Summary
Pegfilgrastim is a pegylated form of the granulocyte‐colony stimulating factor, filgrastim. Herein, we report the results of a multicentre, randomized, double‐blind phase III trial comparing the efficacy and safety of pegfilgrastim with filgrastim in patients with malignant lymphoma. Patients were randomized to receive either a single subcutaneous dose of pegfilgrastim or daily subcutaneous doses of filgrastim on day 4 after the completion of cyclophosphamide, cytarabine, etoposide and dexamethasone ± rituximab (CHASE(R); day 1–3) chemotherapy. The primary endpoint was the duration of severe neutropenia (DSN), defined as the number of days with neutrophil count <0·5 × 109/l in the first cycle of chemotherapy. A total of 111 lymphoma patients were randomized to either the pegfilgrastim or filgrastim group. 109 patients received either pegfilgrastim (n = 54) or filgrastim (n = 55). Efficacy data were available for 107 patients (pegfilgrastim: n = 53, filgrastim: n = 54). Both groups were well balanced in terms of gender, age, performance status and other variables. The mean DSN (±S.D.) was 4·5 (±1·2) and 4·7 (±1·3) d in the pegfilgrastim and filgrastim groups. No significant difference in safety was observed. This trial verified the non‐inferiority of a single subcutaneous dose of pegfilgrastim compared with daily subcutaneous doses of filgrastim, considering DSN as an indicator.
Keywords: pegfilgrastim, CHASE(R) chemotherapy, G‐CSF, febrile neutropenia, malignant lymphoma
Febrile neutropenia (FN) is a condition characterized by the development of fever in patients with neutropenia. A longer duration of neutropenia is associated with a greater risk for FN (Bodey et al, 1966). Salvage chemotherapy is used to treat patients with malignant lymphoma who do not achieve a complete response or who experience recurrence after initial chemotherapy. These salvage chemotherapies often cause long‐lasting severe neutropenia, resulting in an increased risk for FN. In severe cases of FN, patients may also develop sepsis, pneumonia or infection foci, such as cellulitis. Approximately 10% of patients admitted with FN are reported to have serious complications leading to death (Kuderer et al, 2006). The risk for FN associated with intensive chemotherapy can be mitigated by reducing chemotherapeutical doses or extending dosing intervals. However, these measures reduce the relative dose intensity (RDI) of chemotherapy, which may lead to reduced survival rates. Granulocyte‐colony stimulating factor (G‐CSF) is therefore used to reduce the risk of FN. In a systematic review, Kuderer et al (2007) reported that prophylactic use of G‐CSF led to a reduced incidence of infection‐related mortality, early mortality and FN, as well as improved average RDI. The American Society of Clinical Oncology, the National Comprehensive Cancer Network (NCCN), the European Organization for Research and Treatment of Cancer and the Japan Society of Clinical Oncology have established guidelines that recommend the prophylactic use of G‐CSF in accordance with the risk of onset of chemotherapy‐induced FN and patient‐specific risk factors (Smith et al, 2006; Aapro et al, 2011; Japan Society of Clinical Oncology 2013; NCCN 2014).
Pegfilgrastim is a pegylated form of filgrastim. A previous randomized, double‐blind trial demonstrated the non‐inferiority of pegfilgrastim compared with daily filgrastim in breast cancer patients who had received chemotherapy (Green et al, 2003), with the duration of severe neutropenia (DSN) as an indicator. In Japan, a study to determine the dose of pegfilgrastim was conducted in patients with malignant lymphoma using DSN as an endpoint (Miyazaki et al, 2013). Here, we report the results of a double‐blind study in Japanese patients with malignant lymphoma that was conducted to demonstrate the non‐inferiority of a single‐dose pegfilgrastim to daily filgrastim during CHASE(R) (cyclophosphamide, cytarabine, etoposide and dexamethasone ± rituximab) chemotherapy, with DSN as an endpoint.
Patients and methods
Eligibility criteria
The inclusion criteria were as follows: age ≥20 years, diagnosis of non‐Hodgkin lymphoma (NHL) or Hodgkin lymphoma (HL), receiving CHASE(R) chemotherapy, Eastern Cooperative Oncology Group performance status 0–2, absolute neutrophil count (ANC) ≥1·0 × 109/l, platelet count ≥ 75 × 109/l, total bilirubin level ≤1·5 times the upper normal limit in each institute and creatinine level ≤132·6 μmo/l. The exclusion criteria were as follows: history of three or more regimens of salvage chemotherapy, history of radiation therapy within 4 weeks prior to enrolment, history of bone marrow or haematopoietic stem cell transplantation, or presence of multiple primary cancers requiring treatment. The study protocol was approved by the institutional review board at each trial site. Informed consent was obtained from all patients prior to study initiation.
