higher level of contamination than this in our ED. We noted that 34/288 BC taken in the ED were contaminated (11.8%) and 37/288 were genuinely positive (12.8%). Over the same time period, the frequency of contaminated cultures taken throughout the rest of the hospital was 2.5% (39/1,541) and the frequency of genuine pathogens isolated was 9.4% (145/1,541). We concluded that factors specific to the ED were likely to be responsible for the higher incidence of contamination. Noting that one in five significant blood culture isolates in our institution were taken in the ED, we were keen to identify a strategy that reduced BC contamination without prejudicing the diagnosis of true BSI.
Effect of a quality improvement programme on blood culture performance
Having demonstrated that the ED had a higher contamination rate (11.8%) than other clinical areas in the hospital (2.5%), a collaborative programme of change was implemented. Medical staff from the microbiology laboratory first presented results to an ED consultant and invited them to discuss with nursing and medical staff within the department who took BC the possible reasons for the high contamination rates. Three factors were identified: lack of awareness among staff about BC contamination rates in the department; lack of an easily accessible cleaning product to disinfect BC bungs; and lack of awareness of best practice in taking BC.
Setting a <3% contamination rate as our target, in line with Department of Health recommendations, we responded to the three factors previously highlighted. We provided feedback twice monthly to the unit about the quality of BC sampling in the form of a run chart. Run charts were displayed in the ED and results communicated verbally to staff at safety briefings. The ED already used pre-made BC packs. These were supplemented with an insert alerting staff to the high contamination rate and outlining good BC sampling practice, and a second ChloraPrep® applicator for BC bung disinfection. In April 2012, having failed to achieve our target of <3% contamination, we introduced non-judgemental, individualised feedback by an ED consultant to those staff who had taken cultures thought to be contaminated following case note review by the ED consultant. No further reduction in the frequency of contamination was seen.
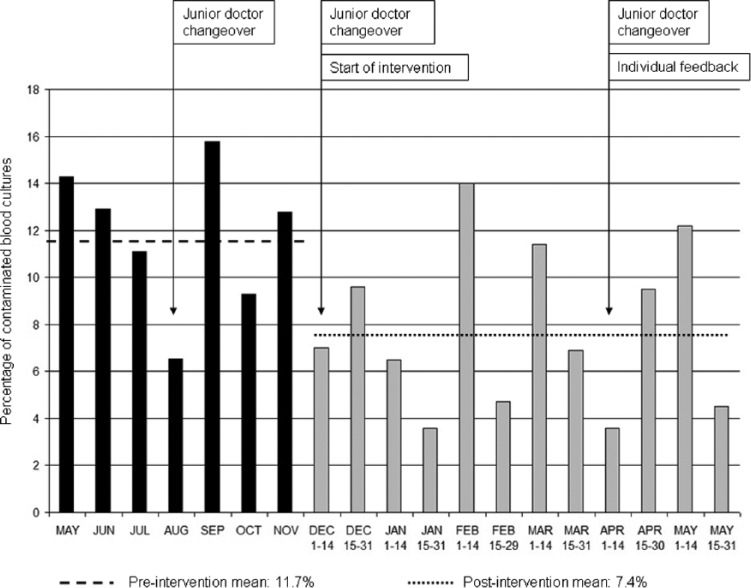
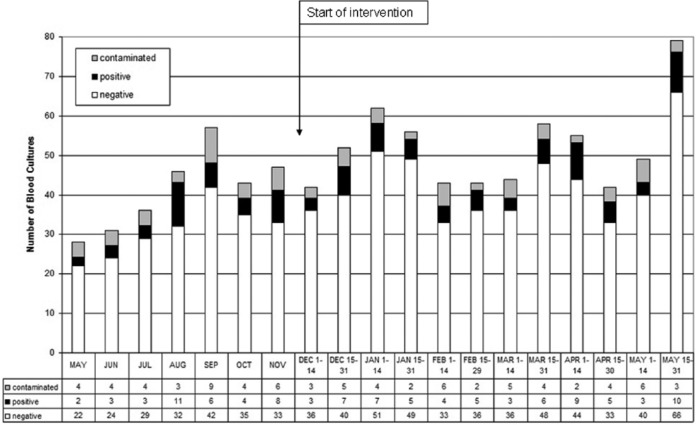
We observed a sustained, significant reduction in BC contamination from 11.8% to 7.4% (46/625) during our intervention (p=0.01) (Figure 1). Three changeovers of new junior medical staff were not associated with increases in the incidence of contamination. The mean number of BC taken per fortnight rose from 41 to 51. This increase was possibly associated with increasing awareness of indications for BC sampling. We found no evidence of a significant change in the frequency of genuine positive BC during the intervention: 12.8% pre-intervention versus 10.7% (67/625) post-intervention (p=0.35) (Figure 2).
