Abstract
Programmed death 1 (PD‐1)/programmed death ligand 1 (PD‐L1) pathway blockade has become a promising therapeutic target in adult cancers. We evaluated PD‐L1 expression and tumor‐infiltrating CD8+ T cells in formalin‐fixed, paraffin‐embedded tumor specimens from 53 untreated pediatric patients with eight cancer types: neuroblastoma, extracranial malignant germ cell tumor, hepatoblastoma, germinoma, medulloblastoma, renal tumor, rhabdomyosarcoma, and atypical teratoid/rhabdoid tumor. One rhabdomyosarcoma with the shortest survival exhibited membranous PD‐L1 expression and germinoma contained abundant tumor‐infiltrating CD8+ T cells and PD‐L1‐positive macrophages. The PD‐1/PD‐L1 pathway tended to be inactive in pediatric cancers.
Keywords: PD‐1/PD‐L1 blockade, pediatric cancer, tumor‐infiltrating lymphocyte
Abbreviations
- AT/RT
atypical teratoid/rhabdoid tumor
- FFPE
formalin‐fixed, paraffin‐embedded
- HPF
high‐power field
- PD‐1
programmed death 1
- PD‐L
programmed death ligand
INTRODUCTION
Studies have historically revealed an immune component of the tumor microenvironment. Recently, several studies showed the effectiveness of blocking the programmed death 1 (PD‐1)/programmed death ligand 1 (PD‐L1) pathway of immune checkpoint inhibitors in adult patients with multiple PD‐L1‐expressing cancers.1, 2, 3, 4 PD‐1, expressed on activated T cells, suppresses T‐cell responses upon binding to PD‐L1 or PD‐L2. Therefore, in PD‐L1 expressing tumors, anti‐PD‐1 antibodies could counteract the inhibitory pathway that blocks effective antitumor T‐cell responses, thus inducing an antitumor effect.5 Furthermore, tumor‐infiltrating lymphocytes (effector cells) are important factors in prognosis, PD‐L1 expression, and the prediction of responses to PD‐1/PD‐L1 blockade.5, 6, 7, 8, 9
Although PD‐1/PD‐L1 blockade is also desirable for patients with pediatric cancers, particularly those with a poor prognosis and long‐term toxic effects of current therapies, little is known about the immunological parameters for PD‐1/PD‐L1 blockade in pediatric cancers.10 Herein, we assessed the intensity of PD‐L1 expression and tumor‐infiltrating CD8+ T cells in pediatric cancers to determine the potential usefulness of PD‐1/PD‐L1 blockade.
METHODS
Patients
This retrospective study was conducted in accordance with the Declaration of Helsinki, with approval from the institutional review board at Saitama Children's Medical Center. We evaluated formalin‐fixed, paraffin‐embedded (FFPE) tumor specimens from patients with pediatric solid tumors who underwent biopsy or resection and were treated at Saitama Children's Medical Center from 2009 to 2011. We excluded patients whose pretreatment paraffin blocks could not be obtained and included two patients with a rhabdomyosarcoma or an atypical teratoid/rhabdoid tumor (AT/RT) because of the small number. We evaluated specimens from 53 patients, including 18 neuroblastomas, eight extracranial malignant germ cell tumors, seven germinomas, seven hepatoblastomas, four renal tumors, four medulloblastomas, three rhabdomyosarcomas, and two AT/RTs. Patients were diagnosed at a median age of 2 years (range: 0 months–16 years), and 17 presented with distant metastases. During a median follow‐up period of 52 months (range: 1–75 months), event‐free survival and overall survival rates were 74% (39/53) and 89% (47/53), respectively. The characteristics of the neuroblastoma cases are shown in Supplementary Table SI.
Immunohistochemistry
Immunohistochemical staining was performed using an automated staining system (XT iVIEW DAB v3 SP; Ventana, Oro Valley, AZ) with anti‐PD‐L1 (E1L3N; Cell Signaling Technology, Danvers, MA), anti‐CD8 antibody (already diluted, Nichirei Biosciences, Tokyo, Japan), and anti‐CD68 antibody (dilution: 1:100, Dako, Glostrup, Denmark). Three micrometers thick FFPE sections were deparaffinized, subjected to heat‐induced antigen retrieval (100ºC for 90 min), and stained with anti‐PD‐L1 antibody (1:200 dilution) for 32 min.
To confirm sensitivity, anti‐PD‐L1 antibody staining was validated using PD‐L1 IHC Controls (Cell Signaling Technology; Fig. 1A) and classical Hodgkin lymphomas (Fig. 1B),3, 11 which were embedded using standard procedures at Saitama Children's Medical Center.
Figure 1.
