Abstract
Background
Elevated red blood cell distribution width (RDW) is a valid predictor of outcome in acute heart failure (AHF). It is unknown whether elevated RDW remains predictive in AHF patients with either preserved left ventricular ejection fraction (LVEF) ≥50% or reduced LVEF (<50%).
Methods and results
Prospective local registry including 402 consecutive hospitalized AHF patients without acute coronary syndrome or need of intensive care. The primary outcome was all‐cause mortality (ACM) at 1 year after admission. Demographic and clinical data derive from admission, echocardiographic examinations (n = 269; 67%) from hospitalization.
The Cox proportional hazard model including all patients (P < 0.001) was adjusted for age, gender, and RDW quartiles. Independent predictors of 1‐year ACM were cardiogenic shock (HR 2.86; CI: 1.3–6.4), male sex (HR 1.9; CI: 1.2–2.9), high RDW quartile (HR 1.66; CI: 1.02–2.8), chronic HF (HR 1.61; CI: 1.05–2.5), valvular heart disease (HR 1.61; CI: 1.09–2.4), increased diastolic blood pressure (HR 1.02 per mmHg; CI: 1.01–1.03), increasing age (HR 1.04 by year; CI: 1.02–1.07), platelet count (HR 1.002 per G/l; CI: 1.0–1.004), systolic blood pressure (HR 0.99 per mmHg; CI: 0.98–0.99), and weight (HR 0.98 per kg; CI: 0.97–0.99). A total of 114 patients (28.4%) died within the first year; ACM of all patients increased with quartiles of rising RDW (χ2 18; P < 0.001). ACM was not different between RDW quartiles of patients with reduced LVEF (n = 153; χ2 6.6; P = 0.084). In AHF with LVEF ≥50% the probability of ACM increased with rising RDW (n = 116; χ2 9.9; P = 0.0195).
Conclusions
High RDW is associated with increased ACM in AHF patients with preserved but not with reduced LVEF in this study population.
Keywords: Red cell distribution width, Prognosis, Acute heart failure, Left ventricular ejection fraction
Background
Acute heart failure (AHF) is associated with an adverse prognosis with an all cause mortality (ACM) of 10–20% at 2–3 months and 17–37% at 1 year.1, 2, 3, 4, 5 Single biomarkers on top of clinical assessment are useful for risk stratification in patients with chronic heart failure (HF).6, 7 However in AHF, the incremental predictive value of a single biomarker is small when added to a clinical score. Results from the Multinational Observational Cohort on Acute Heart Failure (MOCA) study suggest improved risk prediction when miscellaneous biomarkers are assessed simultaneously.5 This concept accounts for the absence of one single relevant pathomechanism in AHF while accepting that interpretation of test results is more difficult. Red cell distribution width (RDW) is a readily available biomarker increasing with cytokine activation, impaired iron mobilization, and decreasing haemoglobin level.8, 9, 10 RDW, thus, integrates various pathophysiological mechanisms associated with HF progression, which can explain why elevated RDW is a robust marker of risk for mortality in acute and chronic HF.11, 12, 13 This prospective registry of consecutive patients hospitalized for AHF investigates whether elevated RDW remains predictive in AHF patients when patients are grouped by LVEF ≥50% or LVEF <50%.
Methods
This is a prospective registry of consecutive patients (n = 408) hospitalized for AHF at a tertiary centre. Patients presenting with AHF at the Emergency Wards were included when hospitalized for heart failure at our institution and after signing the consentment. Patients with acute coronary syndrome, or cardiogenic shock in need of intensive care were not included. Outcome parameter of this registry was 1‐year all cause mortality (ACM). Patients without outcome (n = 6) were excluded from final analysis. The local ethical committee approved the study protocol.
Demographic, clinical, and laboratory data were obtained at admission. Echocardiographic data derive from standard transthoracic studies signed by a board‐certified cardiologist at our institution; all exams were performed during index hospitalization. Physicians' diagnosis of co‐morbidity followed the respective guidelines.14, 15, 16 RDW was measured using the Advia 120 Hematology Analyzer (Siemens Healthcare Diagnostics). RDW was reported as coefficient of variation (in percent) of erythrocyte cell volume.
Statistical analysis
Continuous variables are presented as mean (± SD) or median (± interquartile range; IQR). Categorical variables are presented as numbers and percentages. Analysis of variance compared continuous variables, and χ2‐statistic compared categorical variables.
RDW quartiles were defined in accordance with the literature13, 17, 18 with the lowest quartile serving as the reference group. The distribution of RDW was skewed; therefore, logarithmically transformed values of RDW (ln RDW) were used as dependent variable for multiple linear regressions as reported elsewhere.13 Age, gender, and RDW quartiles were forced into the model predicting ACM; independent explanatory variables were identified by backward elimination of variables associated with RDW at a probability (P) of <0.1 in univariate analysis.
