Abstract
Background:
Healthcare industry representatives (HCIR) visit multiple hospitals every day. Most enter hygiene sensitive areas and work in close proximity to caregivers and patients.
Objective:
The objective of the present study was to evaluate the HCIRs’ current status in hygiene training and vaccination.
Methods:
An anonymous walking intercept study was used based on questionnaires to evaluate industry representatives in comparison to physicians and nurses (n = 311 participants, participation rate 30.2%) after their visit to the MEDICA Congress. The valid participants consisted of HCIR (n = 208), hospital nurses (n = 49) and physicians (n = 41). A total of 82.2% (n = 171) HCIR worked in varying hospitals.
Results:
They frequently request access to hygiene and data-privacy sensitive areas: Among them 51.9% (n = 108) accessed the outpatient clinic, 41.8% (n = 87) the operating room (OR), 33.7% (n = 70) the central supply and sterilisation department (CSSD), and 32.7% (n = 68) the intensive care unit. HCIR requesting access to hygiene sensitive areas showed the lowest scores in hygiene training and a significantly lower Hepatitis B vaccination status, i.e. 37.5% compared to 70.7% for physicians and 53.1% for nurses.
Discussion:
Status of HCIR hygiene training was inadequate – as was vaccination and contamination control. Therefore, HCIR are exposed to increased infection risk and may unknowingly act as infection vector between different hospitals.
Keywords: Compliance, cross-hospital contamination, occupational safety, risk management, vaccination, vector
Background
According to the World Health Organization (WHO) ‘in Europe, Healthcare-Associated Infections (HCAI) cause 16 million extra-days of hospital stay, 37,000 attributable deaths and contribute to an additional 110,000 deaths every year. Annual financial losses are estimated at approximately €7 billion when including direct costs only’ (World Health Organization, 2011). In the USA, approximately ‘99,000 deaths were attributed to HCAI in 2002 and the annual economic impact was estimated at approximately US $6.5 billion back in 2004’ (World Health Organization, 2011). Consequently, a vast amount of research has focused on measuring and improving internal hospital contamination processes and hygiene compliance of healthcare providers (HCP) – primarily physicians and nurses.
On the other hand, the single largest group of professionals who is in close contact with HCP are healthcare industry representatives (HCIR). According to a position statement in October 2011 by EUCOMED, an industry association representing the medical technology industry in Europe (EUCOMED), HCIR personnel traditionally train, educate and support hospitals in various roles onsite during the delivery of hospital services to patients. Requests ‘to have a representative of the company present during surgical or other medical procedures’ (World Health Organization, 2011) are reported to be frequent (Bonten et al., 2001, Bedard et al., 2014). Bedard et al. report that ‘37 percent of HCIR had participated in a surgery in which they felt that their involvement was excessive’. A significant portion of those employees visit more than one hospital during their workday. Personal communication with colleague physicians further confirms the impression that interaction with industry representatives during surgical procedures is non-standardised.
In a prior study we revealed that HCIR did acquire similar contamination patterns as HCP while on site (Schiffers et al., 2014). The findings point to the risk of HCIR serving as vectors and cross-contaminating pathogens such as S. aureus or methicillin-resistant S. aureus (MRSA) between remote healthcare facilities and their HCP.
The potential of HCIR to contribute to cross-contamination in hospitals has far-reaching implications including infection prevention of patients, HCP as well as the related policies, procedures and regulations.
Objective of the study
Prior publications found that staff were commonly implicated in the transmission of HCAI and that in 10% of outbreaks staff was the primary source (Weinstein, 1991; Gastmeier et al., 2005). Other studies have enhanced understanding of contamination models and efforts to optimise hygiene compliance of healthcare providers (HCP) (Pittet et al., 1999; Bonten et al., 2001; Lam et al., 2004; Girou et al., 2006; Pessoa-Silva et al., 2007). A recently published report indicated that patients were ‘visited up to 28 times by as many as 18 different people per hour’ (Cohen et al., 2012). During those visits, contact with the patient environment was made in 33.5% of cases such that contamination of inanimate surfaces occurred. In addition, a reported 8% of those visits in patient rooms were performed by non-clinical staff (Bonten et al., 2001; Cohen et al., 2012). Past research has provided robust data regarding the ability of various pathogens to persist for prolonged times on skin and the surface of inanimate objects (Kramer et al., 2006).