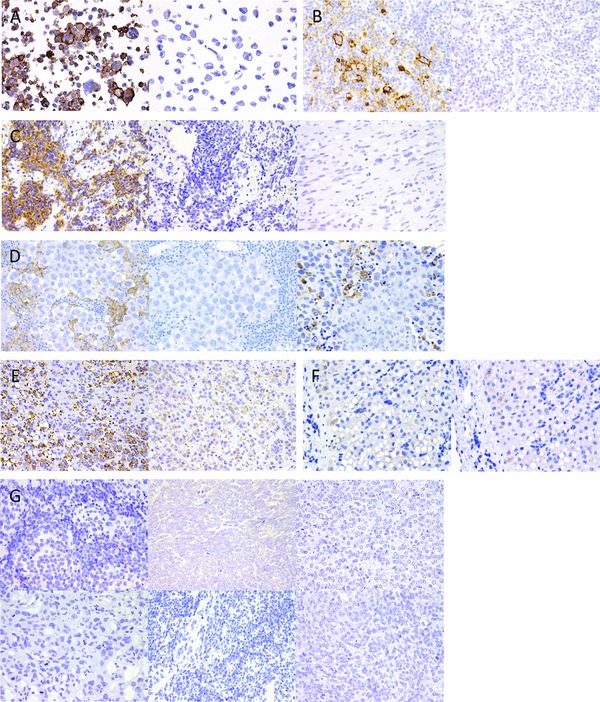
Images of commercially available control tissue sample and pediatric cancers stained to detect PD‐L1. (A) Images of SignalSlide® PD‐L1 IHC Controls (Cell Signaling Technology): Left, positive (E1L3N); right, negative (E1L3N). (B) Classical Hodgkin lymphoma: Left, positive (E1L3N); right, negative (isotype). (C) Rhabdomyosarcoma: Left, positive (E1L3N); middle, negative (isotype); right, negative (E1L3N). (D) Germinoma: Left, negative (E1L3N); middle, negative (isotype); right, CD68 staining. (E) Hepatoblastoma: left, granularly positive (E1L3N); right, negative (isotype). (F) Normal hepatocytes: Left, negative (E1L3N); right, negative (isotype). (G) Upper left, neuroblastoma: negative (E1L3N); upper middle, Wilms tumor: negative (E1L3N); upper right, clear cell sarcoma of the kidney: negative (E1L3N); lower left, yolk sac tumor: negative (E1L3N); lower middle, medulloblastoma: negative (E1L3N); lower right, atypical teratoid/rhabdoid tumor: negative (E1L3N). Original magnification, 400×.
Sample Evaluation
Pathologic diagnoses were confirmed by a pathologist (HK) who reviewed FFPE tissue sections stained with hematoxylin–eosin and selected a representative paraffin block from each specimen for immunohistochemical analysis. PD‐L1 expression was evaluated independently by TA and KH. Specimens were considered PD‐L1 positive when >5% tumor cells exhibited membrane staining.1, 4, 12 Tumor‐infiltrating CD8+ T cells were counted manually by TA in 10 high‐power fields (HPFs; area = 0.075 mm2) per specimen at 400× magnification;12 five HPFs were counted in small specimens.
Statistical Analysis
Tumor‐infiltrating CD8+ T‐cell counts were analyzed using the t‐test. All probability (P) values were two‐sided and the significance level was set at α = 0.05.
RESULTS
PD‐L1 Expression
Table I presents the study results. One rhabdmyosarcoma specimen exhibited membranous PD‐L1 expression (Fig. 1C). Although the patient's histological subtype could not be specified, he presented with distant metastasis and died within 6 months despite chemotherapy, the shortest survival duration in this cohort. The remaining 52 pediatric solid tumors did not express membranous PD‐L1. Although germinomas contained PD‐L1+ cells, the tumor cells did not directly express PD‐L1; rather, PD‐L1+ cells were identified as CD68‐positive macrophages 13 (Fig. 1D). Although tumor cells in two hepatoblastomas exhibited strong PD‐L1 staining (Fig. 1E) compared with normal hepatocytes (Fig. 1F), PD‐L1 staining was granular and restricted to the cytoplasm. The other hepatoblastomas exhibited weak cytoplasmic PD‐L1 expression, similar to normal hepatocytes. The remaining cancer types did not express PD‐L1 (Fig. 1G).
Table I.
