Abstract
Aim
To evaluate the efficacy and safety of adding insulin degludec (IDeg) to treatment in patients with type 2 diabetes receiving liraglutide and metformin and qualifying for treatment intensification because of inadequate glycaemic control.
Methods
In this 26‐week, double‐blind trial, patients who still had inadequate glycaemic control after a 15‐week run‐in period with initiation and dose escalation of liraglutide to 1.8 mg in combination with metformin (≥1500 mg) were randomized to addition of once‐daily IDeg (‘IDeg add‐on to liraglutide’ arm; n = 174) or placebo (‘placebo add‐on to liraglutide’ arm; n = 172), with dosing of both IDeg and placebo based on titration guidelines.
Results
At 26 weeks, the mean change in glycated haemoglobin level was greater in the IDeg add‐on to liraglutide arm (−1.04%) than in the placebo add‐on to liraglutide arm (−0.16%; p < 0.0001). Similarly, the mean fasting plasma glucose reduction was greater, and self‐measured plasma glucose values were lower at all eight time points, with IDeg add‐on versus placebo add‐on (both p < 0.0001). At 26 weeks, the IDeg dose was 51 U (0.54 U/kg). During the run‐in period with liraglutide, body weight decreased by ∼3 kg in both groups. After 26 weeks, the mean change was +2.0 kg (IDeg add‐on to liraglutide) and −1.3 kg (placebo add‐on to liraglutide). Confirmed hypoglycaemia rates were low in both groups, although higher with IDeg than with placebo (0.57 vs. 0.12 episodes/patient‐years of exposure; p = 0.0002). Nocturnal confirmed hypoglycaemia was infrequent in both groups, with no episodes of severe hypoglycaemia, and no marked differences in adverse events with either treatment approach.
Conclusion
The addition of liraglutide and IDeg to patients sub‐optimally treated with metformin and liraglutide and requiring treatment intensification was found to be effective and well‐tolerated.
Keywords: GLP‐1, glycaemic control, type 2 diabetes
Introduction
Current guidelines on the management of type 2 diabetes (T2D) recommend metformin as the initial pharmacological therapy, followed by combination therapy with other oral antihyperglycaemic agents, glucagon‐like peptide‐1 (GLP‐1) receptor agonists (RAs) or insulin 1, 2. In patients who do not achieve control with metformin, one possible strategy is to add a GLP‐1 RA, followed by a basal insulin analogue if required 3, 4. GLP‐1 RAs and basal insulin address different but complementary physiological approaches to treating hyperglycaemia. GLP‐1 RAs mimic the effect of native GLP‐1 by stimulating insulin secretion in response to the absorption of orally ingested glucose (the so‐called incretin effect) 5. Treatment with GLP‐1 RAs benefits both postprandial and fasting plasma glucose (FPG) levels. Basal insulin addresses insufficient pancreatic β‐cell function in T2D by providing a constant concentration of insulin over an extended period, resulting in improved FPG control. In addition, the weight‐lowering effect of GLP‐1 RAs may limit the weight gain associated with insulin 3.
Liraglutide is a long‐acting, once‐daily GLP‐1 RA that offers efficacious glycaemic control with minimal risk of hypoglycaemia accompanied by weight loss. Its efficacy and safety across the continuum of T2D was demonstrated in the LEAD programme of phase III clinical trials 6, 7, 8, 9, 10, 11, 12, 13.
Insulin degludec (IDeg) is a once‐daily basal insulin analogue with a long duration of action 14. In randomized controlled trials, IDeg was as effective in achieving glycaemic control in T2D as insulin glargine, with fewer episodes of nocturnal hypoglycaemia 15, 16, 17, 18, 19, 20, 21, 22.
The aim of the present study (BEGIN: ADD TO GLP‐1) was to determine the efficacy and safety of the addition of IDeg, compared with placebo, in patients who had not reached the glycated haemoglobin (HbA1c) target of <7.0% (<53 mmol/mol) with metformin and maximum‐dose liraglutide (1.8 mg).
Methods
A detailed description of the methods is given in the Supporting Information (Appendix S1).
Trial Design and Participants
This was a randomized (1 : 1), parallel‐group, double‐blind, multinational, controlled trial with a 15‐week run‐in phase, followed by a 26‐week core phase. Patients were eligible if they were aged ≥18 years and had T2D not previously treated with insulin and a body mass index (BMI) ≤45 kg/m2. Patients had to be receiving ongoing therapy with metformin ± a sulphonylurea, glinide, a dipeptidyl peptidase‐4 inhibitor or exenatide (twice daily only; details of regimens are provided in Table S1, Supporting Information), and had to have an HbA1c level of 7.5–10.0% (58–86 mmol/mol) inclusive (patients on metformin monotherapy) or 7.0–9.0% (53–75 mmol/mol) inclusive (patients on metformin combination therapy). At entry into the run‐in period, all previous antihyperglycaemic therapies except for metformin were discontinued. Key exclusion criteria were: a calcitonin level ≥50 ng/l; a history of chronic pancreatitis or idiopathic acute pancreatitis; and a current or past malignant neoplasm (except basal cell and squamous cell carcinoma).