Study design
This was a phase III, multicentre (47 sites), double‐blind, randomized trial conducted in Japan. Patients were stratified by trial site and age (<65 or ≥65 years), and then randomized to receive either pegfilgrastim or filgrastim at a 1:1 ratio (Fig 1).
Figure 1.
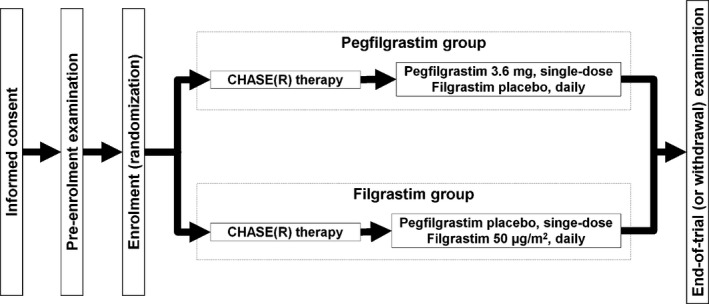
Trial design. CHASE(R), cyclophosphamide, cytarabine, etoposide and dexamethasone ± rituximab.
Procedures
The CHASE(R) chemotherapy consisted of intravenous doses of cyclophosphamide on day 1 (1200 mg/m2), etoposide and dexamethasone on days 1–3 (100 mg/m2 and 40 mg, respectively) and cytarabine on days 2–3 (2000 mg/m2). The dose of dexamethasone was reduced if clinically indicated. The dosing schedule and dosage of rituximab were not specified in the protocol. Pegfilgrastim and filgrastim were both supplied as clear, colourless solutions and identical placebos were provided for each study drug. On day 4 of cycle 1 (≥24 h after chemotherapy), patients in the pegfilgrastim group received 3·6 mg pegfilgrastim plus a filgrastim placebo subcutaneously, followed by once‐daily doses of the filgrastim placebo from day 5 onward. The patients in the filgrastim group received the pegfilgrastim placebo plus filgrastim at 50 μg/m2 on day 4, followed by daily filgrastim at 50 μg/m2 from day 5 onward. Administration of filgrastim was continued until the neutrophil count returned to ≥0·5 × 109/l. The pegfilgrastim dose (3·6 mg) was based on the results of a phase II trial of CHASE(R) chemotherapy in Japanese patients.
After enrolment, patients were not allowed to receive radiation therapy until the end‐of‐trial examination. The use of anti‐cancer chemotherapy other than CHASE(R), as well as the use of any other G‐CSF or macrophage‐colony stimulating factor, was also prohibited. The use of corticosteroids was limited to topical application and treatment of adverse events (AEs).
Complete blood count was measured before administration of the study drug regimen on days 1 and 4, and daily thereafter until the neutrophil count recovered to ≥1·0 × 109/l.
Efficacy and safety measurements
The primary endpoint was DSN, defined as the number of days with neutrophil count <0·5 × 109/l in the first cycle of CHASE(R) therapy. The secondary endpoints were duration of neutropenia (DN), defined as the number of days with ANC <1·0 × 109/l, ANC nadir and incidence of FN. FN was defined as an axillary temperature of 37·5°C and ANC <0·5 × 109/l, according to the definition of FN in Japanese guidelines of antimicrobial use (Masaoka, 2004).
Safety assessments were based on AE reports, abnormal laboratory values and vital signs. AEs were defined by the Japanese version of the Medical Dictionary for Regulatory Activities and graded according to the National Cancer Institute Common Toxicity Criteria (version 4.0) (Japan Clinical Oncology Group 2010).
Data sets and statistical analysis
The target sample size was determined to be 47 patients per group, based on the ability to detect a statistically significant difference with a power of 80% and a one‐sided significance level of 2·5% using the Student's t‐test, a non‐inferiority margin of 1·0 d, and a standard deviation of DSN in the filgrastim group of 1·7 d based on previous results (unpublished data). We also expected approximately 10% of patients to discontinue or withdraw from the study. Thus, the target sample size was set at 52 patients per group (104 patients in total).