PD‐L1 Expression and Tumor‐Infiltrating CD8+ T‐Cell Intensity
| Cancer type | Patient number (N = 53) | Distant metastasis (N = 17) | PD‐L1+ tumor (N = 1) | PD‐L1+ immune cell containing tumor (N = 7) | Tumor‐infiltrating CD8+ T‐cell intensitya | CD8+ T‐cell count/HPF | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | Mean | Range | |||||
| Neuroblastoma | 18 | 12 | 0 | 0 | 2 | 1 | 10 | 5 | 15.1 | 0.4–35.3 |
| Extracranial germ cell tumor | 8 | 2 | 0 | 0 | 4 | 3 | 1 | 0 | 2.5 | 0.2–8.9 |
| Yolk sac tumor | 4 | 1 | 0 | 0 | 2 | 2 | 0 | 0 | ||
| Teratoma and Yolk sac tumor | 3 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | ||
| Teratoma and mixed germ cell tumor | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | ||
| Germinoma | 7 | 0 | 0 | 7 | 1 | 0 | 1 | 5 | 34.2 | 0.8–110.3 |
| Hepatoblastoma | 7 | 1 | 0 | 0 | 0 | 3 | 4 | 0 | 5.2 | 2.0–10.1 |
| Medulloblastoma | 4 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 1.1 | 0.1–2.3 |
| Renal tumor | 4 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 2.7 | 0.6–5.6 |
| Wilms tumor | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | ||
| Clear cell sarcoma of the kidney | 2 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | ||
| Rhabdomyosarcoma | 3 | 1 | 1 | 0 | 0 | 1 | 2 | 0 | 8.5 | 2.7–13.9 |
| Atypical teratoid/rhabdoid tumor | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 3.7 | 1.4–6.0 |
Under the column titled “Tumor‐infiltrating CD8+ T‐cell intensity,” the numbers 0, 1+, 2+, and 3+ represent <2, 2–5, 5–20, and ≥20 CD8+ T‐cell count/HPF, respectively.
Tumor‐Infiltrating CD8+ T‐Cell Intensity
Table I also presents the mean numbers of tumor‐infiltrating CD8+ T cells per HPF in each cancer type. Germinomas had the highest numbers of tumor‐infiltrating CD8+ T cells, followed by neuroblastomas. Excluding patients with primary brain tumors, patients with distant metastases had higher numbers of tumor‐infiltrating CD8+ T cells (P = 0.019, Supplementary Table SII).
DISCUSSION
We showed a low frequency of PD‐L1 expression in pediatric cancers, which is consistent with two previous reports. Routh et al. described PD‐L1 expression in 11 (14%) of 81 Wilms tumors; moreover, only tumors from seven (10%) of 71 patients with favorable histology expressed PD‐L1.14 Uehara et al. also reported a low frequency of PD‐L1 expression in neuroblastomas [five (12%) of 41 pretreatment specimens].15 In contrast, Chowdhury et al. reported membranous PD‐L1 expression in 66 (57%) of 115 pediatric solid tumors, including high‐risk neuroblastomas, rhabdomyosarcomas, Ewing sarcomas, and osteosarcomas.12 Differences in staining antibodies, staining procedures, and antigen retrieval techniques could explain this discrepancy; however, 10 high‐risk neuroblastomas in our study were also negative when stained with another anti‐PD‐L1 antibody (ab58810; abcam, Cambridge, UK) at the dilution reported by Chowdhury et al. As our PD‐L1‐positive rhabdomyosarcoma exhibited the most aggressive clinical course, and Wilms tumors and rhabdomyosarcomas with unfavorable histologies exhibited stronger PD‐L1 expression,12, 14 PD‐L1 expression may correlate with a poor prognostic subtype.
Immune cells including macrophages and lymphocytes that infiltrated germinomas expressed PD‐L1, a phenomenon previously described as a predictive factor of response to anti‐PD‐L1 antibody therapy.16 This abundance of immune cells indicates that immunomodulatory drugs may play an important role in future germinoma treatment strategies. Although we observed stronger PD‐L1 staining in hepatoblastoma cells, the staining pattern differed from previous reports of other cancers, including hepatocellular carcinoma;17 therefore, we could not confirm this staining to be meaningful.
Considering neuroblastomas have low to absent MHC class I expression,18, 19 a low frequency of cytotoxic T lymphocyte is suspected, although CD8+ T cells may be present when stimulated by IL‐2 secreted by other immune cells.20 However, if we can create an immunogenic environment, we could use immune checkpoint inhibitors effectively and unleash the T‐cell response.9 For example, treatment of neuroblastoma cell lines with toll‐like receptor 3 ligands or interferon‐gamma induces significant upregulation of PD‐L1 and MHC class I, thereby generating an immunogenic environment when the PD‐1/PD‐L1 pathway is blocked.18
In conclusion, we identified a low frequency of PD‐L1 expression among pretreatment pediatric solid tumors. However, poor prognostic subtypes might express PD‐L1 more frequently. Because the limited in vitro data show that PD‐L1 can be upregulated in neuroblastoma cell lines, it may be possible to create an immunogenic environment in other pediatric tumors, so that the addition of a checkpoint blockade may induce immune responses.