Figure 1.
Percentage of contaminated blood cultures in the emergency department before and after intervention.
Figure 2.
Number of blood cultures taken, contaminated, and genuinely positive in the emergency department before and after intervention.
Time of sampling and blood culture performance
Hypothesising that increased rates of contamination might occur at times of lower staffing and increased fatigue, we examined the results of BC by the time of day taken and whether the culture was taken during the week (Monday to Friday) or at a weekend (Saturday and Sunday). The time of culture was documented in 414 of 625 BC (66%). The mean number of BC taken per week day (57) and per weekend day (64) were similar, with no statistically significant difference in the frequency of contaminated cultures (p=0.56) or genuine positives (p=0.16) (Table 1).
Table 1.
Number and result of blood cultures by day of the week.
| Weekday | Weekend | |
|---|---|---|
| Total | 286 | 128 |
| Positive | 33 (11.5%) | 9 (7.0%) |
| Contaminated | 20 (7.0%) | 11 (8.6%) |
However, we observed a significant increase in the likelihood of contaminated cultures being taken during daytime hours (06:00 to 18:00) (OR 2.7, 95% CI 1.2 to 6.0, p=0.012) when compared to BC taken outside these hours. There was no difference in the frequency of genuine positive cultures between the two time periods (p=0.23). The time periods during which BC contamination was most likely to occur were neither those where most BC sampling occurred nor those where staff might be expected to be most fatigued (Table 2).
Table 2.
Number and result of blood cultures by time of day.
| 00:00 – 05:59 | 06:00 – 11:59 | 12:00 – 17:59 | 18:00 – 23:59 | |
|---|---|---|---|---|
| Total | 66 | 83 | 121 | 144 |
| Positive | 3 (4.5%) | 3 (3.6%) | 14 (11.6%) | 22 (15.3%) |
| Contaminated | 0 (0%) | 12 (14.5%) | 10 (8.3%) | 9 (6.3%) |
Discussion
We describe the quality of BC sampling in a DGH over a seven month period, finding an overall incidence of BC contamination of 4.0%, close to the recommended level of <3%. Excluding our ED, where a higher incidence was found, an acceptable level of 2.5% was identified. We think two factors are responsible for this. First, newly starting foundation doctors undergo practical training on BC sampling during their induction. Second, pre-made blood cultures packs make it easy for practitioners to have available all the relevant equipment for good BC collection. Since BC packs were also available in the ED, the packs alone are not sufficient to achieve low contamination rates. It is possible that patients in clinical areas outwith the ED were more likely to have established intravenous access and therefore receive a separate venepuncture for BC collection, but we have no data to support this.
However, we found a high baseline level of contamination in the ED (1 in 8.5 cultures taken), albeit somewhat lower than the one in four contamination rate previously described by Madeo et al (2005). We also found a high yield of genuine positive results. Significant organisms were identified from one in nine BC taken, much more frequently than the one in 70 positive BC found by Munro et al (2007). Our ED therefore had both an initially high level of contaminated BC but also accounted for a fifth of all significant BC in our hospital.
Using a simple and inexpensive collaborative intervention we reduced BC contamination without adversely affecting the detection of genuine BSI. The reduction in BC contamination failed to meet our intended target of 3% but nevertheless demonstrated evidence of improvement – around 4.5 fewer contaminated BC per week. It is difficult to reliably assess the real cost of contaminated BC and we have not attempted to extrapolate potential cost savings based on data from other studies. The only economic analysis from the UK found surprisingly prolonged stays and additional costs (5.4 days and £3,770) (Almahadi et al, 2011). It is possible that a disproportionate number of intensive care patients in the contaminant group influenced its findings. We consider it likely that the costs attributable to ED BC contamination are substantially less than this. Reasons for failing to meet our target may have included BC taken during resuscitation when aseptic technique is less of a clinical priority and BC taken by visiting specialties within the ED for whom there was no feedback mechanism available. We have no data on the relative frequency of BC taken from fresh venepuncture as opposed to newly inserted peripheral cannulae.
