ABSTRACT
The hippocampus plays critical roles in both object‐based event memory and spatial navigation, but it is largely unknown whether the left and right hippocampi play functionally equivalent roles in these cognitive domains. To examine the hemispheric symmetry of human hippocampal functions, we used an fMRI scanner to measure BOLD activity while subjects performed tasks requiring both object‐based event memory and spatial navigation in a virtual environment. Specifically, the subjects were required to form object‐place paired associate memory after visiting four buildings containing discrete objects in a virtual plus maze. The four buildings were visually identical, and the subjects used distal visual cues (i.e., scenes) to differentiate the buildings. During testing, the subjects were required to identify one of the buildings when cued with a previously associated object, and when shifted to a random place, the subject was expected to navigate to the previously chosen building. We observed that the BOLD activity foci changed from the left hippocampus to the right hippocampus as task demand changed from identifying a previously seen object (object‐cueing period) to searching for its paired‐associate place (object‐cued place recognition period). Furthermore, the efficient retrieval of object‐place paired associate memory (object‐cued place recognition period) was correlated with the BOLD response of the left hippocampus, whereas the efficient retrieval of relatively pure spatial memory (spatial memory period) was correlated with the right hippocampal BOLD response. These findings suggest that the left and right hippocampi in humans might process qualitatively different information for remembering episodic events in space. © 2016 The Authors Hippocampus Published by Wiley Periodicals, Inc.
Keywords: object‐place paired association, hemispheric lateralization, virtual reality, spatial memory, retrieval efficiency
INTRODUCTION
Whether the region‐specific functions are mirrored between the two hemispheres remains as a fundamental question in systems neuroscience (Ehret, 2006). The lateralized effects of memory in humans have long been reported in previous studies, and many studies have suggested the dominance of the right hippocampus in large‐scale, allocentric spatial navigation (Maguire et al., 1998; Spiers et al., 2001a; Burgess et al., 2002; Iaria et al., 2003; Igloi et al., 2010). For example, the activity in the right hippocampus was observed during a spatial navigation task, correlated with navigational accuracy (Maguire et al., 1998). In another study, patients with right temporal lobectomy showed significant impairment in a spatial navigation task (Spiers et al., 2001a). The right hippocampus was also recruited in subjects who used an allocentric strategy in a virtual eight‐arm maze (Iaria et al., 2003) and in a star maze (Iaria et al., 2010). In animal studies, inactivation of the right hippocampus resulted in deficits in spatial memory retrieval in a Morris water maze task in rats (Klur et al., 2009). Also in a study using “split‐brain” mice, the right hippocampus was required for better performance during spatial memory retrieval (Shinohara et al., 2012). Lesion of the right hippocampus also impaired spatial memory retrieval in homing pigeons (Kahn and Bingman, 2004).
Despite the evidence demonstrating strong right hemispheric bias for spatial memory in the hippocampus (Maguire et al., 1998; Spiers et al., 2001a; Burgess et al., 2002; Iaria et al., 2003; Igloi et al., 2010), it remains unknown which cognitive processes require the left hippocampus. Previous studies have suggested that a verbal strategy might be essential for recruiting the left hippocampus (Milner, 1971; Frisk and Milner, 1990; Wagner et al., 1998). However, verbal hemispheric bias was applicable to the entire left hemisphere in general and has seldom been reported as a specific function of the left hippocampus per se (Grasby et al., 1993; Shallice et al., 1994; Buckner et al., 1995; Stern et al., 1996). More importantly, other tasks that seemingly require nonverbal strategies have been reported to successfully recruit the left hippocampus compared to the right hippocampus. Specifically, various cognitive processes (other than spatial navigational memory) require the left hippocampus more dominantly than the right hippocampus, including temporal sequence memory (Schendan et al., 2003; Lehn et al., 2009), match‐mismatch associative memory (Kumaran and Maguire, 2007), egocentric sequence memory (Igloi et al., 2010), and autobiographical event memory (Stern et al., 1996; Maguire and Mummery, 1999; Maguire et al., 2000; Burgess et al., 2001, 2002; Spiers et al., 2001a; Spiers et al., 2001b; Maguire and Frith, 2003).
One of the common aspects of the above studies is that the left hippocampal function is sought mostly in nonspatial memory domain as opposed to the right hippocampal dominance in spatial memory. On a related note, a recent theory for explaining the formation of episodic memory posits two independent information processing streams in the medial temporal lobe for processing spatial memory and nonspatial memory (Knierim et al., 2006). According to the theory, the neocortical regions in the medial temporal lobe (e.g., perirhinal cortex, parahippocampal cortex, medial entorhinal cortex, lateral entorhinal cortex) residing upstream of the hippocampus process spatial memory (e.g., place) and nonspatial memory (e.g., object) independently and these qualitatively different memories are combined in the hippocampus to form an episodic memory. However, this theory has been largely driven by rodent experimental data (Fyhn et al., 2004; Hargreaves et al., 2005; Leutgeb et al., 2005; Deshmukh et al., 2012, Ahn and Lee, 2015) and assumes no functional hemispheric differences. Motivated by the strong emphasis on the right hippocampal dominance in spatial memory domain in humans in the literature and by the relative lack of agreement on the cognitive function of the left hippocampus, we investigated a possibility of lateralized functions of object memory and spatial memory in the human hippocampus in the current study.
In prior studies, the inconsistencies were found with respect to the conditions that functionally recruit the left hippocampus versus the right hippocampus even when seemingly hippocampal‐dependent tasks were used. For example, in a study requiring subjects to remember a lifelike episodic event (composed of object, person, and place) in a virtual reality (VR) town, the hippocampus was mildly recruited because the left hippocampus was only active when the retrieval condition for object‐place paired associate memory was contrasted with the perceptual judgment (i.e., width) condition for objects (Burgess et al., 2001). The left hippocampus was not active in any other contrasting conditions (e.g., place‐person, person‐object, etc.). Furthermore, although place was an important factor in the study, the right hippocampus was not active in any of the conditions. By contrast, another study using a VR task for retrieving object‐cued place memory reported activation in the right hippocampus, but not in the left hippocampus (Doeller et al., 2008). Hartley et al. have also employed an object‐cued (i.e., word sign) spatial memory retrieval task and found that the BOLD activity in the left hippocampus was higher in subjects with better wayfinding abilities (Hartley et al., 2003), whereas the BOLD activity in the right hippocampus reflected trial‐by‐trial wayfinding accuracy within subjects.
The above inconsistencies among prior studies might be attributable to differences in task demands. In the Doeller et al. study (2008), for example, the cueing object was presented for only 2 s and this might have been too short to see the object‐related mnemonic process in the hippocampus (as compared to over 8 s of mean duration of spatial exploration time in the VR arena). In the Hartley et al. study (2003), the cognitive boundary between the cueing phase (with a word sign) and the retrieval phase of spatial memory was ambiguous because the word cue was always present in the lower corner of the screen during the place search. It is also unclear whether the word sign served as a visual object stimulus or a semantic stimulus (or both). Furthermore, the fact that the activity in the left hippocampus was correlated with between‐subject task difference (wayfinding versus trail‐following), but not within‐subject trial accuracy in the wayfinding task, suggests that good and bad performers might have used different strategies.