Stepwise Cox proportional hazards models calculated the hazard ratio (HR) selecting covariables on the basis of backward stepwise analysis with removal set at P < 0.1. Survival curves were calculated using the Kaplan–Meier method; comparison of survival curves used the log‐rank test. All tests were two‐sided and used a significance level of P < 0.05. Analyses were performed using the R statistical software (version R 3.1.0) (R development core team).
Results
Patients with high RDW were older, had a higher incidence of previous HF‐related hospitalizations, and chronic HF was more prevalent by trend (P = 0.0612). Patients in the high RDW quartile had lower LVEF, lower systolic and diastolic blood pressure, and lower haemoglobin; creatinine, C‐reactive protein, and NT‐proBNP levels were higher. Patients with high RDW received more often loop diuretics and oral anticoagulation (Table 1).
Table 1.
Characteristics of RDW quartiles
| Quartiles RDW | [12.2,14.2] | [14.3,15.2] | [15.3,16.6] | [16.7,32.1] | P‐value |
|---|---|---|---|---|---|
| Number (n) | 133 | 91 | 98 | 80 | |
| Demographics | |||||
| Age (y) | 76 (64–83) | 80 (74–85.5) | 78 (71–83) | 79 (74–83) | 0.0093 |
| Male gender (%) | 83 (62.4) | 47 (51.6) | 58 (59.2) | 45 (56.2) | 0.4363 |
| Prev. HHF (%) | 23 (17.3) | 19 (20.9) | 23 (23.5) | 31 (38.8) | 0.0041 |
| CHF (%) | 72 (54.1) | 57 (62.6) | 67 (68.4) | 56 (70) | 0.0612 |
| In hospital mort (%) | 6 (4.5) | 5 (5.5) | 5 (5.1) | 9 (11.2) | 0.2055 |
| LoS (days) | 9 (4–15) | 11 (4–15) | 9 (4–16) | 11 (5–15) | 0.6543 |
| Classification | |||||
| CS (%) | 3.8 | 4.4 | 4.1 | 5 | 0.9768 |
| PE (%) | 28 | 25.3 | 21.4 | 27.5 | 0.6535 |
| RHF (%) | 4.5 | 7.7 | 17.3 | 16.2 | 0.0042 |
| HTN crisis (%) | 14.3 | 13.2 | 7.1 | 5 | 0.0873 |
| Decomp HF (%) | 48.8 | 49.4 | 50 | 46.3 | 0.9637 |
| Characteristics | |||||
| LVEF (%) | 42 (30–60) | 50 (40–60) | 40 (25–60) | 40 (25–60) | 0.0259 |
| HR (bpm) | 91 (77–105) | 87.5 (72–105) | 94 (79–107) | 90(75–106) | 0.6373 |
| SR (%) | 73 (54.9%) | 49 (53.8%) | 50 (51%) | 38 (47.5%) | 0.7207 |
| SBP (mmHg) | 151 (130–168) | 144 (126–171) | 142(128–166) | 129 (114–151) | 0.0001 |
| DBP (mmHg) | 86 (76–96) | 84 (68–97) | 82.5 (76–93) | 77 (64–88) | 0.0008 |
| BMI | 26.9 (24–31) | 26.1 (23–32) | 26 (24–30) | 25 (23–29) | 0.7347 |
| Comorbidities | |||||
| Diabetes (%) | 36.8 | 35.2 | 37.8 | 51.2 | 0.1315 |
| Hypertension (%) | 82.7 | 84.6 | 87.8 | 83.8 | 0.7474 |
| COBP (%) | 18 | 20.9 | 7.1 | 11.2 | 0.0242 |
| Biological test | |||||
| Crea (mmol/l) | 101 (80–128) | 102 (82–137) | 106 (88–152) | 112 (94–157) | 0.0170 |
| CRP (mg/l) | 10 (4–61) | 16 (9–53) | 14.5 (6–30) | 20.5 (10–51) | 0.0816 |
| NT‐proBNPx103 (pg/ml) | 3.8 (1.9–6.8) | 3.0 (1.3–4.4) | 6.8 (3.3–10.1) | 7.8 (3.5–16.2) | 0.0021 |
| Haemoglobin (G/l) | 130 (116–146) | 127 (114–136) | 121 (107–141) | 114 (99–125) | 0.0000 |
| Platelets (G/l) | 219 (173–280) | 225 (190–263) | 233 (190–300) | 225 (169–288) | 0.6993 |
| Medication | |||||
| Betablocker (%) | 36.1 | 38.5 | 39.8 | 43.8 | 0.7367 |
| ACE‐I (%) | 37.6 | 35.2 | 42.9 | 48.8 | 0.2588 |
| ARB (%) | 26.3 | 27.5 | 31.6 | 17.5 | 0.1941 |
| MR‐blocker (%) | 9.8 | 12.1 | 21.4 | 20 | 0.0438 |
| Loop diuretic (%) | 45.9 | 48.4 | 65.3 | 70 | 0.0006 |
| Digoxin (%) | 11.3 | 13.2 | 15.3 | 18.8 | 0.4809 |
| ASA (%) | 51.1 | 53.8 | 52 | 57.5 | 0.8375 |
| OAC (%) | 24.8 | 36.3 | 43.9 | 53.8 | 0.0002 |
| Clopidogrel (%) | 18.8 | 23.1 | 23.5 | 17.5 | 0.6319 |
Prev. HHF, previous hospitalization for heart failure; in hosp mortality, in hospital mortality; LoS, length of stay; CS, cardiogenic shock; PE, pulmonary edema; RHF, right heart failure; HTN, hypertension; decomp HF: decompensated heart failure; LVEF, left ventricular ejection fraction; HR, heart rate; SR, sinus rhythm; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; COPD, chronic obstructive pulmonary disease.