An instance of workplace cross-contamination is the report of a relationship of nasal MRSA colonisation in German veterinarians (2–45%) and the pig farmers they work for (86%) (Cuny et al., 2009). This suggests a risk of cross-contamination of different professional groups due to close collaboration in the same work environment. Literature research revealed that HCP hygiene compliance is far from optimal and colonisation of patient environment and patients appears to be frequent (Bonten et al., 2001; Kramer et al., 2006). As a result, potential carriers with close contact to multiple HCP most likely pose a threat to hospital hygiene and patient safety.
In a prior study, we found that HCP and HCIR attending a convention share approximately the same level of microbiological burden (Schiffers et al., 2014). The demographic composition and hygienic education of attendees was comparable to the hospital workplace and while a convention does not require the same precautionary behaviour, there is also less chance and necessity of hand-to-hand or surface-to-hand contaminations. It is therefore reasonable to suspect HCP and HCIR share a similar microbiological burden from their shared workplace in hospitals.
Therefore, the current status in hygiene training, contamination screening and vaccination in HCIR compared to hospital staff was analysed – thus measuring the HCIR’s awareness and actions in fighting cross-hospital contamination and HCAI as well as their employer’s engagement with regard to this specific topic of occupational safety. Hence, the null hypothesis for the survey was that no statistical difference would be found in Hepatitis B vaccination status of HCIR compared to hospital physicians and nurses and that hygiene training scores would show no difference between these groups.
Methods
Target location and population
An anonymous prospective intercept survey of HCIR, hospital physicians and hospital nurses in conjunction with the MEDICA Congress 2012 was carried out. The location was chosen as it provided us with the ability to measure a sufficient number of individuals from all three professional groups at the same location in 1 day. The study was conducted with the approval by the local ethical committee of the University of Rostock (registration number: A2013-0010).
Participants were interviewed leaving the MEDICA. Participants were segmented by professional group and job title. Detailed data were gathered on the level of required travelling between hospitals, required hospital department access level, hygiene training, vaccination and screening status. Over 65% of individuals progressing past interception points matched exclusion criteria, primarily due to job location being outside Germany. From the resulting potential participants based in Germany, 30.2% volunteered to take the survey. A total of 311 individuals participated in this study, 13 of which matched exclusion criteria. This resulted in 298 valid participants, 63 women (21.1%) and 235 men (78.9%). By job title, 41 (13.8%) were hospital-based physicians, 49 (16.4%) were hospital nurses and 208 (69.8%) were HCIR.
Walking intercept survey
All professionals were distinguished by professional group, type of job, job execution location (e.g. varying hospitals), required departmental access level in the hospital (e.g. operating room [OR], intensive care unit [ICU], central sterile services department [CSSD] and outpatient clinic [OC]), vaccination status and hygienic training status and schedule. Therefore, the anonymous walking intercept survey contained 38 questions focused on:
- Inclusion / Exclusion criteria
- Professional category
- Primary location and location variability of job execution
- Required departmental access levels in hospitals
- Personal hygiene training status
- Personal vaccination status
- Personal contact to animals / livestock.
It was not mandatory to answer all questions, the full questionnaire is provided as supplementary material.
Data analysis and statistics
Statistical and descriptive analysis of data was performed using Excel 2010® and Minitab 16® in cooperation with the Institute for Biostatistics and Informatics in Medicine and Ageing Research, University Medicine of Rostock. For qualitative parameters the Chi-square test was performed. Quantitative data were tested for normality with KS-test and Anderson-Darling and for significance with U-Test from Mann/Whitney. All data with a P value ≤0.05 were considered statistically significant.