Supporting information
Supplemental Table I. Characteristics of neuroblastoma
Supplemental Table II. Comparison of CD8+ T‐cell count with distant metastasis and outcome
Acknowledgments
This study was supported by a Grant from the Kawano Masanori Memorial Public Interest Incorporated Foundation for Promotion of Pediatrics. The funding agency had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Conflict of interest: Nothing to declare.
Grant sponsor: Kawano Masanori Memorial Public Interest Incorporated Foundation for Promotion of Pediatrics.
REFERENCES
- 1. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, et al. Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N Engl J Med 2012;366:2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, et al. Safety and activity of anti‐PD‐L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH, Zhu L, Grosso JF, Kim SY, Timmerman JM, Shipp MA, Armand P. PD‐1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015;372:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD‐1, PD‐1 ligands, and other features of the tumor immune microenvironment with response to anti‐PD‐1 therapy. Clin Cancer Res 2014;20:5064–5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015;348:56–61. [DOI] [PubMed] [Google Scholar]
- 6. Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T, Fujii S. Programmed cell death 1 ligand 1 and tumor‐infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA 2007;104:3360–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, Chen L. Colocalization of inflammatory response with B7‐h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012;4:127ra137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, et al. PD‐1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up‐regulation of PD‐L1, IDO, and Tregs in the melanoma tumor microenvironment is driven by CD8+ T cells. Sci Transl Med 2013;5:200ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Dam LS, de Zwart VM, Meyer‐Wentrup FA. The role of programmed cell death‐1 (PD‐1) and its ligands in pediatric cancer. Pediatr Blood Cancer 2014;62:190–197. [DOI] [PubMed] [Google Scholar]
- 11. Green MR, Monti S, Rodig SJ, Juszczynski P, Currie T, O'Donnell E, Chapuy B, Takeyama K, Neuberg D, Golub TR, Kutok JL, Shipp MA. Integrative analysis reveals selective 9p24.1 amplification, increased PD‐1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B‐cell lymphoma. Blood 2010;116:3268–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chowdhury F, Dunn S, Mitchell S, Mellows T, Ashton‐Key M, Gray JC. PD‐L1 and CD8(+)PD1(+) lymphocytes exist as targets in the pediatric tumor microenvironment for immunomodulatory therapy. Oncoimmunology 2015;4:e1029701. [Google Scholar]
- 13. Vakkila J, Jaffe R, Michelow M, Lotze MT. Pediatric cancers are infiltrated predominantly by macrophages and contain a paucity of dendritic cells: A major nosologic difference with adult tumors. Clin Cancer Res 2006;12:2049–02054. [DOI] [PubMed] [Google Scholar]
- 14. Routh JC, Ashley RA, Sebo TJ, Lohse CM, Husmann DA, Kramer SA, Kwon ED. B7‐H1 expression in Wilms tumor: Correlation with tumor biology and disease recurrence. J Urol 2008;179:1954–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Uehara S, Nakahata K, Kawatsu M, Zenitani M, Miyamura T, Hashii Y, Okuyama H. The PD‐L1 expression increases after consecutive multimodal therapeies in neuroblastoma. Pediatr Blood Cancer 2015;62:S334. [Google Scholar]
- 16. Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, et al. Predictive correlates of response to the anti‐PD‐L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gao Q, Wang X‐Y, Qiu S‐J, Yamato I, Sho M, Nakajima Y, Zhou J, Li B‐Z, Shi Y‐H, Xiao Y‐S. Overexpression of PD‐L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 2009;15:971–979. [DOI] [PubMed] [Google Scholar]
- 18. Boes M, Meyer‐Wentrup F. TLR3 triggering regulates PD‐L1 (CD274) expression in human neuroblastoma cells. Cancer Lett 2015;361:49–56. [DOI] [PubMed] [Google Scholar]
- 19. Wolfl M, Jungbluth AA, Garrido F, Cabrera T, Meyen‐Southard S, Spitz R, Ernestus K, Berthold F. Expression of MHC class I, MHC class II, and cancer germline antigens in neuroblastoma. Cancer Immunol Immunother 2005;54:400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Facchetti P, Prigione I, Ghiotto F, Tasso P, Garaventa A, Pistoia V. Functional and molecular characterization of tumour‐infiltrating lymphocytes and clones thereof from a major‐histocompatibility‐complex‐negative human tumour: Neuroblastoma. Cancer Immunol Immunother 1996;42:170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table I. Characteristics of neuroblastoma
Supplemental Table II. Comparison of CD8+ T‐cell count with distant metastasis and outcome