Table 2 depicts clinical and laboratory parameters independently associated with RDW. The final model explained 37% of the variability in RDW. RDW was independently related with in‐hospital mortality (P = 0.008). Correlation was poor between RDW and LVEF (P = 0.21), eGFR (P = 0.12), or length of stay (P = 0.10). RDW levels and haemoglobin levels correlated with strength of −0.0021 ± 0.0002 (P = 0.021).
Table 2.
Strength of the correlation between RDW (ln RDW) and different variables
| Estimate (SD) | P‐value | |
|---|---|---|
| Age | 0.0016 (0.0005) | 0.002 |
| SBP | −0.0005 (0.0002) | 0.021 |
| Haemoglobin | –0.0021 (0.0002) | <0.0001 |
| Leucocytes | 0.0050 (0.0009) | <0.0001 |
| Oral anticoagulation | –0.0422 (0.0210) | 0.0461 |
| Prothrombin time | –0.0015 (0.0003) | <0.0001 |
| Prev HHF | 0.02448 (0.0131) | 0.0637 |
| Right heart failure | 0.0607 (0.0174) | 0.000556 |
| Diabetes mellitus | 0.0297 (0.0113) | 0.0088 |
See legend Table 1.
Table 3 shows the results of a multivariable Cox model (maximal logistical likelihood −614.37; LR test statistic: 79.74; P = 1.2 × 10−11). The following variables were associated with increased HR (listed in increasing order): platelets, age, diastolic blood pressure, valvular pathology, chronic HF, elevated RDW, male sex, and cardiogenic shock. Variables with a decreased HR are listed in decreasing order: lower RDW, weight, and systolic blood pressure. Haemoglobin level was not retained in the proportional hazards model predicting 1‐year ACM.
Table 3.
Adjusted proportional hazards model for all cause 1‐year mortality
| Covariate | Mean/(%) | HR (97.5% CI) | P‐value |
|---|---|---|---|
| Age (y) | 75.2 | 1.044 (1.02–1.07) | <0.0001 |
| Gender (male) | 58% | 1.904 (1.24–2.93) | 0.030 |
| RDW quartile | |||
| (14.3–15.2) | 0.24 | 0.761 (0.43–1.34) | 0.341 |
| (15.2–16.6) | 0.25 | 0.877 (0.51–1.51) | 0.638 |
| (16.6–32.1) | 0.17 | 1.686 (1.02–2.79) | 0.041 |
| Weight (kg) | 79 | 0.978 (0.97–0.99) | 0.001 |
| Syst. BP (mmHg) | 147 | 0.985 (0.98–0.99) | 0.002 |
| Diast. BP (mmHg) | 85 | 1.020 (1.01–1.03) | 0.011 |
| Card. shock | 3.6% | 2.864 (1.29–6.35) | 0.010 |
| Platelets (G/l) | 235 | 1.002 (1.0–1.004) | 0.018 |
| Chronic HF | 60.9% | 1.611 (1.05–2.48) | 0.030 |
| β‐blocker | 42.5% | 0.735 (0.50–1.09) | 0.128 |
| Valvular HD | 46.4% | 1.606 (1.09–2.38) | 0.018 |
Female gender and lowest RDW group were used as reference; valvular HD, valvular heart disease.