Results
Walking intercept survey
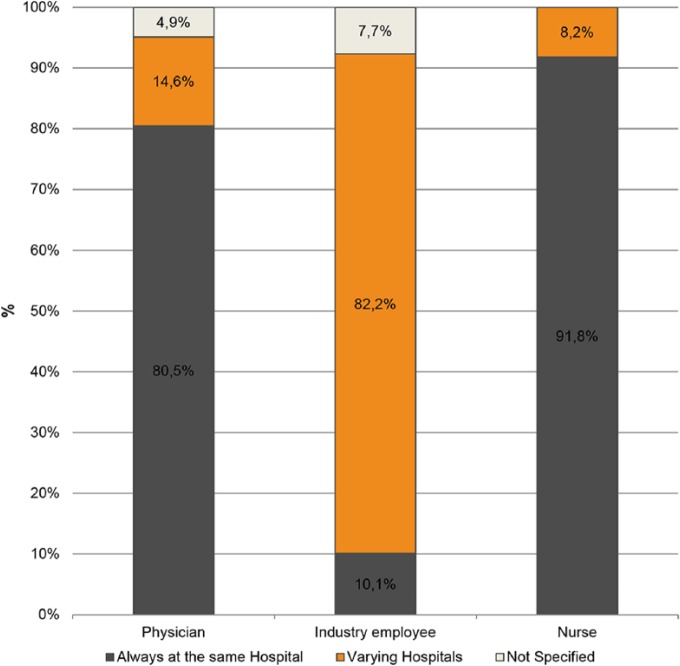
Most physicians (80.5%) and nurses (91.8%) worked in one hospital only. In contrast, it was common for HCIR (82.2%, n = 171) to work in varying hospitals. The percentage of each professional group working in multiple hospitals is shown in Figure 1.
Figure 1.
Location of job execution.
HCIR report that they are frequently requested to access areas that are sensitive in terms of hygiene and privacy: the outpatient clinic (51.9%, n = 108) the OR (41.8%, n = 87), CSSD (33.7%, n = 70) and ICU (32.7%, n = 68). Table 1 contains the complete data on the required access level of the study participants by professional group.
Table 1.
Required level of access at least once a month of the three professional groups.
| Physicians % |
Industry employees % |
Nurses % |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | No answer | Yes | No | No answer | Yes | No | No answer | |
| Operating Room | 58.5 | 29.3 | 12.2 | 41.8 | 38.5 | 19.7 | 38.8 | 32.7 | 28.6 |
| Intensive Care Unit | 48.8 | 29.3 | 22.0 | 32.7 | 44.7 | 22.6 | 53.1 | 30.6 | 16.3 |
| Central Sterile Supply Department | 7.3 | 65.9 | 26.8 | 33.7 | 43.3 | 23.1 | 22.4 | 51.0 | 26.5 |
| Outpatient clinic | 65.9 | 9.8 | 24.4 | 51.9 | 26.0 | 22.1 | 46.9 | 24.5 | 28.6 |
Hygiene training
HCIR displayed the lowest percentages of yearly renewal of hygiene training (HCIR: 15.7% vs. 41.9% physicians and 47.1% nurses) and mandatory attendance to those trainings (HCIR: 10.2% vs. 22.6% physicians and 41.2% nurses). This resulted in the lowest hygiene training scores:
HCIR: 0.01 / 1.0
Physician: 0.03 / 1.0
Nurses: 0.11 / 1.0.
The data used to calculate the hygiene training score are provided in Table 2.
Table 2.
Hygiene training score (HTS) of the three professional groups.
| Physicians % |
Industry employees % |
Nurses % |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | No answer | Yes | No | No answer | Yes | No | No answer | |
| Received hygiene training prior ray first day on the job | 35.5 | 61.3 | 3.2 | 38.0 | 49.1 | 13.0 | 58.8 | 26.5 | 14.7 |
| Received hygiene training every year – as refresher | 41.9 | 41.9 | 16.1 | 15.7 | 73.1 | 11.1 | 47.1 | 32.4 | 20.6 |
| Received hygiene training as mandatory training module | 22.6 | 41.9 | 35.5 | 10.2 | 73.1 | 16.7 | 41.2 | 32.4 | 20.6 |
| Hygiene Training Score | 0.03 | 0.01 | 0.11 | ||||||
Data in this table only regard employees with required monthly need to access OR/ICU or Central Sterile Supplies Department.
