Abstract
Carbapenem-resistant organisms are emerging as a global health threat. The prevalence of CROs in London is largely unknown. A retrospective review of microbiology records indicates an increased in carbapenem-resistant Klebsiella pneumoniae (none in 2011 to 1.3% of 386 in 2013, P = 0.073) and Acinetobacter baumannii (9.1% of 11 in 2011 to 31.2% of 16 in 2013, P = 0.001) in a background of low prevalence at a London hospital. This suggests that CROs may be emerging in our patient population. These increases demand an urgent enhanced surveillance response.
Keywords: Carbapenem-resistant organism (CRO), carbapenem-resistant Enterobacteriaceae (CRE), Acinetobacter baumannii, Klebsiella pneumoniae
Introduction
Carbapenem-resistant organisms (CROs) have emerged in recent years as a significant public health threat (Coelho et al., 2006; Gupta et al., 2011; Higgins et al., 2010; Nordmann et al., 2013). Therapy for CROs relies on older, less effective and less well tolerated antimicrobials such as the polymyxins (Gupta et al., 2011). Carbapenem resistance is most commonly mediated through a combination of impermeability and AmpC (Ampicillinase C) / extended spectrum beta-lactamase (ESBL) activity (AmpC is a betalactamse whose over expression can confer resistance to broad spectrum cephalosporins) or acquired carbapenemase production (Gupta et al., 2011). Carbapenemases are beta-lactamases with the ability to hydrolyse carbapenem antibiotics. The hydrolytic mechanisms require either a serine or zinc molecule on the active site, which forms the basis of their classification; classes A and D have a serine based hydrolytic mechanism and include KPC and OXA-48. Class B, the metallo-beta-lactamases, requires zinc at the active site and include VIM and IMP (Queenan and Bush, 2007). Initially identified on bacterial chromosomal DNA, they are increasingly identified on mobile genetic elements, allowing rapid dissemination in the clinical setting (Canton et al., 2012; Nordmann et al., 2011). Carbapenemase genes can be acquired by the Enterobacteriaceae such as Klebsiella pneumoniae and non-fermenters such as Acinetobacter baumannii and Pseudomonas aeruginosa (Coelho et al., 2006; Higgins et al., 2010; Nordmann et al., 2011).
CROs are emerging worldwide (Gupta et al., 2011; Higgins et al., 2010; Nordmann et al., 2011). The European surveillance network EARS-Net (European Antimicrobial Surveillance Network) collects antimicrobial resistance data from invasive isolates reported to EARS-Net by 29 EU/EEA countries. These data suggest that the prevalence of carbapenem-resistant Enterobacteriaceae (CRE) is low in invasive isolates throughout most of Europe (European Centre for Disease Prevention and Control, 2014). However, the rate of carbapenem-resistant Klebsiella pneumoniae increased from 28% of invasive K. pneumoniae isolates in 2005 to 68% in 2011 in Greece, and from 1% in 2009 to 27% in 2011 in Italy. Rates of carbapenem resistance in invasive Pseudomonas aeruginosa also vary across Europe, with high rates in Greece (54% of all invasive isolates in 2011) and Cyprus (43% in 2011). The rate of carbapenem resistance among P. aeruginosa invasive isolates in the UK has been fairly stable at around 5% and the prevalence of carbapenem-resistant Klebsiella pneumoniae is low at <1% (European Centre for Disease Prevention and Control, 2014). However, outbreaks of CROs have been reported (Coelho et al., 2006; Drew et al., 2013; Higgins et al., 2010) and Public Health England reports an increasing number of carbapenemase-producing Enterobacteriaceae referrals from <100 in 2009 to 800 in 2012 (Public Health England, 2011). In light of the increasing global and national carbapenem resistance trends, the prevalence of CROs was evaluated in recent years at a large London hospital.
Methods
The study investigated the prevalence of CROs in the microbiology database from a large London NHS teaching hospital, which comprises two sites with approximately 1200 beds. The microbiology laboratory processes samples from inpatients, specialist referrals from across the region, and patients seen in primary care facilities in the community. Culture results of all samples from which Enterobacteriaceae or non-fermenting Gram-negative bacteria identified between April 2011 and June 2013 were downloaded into a database. The study was restricted to April 2011 to June 2013 because prior to April 2011 the hospital had a different laboratory system, and changes in standard antimicrobial testing protocols and methods, including a change from disc diffusion to semi-automated broth microdilution (Vitek), meant that meropenem susceptibility was rarely tested in Gram-negative bacteria prior to 2011. After June 2013, enhanced surveillance for CRE began, which would skew the findings. Samples from Enterobacteriaceae or non-fermenting Gram-negative bacteria comprised mainly clinical cultures (99.5%) and some screens (0.5%). During the study period, the routine hospital surveillance programme for resistant organisms included rectal swabs on admission and throughout the stay of patients on ICU and HDU cultured using gentamicin as a selective agent. Antimicrobial susceptibility was determined through semi-automated broth micro-dilution (Vitek) or disc diffusion according to British Society Antimicrobial Chemotherapy (BSAC) guidelines, depending on the specimen type. Isolates resistant to meropenem were considered carbapenem-resistant, according to EUCAST recommendations (2013), regarding meropenem as the most useful carbapenem in the detection of carbapenemase production. Data collected included the date of collection, specimen type, the organism grown and the antibiogram. All Enterobacteriaceae and non-fermenting Gram-negative bacteria tested for meropenem sensitivity (comprising 99.6% of the total) were included in the analysis. Enterobacteriaceae and non-fermenting Gram-negative bacteria were analysed separately. Trends were analysed using Chi-squared tests.