During follow‐up 114 patients (28.4%) died. The Kaplan–Meier analysis including all patients showed a graded increased probability of mortality with increasing quartile of RDW (Figure 1 (A); χ2 18; P = 0.0004). In patients with reduced LVEF, mortality was not significantly different between RDW quartiles (Figure 1 (B); χ2 6.6; P = 0.084). Study patients with preserved LVEF showed a graded increased probability of mortality with rising RDW quartile (Figure 1 (C); χ2 9.9; P = 0.0195).
Figure 1.
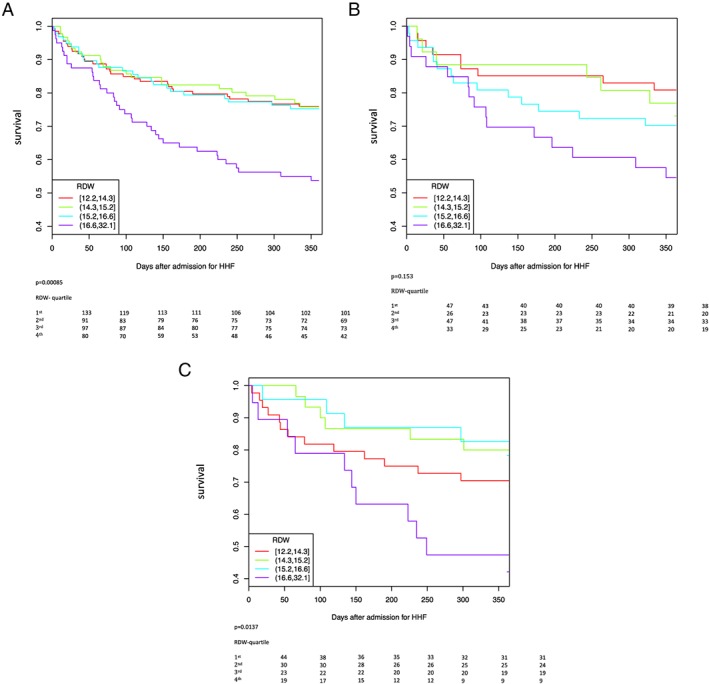
(A) RDW quartiles and 1‐year survival of all AHF patients. (B) RDW quartiles and 1‐year survival in AHF patients with LVEF <50%. (C) RDW quartiles and 1‐year survival in AHF patients with LVEF ≥50%.
Table 4 compares characteristics of AHF patients with preserved and reduced LVEF. AHF patients with preserved LVEF were older, the number of previous hospitalizations for HF was lower, and the prevalence of female gender and diagnosis of de novo HF was higher. AHF patients with preserved LVEF more often presented with hypertensive crisis whereas prevalence of ischemic aetiology and diabetes was lower. The prevalence of valvular pathology was not significantly different between HF patients with preserved and reduced systolic ejection fraction. In AHF patients with preserved LVEF systolic blood pressure was higher while heart rate and NT‐proBNP levels were lower.
Table 4.
Demographics and characteristics of AHF patients with reduced and preserved ejection fraction
| All | LVEF ≥ 50% | LVEF < 50% | P‐value | |
|---|---|---|---|---|
| Number (n) | 402 | 117 | 154 | |
| Demographics | ||||
| Age (y) | 78 (70–84) | 79 (74–84) | 73 (64–81) | <0.0001 |
| Gender (male, %) | 234 (57.4) | 48 (41) | 110 (71.4) | <0.0001 |
| Previous HHF (%) | 96 (23.5) | 22 (18.8) | 49 (31.8) | 0.023 |
| Hx of CHF (%) | 252 (61.8) | 52 (44.4) | 113 (73.4) | <0.0001 |
| LVEF | 40 (30–60) | 60 (55–65) | 30 (25–40) | <0.0001 |
| ICD (%) | 19 (4.7) | 0 (0) | 15 (9.7) | 0.001 |
| Pacemaker (%) | 49 (12) | 10 (8.5) | 24 (15.6) | 0.12 |
| Phenotype of AHF | ||||
| Cardiogenic shock | 17 (4.2) | 2 (1.7) | 11 (7.1) | 0.07 |
| Pulmonary oedema | 104 (25.5) | 30 (25.6) | 45 (29.2) | 0.61 |
| RH decompensation | 44 (10.8) | 10 (8.5) | 20 (13) | 0.34 |
| Hypertensive crisis | 42 (10.3) | 21 (17.9) | 3 (1.9) | <0.0001 |
| Global decompensation | 193 (49.2) | 54 (46.3) | 75 (48.8) | 0.12 |
| Cardiac pathology | ||||
| CAD (%) | 232 (56.9) | 53 (45.3) | 107 (69.5) | <0.0001 |
| LV dilatation (%) | 6 (5.1) | 81 (52.6) | <0.0001 | |
| Mild MR (%) | 88 (75.2) | 146 (94.8) | 0.16 | |
| Moderate MR (%) | 9 (7.7) | 8 (5.2) | ||
| Mild AR (%) | 51 (43.6) | 81 (52.3) | 0.37 | |
| Moderate AR (%) | 2 (1.8) | 2 (1.3) | ||
| Comorbidity | ||||
| Diabetes mellitus (%) | 159 (39) | 35 (29.9) | 73 (47.4) | 0.005 |
| Hypertension (%) | 341 (83.6) | 100 (85.5) | 125 (81.2) | 0.44 |
| COBP (%) | 159 (14.5) | 3 (11.1) | 23 (14.9) | 0.46 |
| BMI | 26 (24–31) | 26 (24–29) | 26 (24–31) | 0.56 |
| Biological parameters | ||||
| SBP (mmHg) | 142 (124–164) | 150 (132–170) | 133 (120–151) | <0.0001 |
| DBP (mmHg) | 84 (72–94) | 81 (74–93) | 84 (72–92) | 0.76 |
| Heart rate (bpm) | 90 (75–105) | 83 (70–100) | 97 (84–117) | <0.0001 |
| Sinus rhythm (%) | 211 (51.7) | 67 (57) | 82 (53) | 0.59 |
| Creatinine (mmol/l) | 105 (84–141) | 97 (81–134) | 109 (86–151) | 0.054 |
| NT‐proBNPx103 (pg/ml) | 4.5 (2.1–9.5) | 3.3 (1.7–7.8) | 6.8 (3.9–13.4) | 0.002 |
| CRP (mg/l) | 15(6–47) | 14.5 (6–54) | 13 (6–44) | 0.54 |
| NIPV (%) | 138 (33.8) | 39 (33.3) | 60 (39%) | 0.41 |
| In hosp mortality n (%) | 26 (6.4) | 5 (4.3) | 10 (6.5) | 0.60 |
| LoS (days) | 10 (4–15) | 10 (6–17) | 11 (7–16) | 0.62 |