The hygiene training score is defined as the product of percentages of ‘prior my first day’, ‘yearly refresher’ and ‘mandatory’. Denotes the chance that every deviation from scheme would be detected best = 1.0, worst = 0.0.
MRSA testing and Hepatitis B vaccination status
When asked about previous MRSA testing, only 8.7% (n = 18) of HCIR reported previous evaluation compared to 26.8% (n = 11) of physicians and 26.5% (n = 13) of nurses. A total of 20.2%, 7.3% and 10.2% of HCIR, physicians and nurses, respectively, did not answer.
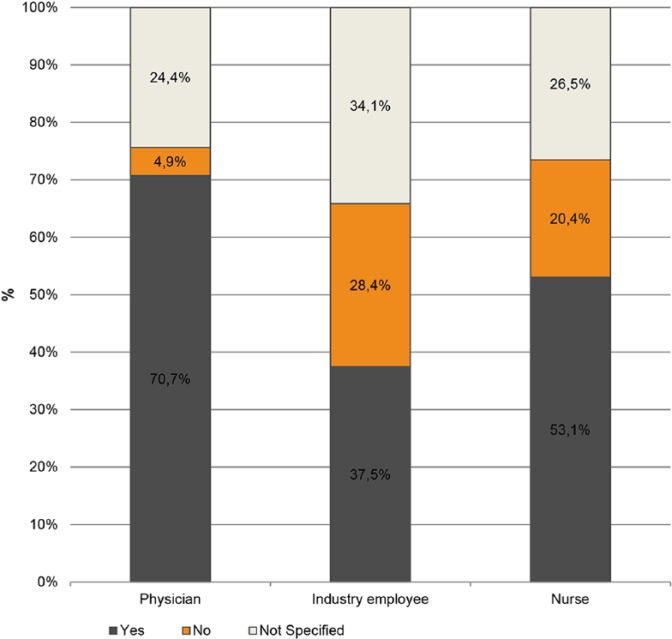
In 62.5% (n = 130) of cases, HCIR employers did not monitor their vaccination status on a yearly basis. In addition, 56.7% (n = 118) of HCIR employers did not mandate Hepatitis B vaccination at all according to survey answers, see Figure 2. A total of 37.5% of HCIR reported an active Hepatitis B vaccination. However, 28.4% knew they were not vaccinated against Hepatitis B. In contrast, 70.7% of physicians and 53.1% of nurses specified a current active Hepatitis B vaccination (see Table 3).
Figure 2.
Hepatitis B immunisation as percentage of professional category.
Table 3.
Vaccination status for the three professional groups.
| Physicians % |
Industry employees % |
Nurses % |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | No answer | Yes | No | No answer | Yes | No | No answer | |
| Hepatitis B vaccination | 70.7 | 4.9 | 24.4 | 37.5 | 28.4 | 34.1 | 53.1 | 20.4 | 26.5 |
| Immunisation is monitored on yearly basis by employer | 41.5 | 46.3 | 12.2 | 16.3 | 62.5 | 21.2 | 28.6 | 28.6 | 42.9 |
| Immunisation is initiated by employer | 43.9 | 34.1 | 22.0 | 16.3 | 56.7 | 26.9 | 36.7 | 20.4 | 42.9 |
Hepatitis B vaccination is listed only regarding employees with required monthly need to access OR/ICU or Central Sterile Supplies Department.
Statistical analysis of hygiene training scores was performed with Chi-square test for qualitative data. No statistical significant difference was found between HCIR and physicians, both being at a significantly lower level than the nurses.
Discussion
In the walking intercept survey a high percentage of HCIR were found to require access to hygiene sensitive hospital areas (64.9%, n = 135). This is consistent with EUCOMEDs statement that ‘Medical Device companies are frequently requested to have a representative of the company present during surgical or other medical procedures as an advisor to the medical team’ (Bonten et al., 2001). In the survey 43.3% (n = 90) of HCIR were performing a sales representative role in hospitals. Within this subgroup 87.8% (n = 79) were performing their roles in varying hospitals, with 60.0% (n = 54) in more than one hospital during the same day on at least 5 days per month.