Efficacy was primarily analysed using the Per Protocol Set (PPS) and additionally using the Full Analysis Set (FAS). Analysis of the primary endpoint was performed using the Student's t‐test, with a one‐sided significance level of 2·5% with respect to a non‐inferiority margin of 1 d. The P‐value was calculated based on the test statistic obtained as a result of adding 1 d, the non‐inferiority margin, to the mean value of DSN in the filgrastim group. Safety was analysed for all patients treated with the study drug. A safety analysis was conducted based on the number of patients with AEs or adverse drug reactions in each group.
All analyses were performed using the SAS software (version 9.2; SAS Institute Inc., Cary, NC).
Results
Patients
One hundred and eleven patients were randomized between February and December 2011, one patient from each group withdrew from the study before study drug administration due to rapid aggravation of the primary disease. The remaining 109 patients (pegfilgrastim group: 54, filgrastim group: 55) were included in the FAS and safety evaluation. Of 109 patients, 107 (pegfilgrastim group: 53, filgrastim group: 54) were included in the PPS after excluding one patient in the pegfilgrastim group, who had a neutrophil count >0·5 × 109/l at the time of discontinuation, and one patient who received an overdose of filgrastim (150 μg instead of 80 μg) at day 4 and was excluded from PPS. This patient was included in the original FAS and safety analysis set. A flow chart of patient allocation is shown in Fig 2.
Figure 2.
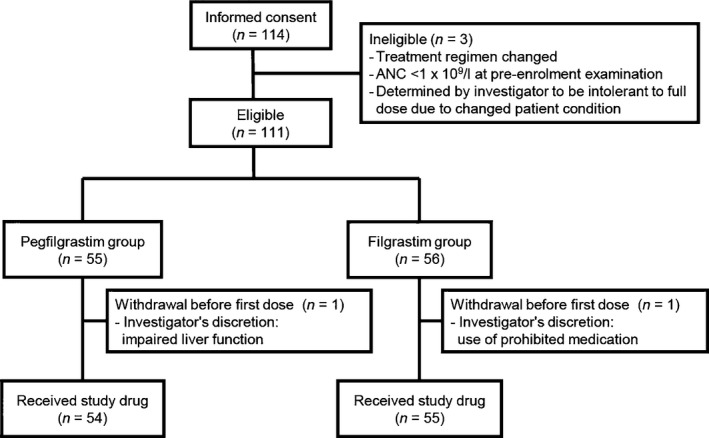
Patient allocation and disposition. ANC, absolute neutrophil count.
The background characteristics of the patients treated with the study drug are summarized in Table 1. The overall demographics were well balanced between the two groups. The number of male and female patients was 35 and 18 in the pegfilgrastim group, and 31 and 23 in the filgrastim group, respectively. The median age (range) in the pegfilgrastim and filgrastim group was 61·0(28–74) years and 60·5 (24–79) years and the mean body weight (±SD) was 60·85 (±11·01) kg and 61·94 (±15·29) kg, respectively. The number of patients with NHL as the primary disease was 50 (94·3%) in the pegfilgrastim and 50 (92·6%) in the filgrastim group. The number of patients with HL as the primary disease was 3 (5·7%) in the pegfilgrastim group and 4 (7·4%) in the filgrastim group. The mean (±SD) number of doses of filgrastim was 11·0 (±1·3). One patient had a cancer (hepatocellular carcinoma) other than the primary disease, which did not require treatment at the time of study.
Table 1.