Treatment
During the 15‐week run‐in period, liraglutide was initiated at 0.6 mg daily and increased to 1.8 mg daily over 2 weeks as recommended in the prescribing information 23. Metformin was continued at ≥1500 mg daily or the maximum tolerated dose (Figure 1). During run‐in and treatment, the daily doses of metformin and liraglutide (after the dose increase to 1.8 mg) were to remain unchanged. Patients were discontinued during run‐in if they could not tolerate liraglutide 1.8 mg daily.
Figure 1.
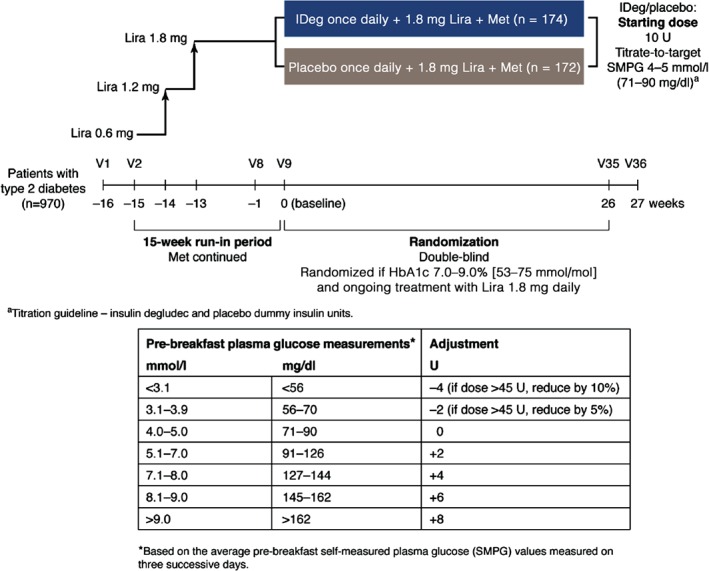
Trial design. FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; IDeg, insulin degludec; Lira, liraglutide; Met, metformin; SMPG, self‐measured plasma glucose; V, visit.
After completion of run‐in, patients whose HbA1c was still 7.0–9.0% (53–75 mmol/mol) were randomized to receive IDeg 10 U or placebo once daily subcutaneously in addition to liraglutide and metformin. IDeg was adjusted according to a titration guideline (Figure 1), aiming at self‐measured plasma glucose (SMPG) values of 4.0–5.0 mmol/l. Patients receiving placebo followed the same titration guideline, with placebo dispensed from an identical pen and measured in ‘dummy units’ as if it were insulin. Withdrawal criteria were prescribed in case of hyperglycaemia (Appendix S1, Supporting Information).
Endpoints and Assessments
The primary endpoint was change from baseline in HbA1c after 26 weeks of randomized treatment. Secondary efficacy endpoints at 26 weeks included: change in FPG; percentage of responders achieving HbA1c <7.0% (<53 mmol/mol); and change from baseline in the following: mean pre‐breakfast SMPG measurements used for titration of IDeg/placebo dose, 8‐point SMPG profile, and mean of the 8‐point profile. Changes from baseline in body weight and health‐related quality of life (HRQoL), and dose of IDeg/placebo, were also assessed.
Safety assessments included adverse events (AEs), hypoglycaemic episodes and changes from baseline in clinical evaluations and central laboratory assessments. Confirmed hypoglycaemic episodes included asymptomatic and symptomatic episodes confirmed by a measured plasma glucose value <3.1 mmol/l or severe episodes requiring assistance. Hypoglycaemic episodes occurring from 00:01 to 05:59 hours (inclusive) were classified as nocturnal.
Prespecified medical events of special interest included thyroid disease, any confirmed episode of calcitonin levels ≥20 ng/l, all neoplasms (excluding thyroid neoplasms), pancreatitis or clinical suspicion of pancreatitis, lipase and/or amylase >3 × upper limit of normal range, and AEs leading to withdrawal (for a full list see Table S2, Supporting Information). Selected AEs as follows underwent adjudication by an independent External Adjudication Committee: neoplasms; thyroid disease; all types of stroke; acute coronary syndrome; hospitalization for unstable angina pectoris; all types of myocardial infarction; and fatal events.
Statistical Analysis
The sample size required to meet the primary objective with ≥90% power, using an assumed mean treatment difference of 0.4% and an estimate for the standard deviation (s.d.) of 1.1% for HbA1c (based on previous phase III trials in patients with T2D) was calculated as 320 participants.
Changes from baseline in HbA1c, FPG, mean of the 8‐point SMPG profile, mean pre‐breakfast SMPG and HRQoL scores were analysed using analysis of covariance, with treatment, sex and region as fixed factors, and age and baseline value of the relevant variable as covariates. For change in HbA1c, superiority was to be considered confirmed if the upper bound of the two‐sided 95% confidence interval (CI) was below 0%. The percentages of responders achieving HbA1c <7.0% [<53 mmol/mol] were analysed based on a logistic regression model using the same fixed factors and covariates.
The number of treatment‐emergent hypoglycaemic episodes was analysed using a negative binomial regression model with a log‐link function and the logarithm of the time period for which a hypoglycaemic episode is considered treatment‐emergent as offset. The model included treatment, sex and region as fixed factors, and age as a covariate. Other safety data were compared using descriptive statistics.