As a result, a significant portion of HCIR travelled on a regular basis between hospitals on the same day. With this in mind, the interaction of physicians and nurses with HCIR in hygiene sensitive areas should be analysed under strict risk benefit aspects.
Employee’s status of prevention and vaccination
Healthcare personnel are known to be at increased riskof occupational acquisition of Hepatitis B virus (Thomas et al., 1993). Vaccination of HCP against Hepatitis B should therefore be vigorously pursued (Thomas et al., 1993). Against this background it is concerning that only 16.3% (n = 34) of HCIR employers checked the vaccination status of their staff on a yearly basis and only 37.5% (n = 78) of HCIR indicated being vaccinated against Hepatitis B.
This is despite the 2011 EUCOMED recommendations to its members that ‘every employee must be reasonably sure that they are not carrying any contagious diseases’ (Bonten et al., 2001).
Our prior study revealed that HCIR and HCP are likely to share a comparable microbiological burden, based on a shared working environment (Schiffers et al., 2014). HCAI are a longstanding prevalent problem. To alleviate this, procedural standards, vaccination and hygiene training are introduced. HCIR are, according to their own account, the least equipped with respect to those prevention methods. As shown in the results (see above), HCIR rank either as the lowest, or share the lowest rank in all three analysed aspects. Having the same microbiological burden, it is reasonable to assume that the HCIR contribute a significant risk factor for HCAI in the hospital environment. This risk probably does not stem not from patient interactions, since HCIR have only little interaction with them (Cohen et al., 2011). The source might be the shared work environment, as shown in our study on colonisation of convention attendees (Schiffers et al. 2014). Furthermore, the fact that a majority of HCIR travels between hospitals frequently exacerbates the risk they pose.
Mielke et al. suggests that the critical factor to succeed in infection prevention is awareness (Mielke et al., 2008). To conclude, we advise increased awareness towards this potential infection vector and to routinely incorporate the risk group of HCIR in designing standards and regulations.
Limitations
A medical trade fare location was chosen to recruit a high number of participants in a short period of time. Study bias may have occurred because the participants of the different professional groups could have thought that their employers may expect them to answer question in a specific direction. Without substantial reason to assume that HCIR or hospital staff are impacted differently, the differences in the data regarding the status in hygiene training, contamination screening and vaccination seem strong enough to draw the conclusions we made. However, it was beyond the scope of the present study to verify both the real vaccination status of the participants and the execution of hygiene training and contamination control on their workplace. This study did not distinguish between subgroups of the HCIR, for instance examining their industrial branch or education level or other potential influence factors. To obtain meaningful data at a higher resolution a larger sample size would be required. Given the substantially higher cost and effort of experimentally verifying the variety and depth of data acquired here, a survey seems to be the most effective method.
Conclusion
Fighting HCAI is a ubiquitous problem in the healthcare industry. A walking intercept study was performed to verify our hypothesis about a neglected possible source of such infections. Thereby, the self-reported status of hygiene training, vaccination and contamination control for HCIR was inadequate. Given the significance of frequent patient and HCP interaction, as well as HCP and HCIR interaction, there currently might be a legitimate interest to re-evaluate industry contacts with HCP. Therefore, HCIR are exposed to increased infection risk and may unknowingly act as infection vector between different hospitals.
The presented data are meant to raise awareness and establish HCIR as a risk group in the hospital environment. Providing approaches to regulate and prevent this risk source is beyond the scope of this study. It can serve, however, as a catalyst for more in-depth investigations in which HCIR may be followed and measured as all of their activities in hospitals are tracked and recorded. Such studies are logistically challenging and costly but may reduce the risk for patients, HCP and HCIR.
Acknowledgments
Execution of the tests would have been impossible without the engagement of the nursing students and teachers of Kaiserswerther Diakonie, Düsseldorf, Germany. Kind thanks to Kelly Carden, MD, MBA for her support. Thanks to Prof. Dr. Günter Kundt, Institute for Biostatistics and Informatics in Medicine and Ageing Research, University Medicine of Rostock for assisting the statistical analysis. The required equipment and single use materials for this work were privately funded by David E. Halliday.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Peer review statement: Not commissioned; blind peer-reviewed.