Overall 19.9% of all patients presented with high RDW, 65% of these patients had a transthoracic echocardiography during hospitalization; 63.4% of these presented a LVEF <50%. Patients with LVEF ≥50% and high RDW were older (80 vs. 75 y; P = 0.013), heart rate was lower (80 vs. 90 bpm P = 0.04), and history of HF was less frequent (47 vs. 79%, P = 0.04). The burden of comorbidities (i.e. diabetes, arterial hypertension, chronic obstructive bronchopulmonary disease, BMI, renal dysfunction by eGFR) was not significantly different between patients with high RDW and LVEF of <50% or ≥50%.
Discussion
The results of the present study add to the available data regarding the predictive relevance of elevated RDW in acute HF. First, the results confirm the relevance of high RDW for prediction of mortality in AHF.11, 12, 13 Second, the present study population shows that high RDW is associated with increased mortality in AHF patients with LVEF ≥50% while there is no interaction between high RDW and mortality in AHF patients with LVEF < 50%.
The small size of the present study population raises the concern whether the sample is representative. However, demographic, clinical, and biological characteristics of the present study collectively correspond with respective characteristics reported from larger studies in AHF.19, 20 In addition, the present study replicates associations between RDW and outcome as described elsewhere, in particular, the negative correlation of RDW with haemoglobin levels as reported in the Candesartan in Heart Failure: Assessment of the Reduction in Mortality and Morbidity (CHARM),8 and in the STAMINA‐HFP (Study of Anemia in HF Population) or the UNITE‐HF (United Investigators to Evaluate Heart Failure) registry.9 In addition, this study replicates the relation of high RDW with increased mortality already described in other AHF study populations.11, 12, 13 Finally, high RDW was retained in the model for prediction of ACM similar to other studies in acute or chronic HF.8, 13, 17
In the general population, RDW increases with conditions of ineffective red cell production (such as iron deficiency, folate or B12 deficiency, and hemglobinopathy), increased red cell destruction, or after blood transfusion. Anaemia is a strong predictor of mortality in heart failure suggesting that RDW is a strong predictor for mortality and morbidity8, 10, 12 because of the high prevalence of anaemia.8 In fact, some heart failure studies link elevated RDW with inflammation, malnutrition, and renal dysfunction that may all impact on erythropoiesis.18, 21 However, results from the CHARM North America study cohort (n = 2697) indicate a prognostic role of RDW that is independent from haemoglobin level based on the observation that both parameters were retained in the final model for prediction of ACM but without reciprocal interaction.8 This observation was replicated in the Duke Databank cohort providing supportive evidence.8 Additional evidence for a distinct role of RDW derives from the European Prospective Investigation into Cancer and Nutrition (EPIC) Norfolk study and the Malmö Cancer and Diet study which show an association of RDW with incident heart failure while RDW was in this middle‐aged healthy population without relation to iron metabolism or inflammation.22, 23
