Abstract
A 350-bed Sydney hospital noted excessive container-associated sharps injuries (CASI) using small sharps containers and compared the effect from 2004 to 2010 of using a larger container engineered to reduce CASI. In Phase 1 (Ph1), disposable 1.4L containers (BD Australia) were carried to/from patients’ rooms. In Phase 2 (Ph2), this stopped and a safety-engineered 32L reusable container (the Device; Sharpsmart, SteriHealth) was mounted in medication stations only and sharps were carried to and from patient rooms using kidney dishes. In Phase 3 (Ph3), the Device was wall-mounted in patient rooms. Sharps injuries were categorised as ‘during-procedure’, ‘after-procedure but before disposal’, ‘CASI’, and ‘improper disposal SI’. Disposal-related SI comprised CASI plus improper-disposal SI. Injuries per 100 full-time-equivalent staff were analysed using Chi2; p ≤ 0.05; and relative risk and 95% confidence limits were calculated. In Ph1 (small containers) 19.4% of SI were CASI and transport injuries were zero. In Ph2 (Device in medication station) CASI fell 94.9% (p <0.001); Disposal-related SI fell 71.1% (p=0.002) but transport injuries rose significantly. In Ph3 (Device in patient room) zero CASI occurred (p<0.001); Disposal-related SI fell 83.1% (p=0.001). Recapping SI fell 85.1% (p=0.01) with the Device. The Device’s volume, large aperture, passive overfill-protection and close-at-hand siting are postulated as SI reduction factors.
Keywords: Container-associated, kidney dish, needlestick, patient-room, recapping, reusable, sharps container, sharps injury, Sharpsmart
Introduction
Commercial sharps containers (SC) were first advocated for use in healthcare facilities (HCF) by Osterman (1975), but their adoption created a new sharps injury (SI) hazard: container-associated sharps injuries (CASI). These are sharps injuries sustained while depositing sharps into, or during the handling of, SC (Grimmond et al, 2010).
National annual SI data are unavailable in the UK, but the annual number of healthcare workers sustaining SI is estimated to be between 56,000 (Elder and Paterson, 2006) and 100,000 (Godfrey, 2001). In a Royal College of Nursing (RCN) survey (2005) of 19 trusts, 11.5% of total SI were CASI, which translates to 6,000–12,000 UK healthcare workers sustaining CASI annually. This may indicate that safer SC and SC protocols are needed as previous studies indicate CASI incidence can be significantly reduced with the use of safety-engineered SC (Grimmond et al, 2003, 2010).
Australian federal regulations do not cover the siting of sharps containers, which is regulated by state guidelines that commonly require containers to be sited “as close as practical to the point of use of sharp devices” (NSW Health, 2007) and most Australian hospitals place containers on the wall in the patient’s room (or occasionally on a cart brought to the bedside). In the UK, the Control of Substances Hazardous to Health (COSHH) regulations require that containers “be provided for the disposal of contaminated waste” (COSHH, 2002) and the more recent Sharps Regulations require containers to be, “located close to areas where medical sharps are used” (Health and Safety, 2013; Health and Safety Executive, 2013). In UK hospitals this is commonly achieved by transporting small containers to and from patients’ rooms so that the container is ‘within arm’s reach’ as recommended by the Royal College of Nursing (2011). In the USA, federal law does not mention SC siting, but it is nationally recommended that SC are placed “within easy horizontal reach of the user” (National Institutes for Occupational Safety and Health (NIOSH), 1998), and fixation on patient-room walls is the norm.
Sydney Adventist Hospital (SAH) is a 350-bed, acute-care private hospital in which, as in many UK hospitals, small SC were carried to and from patients’ rooms. Prior to intervention, sharps containers were not fixed on patient-room walls for reasons of aesthetics and visitor safety. Although safety engineered devices were used at SAH, the incidence of CASI was disturbingly high. At incident follow ups, staff commented to one of the authors (WN) that the increased SI with small SC was likely due to their small aperture, limited capacity and temptation to push more into them. The hospital became aware of an international study reporting significant SI reductions with a large engineered SC with enhanced safety features (the Device) (Grimmond et al, 2003) and, after clinical evaluation revealed high staff acceptance, the Device was adopted. This study formally evaluated the hypothesis that the Device’s size and engineered safety features together with ergonomic placement would reduce the high rate of CASI occurring with small portable SC used at the bedside and that the Device’s size may also reduce improper disposal SI.