Ethical standards: The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals. Refer to the Ethics Commission of University Medicine Rostock, trial identification number: A2013-0010.
References
- Bedard J, Moore CD, Shelton W. (2014) A survey of healthcare industry representatives’ participation in surgery: Some new ethical concerns. Journal of Clinical Ethics 25: 238–244. [PubMed] [Google Scholar]
- Bonten MJ, Austin DJ, Lipsitch M. (2001) Understanding the spread of antibiotic resistant pathogens in hospitals: Mathematical models as tools for control. Clinical Infectious Diseases 33: 1739–1746. [DOI] [PubMed] [Google Scholar]
- Cohen B, Hyman S, Rosenberg L, Larson E. (2012) Frequency of patient contact with health care personnel and visitors: Implications for infection prevention. Joint Commission Journal on Quality and Patient Safety / Joint Commission Resources 38: 560–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuny C, Nathaus R, Layer F, Strommenger B, Altmann D, Witte W. (2009) Nasal colonization of humans with methicillin-resistant Staphylococcus aureus (MRSA) CC398 with and without exposure to pigs. PLoS One 4: e6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastmeier P, Stamm-Balderjahn S, Hansen S, Nitzschke-Tiemann F, Zuchsneid I, Groneberg K, Ruden H. (2005) How outbreaks can contribute to prevention of nosocomial infection: Analysis of 1,022 outbreaks. Infection Control and Hospital Epidemiology 26: 357–361. [DOI] [PubMed] [Google Scholar]
- Girou E, Legrand P, Soing-Altrach S, Lemire A, Allaire A, Tkoub-Scheirlink L, Chai SH, Dupeyron C, Loche CM. (2006) Association between hand hygiene compliance and methicillin-resistant Staphylococcus aureus prevalence in a French rehabilitation hospital. Infection Control and Hospital Epidemiology 27: 1128–1130. [DOI] [PubMed] [Google Scholar]
- Kramer A, Schwebke I, Kampf G. (2006) How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infectious Diseases 6: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam BCC, Lee J, Lau YL. (2004) Hand hygiene practices in a neonatal intensive care unit: A multimodal intervention and impact on nosocomial infection. Pediatrics 114: e565–571. [DOI] [PubMed] [Google Scholar]
- Mielke M. (2008) “Das Problem der nosokomialen Infektionen und Antibiotikaresistenz aus mitteleuropäischer Sicht.” http://www.rki.de/DE/Content/Infekt/Krankenhaushygiene/Nosokomiale_Infektionen/nosok_infektionen_pdf3.pdf?__blob=publicationFile (accessed 7th January 7 2013).
- Pessoa-Silva CL, Hugonnet S, Pfister R, Touveneau S, Dharan S, Posfay-Barbe K, Pittet D. (2007) Reduction of health care associated infection risk in neonates by successful hand hygiene promotion. Pediatrics 120: e382–390. [DOI] [PubMed] [Google Scholar]
- Pittet D, Mourouga P, Perneger TV. (1999) Compliance with handwashing in a teaching hospital. Annals of Internal Medicine 130: 126–130. [DOI] [PubMed] [Google Scholar]
- Schiffers H, Zaatreh S, Mittelmeier W, Bader R. (2014) Examination of cross contamination risks between hospitals by external medical staff via cross-sectional intercept survey of hand hygiene. GMS Hygiene and Infection Control 9: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DL, Factor SH, Kelen GD, Washington AS, Taylor E, Quinn TC. (1993) Viral hepatitis in health care personnel at The Johns Hopkins Hospital. The seroprevalence of and risk factors for hepatitis B virus and hepatitis C virus infection. Archives of Internal Medicine 153: 1705–1712. [PubMed] [Google Scholar]
- Weinstein RA. (1991) Epidemiology and control of nosocomial infections in adult intensive care units. American Journal of Medicine 91 (Suppl. 2): S179–S184. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2011) Report on the burden of endemic health care-associated infection worldwide. Geneva: World Health Organization; http://apps.who.int//iris/handle/10665/80135 (accessed 15th September 2014). [Google Scholar]