Rapid staff turnover, highly variable workload and the high clinical urgency of some cases make ED difficult places to take BC consistently well (Madeo et al, 2005). We were intrigued that most contamination occurred during regular daytime hours, rather than during night shifts. Other studies have found an association between increased departmental activity and BC contamination (Lee et al, 2012; Halverson et al, 2013). We did not match BC sampling times with departmental activity in this study, but this would be a useful component of future work. Our data suggest no negative impact on BC contamination from new influxes of junior doctors to the department.
Recent evidence has shown that fewer than a third of patients with community-acquired severe sepsis have BC taken in a timely manner (Scottish Trauma Audit Group, 2010). BC therefore present something of a conundrum to an ED. On one hand there is strong national and international support for BC sampling in severe sepsis, although implementing this appears to be difficult. Yet, on the other hand, around 90% of BC taken in our study were negative, suggesting a limited ability to identify patients with BSI using clinical judgement. This difficulty of clinically predicting bacteraemia has previously been recognised (Jaimes et al, 2004). Improved systems for identifying and treating sepsis are needed. BC are relatively time-consuming, have no diagnostic or therapeutic benefit in the patient’s initial management, and generate results that are positive only after the patient has left the ED and are therefore rarely acted on by ED staff. It is unsurprising that some nihilism has arisen around their use in the ED (Kelly, 1998; Mountain et al, 2005; Munro et al, 2007). However, identifying the microbiological cause of bloodstream infection serves several important purposes. First, the particular bacterial or fungal isolate provides useful diagnostic information regarding the likely anatomical source of infection – not always apparent following clinical assessment. Second, a positive BC allows in vitro antimicrobial sensitivities to be generated, guiding effective treatment and limiting the unwanted consequences of unnecessarily broad antimicrobial therapy. Third, information obtained from the microbiological identity of the isolate and its antimicrobial sensitivities provides epidemiological information that is vital for guiding local empirical antibiotic policies and informing wider public health policy.
This was a real-life study carried out during routine work at little additional cost (36p per extra ChloraPrep® applicator). Our laboratory already had dedicated laboratory staff time for assembling BC packs (two to three hours of medical laboratory assistant time per week). Data acquisition and processing took around two hours of medical staff time per month at a cost of approximately £45. The time taken for this would be expected to vary depending on the ease of interrogating the laboratory dataset. Cost savings of around £10 per contaminated BC saved could be anticipated, based solely on materials required to process samples. Over a four week period, assuming 4.5 contaminated BC prevented per week, this saving would amount to around £180 – offsetting the cost of medical staff time and additional ChloraPrep® applicators. We believe our framework of first identifying and targeting clinical areas with a high frequency of contamination, then applying collaborative problem-solving using local data, and finally implementing non-restrictive, educational change could be readily applied to other hospitals. The exact methodology may need to vary depending on the local cultural factors thought to contribute to higher contamination rates (De Bono et al, 2014).
In summary, we outline a framework that identified and addressed, with evidence of some success, a high BC contamination rate in an ED using a simple intervention without adversely affecting its ability to diagnose bloodstream infection. More collaborative, evidence-based working between emergency departments and microbiology departments is needed to better identify those patients who most benefit for BC sampling in the ED and to explore factors associated with BC contamination.
Acknowledgments
We wish to thank the Microbiology laboratory staff involved in data generation and formulation of BC packs.
Footnotes
Conflict of interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Peer review statement: Not commissioned, blind peer-reviewed.