Analyses of all efficacy endpoints and hypoglycaemia were based on the full analysis set (all randomized patients). Safety endpoints were summarized using the safety analysis set (all patients receiving at least one dose of study drug). Missing values were imputed using the last observation carried forward method, as recommended in US Food and Drug Administration (FDA) guidance 24.
Results
Patient Disposition and Characteristics
Of 1504 patients screened, 970 met the selection criteria and entered the run‐in period (Figure 2). During the run‐in period, 389 patients (40% of those who entered run‐in) reached an HbA1c level of <7.0% (<53 mmol/mol); among these, 181 patients had previously been treated with metformin only, and 208 had been treated with combination therapy. After the run‐in, the 346 patients meeting HbA1c eligibility criteria were randomized to receive IDeg (n = 174) or placebo (n = 172), both added on to liraglutide 1.8 mg and metformin. Of these, 173 and 170 patients, respectively, received at least one dose of study drug, and 160 and 131 patients, respectively, completed the study. The withdrawal rate was greater with placebo (23.8%) than with IDeg (8.0%; Figure 2).
Figure 2.
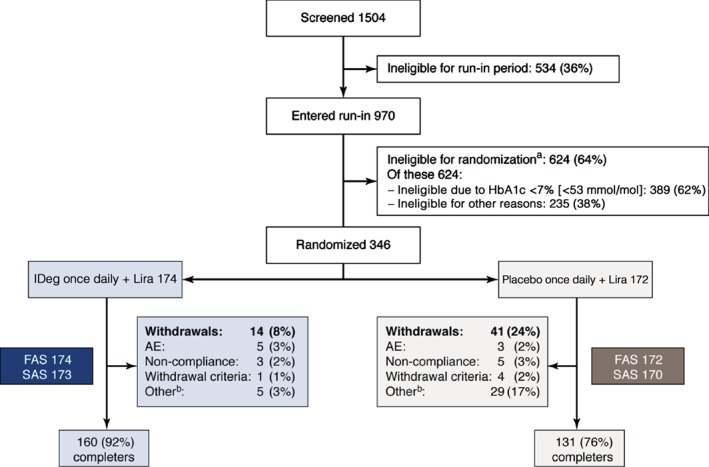
Patient disposition. AE, adverse event; FAS, full analysis set; HbA1c, glycated haemoglobin; IDeg, insulin degludec; Lira, liraglutide; SAS, safety analysis set. FAS: all randomized patients. SAS, all patients receiving at least one dose of study drug. aDuring the run‐in period patients were ineligible for randomization for the following reasons: AE, n = 76; non‐compliance with protocol, n = 29; randomization criteria (including HbA1c <7.0% [<53 mmol/mol]), n = 426; withdrawal criteria, n = 2; other, n = 91. bDuring the treatment phase: withdrawals due to ‘other’ reasons were caused by erroneous randomization, inefficient therapy (only in placebo + liraglutide group) and personal reasons such as patient not able to attend visits or unspecified withdrawn consent.
The characteristics of the two study groups were well matched at week 0 of the study (i.e. after the run‐in period; Table 1). The two study groups were also generally well matched with regard to antihyperglycaemic treatment at screening (Table S1, Supporting Information). At screening, ∼50% of patients were receiving a combination of metformin and sulphonylurea, and 30% metformin only.
Table 1.
Baseline characteristics of participants randomized at week 0.
| Characteristic | IDeg add‐on to liraglutide | Placebo add‐on to liraglutide |
|---|---|---|
| (n = 174) | (n = 172) | |
| Female/male, % | 43.7/56.3 | 39.5/60.5 |
| Race: white/black/Asian/other, % | 80.5/12.6/5.2/1.7 | 87.8/6.4/2.3/3.5 |
| Ethnicity: Hispanic or Latin American, % | 9.8 | 11.0 |
| Age, years | 57.0 (±10.0) | 57.3 (±9.4) |
| Weight, kg | 90.7 (±18.2) | 94.0 (±19.1) |
| BMI, kg/m2 | 32.0 (±5.7) | 32.4 (±5.4) |
| Duration of diabetes, years | 9.7 (±5.8) | 9.3 (±5.4) |
| HbA1c, % | 7.6 (±0.6) | 7.6 (±0.6) |
| HbA1c*, mmol/mol | 59.6 | 59.6 |
| FPG | ||
| mmol/l | 8.7 (±2.1) | 9.1 (±2.2) |
| mg/dl | 156.2 (±37.8) | 164.0 (±39.5) |
BMI, body mass index; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; IDeg, insulin degludec.
Data are mean ± standard deviation unless otherwise indicated.
Calculated values.
The 389 patients who successfully achieved an HbA1c level <7.0% (<53 mmol/mol) during run‐in had the following characteristics at initial screening (week –16): mean (s.d.) HbA1c 8.1 (0.7)% [65 (8) mmol/mol], weight 95.9 (17.7) kg, BMI 33.6 (5.5) kg/m2 and duration of diabetes 7.9 (5.3) years. Values for the patients who did not achieve HbA1c levels <7.0% and were randomized were similar: HbA1c 8.3 (0.7)% [67 (8) mmol/mol], weight 95.4 (18.6) kg, and BMI 33.2 (5.5) kg/m2. Only duration of diabetes differed: 9.5 (5.6) years.