To investigate the contributions of nonspatial and spatial memory to the functional lateralization in the hippocampus, we designed a VR task in which discrete mnemonic periods were present in a given trial for remembering a nonspatial item (object cue) with its paired‐associate place and for spatial navigation. To make the task more hippocampal dependent, object‐cued place recognition was required in‐between the nonspatial memory and spatial navigation phases in our task. If the left hippocampus is more important in object‐place paired associate memory as in the Burgess et al. study (2001), the left hippocampus should be more active in the object‐cueing period in our task, compared to the relatively pure spatial navigation period. If the right hippocampal dominance is a universal phenomenon in spatial tasks, we should also observe a more dominant involvement of the right hippocampus than the left hippocampus in the spatial navigation phase compared to the object‐cueing period. We had no a priori expectation of the functional hemispheric bias in the object‐cued place recognition period largely because of the inconsistencies observed in the literature. Our results showed that the BOLD response was related with the right hippocampal activity overall, but the efficiency in performance was related with the left hippocampal activity.
MATERIALS AND METHODS
Subjects
Twenty‐two right‐handed subjects (7 females and 15 males) with normal or corrected‐to‐normal vision participated in the experiment. The subjects were undergraduate and graduate students from different universities in Seoul. Six subjects were removed from the second session, reflecting claustrophobia (n = 2), excessive head movement (head movements > 6 mm, n = 2) inside the scanner, or low performance on the first session (below 70% correct performance in a novelty detection task for objects, n = 2). The remaining sixteen subjects (5 females and 11 males) averaged 22.1 years of age, ranging from 19 to 25 years old. Prior to participation, all subjects provided written informed consents and all protocols were approved by the Institutional Review Board of the Seoul National University.
Stimuli
The VR environment was built using commercial software (Unreal Development Kit, Epic Games, Cary, NC) and was implemented on a high‐performance gaming laptop computer. The VR environment was a circular environment (∼400‐m radius space when translated to real space) with a virtual plus maze (40 × 40 m2 when translated to real dimensions) located in the center, elevated ∼1.5‐m above the ground level. Transparent walls along the boundaries of the plus maze prevented subjects from exploring outside the maze boundaries. Distinct natural landmarks (green hill, farm, lake and a mountain; Fig. 1A) surrounding the maze were used as distal cues during the tasks. Four identical buildings were located at the ends of the four arms of the maze (Figs. 1A,B). Each building contained 20 objects (5 objects per corner in each building; thus, a total of 80 objects were viewed within the four buildings; Fig. 1C). On the first day, half of 80 objects (10 objects from each building) were used in a novelty detection task with additional 40 new objects that had not been seen in four buildings. The other half of 80 objects were used as object cues on the second day. In control trials, a pear‐shaped object in a blue‐white checker pattern, unseen during the study phase, was consistently used as an object cue.
Figure 1.
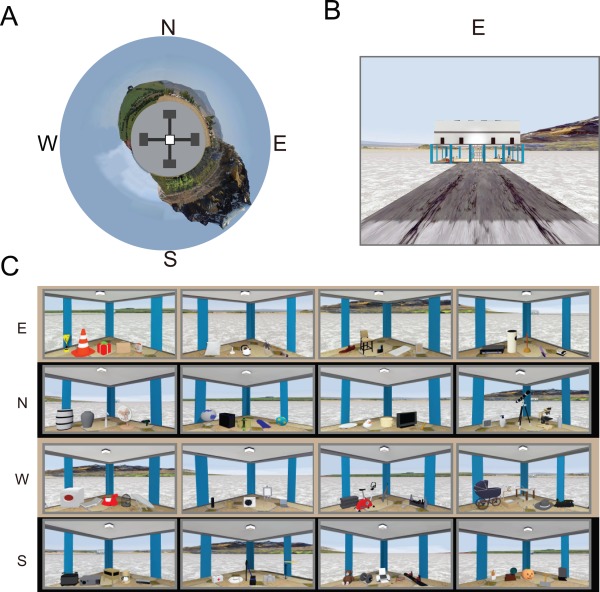
VR Environment. (A) Bird's eye view of the VR environment. N: North, E: East, S: South, W: West. (B) Front view of the east building. (C) Objects in the four corners inside each building. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Experimental Procedures
The experiment comprised two sessions for two consecutive days with a 24‐hr interval between the sessions. Prior to the first session, the subjects practiced navigation in the VR environment using a button box for 5–10 min, depending on whether the subject could navigate smoothly along a circular path in a practice environment. In the first session, performed outside the scanner, the subjects were instructed to explore the environment when visiting each building twice in the order of his or her preference, but to avoid visiting the same building consecutively. Once entering a building, the subjects were instructed to visually sample a set of five objects located in each corner in a pseudorandomized order. The experimenter predetermined the sampling order and signaled the visiting sequence of the corners by turning on and off the green ceiling light in each corner (Fig. 1C). Ten seconds after the subject approached the corner and visually sampled the objects, the green light was turned off, and the subject turned around to look for the next lit green lamp. After visiting each place twice, the subject performed an object recognition task. In the task, ten objects from each building and forty novel objects were shown in a pseudorandomized order against a grey wall that prevented the subjects from viewing the surrounding environment. When presented with an object, the subject indicated whether the object was a novel or old one by a binary button response. Only those individuals who performed above 70% correct participated in the second session on the next day. In the second session conducted inside the scanner, the subject performed an object‐place paired associate memory task followed by a simple navigation task (http://inahlee.org/fmri-vr-experiment-sample.html, Fig. 2A). When the second session began, the subjects visited each building to study object‐place paired associations as in the previous session. Once the subject visited all the buildings, the subject received eighty trials as a memory test, alternating between experimental and control trials. The experimental trial was divided into three event periods (Fig. 2A). In the first period (object‐cueing period), the subject passively viewed an object in the center of the maze and was required to press a button when he/she recognized the object. We did not use a traditional novelty detection task or object recognition task to prevent uncontrolled heterogeneous cognitive processes (e.g., novelty detection, familiarity judgment, and cued recall, etc.) from complicating the interpretation of the functional imaging data when contrasted to the experimental trials. The emphasis on object‐based memory in the object‐cueing period was mainly to discourage the hippocampal involvement during control trials (Astur et al., 2002; Morris et al., 1982).
Figure 2.
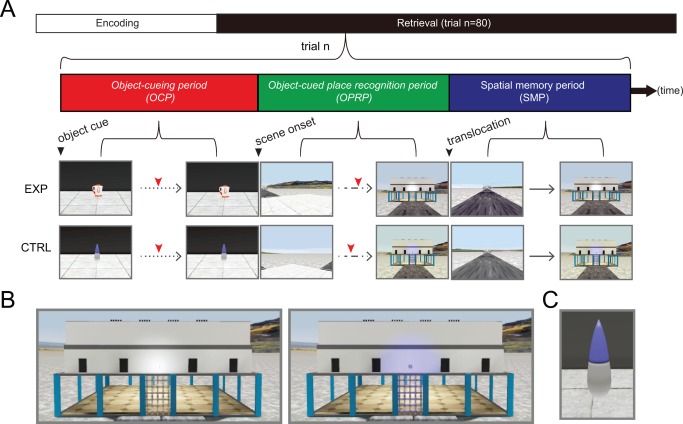
Experimental design. (A) After the encoding period, fMRI scanning was conducted for testing memory retrieval. The structure of a single trial is shown (trial n). Red tick marks denote button responses for recognition. EXP and CTRL denote experimental and control conditions, respectively. Dotted, dashed, and continuous lines denote three types of movement allowed for each period: passive viewing, rotation at a fixed position, and free navigation, respectively. (B) Close‐up views of the building in experimental (left) and control (right) conditions. Note that the light above the entrance door was lit only in the control condition. (C) The control object used throughout all control trials. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In this object‐cueing period, the background landscape was occluded from the subject's view by high walls. At 11.2 s (4 TRs) after the object onset, the cueing object disappeared and the gray walls were removed to expose the subject to the surrounding environment. At the beginning of the second event period (11.2 s, object‐cued place recognition period), subjects were still located in the center of the maze, but facing a pseudorandomly chosen angle. The subject was required to rotate (at a fixed angular speed of 45°/s) until recognizing the building associated with the cued object. As soon as the subject recognized the building, the subject pressed a button.