Methods
The six-year study (2004 to 2010) utilised a three-phase, before-after intervention model. In phase 1 (Ph1) prior to the intervention, healthcare workers at SAH carried disposable 1.4L SC (Tray Collectors, BD Australia) to and from patient rooms in a tray along with injection items. Used sharps too large for the small SC were transported in the tray to each ward’s medication station (med room) for disposal into a single-use 22.7L SC (Nestable Collectors, BD Australia).
In phase 2 (Ph2), use of small SC ceased and a 32L reusable SC with large aperture, counterbalanced door and passive overfill protection (‘the Device’; Daniels Sharpsmart, SteriHealth Ltd) was placed in all med rooms (one per ward) and staff received mandatory in-service training in the use and handling of the Device. In Ph2 the device was not sited in patient rooms for reasons of aesthetics and safety. Sharps were transported to and from patients’ rooms using kidney dishes or trays. In phase 3 (Ph3) of the study, a 22L model of the Device was wall-mounted in patient rooms at an ergonomic height and as close as practical to patients’ beds. Employee descriptions of their SI were obtained retrospectively from the hospital’s SI log during Ph1 (20 months) and Ph2 (30 months) and prospectively in Ph3 (20 months). Data in each change-over month was excluded to avoid the possibility of placing SI in an incorrect study phase.
Sharps injuries were categorised as follows:
During-procedure (these SI are unrelated to sharps containers but included to calculate the total SI)
After-procedure but before disposal (‘before-disposal’), e.g. device activation, reprocessing, transporting to SC, removing needle from syringe/holder, placing/removing sharp in kidney dish, transfer after surgical procedure, recapping
- CASI:
- While placing sharp into container, injury caused by:
- sharp being disposed
- sharp already in container (container not overfilled)
- overfilled sharps container
- sharp (unclear how)
- Sharp protruding from container (but not overfilled)
- Sharp bouncing out of container
- While manipulating container (closing, moving, handling, shaking, entering)
- Sharps puncturing container
Improper-disposal SI: e.g. sharps left on bedside cabinet, overway table, food tray, floor, bed, in linen, or discarded into trash bags or incorrect bins.
CASI and improper-disposal SI were combined as ‘Disposal-related SI’. When SI classification was not clear from the SI log (e.g. stated as ‘during disposal’), clarification was sought from the original incident form or if needed, from the injured staff member. Annual full-time-equivalent staff (FTE) data were obtained, apportioned for the number of months in each study phase, and data normalised by calculating SI per 100 FTE for each phase. The study was reviewed, approved and monitored by the SAH Human Research Ethics Committee. WinPepi v11.26 was used to calculate Chi2 and MedCalc used to calculate relative risk (RR) and 95% confidence limits (CL), on SI rate/100 FTE phase comparisons. In calculating RR, if SI incidence was zero, 0.5 was added to all cells. Statistical significance was set at p≤ 0.05. During the study, no other hospital-wide educational strategy other than in-service training for the Device, or sharps safety device other than the Device, was introduced.
Results
During the six-year study, annual FTE numbers rose 27.1% from 1,016 to 1,291. In Ph1, 2 and 3, the FTE denominators (reflecting the number of months in each phase) were 1,707, 2,785 and 1,786, respectively. Despite annual FTE increases, total SI incidence fell 15.2% from 3.63 to 3.08 SI/100 FTE from Ph1 to Ph3 (not significant) (see Table 1).
Table 1.