Glycaemic Control
The observed mean (s.d.) change in HbA1c values from baseline to week 26 was −1.04 (0.89) percentage points with IDeg and −0.16 (0.86) percentage points with placebo, both on a continued background of liraglutide and metformin (HbA1c values over time are shown in Figure 3a). This resulted in an estimated treatment difference (ETD) of −0.92% (95% CI −1.10; −0.75; p < 0.0001). Thus, treatment with IDeg was confirmed to be superior to placebo for reduction in HbA1c.
Figure 3.
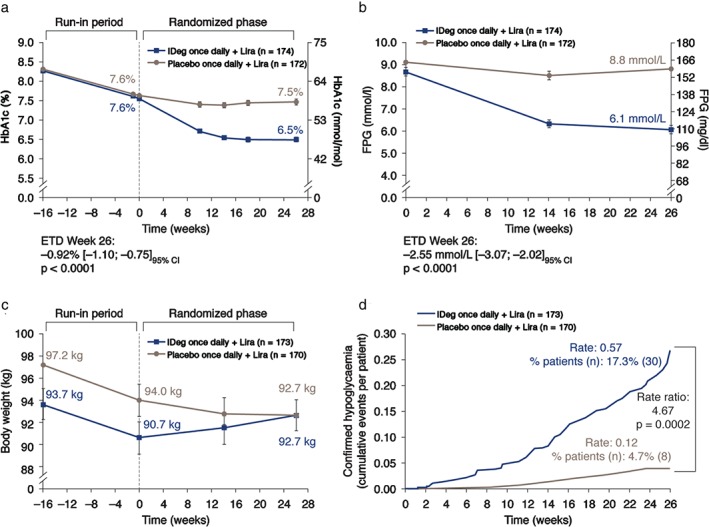
(a) Glycated haemoglobin (HbA1c), (b) fasting plasma glucose (FPG), (c) body weight and (d) hypoglycaemia over time. (FPG values were not available for week −16.) CI, confidence interval; ETD, estimated treatment difference; FPG, fasting plasma glucose; IDeg, insulin degludec; Lira, liraglutide; n, number of patients with events; Rate, number of events per patient‐year of exposure; % patients, proportion of patients with events. HbA1c and FPG are mean values ± standard error (s.e.). Full analysis set: last observation carried forward. Comparisons: estimates adjusted for multiple covariates. Body weight (mean values ± s.e.) and hypoglycaemia are safety analysis set. The statistical comparisons for hypoglycaemia are based on the full analysis set.
At 26 weeks, 77.6% of patients receiving IDeg and 35.5% of those receiving placebo had achieved an HbA1c level <7.0% (<53 mmol/mol); the likelihood of achieving HbA1c <7.0% was significantly higher for participants in the IDeg add‐on group [odds ratio 7.79 (95% CI 4.57; 13.27); p < 0.0001].
The mean reduction from baseline in FPG at week 26 was greater with IDeg than with placebo [ETD –2.55 mmol/l (95% CI −3.07; −2.02); p < 0.0001 (Figure 3b)].
After 26 weeks, pre‐breakfast SMPG values were significantly lower with IDeg than with placebo [ETD –2.34 mmol/l (95% CI −2.67; −2.01); p < 0.0001, for the mean SMPG levels used for dose adjustment (Figure S1, Supporting Information)]. At 26 weeks, plasma glucose levels at all eight time points were significantly lower with IDeg versus placebo (p < 0.0001; Figure S2, Supporting Information). The mean of the 8‐point SMPG profile was significantly lower at week 26 with IDeg versus placebo, with an ETD of –1.95 mmol/l (95% CI −2.29; −1.60; p < 0.0001).
Other Assessments
Physical and mental HRQoL patient‐reported outcome scores changed marginally in both treatment groups, with no statistically significant differences between groups from baseline to week 26.
At screening, body weight in patients subsequently randomized to IDeg [mean (s.d.) 93.7 (18.1) kg] was lower than in those subsequently randomized to placebo [97.2 (19.0) kg]; mean body weight decreased by ∼3 kg during the liraglutide run‐in in both groups (Figure 3c). During randomized treatment, mean body weight increased by 2.0 kg in the IDeg group and decreased by 1.3 kg in the placebo group.
The mean dose of IDeg and placebo was 10 U (0.11 U/kg) at baseline in both groups. Mean doses increased steadily during the initial weeks in both groups, but to a greater extent with placebo (Figure S3, Supporting Information). After 10–12 weeks of treatment, the increase in mean daily dose of IDeg levelled off, reaching 51 U (0.54 U/kg) at week 26. The dose of placebo continued to increase and reached 105 U (1.18 U/kg) at week 26.