This work was classified as service evaluation and exempt from Research Ethics Council review.
Results
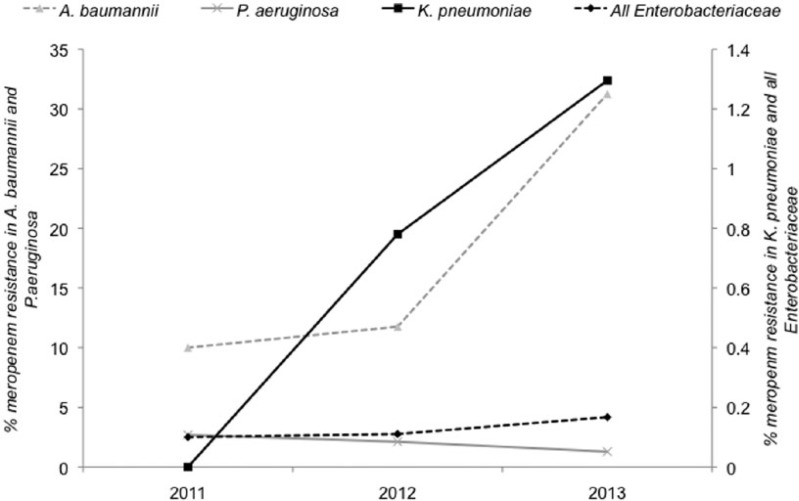
CRE were identified from 23 (0.14%) of 18,279 patients who had a positive culture for Enterobacteriaceae. The prevalence of CRE increased from 15 (0.1%) of 12,931 in 2011–2012 to eight (0.15%) of 5348 in 2013 (P = NS). Importantly, the prevalence of carbapenem-resistant Klebsiella pneumoniae increased from none of 82 in 2011 to five (1.3%) of 386 in 2013 (P = 0.073) (Figure 1). The characteristics of the 23 CRE isolates are summarized in Table 1. The most common CRE identified was Klebsiella species (12/23, 52.2%). CRE were identified from surgery, medicine, critical care, paediatrics and the community. Only two CRE were identified on screens alone; 9/23 were from urine samples. No epidemiological links were obvious between the isolates. Of the 16 meropenem-resistant Enterobacteriaceae detected between 2002 and 2009, 88% were susceptible to another carbapenem (imipenem); in contrast, none of the eight meropenem-resistant Enterobacteriaceae identified between 2011 and 2013 that were tested for susceptibility were sensitive to another carbapenem (ertapenem). This supports an increase in carbapenemase-producing Enterobacteriaceae in recent years.
Figure 1.
The prevalence of carbapenem (meropenem) resistance in key organisms and organism groups, 2011–2013.
Table 1.
Characteristics of the 25 carbapenem-resistant Enterobacteriaceae detected.