Sharps injuries per 100 full-time-equivalent staff by category in Phases 1, 2 and 3
| SI Category | Phase 1 Small SC only (20 months) | Phase 2a The Device in medication station (30 months) | Phase 3a The Device in patient room (20 months) |
|---|---|---|---|
| During procedure (A) | 1.76 | 1.58 | 1.34 |
| After procedure/before disposal (B) | 0.88 | 1.54 (p=0.06; RR=1.7; CL=0.97–3.13) | 1.57 (p=0.07; RR=1.8; CL=0.95–3.31) |
| (SI during transport to SC) | 0.0 | 0.11 (p=0.18; RR=4.3; CL=0.22–82.9) | 0.06 (p=0.33; RR=2.9; CL=0.02–70.30) |
| (SI placing/removing sharps in dish) | 0.0 | 0.32 (p=0.02; RR=11.7; CL=0.67–199.23) | 0.17 (p=0.09; RR=6.7; CL=0.35–129.22) |
| (Combined transport SI + dish SI) | 0.0 | 0.43 (p=0.02; RR=15.3; CL=0.90–257.60) | 0.22 (p=0.051; RR=8.6; CL=0.46–159.30) |
| (Recapping SI) | 0.29 | 0.04 (p=0.02; RR=0.1; CL=0.01–1.05) | 0.06 (p=0.09; RR=0.2; CL=0.61–44.73) |
| Container-associated (C ) | 0.70 | 0.04 (p<0.001; RR=0.05; CL=0.10–0.40) | 0 (p<0.001; RR=0.03; CL=0.002–0.65) |
| Improper disposal (D) | 0.29 | 0.25 (p=0.79; RR=0.9; CL=0.27–2.70) | 0.17 (p=0.44; RR=0.6; CL=0.14–2.40) |
| Total SI (A+B+C+D) | 3.63 | 3.41 (p=0.70; RR=0.9; CL 0.69–1.29) | 3.08 (p=0.37; RR=0.8; CL=0.60–1.22) |
| Total disposal-related SI (C+D) | 1.00 | 0.29 (p=0.002; RR=0.3; CL=0.13–0.67) | 0.17 (p=0.001; RR=0.2; CL=0.05–0.58) |
| Disposal-related + transport + dish SI | 1.00 | 0.72 (p=0.32; RR=0.7; CL=0.38–1.38) | 0.39 (p=0.03; RR=0.4; CL=0.17–0.95) |
| CASI as % of total SI | 19.4% | 1.2% | 0.0% |
| Disposal-related SI as % of total SI | 27.4% | 8.5% | 5.5% |
statistical data refer to comparisons with Ph1
SI=sharps injuries; CASI=container-associated SI; SC=sharps container; RR=relative risk; CL=confidence limits.
In Ph1 with the use of small SC at the bedside, 19.4% of total SI were CASI. The 12 CASI fell within three of the eight CASI sub-categories (own sharp during deposition; overfilling; bounce-out) (see Table 2).
Table 2.
Details of container-associated sharps injuries in Phases1, 2 and 3
| CASI category | Ph1 |
Ph2 |
Ph3 |
|||
|---|---|---|---|---|---|---|
| SI | SI/100 FTE | SI | SI/100 FTE | SI | SI/100 FTE | |
| While placing sharp in container: | ||||||
| – injured by sharp being disposed | 6 | 0.35 | 1 | 0.04 | 0 | 0.0 |
| – injured by sharp already in container | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| – injured via overfilled sharps container | 4 | 0.23 | 0 | 0.0 | 0 | 0.0 |
| – injured by sharp (unclear how) | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Sharps protruding from containera (injury not during deposit) | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Bounce-out during/after disposal | 2 | 0.12 | 0 | 0.0 | 0 | 0.0 |
| Manipulating container | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Sharps puncturing container | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Total CASI | 12 | 0.70 | 1 | 0.04 | 0 | 0.0 |
Container not overfilled
SI=sharps injuries; CASI=container-associated SI; FTE=full-time-equivalent; Ph=phase
In Ph2 (Device in med room) Table 1 shows: significant decreases occurred in the incidence of CASI (94.9%), disposal-related SI (71.1%) and recapping SI (87.7%); and non-significant decreases in improper-disposal SI (14.2%) and total SI (6.0%). In Ph2, ‘before-disposal’ SI increased (non-significant) and although the majority of these SI were after surgical procedures (transfer of sharps between staff, clean-up, and scalpel blade removal – all unrelated to SC), of note was that ‘transport + dish’ SI rose significantly (see Table 1).
In Ph3 (Device in patient room), zero CASI were reported. Table 1 shows that compared to Ph1, Ph3 incidence of: Disposal-related SI decreased significantly by 83.1%; improper-disposal SI decreased 42.7% (non-significant); total SI decreased 15.2% (non-significant); recapping SI decreased 80.9% (non-significant); and the combined ‘transport’ and ‘dish’ SI incidence (one transport and three dish SI) increased (non-significant). The three ‘dish’ SI in Ph3 occurred at the bedside. The incidence of recapping SI when the larger SC was used (Ph1 vs Ph2 + Ph3) was significantly reduced (p=0.01; RR=0.1; CL=0.03–0.78). Figure 1 depicts the incidence of CASI sub-categories over the three study periods.