High RDW is associated with increased interleukin‐6 levels (IL‐6) as shown in the STAMINA‐HFP and the UNITE‐HF registries.9 In patients with mild or moderate HF with reduced LVEF, increased IL‐6 levels are associated with increased risk of disease progression.24 Furthermore, IL‐6 are likely to be relevant in HF with preserved HF as suggested in a study by Collier et al. which showed an increase of IL‐6 levels in patients with LVEF ≥50% and heart failure symptoms while IL‐6 was low in patients without HF symptoms.25, 26 Complementary evidence of a pathophysiological role of IL‐6 in HF derives from the Health, Aging, and Body Composition (ABC) study that showed a correlation between increased IL‐6 levels and incident HF with preserved LVEF.27 Because blood samples were not retained from the present study population, we cannot correlate RDW with IL‐6 levels; however, increased CRP levels in patients with high RDW suggest increased IL‐6 levels because IL‐6 levels correlate with CRP levels patients with AHF and chronic HF.24, 28
In the present study, high RDW was associated with increased mortality in patients with LVEF ≥ 50% while mortality was not different between RDW groups of patients with reduced LVEF. Several characteristics of the two subgroups may explain this finding: first, evidence‐based pharmacological treatment6 was increased in the majority of patients with reduced LVEF <50% during hospitalization (inhibitors of the renin–angiotensin system: 70% to 92%; β‐blocker treatment: 35 to 58%; antagonists of the mineralocorticoid receptor: 21 to 38%). It is well known that increase of pharmacological HF treatment improves 1‐year survival;6, 13 therefore, mortality difference between the RDW quartiles in the present study may have decreased because of improved drug therapy; second, more patients with preserved LVEF and low RDW had developed AHF because of hypertensive crisis. Hypertensive crisis is associated with a low mortality29 suggesting accentuation of the mortality difference between low and high RDW quartiles; third, recent results from the Swedish Heart failure registry suggest that β‐blocker treatment reduces ACM in HF patients with preserved LVEF.30 Only 42% of the study patients with preserved LVEF ≥ 50% were on β‐blocker treatment suggesting an increased hazard for mortality in patients without β‐blocker in accordance with the proportional hazards model of this study. In fact, β‐blocker treatment decreased from 52% to 41% in patients with preserved LVEF and high RDW.
Study limitations
The single‐centre study design, the sample size, and the outcome parameter of 1‐year all‐cause mortality without consideration of cardiovascular mortality or rehospitalization rate represent limitations of the study. Furthermore, echocardiograms were not obtained in all patients, which might have introduced a selection bias. However, characteristics of patients with or without echocardiogram were not significantly different.
Conclusions
We have shown for the first time the prognostic relevance of RDW in AHF with preserved ejection suggesting that RDW may help to stratify risk already at admission. In a next step, this observation should be confirmed in a larger AHF population with echocardiographic exams obtained during index hospitalization. In addition, the results of this study merit further work‐up of the interaction of RDW and IL‐6 in AHF with preserved ejection fraction, and thus, increase our understanding of clinically relevant pathomechanisms in these patients.
Conflict of Interest
None declared.
Funding
CARDIOMET, an initiative of the CHUV to improve quality of care in cardiovascular and metabolic disease; Swiss National Science Foundation (320030_147121/1), SWISSHEART Foundation, to R.H.
Acknowledgement
We acknowledge the support of Veronique Prudent (research nurse).
Sotiropoulos, K. , Yerly, P. , Monney, P. , Garnier, A. , Regamey, J. , Hugli, O. , Martin, D. , Metrich, M. , Antonietti, J. ‐P. , and Hullin, R. (2016) Red cell distribution width and mortality in acute heart failure patients with preserved and reduced ejection fraction. ESC Heart Failure, 3: 198–204. doi: 10.1002/ehf2.12091.