Background characteristics of patients
| Pegfilgrastim group (n = 53) | Filgrastim group (n = 54) | |||
|---|---|---|---|---|
| n | % | n | % | |
| Sex | ||||
| Male | 35 | 66·0 | 31 | 57·4 |
| Female | 18 | 34·0 | 23 | 42·6 |
| Age (years) | ||||
| Median (Range) | 61·0 (28–74) | 60·5 (24–79) | ||
| Body weight (kg) | ||||
| Mean (SD) | 60·85 (11·01) | 61·94 (15·29) | ||
| <60 kg | 25 | 47·2 | 27 | 50·0 |
| ≥60 kg | 28 | 52·8 | 27 | 50·0 |
| Body surface area (m2) | ||||
| Mean (SD) | 1·66 (0·18) | 1·66 (0·23) | ||
| Primary disease | ||||
| Non‐Hodgkin lymphoma | 50 | 94·3 | 50 | 92·6 |
| B‐cell lymphoma | ||||
| Diffuse large B‐cell lymphoma | 30 | 56·6 | 26 | 48·1 |
| Follicular lymphoma | 7 | 13·2 | 9 | 16·7 |
| Mantle cell lymphoma | 2 | 3·8 | 2 | 3·7 |
| Nodal marginal zone B‐cell lymphoma | 1 | 1·9 | 0 | 0 |
| Primary mediastinal large B‐cell lymphoma | 0 | 0 | 1 | 1·9 |
| T/NK‐cell lymphoma | ||||
| Peripheral T‐cell lymphoma, unspecified | 5 | 9·4 | 7 | 13·0 |
| Anaplastic large cell lymphoma | 2 | 3·8 | 3 | 5·6 |
| Other | 3 | 5·7 | 2 | 3·7 |
| Hodgkin lymphoma | 3 | 5·7 | 4 | 7·4 |
| Clinical stage | ||||
| I | 4 | 7·5 | 1 | 1·9 |
| II | 7 | 13·2 | 20 | 37·0 |
| III | 15 | 28·3 | 13 | 24·1 |
| IV | 27 | 50·9 | 20 | 37·0 |
| Bone marrow involvement | ||||
| + | 6 | 11·3 | 5 | 9·3 |
| − | 46 | 86·8 | 47 | 87·0 |
| Unknown | 1 | 1·9 | 2 | 3·7 |
| Other malignancy | ||||
| Absent | 53 | 100·0 | 53 | 98·1 |
| Present | 0 | 0 | 1 | 1·9 |
| Operation prior to primary disease | ||||
| No | 48 | 90·6 | 53 | 98·1 |
| Yes | 5 | 9·4 | 1 | 1·9 |
| Radiotherapy prior to primary disease | ||||
| No | 46 | 86·8 | 48 | 88·9 |
| Yes | 7 | 13·2 | 6 | 11·1 |
| Chemotherapy prior to primary disease | ||||
| No | 1 | 1·9 | 0 | 0 |
| Yes | 52 | 98·1 | 54 | 100·0 |
Efficacy
The mean (±SD) of the primary endpoint (DSN) was 4·5 (±1·2) d in the pegfilgrastim group and 4·7 (±1·3) d in the filgrastim group (P < 0·001), demonstrating the non‐inferiority of pegfilgrastim to filgrastim (Table 2). The time course of the neutrophil count is shown in Fig 3. The neutrophil count reached its peak on the day after the start of study drug administration and day 5 for pegfilgrastim and filgrastim, respectively. The peak of the nadir was between days 8 and 10 for both groups and then returned to ≥1·0 × 109/l by day 16 in all patients.
Table 2.
Study endpoints
| Pegfilgrastim group n = 53 | Filgrastim group n = 54 | |
|---|---|---|
| DSN (d), Mean ± SD | 4·5 ± 1·2 | 4·7 ± 1·3 |
| DN (d), Mean ± SD | 5·2 ± 1·3 | 5·1 ± 1·3 |
| ANC nadir (/×109/l), Mean ± SD | 0.0131 ± 0.0261 | 0.0175 ± 0.0552 |
| FN, n (%) | 30 (56·6) | 30 (55·6) |
DSN, duration of severe neutropenia; DN, duration of neutropenia; ANC, absolute neutrophil count; FN, febrile neutropenia; SD, standard deviation.
Figure 3.
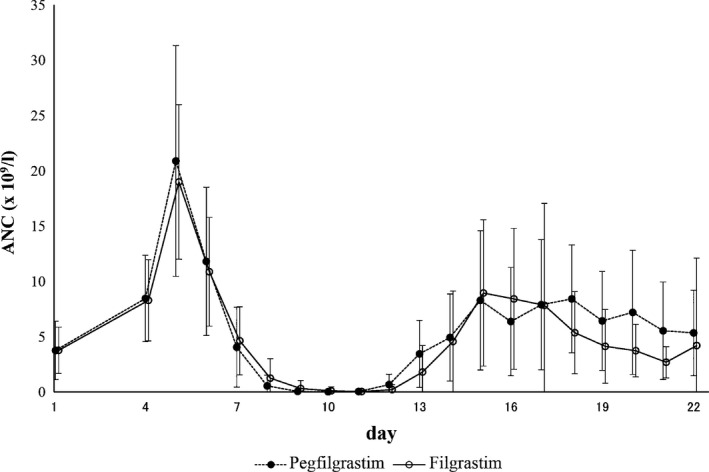
Changes in absolute neutrophil counts during the first cycle of chemotherapy for patients in the per protocol set. ANC, absolute neutrophil count.