Figure 1.
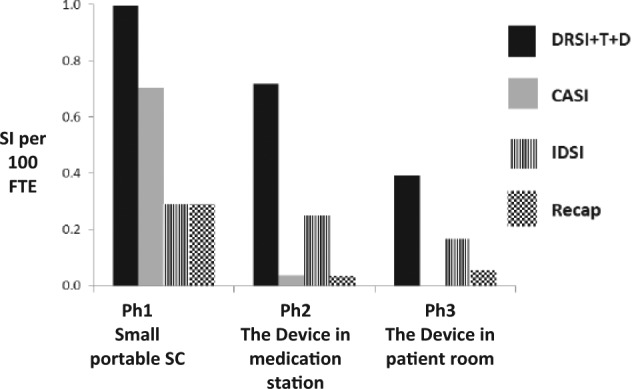
Effect of the Device on relevant sharps injury (SI) categories.
DRSI+T+D=disposal-related SI + transport SI + dish SI; CASI=container-associated SI; IDSI=improper-disposal SI; recap=recapping SI; SC=sharps container; SI = sharps injuries; Ph = phase; CASI = container-associated SI; FTE = full time equivalent
Discussion
The risk of UK healthcare workers contracting diseases from bloodborne pathogens through sharps injuries is real (Health Protection Agency, 2012) and is a major concern for UK nurses (RCN, 2008). Guidelines (RCN, 2011) on the 2013 implementation of the EU Directive on prevention of sharps injuries recommends many measures including disposing of sharps ‘immediately after use’ into ‘adequate numbers of easily accessible, safely constructed SC’. These recommendations are soundly evidence-based as transporting sharps to a distant SC increases SI risk via carrying, dropping, stumbling, momentarily putting sharp down, multi-tasking during transport, impacting with objects and collision with colleagues (Ribner et al, 1987; Hyman, 2002; Grimmond et al, 2003); transporting or placing sharps in kidney dishes is also associated with increased SI (Santhna, at al, 2007; Yoshikawa et al, 2007; Rich, 2012). Taking a small SC to the bedside would appear to meet the RCN recommendations (SC within arm’s reach, immediate deposition) but for several factors which increase SI risk (several mentioned by SAH staff): large sharps needing to be transported back to the med room; and small SC being associated with increased SI because of small apertures, ‘stuffing’, steadying SC with other hand, overfilling and potential for SI during closure (Osterman, 1975; Weltman et al, 1995; Hatcher, 2002; Grimmond et al, 2003).
Phase 1 of the study showed that the use of small SC taken to the bedside was associated with a high CASI rate in three sub-categories: injured by own sharp during deposit; overfilled SC; and bounce-out (see Tables 1 and 2) and, as stated above, these SI modes are frequently associated with ‘point-first’, small SC. The portable SC taken to UK bedsides may in some cases be larger than those used at SAH, however the UK RCN study (2005) showed CASI in one UK trust accounted for 22.5% of total SI, which is not dissimilar from the 19.4% CASI seen with small SC use at SAH.
Use of the Device in med rooms (Ph2) was associated with a significant reduction (94%) in CASI, which understandably also significantly reduced disposal-related SI. As mentioned in the Results section, the ‘before disposal’ SI sub-categories of ‘transport’ and ‘placing/removing sharp in dish’ significantly increased in Ph2. While not CASI, they reflect the risk of transporting sharps to a remote SC and the hazard of using kidney dishes or similar for temporary placement or transport of sharps. In several instances, SI occurred when healthcare workers removed sharps from the dish to place in the SC (rather than decant the dish’s contents directly into SC). Their increased incidence confirms the RCN (2011), UK Health and Safety Executive (2013), USA NIOSH (1998) and NSW Health (2007) recommendations to place SC as close as practical to the point of generation of the sharp and to dispose of the sharp into SC immediately after use, i.e. eliminate transport of sharps and use of temporary dishes or trays. Because total SI had been decreasing in Ph2 (due to decreases in during-procedure SI and CASI), the rise in ‘transport SI’ (average one transport SI every 3 months) went unnoticed until the second year of Ph2. It was at this point that SAH administration deemed the carrying of sharps to a ‘remote SC’ unacceptable and the decision was made to fix the Device in patient rooms (the norm in Australian public hospitals). Confirmation of ‘remote SC’ risk in this study hopefully may serve as evidence-to-change in hospitals and countries where this practice exists (Yoshikawa et al, 2007).