Safety Endpoints
Hypoglycaemia
The number of patients experiencing confirmed hypoglycaemia during randomized treatment was higher with IDeg (17.3%) than with placebo (4.7%; Table S3, Supporting Information). The rate of confirmed hypoglycaemia was significantly higher with IDeg [0.57 episodes/patient‐years of exposure (PYE)] than with placebo [0.12 episodes/PYE; p = 0.0002 (Figure 3d)]. No severe hypoglycaemia was reported during the randomized treatment period. The proportion of patients with, and the rates of, nocturnal confirmed hypoglycaemia episodes during randomized treatment were low, at 1.7% (0.05 episodes/PYE) with IDeg and 1.2% (0.03 episodes/PYE) with placebo (not significant).
During the run‐in period, plasma‐glucose‐confirmed non‐severe hypoglycaemia was reported by six patients (1.7%). Three episodes of severe hypoglycaemia were reported in two individuals: one episode with a blood glucose level of 2.2 mmol/l in a female patient later randomized to IDeg, who subsequently withdrew because of non‐compliance that was not further described; and two episodes that were not supported by blood glucose measurements, occurring in one female patient who was considered ineligible for randomization because she was not testing blood glucose during possible hypoglycaemic events.
Adverse Events
Adverse events reported by ≥5% of patients during the run‐in period were primarily gastrointestinal, with no unexpected clustering of events. Rates of diarrhoea and vomiting were low in both phases. The incidence of nausea during run‐in was 27.2 and 22.9% for participants subsequently randomized to IDeg or placebo, respectively, but decreased to 4.6 and 3.5%, respectively, during the randomized period. During run‐in, 76 patients withdrew because of AEs, of whom 57 withdrew because of gastrointestinal AEs.
During the randomized period, the proportion of patients reporting treatment‐emergent AEs and the rates of treatment‐emergent AEs were similar in the two groups (Table S4, Supporting Information). Proportions reporting treatment‐emergent AEs were 55% (IDeg add‐on to liraglutide) and 52% (placebo add‐on to liraglutide), and rates were 344 and 335 events per 100 PYE, respectively. The most frequently reported AEs were nasopharyngitis, diarrhoea and elevated lipase. Serious AEs were reported by 3.5% of IDeg patients and 5.3% of placebo patients; no deaths were reported. The proportion of patients who withdrew from the trial because of AEs was 2.9% (IDeg) and 1.7% (placebo).
Medical events of special interest were few, with no difference between treatment groups. No pancreatitis or pancreatic neoplasms were reported over the entire trial period, including run‐in, and all pancreas‐related AEs were non‐serious. Mean amylase and lipase values increased in both groups, with wide fluctuations between individual patients. Two patients withdrew from the trial because of elevated amylase and lipase values (both in the IDeg group). Two patients were withdrawn because of increased calcitonin values (Appendix S1, Supporting Information). Calcitonin levels normalized after 3 weeks in one individual; the other had a normal thyroid on examination, and continued to have elevated calcitonin levels. Further information on pancreas‐ and thyroid‐related AEs is available in Appendix S1, Supporting Information. Treatment‐emergent neoplasms were identified in four participants by the External Adjudication Committee (two in each group); none were considered related to study drugs (Table S5, Supporting Information). No event was confirmed by the External Adjudication Committee as a major adverse cardiovascular event.
No clinically relevant differences in mean blood pressure between the two groups were seen at screening or end of treatment. From week 0 to week 26, mean blood pressure decreased slightly in both treatment groups; mean pulse increased slightly with IDeg (2.5 beats/min) but remained almost unchanged with placebo (0.6 beats/min).
Discussion
In the present study we evaluated the sequential addition of a GLP‐1 RA, liraglutide, to metformin, followed by either a basal insulin, IDeg, or placebo in patients requiring treatment intensification after treatment with liraglutide. Adding IDeg resulted in the majority of patients reaching the glycaemic goal within 6 months. HbA1c and FPG levels fell significantly more in patients who received add‐on IDeg than in those who received add‐on placebo. After 26 weeks, 78% of the patients receiving IDeg had achieved HbA1c <7.0% (<53 mmol/mol) versus 36% of patients with placebo. In comparison, a 43% response rate was reported in a study evaluating the addition of insulin detemir to liraglutide plus metformin in patients with T2D and HbA1c levels ≥7.0% (≥53 mmol/mol) 25.
Rates of plasma‐glucose‐confirmed hypoglycaemia were higher with IDeg versus placebo (events/PYE: 0.57 vs. 0.12, respectively); rates of nocturnal confirmed hypoglycaemia were low in both treatment groups (higher with IDeg but not statistically significant). The reduction in HbA1c and FPG observed with IDeg was not accompanied by severe hypoglycaemia. The rates observed are relatively low for insulin‐treated patients when compared with other IDeg trials in the phase IIIa programme (BEGIN). In two trials, confirmed hypoglycaemia rates (using the definition used in the present study) in previously insulin‐naïve patients were 1.2 and 1.5 events/PYE with IDeg and 1.4 and 1.9 events/PYE with insulin glargine, respectively, used at similar insulin dose levels to those in the present study (0.53 and 0.59 U/kg for IDeg, and 0.60 U/kg for insulin glargine in both trials) 15, 18.