References
- 1. Curtis LH, Greiner MA, Hammill BG, Kramer JM, Whellan DJ, Schulman KA, Hernandez AF. Early and long‐term outcomes of heart failure in elderly persons. Arch Intern Med 2008; 168: 2481–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. O'Connor CM, Stough WG, Gallup DS, Hasselblad V, Gheorghiade M. Demographics, clinical characteristics, and outcomes of patients hospitalized for decompensated heart failure: observations from the IMPACT‐HF registry. J Card Fail 2005; 11: 200–205. [DOI] [PubMed] [Google Scholar]
- 3. Gheorghiade M, Abraham WT, Albert NM, Greenberg BH, O'Connor CM, She L, Gattis Stough W, Yancy CW, Young JB, Fonarow GC, for the OPTIMIZE‐HF Investigators and Coordinators et al. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA 2006; 296: 2217–2226. [DOI] [PubMed] [Google Scholar]
- 4. Maggioni AP, Dahlstrom U, Filippatos G, Chioncel O, Crespo Leiro M, Drozdz J, Fruhwald F, Gullestad L, Logeart D, Fabbri G, Urso R, Metra M, Parissis J, Persson H, Ponikowski P, Rauchhaus M, Voors AA, Wendelboe Nielsen O, Zannad F, Tavazzi L, on behalf of the Heart Failure Association and the European Society of Cardiology. EURObservational Research Program: regional differences and 1‐year follow‐up results of the Heart Failure Pilot Survey (ESC‐HF Pilot). Eur J Heart Fail 2013; 15: 808–817. [DOI] [PubMed] [Google Scholar]
- 5. Lassus J, Gayat E, Mueller C, Peacock WF, Spinar J, Harjola VP, van Kimmenade R, Pathak A, Mueller T, di Somma S, Metra M, Pascual‐Figal D, Laribi S, Logeart D, Nouira S, Sato N, Potocki M, Parenica J, Collet C, Cohen‐Solal A, Jauzzi JL, Mebazaa A, for the GREAT‐network. Incremental value of biomarkers to clinical variables for mortality prediction in acutely decompensated heart failure: The Multinational Observational Cohort on Acute Heart Failure (MOCA) study. Int J Cardiol 2013; 168: 2186–2194. [DOI] [PubMed] [Google Scholar]
- 6. McMurray JJ, Adomopoulos S, Anker SD, Aurricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Kober L, Lip GYH, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A. ESC guidelines for diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2012; 33: 1787–1847. [DOI] [PubMed] [Google Scholar]
- 7. Hartmann F, Packer M, Coats AJS, Fowler MB, Krum H, Mohacsi P, Rouleau JL, Tendera M, Castaigne A, Anker SD, Amann‐Zalan I, Hoersch S, Katus HA. Prognostic impact of plasma N‐terminal pro–brain natriuretic peptide in severe chronic congestive heart failure: a substudy of the carvedilol prospective randomized cumulative survival (COPERNICUS) trial. Circulation 2004; 110: 1780–1786. [DOI] [PubMed] [Google Scholar]
- 8. Felker GM, Allen LA, Pocock SJ, Shaw LK, McMurray JJ, Pfeffer MA, Swedberg K, Wang D, Yusuf S, Michelson EL, Granger CB, for the CHARM Investigators. Red cell distribution width as a novel prognostic marker in heart failure. Data from the CHARM program and the Duke databank. J Am Coll Cardiol 2007; 50: 40–47. [DOI] [PubMed] [Google Scholar]
- 9. Allen LA, Felker GM, Mehra MR, Chiong JR, Dunlap SH, Ghali JK, Lenihan DJ, Oren RM, Wagoner LE, Schwartz TA, Adams KF. Validation and potential mechanisms of red cell distribution width as a prognostic marker in heart failure. J Card Fail 2010; 16: 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jenei ZM, Förhécz Z, Gombos T, Pozsonyi Z, Janoskuit L, Prohaszka Z. Red cell distribution width as predictive marker in CHF: testing model performance by reclassification methods. Int J Cardiol 2014; 174: 783–785. [DOI] [PubMed] [Google Scholar]
- 11. Pascual‐Figal DA, Bonaque JC, Redondo B, Manzano‐Fernandez S, Fernandez A, Garrido IP, Pastor‐Perez F, Lax A, Valdes M, Januzzi JL. Red blood cell distribution width predicts long‐term outcome regardless of anemia status in acute heart failure patients. Eur J Heart Fail 2009; 11: 840–846. [DOI] [PubMed] [Google Scholar]
- 12. van Kimmenade RR, Mohammed AA, Uthamalingam S, van der Meer P, Felker GM, Januzzi JL. Red blood cell distribution width and 1‐year mortality in acute heart failure. Eur J Heart Fail 2010; 12: 129–136. [DOI] [PubMed] [Google Scholar]
- 13. Makhoul BF, Khourieh A, Kaplan M, Badouth F, Aronson D, Azzam ZS. Relation between changes in red cell distribution width and clinical outcomes in acute decompensated heart failure. Int J Cardiol 2013; 167: 1412–1416. [DOI] [PubMed] [Google Scholar]