After the object‐cued place recognition period, both the viewing angle and the position of the subject were suddenly changed, resulting in a pseudorandom shift of the subject's location and viewpoint to one of the entrances of four arms, facing the building associated with the newly chosen arm. Subsequently, the subject was required to navigate (both rotational and translational movements allowed) to the arm previously selected during the object‐cued place recognition period, irrespective of whether the arm previously chosen was correct or incorrect in association with the cueing object. This third event period was called a spatial memory period. Once the subject entered the chosen arm and reached the half point of the arm, further movement was blocked by a transparent wall and the subject pressed a button to end the trial (average duration of 11.1 s). When the button was pressed, regardless of whether the subject entered the correct or an incorrect arm, the subject was relocated to the center of the maze and spent an intertrial interval period ranging from 2.8 to 5.6 s (1–2 TRs) inside the black box before the next trial began. Control trials were identical to the experimental trials described above, except that the target building was explicitly marked by a light cue above the building entrance (Fig. 2B), and the same object was presented in all trials during the object‐cueing period (Fig. 2C). The start of each period was synchronized with an fMRI trigger signal in each trial. For all three periods, the timestamps associated with button presses were used to measure trial latency, which was subsequently used in regression analysis. A post‐scan interview was conducted to record the strategy used by each subject.
Behavioral Analysis
The behavioral analysis was conducted on the log file produced by UDK, which included locations, viewing perspectives, and timestamps for task‐relevant events. Matlab (MathWorks, Waltham, MA) and JMP10 (SAS, Cary, NC) were used to analyze the response accuracy and reaction time for each period. The binomial threshold (n = 40, α = 0.001) was calculated to determine whether each subject performed significantly higher than chance. Additionally, the cumulative amount of angular rotation in the object‐cued place recognition period and the spatial memory period was measured. To determine how effectively the subject identified the target building, we calculated the efficiency index, dividing the standard angular distance (i.e., the shortest distance between starting angle and the angle of the target building) by the cumulative angular distance. Individual efficiency was determined after dividing the average efficiency index for experimental trials by that for control trials. This normalization removed potentially confounding motor skill‐related factors in search behavior.
There are several advantages in using the cumulative angular rotation over the final angle of view as accuracy measure. Most of all, since most subjects chose one of the four buildings, the final angles of choices were non‐linearly distributed around discrete angles (0°, 90°, 180°), whereas the cumulative angular rotation and the efficiency index provided more continuous distribution of angles. Such distribution was well suited as a regressor for the GLM analysis for analyzing parametric modulation. Furthermore, cumulative angular rotation allowed us to dissociate trials in which the subjects immediately chose a building (presumably based on object‐cued recall) from trials in which the subjects had to turn around multiple times to choose a building (presumably based on place recognition). In a sense, the duration of stay at particular angles incorporated to the cumulative index reflected the confidence of the subject when making choices. For these reasons, we prefer to use the cumulative rotational angle as an index instead of the final angle of choice. Unless otherwise stated, all of the correlations were calculated using Pearson's rho.
Acquisition of Functional MRI (fMRI) Data
Scanning was performed using a 3T Siemens Tim trio MRI system with a 32‐channel whole‐head coil. Functional images were acquired using a gradient echo EPI sequence [field of view (FOV) =180 mm, image matrix = 72 × 72, repetition time (TR) = 2.8 s, time to echo (TE) = 33 ms, 36 interleaved slices parallel to the hippocampal axis, 2.5 × 2.5 × 2.5 mm3 resolution], and T1‐weighted in‐plane images in identical slice prescriptions (FOV = 180 mm, image matrix =192 × 192, TR = 1.2 s, TE = 2.85 ms, 0.9 × 0.9 × 2.5 mm3 resolution) were acquired for image registration. Once the session was completed, high‐resolution T1‐weighted structural images (MPRAGE, FOV = 256 mm, image matrix =256 × 256, TR = 1.9 s, TE = 2.36 ms, 1 × 1 × 1 mm3 resolution) were acquired. The VR stimuli were projected onto a screen using an LCD projector (Canon XEED SX60), and the subjects viewed the screen through mirrors on goggles. All responses were collected using an MRI‐compatible button box with four buttons. To ensure the safety of MRI data storage, each run typically ended after the 9th trial, depending on the response time for the spatial memory period. For each subject, twelve runs ranging from 12 to 14 runs were acquired on average.
Preprocessing of fMRI Data
The fMRI data were preprocessed using SPM8 toolbox (http://www.fil.ion.ucl.ac.uk/spm). First, the functional images were corrected for different slice timing in each frame, and subsequently, the images were realigned to the first frame to correct for head motion. After realignment, the functional images were co‐registered to structural images and normalized to the MNI template with the same parameters used to normalize the structural images. The normalized functional images were spatially smoothed using a 6‐mm full‐width‐at‐half‐maximum isotropic Gaussian kernel. To remove global fluctuations resulting from unknown sources in BOLD signals, the across‐voxel average time course was subtracted from the raw responses of each voxel (Fox et al., 2006; Pestilli et al., 2011; Cardoso et al., 2012; Choe et al., 2014). In addition, voxels with a response variance exceeding the smallest 90th percentile range of the entire pool of voxels in the region were excluded from analysis to remove blood vessel‐clamping effects (Olman et al., 2007; Shmuel et al., 2007).
For anatomical data, the images were processed using FREESURFER software (http://surfer.nmr.mgh.harvard.edu, ver. 5.1.0) to generate anatomical ROIs, including the hippocampus. After automatic segmentation using FREESURFER (Fischl et al., 2002; Desikan et al., 2006), the hippocampal volume was manually edited according to previously established protocols (Pruessner et al., 2000).
Statistical Analysis of the fMRI Data
Individual statistical tests were conducted using the general linear model (GLM) procedures included in SPM8 toolbox (http://www.fil.ion.ucl.ac.uk/spm), which was implemented in Matlab. To determine how the activity associated with each voxel changed across different periods and conditions, a 2 × 3 factorial design was used (with two levels of trial type and three levels of event period). Later, in the second‐level analysis, contrast images between experimental and control trial types were used for each period to run a one‐way repeated‐measures ANOVA. For both the experimental and control conditions, GLM regressors were generated after convolving the response latency of each condition with the canonical hemodynamic response function. For each subject, total of 80 trials, regardless of their correctness, were included in GLM analysis. For the object‐cued place recognition period and the spatial memory period, the trial‐by‐trial efficiency index for each event period was used as the first‐order modulatory regressor. In addition, regressors for each run and head movements were included in the GLM analysis. A high‐pass filter with a cut‐off time of 128 s was applied to remove slow drifts in the signal irrelevant to the task. The contrast images between resulting beta coefficients for experimental and control conditions were calculated for each period and subsequently used for the second‐level random effect analysis.
A one‐way within‐subject ANOVA with the period as a factor with three levels (the object‐cueing period, the object‐cued place recognition period, and the spatial memory period) was conducted using the SPM8 toolbox. One‐sample t tests were used to analyze the modulatory regressors derived from the efficiency indices of the object‐cued place recognition period and the spatial memory period. Because the region of interest of the current study was the hippocampus, we used a small volume correction approach with anatomically segmented hippocampal volume applied as a mask. A significance threshold of P < 0.05 (FWE‐corrected for multiple comparisons) for the peak activity was applied for the hippocampus. Brain areas showing significant activities at the threshold of P < 0.001 (uncorrected for multiple comparison) were also reported in Table 2.