The present study also confirmed the efficacy of liraglutide as add‐on to metformin 6: among the 624 patients ineligible for randomization after the run‐in period, 389 [62%; 40% of all 970 run‐in patients (Figure 2)] had reached HbA1c <7.0% (<53 mmol/mol) with the addition of liraglutide. This is a notable result given that, among these 389 patients, 208 (53%) had discontinued an additional oral antihyperglycaemic drug or twice‐daily exenatide from their metformin combination therapy before entering the run‐in phase. In the present trial, patients also lost ∼3 kg during the run‐in period and there was no unexpected clustering of AEs. The low incidence of hypoglycaemia during run‐in is consistent with rates observed in the LEAD development programme for liraglutide, and specifically in LEAD‐2 6.
Patients who successfully reached HbA1c levels <7.0% (<53 mmol/mol) at the completion of the run‐in period had a mean HbA1c of 8.1% (65 mmol/mol) compared with 8.3% (67 mmol/mol) in those who did not reach target, and a shorter disease duration of 7.9 years (vs. 9.5 years), while mean weight and BMI did not differ.
A run‐in period of 15 weeks was chosen to allow the initiation and dose escalation of liraglutide to the maximum dose of 1.8 mg daily. This was long enough to identify a population qualifying for treatment intensification in accordance with current guidelines 1, while also avoiding a confounding effect of any additional increase in dose of liraglutide during the trial. In order to justify the addition of IDeg or placebo, only patients who failed to reach the glycaemic target on the highest dose of liraglutide (1.8 mg), and therefore needed treatment intensification, were included.
Weight increased slowly over 26 weeks in patients receiving IDeg, by a mean of 2.0 kg; however, final mean weight did not reach the pre‐run‐in value, suggesting that the weight gain associated with insulin may have been mitigated by the concomitant use of liraglutide. In this regard, it is of interest to note the results of a recent study, BEGIN VICTOZA ADD‐ON, which employed IDeg and liraglutide in the opposite sequence to that used in the present study 26. Patients with HbA1c >7.0% (>53 mmol/mol) after 104 weeks of treatment with metformin + IDeg were randomized to add either liraglutide once daily (dose‐escalated to 1.2 or 1.8 mg) or insulin aspart once daily (starting at 4 U and titrated as needed). The mean weight change at 26 weeks was −2.8 kg (liraglutide) and +0.9 kg (insulin aspart; p < 0.0001). While the patients in this study had more advanced diabetes, and the comparator group included a prandial insulin, both studies show a weight‐mitigating effect of liraglutide administered together with IDeg.
In the placebo group, HbA1c remained more or less stable [mean value decreased from 7.6 to 7.5% (60–58 mmol/mol)] and weight decreased from 94.2 to 92.7 kg. These favourable results can be attributed to ongoing treatment with liraglutide.
In the present study, there were no confirmed cases of pancreatitis or pancreatic cancer. Other AEs reported in this study were as expected considering the component drugs of the regimen, and patterns of AEs were similar in the two treatment groups. The AEs reported during the run‐in period were primarily gastrointestinal‐related, as was seen in the liraglutide development programme 5, but the relatively high incidence of these gastrointestinal AEs decreased over time, as has previously been reported with GLP‐1 RAs 25.
The present carefully designed study had the advantage of being double‐blinded, unlike many studies using insulin. Limitations include the fact that placebo rather than an active drug was the comparator; this was decided so that the absolute effect of adding IDeg to liraglutide could be assessed, in line with FDA guidance that phase III studies of investigational agents as add‐on therapy are typically designed as placebo‐controlled superiority or active‐controlled non‐inferiority trials 24. Other limitations were the fact that only patients able to tolerate the maximum dose of liraglutide were randomized, and the withdrawal rate in the placebo add‐on to liraglutide arm was notably greater than that in the IDeg add‐on to liraglutide arm – driven in part by withdrawals as a result of inefficient therapy in the placebo arm.
In conclusion, physicians can now choose from a number of strategies that involve the complementary actions of a GLP‐1 RA and basal insulin, according to the individual patient's needs: GLP‐1 RA followed by basal insulin; or basal insulin followed by GLP‐1 RA; or use of the two in combination. In this multicentre, randomized, placebo‐controlled trial, the sequential addition of a GLP‐1 RA, liraglutide, followed by a basal insulin, IDeg, to patients with T2D requiring treatment intensification was found to be an effective and well‐tolerated treatment regimen.