| Patient number | Age (years) | Sex | Year of sample | Wards | Specimen location | Subsequent clinical sample | Organism | Antibiogram |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMC | AMO | CAZ | CTX | CXM | ERT | FEP | FOX | MER | TAZ | ||||||||
| 1 | 83 | M | 2011 | Outpatient | Urine | E. cloacae | R | R | R | R | R | R | R | R | R | R | |
| 2 | 62 | M | 2011 | Haemoncology | Sputum | E. cloacae | R | R | R | R | R | R | R | R | R | R | |
| 3 | 67 | F | 2012 | Surgery | Drain fluid | Enterobacter sp | R | R | R | – | R | – | – | R | R | ||
| 4 | 80 | M | 2012 | ICU | Resistance screen | Yes | K. pneumoniae | R | R | R | R | R | R | R | R | R | R |
| 5 | 57 | F | 2012 | Community | Urine | Klebsieíla sp | R | R | R | R | R | R | R | R | R | R | |
| 6 | 75 | m | 2012 | CU | Sputum | K. pneumoniae | R | R | R | R | R | R | R | R | R | ||
| 7 | 86 | M | 2012 | ICU | Sputum | S. marcescens | R | R | R | R | – | – | R | R | |||
| 8 | 66 | M | 2012 | Surgery | Urine | K-Oxytoca | R | R | R | R | R | R | R | R | R | ||
| 9 | 53 | F | 2012 | Surgery | Unknown | K. pneumoniae | R | R | R | R | R | R | R | R | R | ||
| 10 | 42 | F | 2012 | Medicine | Urine | K. pneumoniae | R | R | R | R | R | R | R | R | R | R | |
| 11 | 89 | F | 2012 | Surgery | Urine | M. morganii | R | R | R | R | – | – | R | R | |||
| 12 | 46 | F | 2012 | Surgery | Abdominal swab | K. pneumoniae | R | R | R | R | R | R | R | R | R | R | |
| 13 | 88 | M | 2012 | Surgery | Specimen | E. coli | R | R | R | R | R | – | R | R | |||
| 14 | U | U | 2012 | Unknown | Lung | E. coli | R | R | R | R | R | R | R | R | R | ||
| 15 | 0.92 | M | 2012 | Paediatrics | Resistance screen | Yes | K. pneumoniae | R | R | R | R | R | R | R | R | R | |
| 16 | 33 | M | 2013 | Haemoncology | Blood - culture | E. coli | R | R | R | R | R | R | R | R | R | ||
| 17 | U | U | 2013 | Community | Urine | K. pneumoniae | R | R | R | R | R | R | R | R | R | R | |
| 18 | U | U | 2013 | Community | Urine | E. coli | R | R | R | R | R | R | R | R | R | ||
| 19 | 1 | M | 2013 | PICU | Resistance screen | No | K. pneumoniae | R | R | R | R | – | R | R | |||
| 20 | 51 | F | 2013 | Surgery | Hip swab | K. pneumoniae | R | R | R | R | R | – | R | R | R | R | |
| 21 | 2 | F | 2013 | PICU | Resistance screen | No | K. pneumoniae | R | R | R | R | R | – | R | R | R | R |
| 22 | 73 | F | 2013 | Surgery | Urine | K. pneumoniae | R | R | R | R | R | R | R | R | R | R | |
| 23 | U | U | 2013 | Community | Urine | E. coli | _R | R | R | R | R | R | R | R | R | R | |
Carbapenem-resistant non-fermenters were identified from 129 (2.6%) of 4779 patients. The prevalence of carbapenem resistance among the non-fermenters did not increase over the study period overall but carbapenem-resistant A. baumannii increased from three (10.3%) of 29 in 2011 to five (31.2%) of 16 in 2013 (P = 0.001) (Figure 1). Analysis of the carbapenem-resistant A. baumannii isolates from 2013 indicated a range of specialties and antibiograms, indicating that a clonal outbreak was not responsible for the increase.
Discussion
The study confirms the low prevalence of CROs in the population studied amounting to <1% of all Enterobacteriaceae and <5% of non-fermenters. However, it has demonstrated an apparent increase in the proportion of Enterobactericae that are CRE in recent years, particularly in K. pneumoniae. Although these increases were not statistically significant due to the low number of cases, the trends are concerning given rapid increases of CRE in Greece, Italy and Israel, and suggests that the increase in CRE reported by Public Health England is not solely explained by ‘referral bias’ (Canton et al., 2012; ; Public Health England, 2011 Schwaber et al., 2011). Only two CRE cases were reported in 2011 compared with eight cases in the first half of 2013, suggesting an increase. CRE were most frequently identified from clinical cultures, indicating that they are likely to cause infection, and represented sporadic cases. The discovery of CRE in a range of species and specialties suggests repeated introduction with limited horizontal transmission. There is some evidence of a community reservoir, with 30% of cases from general practice/outpatient clinics. However, many of these patients are likely to have had healthcare contact. While there is some evidence that CRE are emerging globally and nationally in the UK, prevalence studies are lacking (Canton et al., 2012; Drew et al., 2013; Nordmann et al., 2011).
The epidemiology of non-fermenting Gram-negative bacteria such as A. baumannii is distinct from CRE. While resistance to carbapenems in these organisms is problematic and has been associated with outbreaks, particularly in critical care, the explosive spread associated with CRE has not been reported (Coelho et al., 2006; Higgins et al., 2010; Nordmann et al., 2011). We detected a significant increase in the rate of carbapenem resistance in A. baumannii, reaching more than 30% in 2013. A. baumannii is an uncommon cause of serious infection in the UK, and tends to cause clinical infection in the immunocompromised or those in the intensive care units. While the emergence of carbapenem resistance in A. baumannii is consistent with global trends, we cannot explain the rapidity of the increase over the past few years since the antibiogram and epidemiology of individual cases did not appear to be linked (Higgins et al., 2010).