Table 2.
Brain Areas Showing Significant Increase in BOLD Activity During Each Event Period Compared to the Control Baseline
| Area | Left | Right | |||||
|---|---|---|---|---|---|---|---|
| t value | MNI x,y,z | N | t value | MNI x,y,z | N | ||
| A. Object‐cueing period. | |||||||
| Superior occipital gyrus | 4.23 | −26 −70 35 | 1 | 5.27 | 20 −64 45 | 43 | |
| Precuneus | 4.77 | −10 −72 42 | 26 | 5.24 | 12 −67 48 | 30 | |
| – | – | – | 5.06 | 12 −72 42 | 4 | ||
| Superior parietal lobule | 5.09 | −23 −70 45 | 34 | 3.75 | 30 −70 50 | 1 | |
| Angular gyrus | – | – | – | 4.44 | 37 −67 45 | 16 | |
| Middle occipital gyrus | 4.01 | −26 −77 38 | 5 | – | – | – | |
| Hippocampus | 3.97 | −28 −27 −15 | 1 | – | – | – | |
| Inferior parietal lobule | 3.97 | −36 −50 48 | 1 | – | – | – | |
| 3.96 | −33 −52 50 | 1 | – | – | – | ||
| Area | Left | Right | ||||
|---|---|---|---|---|---|---|
| t value | MNI x, y, z | N | t value | MNI x, y, z | N | |
| B. Object‐cued place recognition period. | ||||||
| Hippocampus | – | – | – | 5.33 | 32 −14 −22 | 3 |
| Thalamus | 5.21 | −16 −7 0 | 2 | 3.87 | 4 −12 2 | 1 |
| 4.07 | −16 −24 15 | 7 | – | – | – | |
| 3.81 | −6 −12 0 | 1 | – | – | – | |
| Inferior temporal gyrus | – | – | – | 4.80 | 47 −10 −28 | 1 |
| – | – | – | 3.78 | 54 −14 −22 | 1 | |
| Heschl's gyrus | – | – | – | 4.04 | 42 −24 12 | 3 |
| Inferior parietal lobule | – | – | – | 3.88 | 47 −52 48 | 2 |
| Area | Left | Right | |||||
|---|---|---|---|---|---|---|---|
| t value | MNI x, y, z | N | t value | MNI x, y, z | N | ||
| C. Spatial memory period. | |||||||
| Superior temporal gyrus | 7.70 | −63 −34 20 | 182 | 4.27 | 64 −30 20 | 1 | |
| 6.02 | −43 −14 −5 | 74 | 4.17 | 57 −20 10 | 4 | ||
| 4.64 | −43 −34 15 | 11 | – | – | – | ||
| 3.95 | −66 −47 15 | 1 | – | – | – | ||
| 3.77 | −50 3 −2 | 1 | – | – | – | ||
| Middle temporal gyrus | 5.63 | −56 −17 −20 | 14 | 7.21 | 64 −17 −22 | 23 | |
| 5.28 | −63 −14 −20 | 12 | 4.46 | 67 −32 0 | 10 | ||
| 4.77 | −56 −22 −15 | 4 | 4.29 | 60 −40 −2 | 10 | ||
| 4.01 | −50 −60 22 | 3 | 4.19 | 54 0 −30 | 2 | ||
| 3.86 | −68 −30 −2 | 3 | 4.06 | 52 −30 −5 | 8 | ||
| 3.86 | −66 −42 2 | 2 | – | – | – | ||
| 3.85 | −53 −52 22 | 1 | – | – | – | ||
| Lingual gyrus | 4.65 | −8 −70 −2 | 25 | 6.46 | 12 −70 −5 | 24 | |
| – | – | – | 4.24 | 20 −52 −10 | 5 | ||
| – | – | – | 3.98 | 10 −67 0 | 3 | ||
| Angular gyrus | – | – | – | 6.11 | 62 −50 35 | 188 | |
| Cuneus | 5.38 | −3 −80 18 | 38 | – | – | – | |
| 4.81 | 2 −77 18 | 21 | – | – | – | ||
| Insula | – | – | – | 4.94 | 42 −10 −5 | 34 | |
| – | – | – | 4.44 | 34 −22 5 | 19 | ||
| Supramarginal gyrus | – | – | – | 4.75 | 62 −24 30 | 12 | |
| Hippocampus | – | – | – | 4.68 | 32 −20 −18 | 4 | |
| 4.27 | 20 −12 −18 | 2 | |||||
| Orbitofrontal gyrus | – | – | – | 4.44 | 30 18 −22 | 6 | |
| Rolandic Operculum | – | – | – | 4.43 | 52 −22 15 | 6 | |
| – | – | – | 3.96 | 44 −27 18 | 2 | ||
| Parahippocampal gyrus | – | – | – | 4.32 | 30 −27 −15 | 1 | |
| Inferior temporal gyrus | 4.20 | −48 −10 −30 | 1 | 4.14 | 57 −14 −22 | 3 | |
| Superior occipital gyrus | 3.98 | −10 −92 20 | 4 | – | – | – | |
A–C Brain areas showing significant activity during the object‐cueing period, object‐cued place recognition period and the spatial memory period (P < 0.001, uncorrected, one‐sample t test, df = 15). For each region, t value, MNI coordinates of the peak location and the number of significant voxels in the cluster (denoted as N) are given separately for the left and right hemispheres.
Notably, the experimental and control trials for each task shared similar navigational demands, motor responses, speeds of translational/rotational movement, and optic flow. That is, only the task demands were different between the experimental and control conditions; therefore, the BOLD responses from the control trials were used as a baseline for comparison with the BOLD responses from the experimental trials.
In a separate analytical stream using manual scripting in Matlab, we analyzed the responses of the entire hippocampal voxels across time for each subject. Using a common template (based on the MNI template), BOLD responses in voxels included in the hippocampal template were extracted across time. After removing slow drift in the signal using a Butterworth filter (N = 4 and FL = 2.8), the time series in each run was converted to %BOLD signal. Using %BOLD signal, we calculated the normalized difference between the mean responses across time for each individual in the experimental and control trials of all the hippocampal voxels. This line of analysis was intended to check whether hippocampal BOLD activities showed sustained response that might have been undetected by the GLM‐based analysis (Henson, 2003). Using the normalized difference between the experimental and control trials, we calculated the hemispheric bias for each event period after subtracting the average normalized differences across the left hippocampal voxels from that of the right hippocampal voxels, followed by a repeated‐measures ANOVA to confirm whether the assumptions of the GLM affected hemispheric specialization. To examine whether differences in difficulty between object‐cued place recognition period and spatial memory period affected lateralization, we divided the trials into two groups based on object‐specific accuracies during the object‐cued place recognition period across participants. Subsequently, the procedures described above were repeated for trials with high and low accuracies. Using the event period, hemisphere and accuracy group as factors, a three‐way repeated‐measures ANOVA was conducted. In addition, same analysis approach was conducted separately for male and female groups to examine the effect of gender on hemispheric bias.
RESULTS
Successful Behavioral Performance in the VR Task
In the VR task, most subjects successfully located the target building when cued by an object during the object‐cued place recognition period (P < 0.001, Rayleigh's test; Fig. 3A). Average and standard deviation of individual accuracies and latencies for three periods are listed in Table 1. Although some individual differences were observed during the retrieval period in terms of accuracy for retrieving object‐place paired associate memory, the performance of most subjects exceeded chance level (25%) in the object‐cued place recognition period (t (15) = 4.44, P < 0.001, one‐sample t test; Fig. 3B). During the spatial memory period, all subjects successfully navigated to the target building when shifted to a random position in the central platform of the maze (t (15) = 26.25, P < 0.001, one‐sample t test; Fig. 3C). Performance was more variable during the object‐cued place recognition period than during the spatial memory period (Fig. 3B, C), and this may reflect difference in task difficulty between the two event periods. Higher performance levels under the control conditions compared to the experimental conditions demonstrate that the sensory‐motor skills were normal in all subjects (Fig. 3B,C). When the performance levels for individual objects were analyzed for the object‐cued place recognition period, object cues with seemingly distinct colors and shapes (e.g., color ball, gift box, or exercise bicycle) tended to result in more accurate target place searches, while objects with gray or black‐and‐white colors with simple shapes (e.g., data projector, hat) were associated with low target search accuracies (Fig. 4).