Although the SC the Device replaced in the med room was also large (22.7L), the reduction in CASI with the use of the Device in med rooms (Ph2) may be due to its larger size (32L) and because it replaced all small SC, but previous studies confirm the Device’s increased safety over a range of SC designs and sizes (Grimmond et al, 2003, 2010). The high incidence of recapping SI observed in Ph1 was significantly lower with Device use (Ph2 and Ph3) and it is postulated that, despite a non-recapping policy at SAH, staff may have perceived a ‘self-safety’ need to recap (Jagger et al, 1988) when using small SC but not when using a safer SC system (Gershon et al, 1999).
The placement of the Device in patient rooms (Ph3) was associated with zero CASI and slightly lower incidences (non-significant) of improper-disposal SI and total SI than in Ph2. Analysis of ‘before-disposal’ SI incidents in Ph3 showed that one transport SI and three ‘bedside dish’ SI still occurred and although the low numbers did not enable statistical significance to be shown (p=0.51), these injuries were not evident in Ph1 and they confirm the hazard of any form of sharp ‘transport’ or placement in dishes. We hypothesised that correct use and siting of a safer container should not only decrease CASI, but should also decrease improper-disposal SI as well as ‘transport’ and ‘dish’ SI. These four categories of SI should reflect the impact of a safe, adequately sized, nearby, highly visible SC into which sharps are immediately placed. The results of this study support this hypothesis in that when the first-year incidence of CASI, improper-disposal SI, ‘transport’ and ‘dish’ SI were extrapolated using the increased FTE over the six years of the study, the incidence of these SI was projected to be 15 in the final year of the study, when in fact only three injuries occurred in these combined categories, thus 12 fewer staff could have sustained disposal-related SI with use of the Device in the final year.
The elimination of CASI with use of the Device in patient rooms confirms the beneficial effect of using a larger, close-at-hand, well-engineered SC and confirms the results of previous studies of the Device (Grimmond et al, 2003, 2010). Small containers carry an associated SI risk through small apertures impeding sharps deposition (Weltman et al, 1995); small volumes causing rapid filling and staff sustaining SI trying to ‘put one last sharp in’ (Grimmond et al, 2010); staff trying to put too large a sharp in a small SC (NIOSH, 1998); ‘straight-drop’ deposition allowing easy overfilling (Hatcher, 2002); ‘pressure-fit’ lids on small SC requiring closure by pressing down on the lid, which may have upturned sharps beneath it (Weltman et al, 1995); staff grasping small SC with one hand while depositing sharp with the other thus increasing SI risk by bringing the deposition of the sharp into the same plane as the other hand (safe sharps deposition is one-handed (NIOSH, 1998)); the risk of sharps puncturing the wall in smaller, thinner-wall containers (Horwood, 2007); and the need to transport sharps (Yoshikawa et al, 2007) to a larger SC. Placement of SC on mobile trolleys as advised by the Health and Safety Executive (2013) would enable larger SC to replace small SC even in space-restricted patient rooms and it is proposed that, although the Device was associated with zero CASI, the Device’s use on trolleys taken to the bedside could result in further reductions in improper disposal SI and transport SI than those observed with patient-room wall-mounting of the Device in this study.
All models of the Device have identical safety features and represent an engineering departure from standard SC design. The Device was developed using human factors engineering to accommodate a broader spectrum of user behaviour (US Food and Drug Administration, 1996; Hyman, 2002). Using this concept, clinical user groups expressed their need for a larger horizontal aperture (the Device’s is 100 mm x 300 mm), deeper atrium (throat of container above counterbalanced tray), more sensitive counterbalanced tray, one-handed deposit, automatic lock-out when full (passive overfill protection), hand entry restriction, pre-assembly, tamper-proof locks, ‘hand-safe’ closures which do not require fingers to be placed near the aperture for activation, and walls with higher puncture-resistance.
We postulate that the elimination of CASI at SAH was due to the Device’s large aperture, large atrium, one-handed deposit and passive overfill prevention, and the decrease in improper-disposal SI was due to the use of a large SC that is visible and proximal, reminding staff of ‘proper and immediate’ disposal.
Notwithstanding the EU Directive requiring greater use of safety engineered devices, the use of large, well-engineered, close-at-hand SC will remain a necessity (Jagger and Bentley, 1995; Grimmond et al, 2010).