The mean (±SD) DN, a secondary endpoint, was 5·2 (±1·3) d in the pegfilgrastim group and 5·1 (±1·3) d in the filgrastim group. The mean (±SD) neutrophil count at the nadir was 0·013 (±0·026) × 109/l and 0·017 (±0·055) × 109/l, and the incidence of FN was 56·6% (30/53) and 55·6% (30/54) in the pegfilgrastim and filgrastim groups, respectively. The efficacy end points using the FAS are shown in Table SI.
Safety
All patients suffered AEs, most of which were related to the chemotherapy regimen. Two patients died; one patient in the pegfilgrastim group due to a malignant neoplasm and one patient in the filgrastim group due to septic shock. Both events were determined to be unrelated to the study drug. Other serious AEs included pneumonia and bacterial gastritis in one patient each in the pegfilgrastim group and gastrointestinal haemorrhage in one patient in the filgrastim group. The bacterial gastritis event in the pegfilgrastim group was diagnosed as acute gastritis based on the endoscopic findings of extensive mucosal inflammation.
AEs were reported in ≥10% patients and are shown in Table 3. Of 54 patients in the pegfilgrastim group, platelet count decreased in 53 (98·1%) patients, neutropenia developed in 52 (96·3%) patients and haemoglobin level decreased in 23 (42·6%) patients. Vomiting was the AE more frequently reported in the pegfilgrastim group (8 patients, 14·8%) than in the filgrastim group (1 patient, 1·8%). One case of grade 1 vomiting occurred in the pegfilgrastim group at the same day of pegfilgrastim administration and recovered in the same day. Except for this case, in the pegfilgrastim group, most cases of vomiting were related to the chemotherapy regimen and assessed as unrelated to the study drug. None of the other AEs were reported to be significantly higher in frequency in the pegfilgrastim than in the filgrastim group.
Table 3.
Subjects with adverse events
| Treatment emergent adverse events | ||||
|---|---|---|---|---|
| Pegfilgrastim group n = 54 | Filgrastim group n = 55 | |||
| n | (%) | n | (%) | |
| Overall | 54 | 100 | 55 | 100 |
| Platelet count decreased | 53 | 98·1 | 55 | 100 |
| Neutrophil count decreased | 52 | 96·3 | 55 | 100 |
| Haemoglobin decreased | 23 | 42·6 | 25 | 45·5 |
| Febrile neutropenia | 20 | 37·0 | 12 | 21·8 |
| Back pain | 12 | 22·2 | 16 | 29·1 |
| Pyrexia | 12 | 22·2 | 14 | 25·5 |
| Blood lactate dehydrogenase increased | 11 | 20·4 | 18 | 32·7 |
| Malaise | 10 | 18·5 | 5 | 9·1 |
| Nausea | 8 | 14·8 | 7 | 12·7 |
| Stomatitis | 8 | 14·8 | 8 | 14·5 |
| Vomiting | 8 | 14·8 | 1 | 1·8 |
| Decreased appetite | 8 | 14·8 | 4 | 7·3 |
| Urticaria | 6 | 11·1 | 5 | 9·1 |
| Alopecia | 5 | 9·3 | 8 | 14·5 |
| Headache | 5 | 9·3 | 6 | 10·9 |
| Blood alkaline phosphatase increased | 3 | 5·6 | 7 | 12·7 |
The use of G‐CSF is known to be associated with back and bone pain, as the typical adverse drug reactions. Back pain was observed in 12 (22·2%) patients in the pegfilgrastim group and 16 (29·1%) patients in the filgrastim group. Bone pain was observed in no patient in the pegfilgrastim group and 5 (9·1%) patients in the filgrastim group. All these events were grade 2 or lower in severity. No safety issue was identified in the patient given an overdose of filgrastim.
Neither anti‐pegfilgrastim nor anti‐filgrastim antibodies were detected in either group.
Discussion
The NCCN guideline (Version 1.2014) recommends the use of chemotherapy regimens, such as dexamethasone, cytarabine and cisplatin (DHAP) and rituximab, etoposide, methylprednisolone, cytarabine and cisplatin ((R)ESHAP), for the treatment of recurrent or refractory NHL (NCCN 2014). The guideline also recommends the use of G‐CSF during these chemotherapy regimens due to its strong myelosuppressive effect and the associated increase in the FN risk. In addition to these regimens, a highly intensive chemotherapy regimen, referred to as CHASE(R), is also used in Japan (Ogura et al, 2003).