Figure 3.
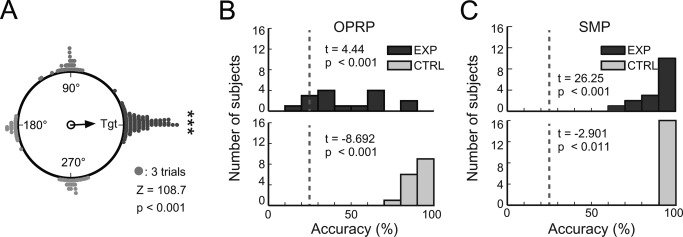
Task performance. (A) Cumulative choices for target buildings (Tgt) in the object‐cued place recognition period (all targets aligned to 0°). The vector arrow indicates the strength of the target response. (B–C) Distributions of response accuracies in the object‐cued place recognition period (OPRP) (B) and the spatial memory period (SMP) (C) for individual subjects. Dashed lines denote chance level (25%). For each period, results of one‐sample t tests for comparing average retrieval accuracy during the experimental condition (top) and paired t test for comparing average retrieval accuracies between experimental and control conditions (bottom) are shown.
Table 1.
Performance‐related Descriptive Statistics for Each Period
| OCP | OPRP | SMP | |
|---|---|---|---|
| EXP accuracy | – | 50% (22.1%) | 91% (10%) |
| CTRL accuracy | – | 92% (6%) | 98% (2%) |
| EXP latency | 4.2s (1.6 s) | 5.4s (0.8 s) | 11.1s (2.2 s) |
| CTRL latency | 4.2s (1.6 s) | 5.0s (0.6 s) | 11.2s (2.1 s) |
Mean accuracy and latency for each period are shown. Numbers inside parentheses denote standard deviation for each condition. OCP, OPRP, and SMP denote object‐cueing period, object‐cued place recognition period, and spatial memory period, respectively. EXP and CTRL denote experimental and control conditions, respectively.
Figure 4.
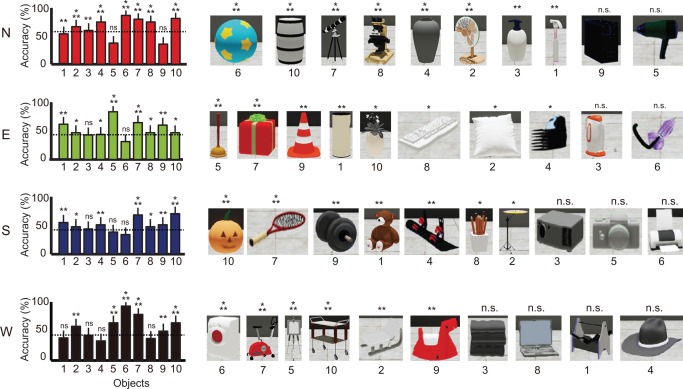
Choice accuracy for individual buildings and objects. Average choice accuracy for all objects associated with the four buildings in the experimental condition in the object‐cued place recognition period. Dotted lines indicate the mean accuracy for the corresponding building. Objects are shown in a descending order based on choice accuracy. Mean ± S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001, n.s. P > 0.05. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Mean accuracy measures for selecting object‐associated target buildings were not significantly different (F (3, 63) = 2.75, P = 0.05, repeated‐measures ANOVA), suggesting that the four buildings were well matched with respect to task difficulty during the object‐cued place recognition period. When the search efficiency was examined (for details, see Materials and Methods) in the object‐cued place recognition period, some subjects were more efficient than others in locating the object‐associated building (Fig. 5). For example, the search trajectories of subjects 8 and 10 showed organized patterns of rotation with minimal directional confusion during the rotational search in both experimental and control trials, whereas those of subjects 5 and 13 were less organized, resulting in more direction changes with longer latencies.
Figure 5.
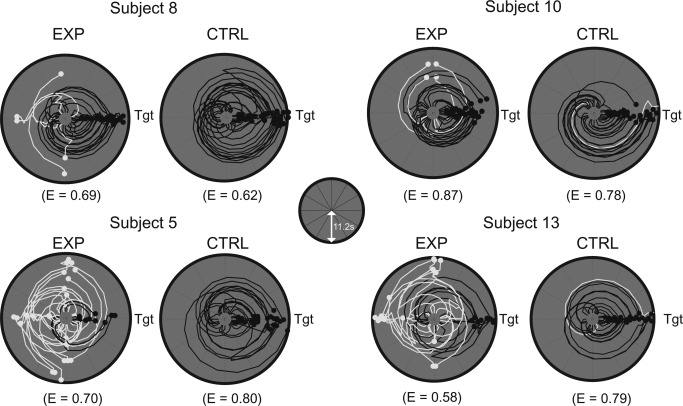
Individual difference in search efficiency. Examples of angular trajectory of four subjects with efficiency indices (E) during the object‐cued place recognition period. For each subject, trajectory data were aligned so that the correct target (denoted as Tgt) was located on the east, separately shown for the experimental and control conditions. Distance from the center of the plot represents the duration of time (maximum of 11.2 s, as depicted in inset) and the azimuth of the point depicts the viewing angle of the subject at that particular time. Black and white lines denote trajectories for correct and incorrect trials, respectively.
BOLD Signal Change during Memory‐based versus Visually Guided Retrieval
The brain areas that showed a significant increase in BOLD activity in the experimental trials, compared with control trials, are listed in Table 2. When the subject viewed only an object cue and indicated object recognition using a button press during the object‐cueing period, the left hippocampus showed increased BOLD activity during experimental trials compared to control trials (P < 0.05, FWE‐corrected, one‐sample t test; Fig. 6A, 6B). Other regions also showed increased BOLD responses, including the superior occipital gyrus, superior parietal lobule, inferior parietal lobule, precuneus, and middle occipital gyrus (P < 0.001, uncorrected for multiple comparisons; Table 2). In the object‐cued place recognition period, the right hippocampus showed a significant increase in BOLD activity (P < 0.05, FWE‐corrected, one‐sample t test; Fig. 6C, 6D) with other regions including the inferior parietal lobule, inferior temporal gyrus and thalamus (P < 0.001, uncorrected for multiple comparisons; Table 2). In the spatial memory period during which subjects were required to navigate to the place selected in the object‐cued place recognition period, the right hippocampus showed a significant increase in BOLD activity (P < 0.05, FWE‐corrected, one‐sample t test; Fig. 6E, 6F). Other brain regions also showed similar BOLD responses, including the superior temporal gyrus, middle temporal gyrus, lingual gyrus, and cuneus (P < 0.001, uncorrected for multiple comparisons; Table 2).
Figure 6.