- 14. Russi EW, Karrer W, Brutsche M, Eich C, Fitting JW, Frey M, Geiser T, Kuhn M, Nicod L, Quadri F, Rochat T, Steuer‐Stey C, Stolz D; Swiss Respiratory Society. Diagnosis and management of chronic obstructive pulmonary disease. Respiration 2013; 85: 160–174. [DOI] [PubMed] [Google Scholar]
- 15. ESC task force for the management of arterial hypertension . 2013 practice guidelines for management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC task force for the management of arterial hypertension. J Hypertens 2013; 31: 1925–1938. [DOI] [PubMed] [Google Scholar]
- 16. World Health Organization . (WHO) consultation: Definition and diagnosis of diabetes mellitus. Geneva: World Health Organisation; 1999, Rapport 99.2. http: //whqlibdoc.who.int/hq/1999/who_ncdx_ncs_99.2pdf. [Google Scholar]
- 17. Zalawadiya SK, Zmily H, Farah J, Daiffallah S, Amaima A, Ghali JK. Red cell distribution width and mortality in predominantly African–American population with decompensated heart failure. J Card Fail 2011; 17: 292–298. [DOI] [PubMed] [Google Scholar]
- 18. Förhecz Z, Gombos T, Borgulya G, Pozsonyi Z, Prohaszka Z, Janoskuti L. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J 2009; 158: 659–666. [DOI] [PubMed] [Google Scholar]
- 19. Fonarow GC, Gattis Stough W, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, MD, O'Connor C, Sun JL, Yancy CW, Young JB, for the OPTIMIZE‐HF Investigators and Hospitals. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure. A report from the OPTIMIZE‐HF registry. J Am Coll Cardiol 2007; 50: 768–777. [DOI] [PubMed] [Google Scholar]
- 20. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AM, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure. J Am Coll Cardiol 2014; 63: 1123–1133. [DOI] [PubMed] [Google Scholar]
- 21. Roland RJ, van Kimmenade RR, Mohammed AA, Uthamalingam S, van der Meer P, Felker GM, Januzzi JL. Red blood cell distribution width and 1‐year mortality in acute heart failure. Eur J Heart Fail 2010; 12: 129–136. [DOI] [PubMed] [Google Scholar]
- 22. Borné Y, Smith JG, Melander O, Hedblad B, Engström G. Red cell distribution width and risk for first hospitalization due to heart failure: a population‐based cohort study. Eur J Heart Fail 2011; 13: 1355–1361. [DOI] [PubMed] [Google Scholar]
- 23. Emans ME, Gaillard C, Pfister R, Tanck MW, Matthijs Boekholdt S, Warham NJ, Khaw K‐T. Red cell distribution width is associated with physical inactivity and heart failure, independent of established risk factors, inflammation or iron metabolism; the EPIC‐Norfolk study. Int J Cardiol 2013; 168: 3550–3555. [DOI] [PubMed] [Google Scholar]
- 24. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection action. J Am Coll Cardiol 2013; 61: 263–271. [DOI] [PubMed] [Google Scholar]
- 25. Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, Harrison DG, Yongmei L, Hoffmann U, Bauer DC, Newmann AB, Kritchevsky SB, Harris TB, Butler J, for the Health ABC Investigators . Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol 2010; 55: 2129–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Collier P, Watson CJ, Voon V, Phelan D, Jan A, Mak G, Martos R, Baugh JA, Ledwidge MT, McDonald K. Can emerging biomarkers of myocardial remodelling identify asymptomatic hypertensive patients at risk for diastolic dysfunction and diastolic heart failure? Eur J Heart Fail 2011; 13: 1087–1095. [DOI] [PubMed] [Google Scholar]
- 27. Tanner H, Mohacsi P, Fuller‐Bicer GA, Rieben R, Meier B, Hess OM, Hullin R. Cytokine activation and disease progression in patients with stable moderate chronic heart failure. J Heart Lung Transplant 2007; 26: 622–629. [DOI] [PubMed] [Google Scholar]
- 28. Milo‐Cotter O, Cotter‐Davison B, Lombardi C, Sun H, Bettari L, Bugatti S, Rund M, Metra M, McMurray JJ, Teerlink JR, Cotter‐Davison G. Neurohumoral activation in heart failure: results from VERITAS. Cardiology 2011; 118: 96–105. [DOI] [PubMed] [Google Scholar]
- 29. Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola V‐K, Hochadel M, Komajda M, Lassus J, Lopez‐Sendon JL, Ponikowski P, Tavazzi L, on behalf of the EuroHeart Survey Investigators. EURO Heart Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J 2007; 27: 2725–2736. [DOI] [PubMed] [Google Scholar]
- 30. Lund LH, Benson L, Dahlström U, Edner M, Friberg L. Association between use of β‐blockers and outcomes in patients with heart failure and preserved ejection fraction. JAMA 2014; 312: 2008–2018. [DOI] [PubMed] [Google Scholar]