The CHASE(R) regimen is highly effective for the treatment of recurrent or refractory NHL, with an overall response rate of 84%. At the same time, the regimen is reported to be highly myelosuppressive and associated with a 78% risk of causing FN (Oki et al, 2008). In Japan, G‐CSF is administered daily during these chemotherapies when treatment‐related neutropenia is decreased. However, the daily use of G‐CSF places a significant burden on patients and their families due to the daily visit to hospital. It also puts some burden on medical staff. These burdens may be reduced by replacing filgrastim by pegfilgrastim, which is administered only once per chemotherapy cycle.
In a randomized, open‐label study of NHL and haemodialysis patients, Vose et al (2003) demonstrated the non‐inferiority of pegfilgrastim to filgrastim (33 patients per group) in terms of DSN following the ESHAP regimen. In a randomized, open‐label study in elderly NHL patients, Grigg et al (2003) demonstrated the non‐inferiority of pegfilgrastim to filgrastim in terms of DSN (13 and 14 patients, respectively) following cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) chemotherapy, despite higher rates of bone marrow involvement (29% vs. 0%) and prior chemotherapy (43% vs. 15%), together with a higher proportion of patients with stage IV disease (57% vs. 31%). The current phase III, double‐blind, randomized trial conducted in Japan demonstrated the non‐inferiority of a single subcutaneous dose of pegfilgrastim to daily subcutaneous doses of filgrastim in reducing duration of severe neutropenia following myelosuppressive chemotherapy. The incidence of FN was comparable in the pegfilgrastim and filgrastim groups (56·5% vs. 55·6%), with the neutrophil count returning to ≥1·0 × 109/l by day 16 in all patients. In the current trial, FN, a secondary endpoint, was defined as an axillary temperature of 37·5°C and ANC <0·5 × 109/l, which is different from the definition of AEs by the CTCAE. Due to higher threshold of body temperature in CTCAE criteria (38·3°C), the incidence of investigator‐reported FN was higher than FN rate calculated for the secondary endpoint. In terms of safety, most AEs were related to the chemotherapy regimen and no new safety concerns were identified. No significant difference was found in the incidence of AEs or adverse drug reactions between the groups. No clinically significant changes were observed in laboratory parameters or vital signs. No significant increase in the incidence of bone and back pain, which are G‐CSF‐related adverse drug reactions, was observed with pegfilgrastim or filgrastim. These observations confirm that single‐dose pegfilgrastim is as effective as daily filgrastim in reducing DSN during a highly myelosuppressive chemotherapy regimen, allowing safe administration of chemotherapy. Though the possibility remains of introducing bias in the analysis data of the PPS, which is the primary analysis set in this study, comparison of the analysis results of the FAS and PPS revealed that the both data were not largely different from each other.
In conclusion, the present results suggest that a single subcutaneous dose of prophylactic pegfilgrastim per chemotherapy cycle is safe and effective in reducing DN and risk of FN in Japanese patients. The reduction in dosing frequency, as compared to daily G‐CSF, leads to a reduction in visit frequency for patients and their families, as well as a reduced workload for the medical staff.
Funding sources
This study was funded by Kyowa Hakko Kirin Co., Ltd. (Tokyo, Japan) and has been registered at www.clinicaltrials.jp as JapicCTI‐ 111394.
Disclosure of conflicts of interests
KT received payment from Kyowa Hakko Kirin Co., Ltd in relation to the role of medical adviser. NU and AU received payment in relation to the role of the safety review committee. TH received payment in relation to the coordinating investigator. RS is employed by and owns stock in Kyowa Hakko Kirin. KK, YM and TM performed research and have no disclosures or financial support to declare.
Author contribution statement
KK, YM, TM performed the research. NU and AU were members of the safety data review committee of the study. TH was the coordinating investigator of the study. KT was the medical reviewer of the study. KK wrote the paper and all the other authors reviewed the paper.
Supporting information
Table SI. Study endpoints (FAS).
Acknowledgements
We would like to thank all of the participating patients and their families as well as investigators, research nurses, study coordinators and operations staff.