Strengths of this study were its three-phase design to examine the effect of the introduction of the same safety device in two scenarios; non-introduction of other SI prevention strategies during the study period; and the blind nature of incident reporting (injured healthcare workers unaware of study). Limitations of the study were that the impact of the Device’s size could not be separated from the impact of its engineered features; retrospective reliance on SI logs (voluntary reporting); reliance on staff incident description records to categorise SI; longitudinal design and assumption that SI reporting rates and procedures were the same throughout the three study phases; omission of a comparison with patient-room use of other large SC; and omission of a comparison with use of the Device or other large SC at the bedside. The length of the study could potentially be a limitation (increased risk of confounding variables), however no institution-wide SI prevention strategies were introduced other than the Device over the length of the study. The increase in ‘before disposal’ SI rates over the length of the study could indicate that other potentially influencing factors (such as increased staff knowledge or safer use of existing safety devices), were not a factor during the study.
Conclusions
This study validates the necessity of the international recommendations that sharps be placed immediately after use into well-engineered, safe SC, sited close to the point of sharps generation. Compared to small portable SC, use of the larger, safety-engineered Device mounted on patient-room walls can result in significantly fewer staff sustaining disposal-related SI annually. The Device’s volume, large aperture, passive overfill protection, one-hand deposit and close-at-hand siting are postulated as contributing to the reduction in disposal-related SI. Further reductions may be possible with use of the device on trolleys taken to the bedside. Use of kidney dishes or trays for the interim storage or transport of used sharps is not recommended.
Footnotes
Declaration of conflicts of interest: None declared for WN. TG is a consultant in sharps injury prevention and the healthcare sustainability and waste sector and one of his clients produces the Device studied in this paper. The client did not initiate, fund, review, sight or have any input into the conduct or write-up of the study.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- Control of Substances Hazardous to Health (COSHH). (2013) The Control of Substances Hazardous to Health Regulations 2002. Approved Code of Practice and guidance. Health and Safety Executive; Available at: http://www.hse.gov.uk/pubns/priced/l5.pdf (accessed 20 May 2014). [Google Scholar]
- Elder A, Paterson C. (2006) Sharps injuries in UK health care: a review of injury rates, viral transmission and potential efficacy of safety devices. Occupational Medicine (London) 56: 566–74. [DOI] [PubMed] [Google Scholar]
- Gershon RRM, Pearse L, Grimes M, Flanagan PA, Vlahov D. (1999) The impact of multifocused interventions on sharps injury rates at an acute care hospital. Infection Control and Hospital Epidemiology 20(12): 806–11. [DOI] [PubMed] [Google Scholar]
- Godfrey K. (2001) Sharp practice. Nursing Times 97(2): 22–4. [PubMed] [Google Scholar]
- Grimmond T, Bylund S, Anglea C, Beeke L, Callahan A, Christiansen E, Flewelling K, McIntosh K, Richter K, Vitale M. (2010) Sharps injury reduction using a sharps container with enhanced engineering: a 28 hospital nonrandomized intervention and cohort study. American Journal of Infection Control 38: 799–805. [DOI] [PubMed] [Google Scholar]
- Grimmond T, Rings T, Taylor C, Creech R, Kampen R, Kable W, Mead P, Mackie P, Pandur R. (2003) Sharps injury reduction using Sharpsmart – a reusable sharps management system. Journal of Hospital Infection 54(3): 232–8. [DOI] [PubMed] [Google Scholar]
- Hatcher I. (2002) Reducing sharps injuries among health care workers: a sharps container quality improvement project. Joint Commission Journal on Quality Improvement 28: 410–14. [DOI] [PubMed] [Google Scholar]
- Health and Safety. (2013) The Health and Safety (Sharp Instruments in Healthcare) Regulations 2013. Statutory Instrument No. 645. Available at: http://www.legislation.gov.uk/uksi/2013/645/pdfs/uksi_20130645_en.pdf (accessed 12 April 2014).
- Health and Safety Executive. (2013) Health and Safety (Sharp Instruments in Healthcare) Regulations 2013. Guidance for employers and employees. Available at: http://www.hse.gov.uk/pubns/hsis7.pdf (accessed 20 May 2013).