Conflict of Interest
V. R. A. has received research grants from Amylin, AstraZeneca, Boehringer Ingelheim, Hanmi, Eisai, GI Dynamics, Novo Nordisk, Sanofi‐Aventis and Takeda (all to employer institution) and served as a consultant for Janssen, Novo Nordisk, Sanofi (to employer institution). T. B. has received research grants from Abbott, ACON, Alere, Animas, Bayer, BD, Cebix, Bristol‐Myers Squibb, Dexcom, GlaxoSmithKline, Halozyme, Insulet, Lifescan, Eli Lilly & Co, Mannkind, Medtronic, Merck, Novo Nordisk, Orexigen, Sanofi and Tandem, has acted as a consultant for Bayer, BD, Medtronic, Novo Nordisk and Sanofi, and attended speakers' bureaux for Novo Nordisk. B. C. has received research grants from Sanofi‐Regeneron and acted as a consultant for Amgen, AstraZeneca, Debiopharm, Janssen, Eli Lilly & Co., Genfit, Novo Nordisk and Sanofi‐Regeneron. S. K. has received research grants from, and attended advisory boards for Novo Nordisk. L. A. L. has received research grants from AstraZeneca Pharmaceuticals LP, Boehringer Ingelheim Pharmaceuticals, Inc., Eli Lilly & Co., GlaxoSmithKline, Janssen Pharmaceuticals, Merck, Novo Nordisk, Pfizer, Sanofi, Servier; attended advisory boards for AstraZeneca Pharmaceuticals LP, Boehringer Ingelheim Pharmaceuticals, Inc., Eli Lilly & Co., Janssen Pharmaceuticals, Merck, Novo Nordisk, Sanofi, Servier, and attended speakers' bureaux for AstraZeneca Pharmaceuticals LP, Boehringer Ingelheim Pharmaceuticals, Inc., Eli Lilly & Co., Janssen Pharmaceuticals, Merck, Novo Nordisk and Sanofi. P. R. has received research grants from Novo Nordisk, Eli Lilly & Co., Sanofi, Pfizer, AstraZeneca Pharmaceuticals LP, Boehringer Ingelheim Pharmaceuticals, Inc., Gilead Sciences, Inc., Intarcia; acted as a consultant for Janssen Pharmaceuticals, Inc., GlaxoSmithKline Pharmaceuticals, and attended speakers' bureaux for Janssen. J. Z. is an employee of Novo Nordisk and holds shares in the company. T. H. A. was an employee of Novo Nordisk at the time of this study and holds shares in the company. A. P.‐T. has received research grants from Merck, Novo Nordisk, Sanofi, Lilly, Amylin, AstraZeneca, Pfizer, Janssen and Genentech, and acted as an advisory panel member for Lilly, Novo Nordisk and Sanofi on behalf of her employer institution.
All authors (V. R. A, T. B., B. C, S. K., L. A. L, P. R., J. Z., T. H. A and A. P. ‐T.) were involved in critical analysis and interpretation of the data, drafting/critically revising the article and shared in the final responsibility for the content of the manuscript and the decision to submit it for publication.
Supporting information
Appendix S1. Supplementary methods.
Table S1. Antihyperglycaemic treatment at screening.
Table S2. Adverse events classified as medical events of special interest.
Table S3. Hypoglycaemic episodes.
Table S4. Treatment‐emergent adverse events.
Table S5. Neoplasms by system organ class and preferred term: safety analysis set.
Figure S1. Mean pre‐breakfast self‐measured plasma glucose for dose adjustment by treatment week: mean plot, full analysis set.
Figure S2. Eight‐point self‐measured plasma glucose profile at week 26: mean plot, full analysis set.
Figure S3. Insulin doses.
Acknowledgements
The authors would like to thank Grace Townshend for medical writing support and Daria Renshaw for styling/submission support, both from Watermeadow Medical Ltd, an Ashfield company. This support was funded by Novo Nordisk A/S.
The study was funded by Novo Nordisk A/S. ClinicalTrials.gov: NCT01664247. The results of this study were presented in poster form at the 10th International Diabetes Federation, Western Pacific Region Congress, 21–24 November 2014, Singapore.
References
- 1. Inzucchi SE, Bergenstal RM, Buse JB et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38: 140–149. [DOI] [PubMed] [Google Scholar]
- 2. Garber AJ, Abrahamson MJ, Barzilay JI et al. AACE/ACE comprehensive diabetes management algorithm 2015. Endocr Pract 2015; 21: 438–447. [DOI] [PubMed] [Google Scholar]
- 3. Vora J. Combining incretin‐based therapies with insulin: realizing the potential in type 2 diabetes. Diabetes Care 2013; 36(Suppl. 2): S226–S232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Balena R, Hensley IE, Miller S, Barnett AH. Combination therapy with GLP‐1 receptor agonists and basal insulin: a systematic review of the literature. Diabetes Obes Metab 2013; 15: 485–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bode B. An overview of the pharmacokinetics, efficacy and safety of liraglutide. Diabetes Res Clin Pract 2012; 97: 27–42. [DOI] [PubMed] [Google Scholar]
- 6. Nauck M, Frid A, Hermansen K et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (Liraglutide Effect and Action in Diabetes)‐2 study. Diabetes Care 2009; 32: 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buse J, Rosenstock J, Sesti G et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26‐week randomised, parallel‐group, multinational, open‐label trial (LEAD‐6). Lancet 2009; 374: 39–47. [DOI] [PubMed] [Google Scholar]
- 8. Buse J, Sesti G, Schmidt WE et al. Switching to once‐daily liraglutide from twice‐daily exenatide further improves glycemic control in patients with type 2 diabetes using oral agents. Diabetes Care 2010; 33: 1300–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marre M, Shaw J, Brandle M et al. Liraglutide, a once‐daily human GLP‐1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD‐1 SU). Diabet Med 2009; 26: 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garber A, Henry R, Ratner R et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD‐3 Mono): a randomised, 52‐week, phase III, double‐blind, parallel‐treatment trial. Lancet 2009; 373: 473–481. [DOI] [PubMed] [Google Scholar]