Study participants: Drs. Mitsutoshi Kurosawa (National Hospital Organization Hokkaido Cancer Centre, Sapporo); Masanobu Nakata (Sapporo Hokuyu Hospital, Sapporo); Kohmei Kubo (Aomori Prefectural Central Hospital, Aomori); Kenichi Ishizawa (Tohoku University Hospital, Sendai); Osamu Sasaki (Department of Internal Medicine, Miyagi Cancer Centre, Natori); Yoshihiro Kameoka (Akita University Hospital, Akita); Hiroyuki Kanbayashi (Ohta Nishinouchi Hospital, Kohriyama); Takuya Komeno (National Hospital Organization Mito Medical Centre, Higashiibaraki); Atsushi Shinagawa (Department of Internal Medicine, Hitachi General Hospital, Hitachi); Norifumi Tsukamoto (Gunma University Hospital, Maebashi); Kyoya Kumagai (Chiba Cancer Centre Hospital, Chiba); Kuniaki Itoh (National Cancer Centre Hospital East, Kashiwa); Kensuke Usuki (NTT Kanto Medical Centre, Tokyo); Kiyohiko Hatake (Japanese Foundation for Cancer Research, Tokyo); Rumiko Okamoto (Tokyo Metropolitan Komagome Hospital, Tokyo); Koji Izutsu (Toranomon Hospital, Tokyo); Kiyoshi Ando (Tokai University Hospital, Isehara); Jun Takizawa (Niigata University Medical & Dental Hospital, Niigata); Takaaki Chou (Niigata Cancer Centre Hospital, Niigata); Shinji Nakao (Kanazawa University Hospital, Kanazawa); Hiroshi Kosugi (Ogaki municipal Hospital, Ogaki); Akihiro Tomita (Nagoya University Hospital, Nagoya); Michinori Ogura (Nagoya Daini Red Cross Hospital, Nagoya); Kazuhito Yamamoto (Aichi Cancer Centre, Nagoya); Yasuhiko Miyata (National Hospital Organization Nagoya Medical Centre, Nagoya); Shigeru Kusumoto (Nagoya City University Hospital, Nagoya); Masashi Sawa (Anjo Kosei Hospital, Anjo); Toshihito Ohno (Tosei General Hospital, Seto); Yoshihisa Morishita (Konan Kosei Hospital, Konan); Isamu Sugiura (Toyohashi Municipal Hospital, Toyohashi); Motoko Yamaguchi (Mie University Hospital, Tsu); Takahiko Utsumi (Shiga Medical Centre for Adults, Moriyama); Yutaka Kobayashi (Kyoto Second Red Cross Hospital, Kyoto); Masafumi Taniwaki (University Hospital, Kyoto Prefectural University of Medicine, Kyoto); Tetsuo Maeda (Osaka University Hospital, Suita); Nobuhiko Uoshima (Matsushita Memorial Hospital, Moriguchi); Hiroshi Kanashima (Osaka City General Hospital, Osaka); Toru Murayama (Hyogo Cancer Centre, Akashi); Mitsune Tanimoto (Okayama University Hospital, Okayama); Isao Yoshida (National Hospital Organization Shikoku Cancer Centre, Matsuyama); Toshihiro Miyamoto (Kyushu University Hospital, Fukuoka); Naokuni Uike (National Hospital Organization Kyushu Cancer Centre, Fukuoka); Jo Tatsuro (Japanese Red Cross Nagasaki Genbaku Hospital, Nagasaki); Kunihiro Tsukasaki (Nagasaki University Hospital, Nagasaki); Michihiro Hidaka (National Hospital Organization Kumamoto Medical Centre, Kumamoto); Kisato Nosaka (Kumamoto University Hospital, Kumamoto); Yasuhiko Miyazaki (Oita Prefectural Hospital, Oita).
References
- Aapro, M.S. , Bohlius, J. , Cameron, D.A. , Dal Lago, L. , Donnelly, J.P. , Kearney, N. , Lyman, G.H. , Pettengell, R. , Tjan‐Heijnen, V.C. , Walewski, J. , Weber, D.C. & Zielinski, C. ; European Organisation for Research and Treatment of Cancer . (2011) 2010 update of EORTC guidelines for the use of granulocyte‐colony stimulating factor to reduce the incidence of chemotherapy‐induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. European Journal of Cancer, 47, 8–32. [DOI] [PubMed] [Google Scholar]
- Bodey, G.P. , Buckley, M. , Sathe, Y.S. & Freireich, E.J. (1966) Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Annals of Internal Medicine, 64, 328–340. [DOI] [PubMed] [Google Scholar]
- Green, M.D. , Koelbl, H. , Baselga, J. , Galid, A. , Guillem, V. , Gascon, P. , Siena, S. , Lalisang, R.I. , Samonigg, H. , Clemens, M.R. , Zani, V. , Liang, B.C. , Renwick, J. & Piccart, M.J. ; International Pegfilgrastim 749 Study Group . (2003) A randomized double‐blind multicenter phase III study of fixed‐dose single‐administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Annals of Oncology, 14, 29–35. [DOI] [PubMed] [Google Scholar]
- Grigg, A. , Solal‐Celigny, S. , Hoskin, P. , Taylor, K. , McMillan, A. , Forstpointner, R. , Bacon, P. , Renwick, J. & Hiddermann, W. ; International Study Group . (2003) Open‐label, randomized study of pegfilgrastim vs. daily filgrastim as an adjunct to chemotherapy in elderly patients with non‐Hodgkin's Lymphoma. Leukemia & Lymphoma, 44, 1503–1508. [DOI] [PubMed] [Google Scholar]
- Japan Clinical Oncology Group . (2010) Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. http://www.jcog.jp/doctor/tool/CTCAEv4J_20100911.pdf. Last accessed 8 December 2010.