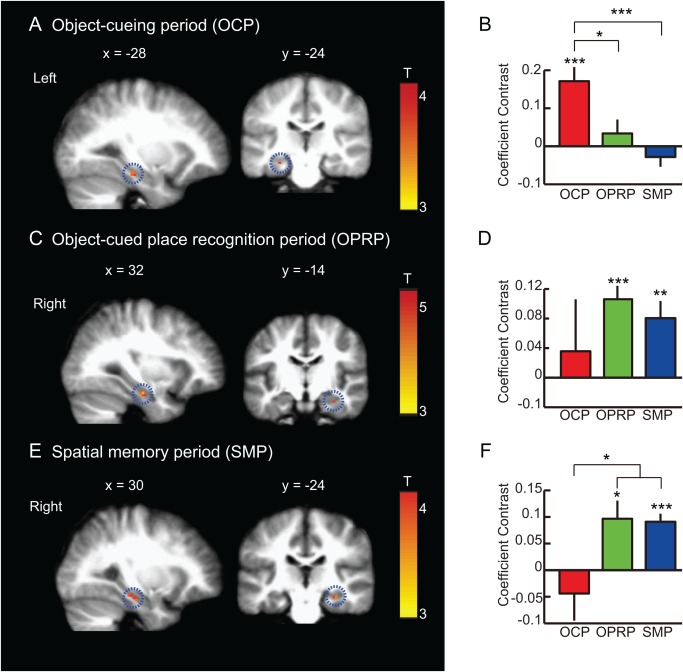
BOLD activity during object‐cueing period, object‐cued place recognition period, and spatial memory period. (A, C, E) A sagittal (left) and a coronal (right) section showing the peak hippocampal activity for each event period (P < 0.05, FWE‐corrected, one‐sample t test). MNI coordinates are shown above the corresponding sections. In each row, the scale bar shows the range of the t‐statistics from GLM analysis for each event period with red color representing higher response. OCP: Object‐cueing period, OPRP: Object‐cued place recognition period, SMP: Spatial memory period. Mean ± S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001. (A) Object‐cueing period (OCP): A subset of voxels in the left hippocampus showing significant activity during the object‐cueing period (highlighted with a dotted circle), compared to the control. (B) Mean coefficient contrasts of the OCP‐responsive voxels in the left hippocampus across three different periods. (C) Object‐cued place recognition period (OPRP): A subset of voxels in the right hippocampus (highlighted with dotted circles) showing significant BOLD activity during the OPRP. (D) Mean coefficient contrasts of the OPRP‐response voxels in the right hippocampus across three different event periods. (E) Spatial memory period (SMP): During the spatial memory period, the right hippocampus (highlighted with a dotted circle) showed significant BOLD activity compared to the control condition. (F) Average coefficient contrasts of the SMP‐responsive voxels in the right hippocampus across three different event periods. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
These results suggest that the left hippocampus was active during object recognition (the object‐cueing period), but that the functional activity focus shifted to the right hippocampus (Fig. 6B, 6D, 6F) (i) as place memory was retrieved in association with object memory (the object‐cued place recognition period) and (ii) when spatial navigation was required (the spatial memory period). In addition, during the object‐cueing period, the areas showing significant BOLD activities included the regions typically associated with the early processing of visual information (e.g., middle occipital gyrus). Areas typically associated with the retrieval of episodic memory, such as the hippocampus and the angular gyrus (Rugg and Vilberg, 2013), were also active during the object‐cueing period. During the object‐cued place recognition period, only a few brain regions, including the right hippocampus, showed significant activity, compared to the other two periods. In the spatial memory period, consistent with prior studies using spatial memory tasks, the right hippocampus was active. In addition, both superior and middle temporal gyri showed a significant increase in BOLD activity.
Contributions of Other Factors to Hemispheric Bias
Other possibilities that might have contributed to the observed hemispheric differences were also examined. Among those, we examined the possibility that the assumptions of regression analysis affected the current findings. For example, a regression‐based analysis might be biased toward selecting voxels showing phasic responses, but might not detect voxels showing sustained responses (Henson, 2003). The hemispheric bias calculated based on normalized activity in the hippocampus using this GLM assumption‐free approach showed results similar to those obtained with the regression analysis (F (2, 47) = 7, P < 0.01, repeated‐measures ANOVA; Fig. 7A).
Figure 7.
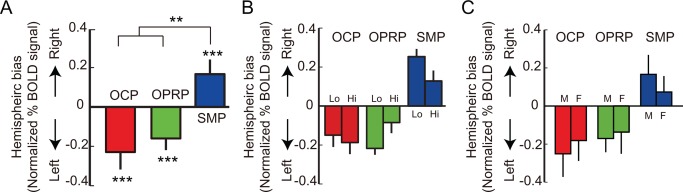
Contributions of object‐associated task difficulty and sex to the hemispheric bias in %BOLD signal change. (A) Hemispheric bias in normalized %BOLD signal change in each event period. (B) Hemispheric bias in normalized %BOLD signal change for each event period, shown separately for the objects with which performance levels were either high (Hi, n = 19) or low (Lo, n = 21). (C) Hemispheric bias in normalized % BOLD signal change for each event period, drawn separately for female (F, n = 5) and male (M, n = 11) subjects. OCP: object‐cueing period, OPRP: object‐cued place recognition period, SMP: spatial memory period. Mean ± S.E.M. **P < 0.01, ***P < 0.001. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Because the performance data indicated that the spatial memory period was easier than the object‐cued place recognition period in the current study in terms of task difficulty (Fig. 3B,C), task difficulty between the object‐cued place recognition period and the spatial memory period might have contributed to the hemispheric differences. The difference in task difficulty may have been caused by the relatively short retention time between the encoding and retrieval of memory in the spatial memory period, compared to the object‐cued place recognition period. Alternatively, in the spatial memory period, the subject was required to simply move to the previously chosen building in the object‐cued place recognition period, irrespective of whether the building was a correct paired associate of the object cue or not. Nonetheless, task difficulty alone could not explain these results because the amount of lateralization observed with the object cues associated with high performance levels in the object‐cued place recognition period was not significantly different from that associated with less difficult object cues although there was a trend (F (2, 47) = 2.86, P = 0.07, repeated‐measures ANOVA; Fig. 7B). Because gender could also have influenced our results (Gron et al., 2000), we examined whether a significant difference was found in hemispheric bias across task periods between male and female subjects. However, we found no significant gender effect (F (2, 47) = 0.06, P = 0.95, repeated‐measures ANOVA; Fig. 7C).
Hemispheric Difference in BOLD Activity Correlated with the Efficient Retrieval of Object‐place Memory and Spatial Memory
We examined whether the BOLD response was significantly correlated with the search efficiency (Fig. 5) of the subject in the event periods involving spatial components (i.e., object‐cued place recognition period and spatial memory period; Fig. 8A, 8B). When comparing the coefficient contrasts of the voxels significantly correlated with search efficiencies in the left and right hippocampi, a significant interaction was found between the hemisphere and the task period (F (1, 15) = 23.8, P < 0.001, repeated‐measures ANOVA; Fig. 8C). The BOLD signals of the voxels in the left hippocampus were significantly modulated by search efficiency in the object‐cued place recognition period (P < 0.05, Bonferroni corrected, post hoc paired t test; Fig. 8A and Table 3), whereas the BOLD responses of the right hippocampal voxels were significantly modulated by search efficiency during the spatial memory period (P < 0.01, Bonferroni corrected, post hoc paired t test; Fig. 8B and Table 3). Although the hippocampus was the only area showing significant correlation between the BOLD response and search efficiency in the left hemisphere (Table 3), in the right hemisphere, the BOLD signals of the voxels in the calcarine sulcus and lingual gyrus were also significantly correlated with search efficiency in addition to the hippocampus (Table 3). These results demonstrate that the left hippocampus was more important than the right hippocampus for efficiently retrieving the object‐associated place memory, whereas the right hippocampus was more important than the left hippocampus for the efficient retrieval of relatively pure form of spatial memory.