References
- Almahadi YM, Aldeyab MA, McEnlay, Scott MG, Darwish Elhajji FW, Magee FA, Dowds M, Edwards C, Fullerton L, Tate A, Kearney MP. (2011) Clinical and economic impact of contaminated blood cultures within the hospital setting. Journal of Hospital Infection 77: 233–236. [DOI] [PubMed] [Google Scholar]
- Bates DW, Goldman L, Lee TH. (1991) Contaminant blood cultures and resource utilization. The true consequences of false-positive results. JAMA 265: 365–369. [PubMed] [Google Scholar]
- De Bono S, Heling G, Borg MA. (2014) Organizational culture and its implications for infection prevention and control in healthcare institutions. Journal of Hospital Infection 86: 1–6. [DOI] [PubMed] [Google Scholar]
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R. (2012) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2012. Critical Care Medicine 41: 580–637. [DOI] [PubMed] [Google Scholar]
- Department of Health (2007) Saving Lives: Reducing Infection, Delivering Clean and Safe Care; Taking Blood Cultures: A Summary of Best Practice. Department of Health: London. [Google Scholar]
- Gander RM, Byrd L, De Cresconzo M, Hirnay S, Bowen M, Baughman J. (2009) Impact of blood cultures drawn by phlebotomy on contamination rates and health care costs in a hospital emergency department. Journal of Clinical Microbiology 47: 1021–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson S, Malani PN, Newton DW, Habicht A, Vander Have K, Younger JG. (2013) Impact of hourly emergency department patient volume on blood culture contamination and diagnostic yield. Journal of Clinical Microbiology 51: 1721–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding AD, Bollinger S. (2013) Reducing blood culture contamination rates in the emergency department. Journal of Emergency Nursing 39: e1–6. [DOI] [PubMed] [Google Scholar]
- Jaimes F, Arango C, Ruiz G, Cuervo J, Botreo J, Velez G, Upegui N, Machado F. (2004) Predicting bacteraemia at the bedside. Clinical Infectious Diseases 38: 357–362. [DOI] [PubMed] [Google Scholar]
- Kelly A-M. (1998) Clinical impact of blood cultures taken in the emergency department. Journal of Accident and Emergency Medicine 15: 254–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C-C, Lee N-Y, Chaung M-C, Chen P-L, Chang C-M, Ko W-C. (2012) The impact of overcrowding on the bacterial contamination of blood cultures in the ED. American Journal of Emergency Medicine 30: 839–45. [DOI] [PubMed] [Google Scholar]
- Madeo M, Jackson T, Williams C. (2005) Simple measures to reduce the rate of contamination of blood cultures in Accident and Emergency. Emergency Medical Journal 22: 810–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini MA, Truog AW. (2012) Reducing false-positive peripheral blood cultures in a pediatric emergency department. Journal of Emergency Nursing. Published online first: 4 May 2012. doi: 10.1016/j.jen.2011.12.017 [DOI] [PubMed] [Google Scholar]
- Mountain D, Bailey PM, O’Brien D, Jelinek GA. (2005) Blood cultures ordered in the adult emergency department are rarely useful. European Journal of Emergency Medicine 13: 76–79. [DOI] [PubMed] [Google Scholar]
- Munro PT, Howie N, Gerstenmaier JF. (2007) Do peripheral blood cultures taken in the emergency department influence clinical management? Emergency Medicine Journal 24: 211–212. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Murillo TA, Vandiver T, English D, Plummer V, Stovall SH. (2011) Reducing contamination of peripheral blood cultures in a pediatric emergency department. Pediatric Emergency Care 27: 918–921. [DOI] [PubMed] [Google Scholar]
- Royal College of Emergency Medicine. (2013) Clinical Standards for Emergency Departments. www.collemergencymed.ac.uk/Shop-Floor/Clinical%20Standards/Sepsis/default.asp (accessed 30 August 2013).
- Schifman RB, Strand CL, Meier FA, Howanitz PJ. (1998) Blood culture contamination. Archives of Pathology and Laboratory Medicine 122: 216–221. [PubMed] [Google Scholar]
- Scottish Trauma Audit Group (2010) Sepsis Management in Scotland. www.stag.scot.nhs.uk/SEPSIS/Main.html (accessed 30 August 2013).
- Self WH, Speroff T, Grijalva CG, McNaughton CD, Ashburn J, Liu D, Arbogast PG, Russ S, Storrow AB, Talbot TR. (2013) Reducing blood culture contamination in the emergency department: an interrupted time series quality improvement study. Academic Emergency Medicine 20: 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S, Cheesbrough J, Plumb S, Bolton L, Wilkinson P, Walmsley J, Diggle P. (2011) Impact of a blood culture collection kit on the quality of blood culture sampling: fear and the law of unintended consequences. Journal of Hospital Infection 78: 256–259. [DOI] [PubMed] [Google Scholar]
- Youssef D, Shams W, Bailey B, O’Neil TJ, Al-Abbadi MA. (2012) Effective strategy for decreasing blood culture contamination rates: the experience of a veterans affairs medical centre. Journal of Hospital Infection 81: 288–291. [DOI] [PubMed] [Google Scholar]
- Zwang O, Albert RK. (2006) Analysis of strategies to improve cost effectiveness of blood cultures. Journal of Hospital Medicine 1: 272–276. [DOI] [PubMed] [Google Scholar]