- Japan Society of Clinical Oncology . (2013) Japan Society of Clinical Oncology Clinical Practice Guideline: GCSF supportive treatment. http://www.jsco-cpg.jp/item/30/index.html. Last accessed 6 June 2014.
- Kuderer, N.M. , Dale, D.C. , Crawford, J. , Cosler, L.E. & Lyman, G.H. (2006) Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer, 106, 2258–2266. [DOI] [PubMed] [Google Scholar]
- Kuderer, N.M. , Dale, D.C. , Crawford, J. & Lyman, G.H. (2007) Impact of primary prophylaxis with granulocyte colony‐stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. Journal of Clinical Oncology, 25, 3158–3167. [DOI] [PubMed] [Google Scholar]
- Masaoka, T. (2004) Evidence‐based recommendations for antimicrobial use in febrile neutropenia in Japan: executive summary. Clinical Infectious Diseases, 39, S49–S52. [DOI] [PubMed] [Google Scholar]
- Miyazaki, Y. , Kubo, K. , Murayama, T. , Usui, N. , Urabe, A. , Tamura, K. & Hotta, T. (2013) A multicenter, double‐blind, randomized Phase III study comparing KRN125 with filgrastim in lymphoma. The Japanese Journal of Clinical Hematology, 54, 1064. [Google Scholar]
- NCCN . (2014) The National Comprehensive Cancer Network (NCCN) Guidelines (2014); Clinical practice guidelines in oncology: myeloid growth factors, version 1. http://www.nccn.org/professionals/physician_gls/pdf/myeloid_growth.pdf. Last accessed 6 June 2014.
- Ogura, M. , Kagami, Y. , Taji, H. , Suzuki, R. , Miura, K. , Takeuchi, T. & Morshima, Y. (2003) Pilot phase I/II study of new salvage therapy (CHASE) for refractory or relapsed malignant lymphoma. International Journal of Hematology, 77, 503–511. [DOI] [PubMed] [Google Scholar]
- Oki, Y. , Ogura, M. , Kato, H. , Kikuchi, Y. , Taji, H. , Kagami, A. , Tsujimura, A. , Yamamoto, K. & Morshima, Y. (2008) Phase II study of a salvage regimen using cyclophosphamide, high‐dose cytarabine, dexamethasone, etoposide, and rituximab in patients with relapsed or refractory B‐cell non‐Hodgkin's lymphoma. Cancer Science, 99, 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, T.J. , Khatcheressian, J. , Lyman, G.H. , Ozer, H. , Armitage, J.O. , Balducci, L. , Bennett, C.L. , Cantor, S.B. , Crawford, J. , Cross, S.J. , Demetri, G. , Desch, C.E. , Pizzo, P.A. , Schiffer, C.A. , Schwartzberg, L. , Somerfield, M.R. , Somlo, G. , Wade, J.C. , Wade, J.L. , Winn, R.J. , Wozniak, A.J. & Wolff, A.C. (2006) 2006 update of recommendations for the use of white blood cell growth factors: an evidence‐based clinical practice guideline. Journal of Clinical Oncology, 24, 3187–3205. [DOI] [PubMed] [Google Scholar]
- Vose, J.M. , Crump, M. , Lazarus, H. , Emmanouilides, C. , Schenkein, D. , Moore, J. , Frankel, S. , Flinn, I. , Lovelace, W. , Hackett, J. & Liang, B.C. (2003) Randomized, multicenter, open‐label study of pegfilgrastim compared with daily filgrastim after chemotherapy for lymphoma. Journal of Clinical Oncology, 21, 514–519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Study endpoints (FAS).