Figure 8.
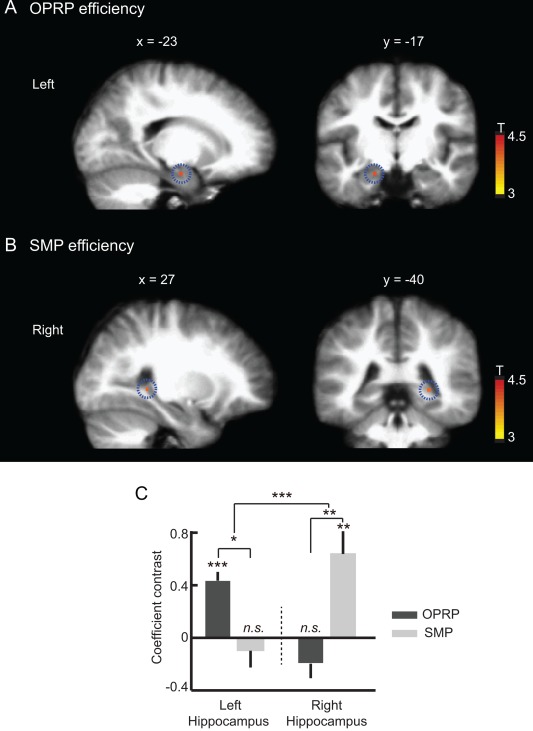
Left and right hippocampal BOLD activity correlated with efficient search for target place during the object‐cued place recognition period (OPRP) and spatial memory period (SMP), respectively. (A,B) Sagittal (left) and coronal (right) hippocampal sections showing the regions significantly modulated by search efficiency during the OPRP and the SMP, two event periods in which spatial memory components were important. Scale bars show the range of the t‐statistics from t test. (A) The BOLD signal in the left hippocampus (highlighted with dotted circles) was significantly modulated by search efficiency during the OPRP, compared to the control baseline (P < 0.001, uncorrected, one‐sample t test). (B) The BOLD response of the right hippocampus (highlighted with dotted circles) was significantly modulated by search efficiency during the SMP, compared to the control baseline (P < 0.001, uncorrected, one‐sample t test). (C) Comparison of the modulatory coefficient contrasts for the left and right hippocampal voxels showed a significant interaction between hemisphere and event period. Mean ± S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001 (Bonferroni corrected, paired t test). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table 3.
Brain Areas Showing Significant Modulation of BOLD Response by Search Efficiency in the Object‐place Recognition Period (OPRP) and Spatial Memory Period (SMP)
| Area | Left | Right | |||||
|---|---|---|---|---|---|---|---|
| t value | MNI x,y,z | N | t value | MNI x,y,z | N | ||
| Hippocampus | 4.57 | −23 −20 −20 | 3 | – | – | – | |
| 4.09 | −18 −12 −20 | 1 | – | – | – | ||
| Area | Left | Right | ||||
|---|---|---|---|---|---|---|
| t value | MNI x,y,z | N | t value | MNI x,y,z | N | |
| B. SMP efficiency. | ||||||
| Calcarine | – | – | – | 4.49 | 12 −90 0 | 5 |
| – | – | – | 4.13 | 17 −84 5 | 1 | |
| Lingual gyrus | – | – | – | 4.32 | 14 −80 −8 | 2 |
| Hippocampus | – | – | – | 3.16 | 27 −40 0 | 1 |
A Brain areas whose BOLD signals were significantly modulated by the search efficiency for target place in the OPRP (P < 0.001, uncorrected, one‐sample t test, d.f. = 15). (B) Brain areas whose BOLD signals were significantly modulated by the search efficiency for target place in the SMP (P < 0.001, uncorrected, one‐sample t test, d.f. = 15).
DISCUSSION
In the current study, we showed how multiple brain areas were differentially recruited as the task demand switched from retrieving object‐place paired associate memory to spatial navigation. The activity foci in the hippocampus shifted from the left hippocampus to the right hippocampus as additional spatial components were required in the task. Outside of the hippocampus, the areas responding to the task shifted from the early visual information processing areas to the higher information processing areas as the task demand changed from object recognition to object‐cued place recognition and subsequently to spatial navigation. Moreover, the left and right hippocampal BOLD activities were modulated by the efficient retrieval of object‐place paired associate memory and spatial memory, respectively. In addition, a comparison of the BOLD response differences in the entire hippocampal voxels between the object‐place paired associate memory and spatial memory revealed that the left hippocampus was important for efficient retrieval of object‐place paired associate memory and the right hippocampus was important for efficient retrieval of spatial memory. Although previous studies used navigation paradigms in VR environments (Burgess et al., 2001, 2002; Brown et al., 2010), the hemispheric dissociation between object‐place paired associate memory and spatial memory remained unclear, and, to our knowledge, our study demonstrates the first within‐subject cross‐hemispheric shift of functional bias in association with a task demand.
What made the left hippocampus more active during the object‐cueing period in our study? One possibility is that the subjects used a verbal strategy (Burgess et al., 2001; Brown et al., 2010) for remembering objects (and possibly their paired‐associate places) because we used common objects. Some studies with verbal components have indeed reported the involvement of the left hippocampus in retrieving remote autobiographical memory (Maguire et al., 1998; Burgess et al., 2001; Spiers et al., 2001a; Spiers et al., 2001b). However, Maguire et al. (1998) observed no correlation between navigational accuracy and the degree of activity in the left hippocampus. Instead, they reported a significant correlation between navigational accuracy and activity in the right hippocampus. Furthermore, the left hemispheric bias typically includes the entire left hemisphere in the literature (Burgess et al., 2001, Spiers et al., 2001), but not specifically localized within the left hippocampus. Also, various tasks, not necessarily requiring verbalization for performance, still successfully activated the left hippocampus more than the right hippocampus (Shallice et al., 1994; Stern et al., 1996; Kumaran and Maguire, 2007; Igloi et al., 2010). For example, Burgess et al. (2002) used a VR town environment that might have involved additional verbal strategies compared to the VR tasks conducted in the present study, as some of the buildings were physically associated with verbal signs, such as “CINEMA” and “BAR.” Moreover, words (e.g., PLACE, OBJECT, PERSON) were used as cues to indicate the episodic task demand in the previous study. Nevertheless, Burgess et al. (2002) showed no clear evidence of direct verbal mediation. It is difficult to unequivocally dispute the involvement of the verbal component and the verbal strategy might have adopted in our study, but the results from the previous studies (Milner, 1971; Frisk and Milner, 1990; Grasby et al., 1993; Buckner et al., 1995) and the current study suggest that there might be other strong functional roles of the left hippocampus in object‐based memory than just verbal information processing.
In regards to gender differences in the hemispheric lateralization, Gron et al. (2000) reported activation in the left hippocampus only for the gender contrast and not for the navigation task itself. However, such gender differences were not found in the current study, with both gender groups showing equal level of hippocampal hemispheric lateralization. Overall, we conclude that gender differences alone may not fully explain the hemispheric bias observed in the current study.