This pragmatic prevalence survey has several important limitations. The investigators were reliant on electronic records that had only limited patient data. Increasing worldwide travel has been associated with CRE (Canton et al., 2012; Nordmann et al., 2011). In this study no data were available regarding foreign travel which is a common feature associated with CRE. Although no changes to the screening policy occurred during the study period, the survey included a small number of resistance screens, which may skew apparent prevalence. Molecular analysis to determine the mechanism of carbapenem resistance was not performed.
Based on the apparent emergence of carbapenem resistance in both Enterobacteriaceae and non-fermenters in our hospitals over the past few years, there is an urgent need to prospectively define the prevalence of CROs in order to evaluate the risk and implement effective interventions to prevent the sporadic cases developing into an epidemic. In response to our findings and the threat of CROs, we are embarking on enhanced surveillance including screening a wider group of patients for CROs to identify the hidden burden. Specifically, all those patients admitted from other hospitals, from overseas, and on admission to high-risk wards (intensive care wards) will be screened for CRE (and VRE). We hope that targeted interventions in our low prevalence setting will prevent the widespread emergence of CROs in our patient population, which would have grave implications.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was classified as service evaluation and exempt from Research Ethics Council review.
Peer review statement: Not commissioned; blind peer-reviewed.
References
- Canton R, Akova M, Carmeli Y, Giske CG, Glupczynski Y, Gniadkowski M, Livermore DM, Miriagou V, Naas T, Rossolini GM, Samuelsen O, Seifert H, Woodford N, Nordmann P; European Network on Carbapenemases. (2012) Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clinical Microbiology and Infection 18: 413–431. [DOI] [PubMed] [Google Scholar]
- Coelho JM, Turton JF, Kaufmann ME, Glover J, Woodford N, Warner M, Palepou MF, Pike R, Pitt TL, Patel BC, Livermore DM. (2006) Occurrence of carbapenem-resistant Acinetobacter baumannii clones at multiple hospitals in London and Southeast England. Journal of Clinical Microbiology 44: 3623–3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew RJ, Turton JF, Hill RL, Livermore DM, Woodford N, Paulus S, Cunliffe NA. (2013) Emergence of carbapenem-resistant Enterobacteriaceae in a UK paediatric hospital. Journal of Hospital Infection 84: 300–304. [DOI] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control. (2014) Antimicrobial resistance interactive database: ERAS-Net. Available at: http://www.ecdc.europa.eu/en/healthtopics/antimicrobial_resistance/database/Pages/database.aspx (accessed 27 November 2014).
- EUCAST subcommittee for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance. (2013) Available at: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Consultation/EUCAST_guidelines_detection_of_resistance_mechanisms_121222.pdf (accessed June 2015).
- Gupta N, Limbago BM, Patel JB, Kallen AJ. (2011) Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clinical Infectious Diseases 53: 60–67. [DOI] [PubMed] [Google Scholar]
- Higgins PG, Dammhayn C, Hackel M, Seifert H. (2010) Global spread of carbapenem-resistant Acinetobacter baumannii. Journal of Antimicrobial Chemotherapy 65: 233–238. [DOI] [PubMed] [Google Scholar]
- Nordmann P, Naas T, Poirel L. (2011) Global spread of Carbapenemase-producing Enterobacteriaceae. Emerging Infectious Diseases 17: 1791–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann P, Poirel L. (2013) Strategies for identification of carbapenemase-producing Enterobacteriaceae. Journal of Antimicrobial Chemotherapy 68: 487–489. [DOI] [PubMed] [Google Scholar]
- Public Health England. (2011) Carbapenemase-producing Entero bacteria ceae: laboratory confirmed cases, 2003 to 2013. Available at: https://www.gov.uk/government/publications/carbapenemase-producing-enterobacteriaceae-laboratory-confirmed-cases/carbapenemase-producing-enterobacteriaceae-laboratory-confirmed-cases-2003-to-2013 (accessed 27 November 2014).
- Queenan AM, Bush K. (2007) Carbapenemases: the versatile beta-lactamases. Clinical Microbiology Reviews 20: 440–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaber MJ, Lev B, Israeli A, Solter E, Smollan G, Rubinovitch B, Shalit I, Carmeli Y; Israel Carbapenem-Resistant Enterobacteriaceae Working Group. (2011) Containment of a country-wide outbreak of carbapenem-resistant Klebsiella pneumoniae in Israeli hospitals via a nationally implemented intervention. Clinical Infectious Diseases 52: 848–855. [DOI] [PubMed] [Google Scholar]