- 11. Zinman B, Gerich J, Buse J et al. Efficacy and safety of the human glucagon‐like peptide‐1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD‐4 Met+TZD). Diabetes Care 2009; 32: 1224–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Russell‐Jones D, Vaag A, Schmitz O et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD‐5 met+SU): a randomised controlled trial. Diabetologia 2009; 52: 2046–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pratley RE, Nauck M, Bailey T et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26‐week, randomised, parallel‐group, open‐label trial. Lancet 2010; 375: 1447–1456. [DOI] [PubMed] [Google Scholar]
- 14. Heise T, Nosek L, Bøttcher SG, Hastrup H, Haahr H. Ultra‐long‐acting insulin degludec has a flat and stable glucose‐lowering effect in type 2 diabetes. Diabetes Obes Metab 2012; 14: 944–950. [DOI] [PubMed] [Google Scholar]
- 15. Zinman B, Philis‐Tsimikas A, Cariou B et al. Insulin degludec versus insulin glargine in insulin‐naïve patients with type 2 diabetes: a 1‐year, randomized, treat‐to‐target trial (BEGIN Once Long). Diabetes Care 2012; 35: 2464–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garber AJ, King AB, Del Prato S et al. Insulin degludec, an ultra‐long‐acting basal insulin, versus insulin glargine in basal‐bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal‐Bolus Type 2): a phase 3, randomised, open‐label, treat‐to‐target non‐inferiority trial. Lancet 2012; 379: 1498–1507. [DOI] [PubMed] [Google Scholar]
- 17. Meneghini L, Atkin SL, Gough SC et al. The efficacy and safety of insulin degludec given in variable once‐daily dosing intervals compared with insulin glargine and insulin degludec dosed at the same time daily: a 26‐week, randomized, open‐label, parallel‐group, treat‐to‐target trial in individuals with type 2 diabetes. Diabetes Care 2013; 36: 858–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gough SC, Bhargava A, Jain R, Mersebach H, Rasmussen S, Bergenstal RM. Low‐volume insulin degludec 200 units/mL once daily improves glycemic control similarly to insulin glargine with a low risk of hypoglycemia in insulin‐naïve patients with type 2 diabetes: a 26‐week, randomized, controlled, multinational, treat‐to‐target trial: the BEGIN LOW VOLUME trial. Diabetes Care 2013; 36: 2536–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Onishi Y, Iwamoto Y, Yoo SJ, Clauson P, Tamer SC, Park S. Insulin degludec compared with insulin glargine in insulin‐naïve patients with type 2 diabetes: a 26‐week, randomized, controlled, Pan‐Asian, treat‐to‐target trial. J Diabetes Investig 2013; 4: 605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rodbard HW, Cariou B, Zinman B et al. Comparison of insulin degludec with insulin glargine in insulin‐naïve subjects with type 2 diabetes: a 2 year randomized, treat‐to‐target trial. Diabet Med 2013; 30: 1298–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ratner RE, Gough SC, Mathieu C et al. Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre‐planned meta‐analysis of phase 3 trials. Diabetes Obes Metab 2013; 15: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heller S, Mathieu C, Kapur R, Wolden ML, Zinman B. A meta‐analysis of rate ratios for nocturnal confirmed hypoglycaemia with insulin degludec vs. insulin glargine using different definitions for hypoglycaemia. Diabet Med 2016; 33: 478–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Victoza 6 mg/ml solution for injection in prefilled pen. Summary of product characteristics. Novo Nordisk A/S, Denmark. March 2015. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Information/human/001026/WC500050017.pdf. Accessed 21 January 2016.
- 24. FDA, USDHHS, and CDER . Guidance for industry: diabetes mellitus: developing drugs and therapeutic biologics for treatment and prevention, 2008. Available from URL: http://www.fda.gov/downloads/Drugs/Guidances/ucm071624.pdf. Accessed 12 October 2015.
- 25. DeVries J, Bain SC, Rodbard HW et al. Sequential intensification of metformin treatment in type 2 diabetes with liraglutide followed by randomized addition of basal insulin prompted by A1C targets. Diabetes Care 2012; 35: 1446–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mathieu C, Rodbard HW, Cariou B et al. A comparison of adding liraglutide versus a single daily dose of insulin aspart to insulin degludec in subjects with type 2 diabetes (BEGIN: VICTOZA ADD‐ON). Diabetes Obes Metab 2014; 16: 636–644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supplementary methods.
Table S1. Antihyperglycaemic treatment at screening.
Table S2. Adverse events classified as medical events of special interest.
Table S3. Hypoglycaemic episodes.
Table S4. Treatment‐emergent adverse events.
Table S5. Neoplasms by system organ class and preferred term: safety analysis set.
Figure S1. Mean pre‐breakfast self‐measured plasma glucose for dose adjustment by treatment week: mean plot, full analysis set.
Figure S2. Eight‐point self‐measured plasma glucose profile at week 26: mean plot, full analysis set.
Figure S3. Insulin doses.