- Health Protection Agency. (2012) Eye of the needle. United Kingdom surveillance of significant occupational exposures to bloodborne viruses in healthcare workers. Public Health England; Available at: http://www.hpa.org.uk/webw/HPAweb&HPAwebStandard/HPAweb_C/1317137291281. (accessed 12 October 2013). [Google Scholar]
- Horwood D. (2007) Small sharps containers. Best practice communique 07-07. Remote Health Branch, Northern Territory Government; Available at: http://remotehealthatlas.nt.gov.au/0707_small_sharps_containers_communique.pdf (accessed 20 May 2014). [Google Scholar]
- Hyman W. (2002) Human factors analysis of needle safety devices. Journal of Clinical Engineering 27: 280–6. [Google Scholar]
- Jagger J, Bentley M. (1995) Disposal-related sharp object injuries. Advances in Exposure Prevention 1: 1,2,6,7,11. [Google Scholar]
- Jagger J, Hunt E, Brand-Elnaggar J, Pearson R. (1988) Rates of needle-stick injury caused by various devices in a university hospital. New England Journal of Medicine 319: 284–8. [DOI] [PubMed] [Google Scholar]
- National Institutes for Occupational Safety and Health (NIOSH). (1998). selecting, evaluating, and using sharps disposal containers. DHHS (NIOSH) Publication No. 97-111. US Department of Health and Human Services, Public Health Service, Centers for Diseases Control and Prevention, National Institutes for Occupational Safety and Health, Atlanta Georgia USA: Available at: http://www.cdc.gov/niosh/docs/97-111/pdfs/97-111.pdf (accessed 10 June 2014). [Google Scholar]
- NSW Health. (2007). Policy and guidelines for the prevention of sharps injuries in the NSW public health system. New South Wales Department of Health Policy Directive PD2007-052; Available at: http://www0.health.nsw.gov.au/policies/pd/2007/pdf/PD2007_052.pdf (accessed 10 June 2014). [Google Scholar]
- Osterman C. (1975) Relation of new disposal unit to risk of needle puncture injuries. Hospital Topics 53: 12–13. [PubMed] [Google Scholar]
- Ribner B, Landry M, Gholson G, Linden L. (1987) Impact of a rigid, puncture resistant container system upon needlestick injuries. Infection Control 8: 63–6. [DOI] [PubMed] [Google Scholar]
- Rich S. (2012) Sharps injuries are a significant occupational health risk. Kai Tiaki Nursing New Zealand 18(10): 26–8. [PubMed] [Google Scholar]
- Royal College of Nursing. (2005) EPINet overview and RCN surveillance project. RCN: London: Available at: http://www.saferneedles.org.uk/?page=41&id=64 (accessed 10 April 2013). [Google Scholar]
- Royal College of Nursing. (2008) Needlestick injury in 2008: results from a survey of RCN members. RCN: London: Available at: http://www.rcn.org.uk/__data/assets/pdf_file/0019/203374/003_304.pdf (accessed 3 June 2014). [Google Scholar]
- Royal College of Nursing. (2011) Sharps Safety. RCN Guidance to support implementation of the EU Directive 2010/32/EU on the prevention of sharps injuries in the health care sector. RCN: London: Available at: http://www.rcn.org.uk/__data/assets/pdf_file/0008/418490/004135.pdf (accessed 3 June 2014). [Google Scholar]
- Santhna L, Samsiah M, Raja Lexshimi R, Roshdinom R, Ho S, Hamidah H. (2007) Sharps injury in Hospital Universiti Kebangsaan Malaysia (HUKM): experiences of health care workers and students. Medicine and Health 2(1): 86–92. [Google Scholar]
- US Food and Drug Administration. (1996) Do it by design. an introduction to human factors in medical devices. Office of Communication, Education, and Radiation Programs, Center for Devices and Radiological Health, US Food and Drug Administration, Washington DC USA: Available at: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm094957.htm (accessed 10 June 2014). [Google Scholar]
- Weltman A, Short L, Mendelson M, Lilienfeld D, Rodriguez M. (1995) Disposal-related sharps injuries at a New York City teaching hospital. Infection Control and Hospital Epidemiology 16: 268–74. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T, Kidouchi K, Kimurs S, Okubo T, Perry J, Jagger J. (2007). Needlestick injuries to the feet of Japanese healthcare workers: a culture-specific exposure risk. Infection Control and Hospital Epidemiology 28: 215–18. [DOI] [PubMed] [Google Scholar]