In our task, the BOLD activity in the left hippocampus was significantly elevated when subjects were cued by objects (compared to control conditions), but not when the subjects looked for the target building associated with the object cue and when they tried to reorient to the target building once disoriented (Fig. 6). The BOLD response of the right hippocampus showed opposite patterns. Our efficiency‐related BOLD data may appear to contradict these results because the left hippocampal voxels showed significant efficiency‐related BOLD activity in the object‐cued place recognition period (Fig. 7A). However, we would like to emphasize that the cognitive components involved in these two analyses (i.e., BOLD activity contrast between experimental and control trials in Fig. 6 and BOLD activity related to more “efficient” spatial search in Fig. 7) are not identical, and the results may not be necessarily interpreted as contradicting. One way to reconcile the two results are as follows: Overall, when a spatial component is added (to the object‐cued period) as in the object‐cued place recognition period, the right hippocampal importance significantly increases compared to the preceding object‐cueing period in which relatively pure object‐recognition memory was required. This may be detected as significantly active voxels residing in the right hippocampus, but not in the left hippocampus in the object‐cued place recognition period. The same interpretation may be applied to the right hemispheric correlates in the spatial memory period. However, unlike the spatial memory period, object memory (which appears to depend more on the left hippocampus) may still play critical roles during object‐associated place search in the object‐cued place recognition period and, importantly, the level of involvement of object memory during this period might determine how efficiently a subject finds the target building. For example, the significant BOLD response correlated with “efficiency” of search in the left hippocampus may be related to the fact that the efficient place searchers in our task might try to actively “recall” the target place based on strong object memory, whereas poor searchers might just try to “recognize” a scene while rotating in the environment. Such assumption is further supported by the fact that ten out of sixteen subjects indicated that they recalled the associated building immediately after an object cue was presented. This type of dissociation between left and right hippocampal responses can also be found in the literature. For example, in the Hartley et al. (2003) study, a subject was required to find a place (in a VR environment) associated with a cueing word displayed at the bottom of the screen, which can be thought of as an overtly verbal version of object cue in our study. In that study, subjects with good wayfinding performance showed a significant involvement of the left hippocampus, but not with the right hippocampus, when compared against a trail‐following control task. However, the within‐subject trial‐by‐trial wayfinding performance was related more with the right hippocampal activity than with the left hippocampal activity in that study. Although the design and task demands of the Hartley et al. study were not identical with our task (e.g., object cue disappearing in our task versus the word cue remaining throughout a trial in the Hartley et al. study, etc.), the results nonetheless point to the possibility of observing functional dissociation between the left and right hippocampi when analyzing brain activity in an event period irrespective of efficiency versus focusing more on the efficiency of performance in that period.
Although we showed that the amount of lateralization associated with higher performance was not significantly different from that associated with less difficult object‐place paired association in the object‐cued place recognition period, we could not completely rule out the possibility that the level of task difficulty contributed to the hemispheric difference between the object‐cued place recognition period and the spatial memory period. For instance, the performance data clearly indicate that subjects showed higher accuracy in the spatial memory period than in the object‐cued place recognition period. Moreover, there were only four places (i.e., buildings) to recognize in the spatial memory period, but there were eighty different objects to remember in association with the four buildings in the object‐cued place recognition period. Therefore, memory load and mnemonic interference were presumably higher in the object‐cued place recognition period than in the spatial memory period. Associating more places with the objects in the task might have reduced the differences in the task difficulties between the object‐cued place recognition period and the spatial memory period, but we avoided this design because it might overly complicate the task. In addition, during the spatial memory period, the subject had to navigate toward the target immediately after being disoriented, whereas in the object‐cued place recognition period, there was a 2‐ to 40‐min delay period prior to searching for the target building associated with the object. Therefore, a delay‐dependent mismatch might have influenced the task difficulty between the object‐cued place recognition period and the spatial memory period. However, if task difficulty is the dominant underlying factor for the left‐to‐right hemispheric shift of activity loci in the hippocampus, then it would be more reasonable to expect the initial activation of a higher network volume in the hippocampus when the task was more difficult (i.e., the object‐cued place recognition period). Then, the proportion of active hippocampus would decrease as the task became easier (i.e., the spatial memory period). For example, both left and right hippocampi would be active during the object‐cued place recognition period, whereas only either the left or the right hippocampus would be more active during the spatial memory period, which was not the case in the present study.
Along the anteroposterior axis in the hippocampus, the posterior hippocampus was modulated according to the efficiency of spatial navigation. Similar posterior dominance in spatial memory has been reported in previous human (Maguire et al., 2000; Iaria et al., 2003) and animal studies (Morris et al., 1982; Moser et al., 1993). The results might reflect the fact that the human posterior hippocampus is anatomically equivalent to the dorsal hippocampus in rodents. In rodents, neurons in the dorsal hippocampus show more spatial firing than those in the ventral hippocampus (Kjelstrup et al., 2008), presumably because the dorsal hippocampus is connected strongly to the dorsolateral MEC in which grid cells were detected (Amaral and Witter, 1989; Strange et al., 2014).
The hippocampus has long been hypothesized to process different types of information, regarding items and events episodically encountered in an environment and information about spatial positions in the environment (Milner, 1971). How these two types of representations are merged to render autobiographical memory experience remains largely unknown and is an active area of investigation (O'Keefe, 1976; Wagner et al., 1998; Davachi, 2006; Eichenbaum and Lipton, 2008; Brown et al., 2010). Previous studies and the results of the present study suggest that, at least in humans, the left and right hippocampi might perform fundamentally different computations as episodic memory is interweaved with spatial memory. Understanding the neurobiological mechanisms underlying the hemispheric asymmetry in synaptic plasticity (Kawakami et al., 2003; Knierim et al., 2006; Henriksen et al., 2010) might provide deeper insights into how the hippocampus processes information in mammals.
Acknowledgments
The authors thank James Knierim and Randolph Blake for helpful comments and discussions on earlier versions of the manuscript. The authors declare no competing financial interests.
REFERENCES
- Ahn JR, Lee I. 2015. Neural correlates of object‐associated choice behavior in the perirhinal cortex of rats. J Neurosci 35:1692–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Witter MP. 1989. The three‐dimensional organization of the hippocampal formation: A review of anatomical data. Neuroscience 31:571–591. [DOI] [PubMed] [Google Scholar]
- Astur RS, Taylor LB, Mamelak AN, Philpott L, Sutherland RJ. 2002. Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behav Brain Res 132:77–84. [DOI] [PubMed] [Google Scholar]
- Brown TI, Ross RS, Keller JB, Hasselmo ME, Stern CE. 2010. Which way was I going? Contextual retrieval supports the disambiguation of well learned overlapping navigational routes. J Neurosci 30:7414–7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Petersen SE, Ojemann JG, Miezin FM, Squire LR, Raichle ME. 1995. Functional anatomical studies of explicit and implicit memory retrieval tasks. J Neurosci 15:12–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, Spiers HJ, O'Keefe J. 2001. A temporoparietal and prefrontal network for retrieving the spatial context of lifelike events. Neuroimage 14:439–453. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O'Keefe J. 2002. The human hippocampus and spatial and episodic memory. Neuron 35:625–641. [DOI] [PubMed] [Google Scholar]
- Cardoso MMB, Sirotin YB, Lima B, Glushenkova E, Das A. 2012. The neuroimaging signal is a linear sum of neurally distinct stimulus‐ and task‐related components. Nat Neurosci 15:1298‐ U172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe KW, Blake R, Lee SH. 2014. Dissociation between neural signatures of stimulus and choice in population activity of human V1 during perceptual decision‐making. J Neurosci 34:2725–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L. 2006. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol 16:693–700. [DOI] [PubMed] [Google Scholar]
- Deshmukh SS, Johnson JL, Knierim JJ. 2012. Perirhinal cortex represents nonspatial, but not spatial, information in rats foraging in the presence of objects: Comparison with lateral entorhinal cortex. Hippocampus 22:2045–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31:968–980. [DOI] [PubMed] [Google Scholar]
- Doeller CF, King JA, Burgess N. 2008. Parallel striatal and hippocampal systems for landmarks and boundaries in spatial memory. Proc Natl Acad Sci USA 105:5915–5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret G. 2006. Hemisphere dominance of brain function‐which functions are lateralized and why? In: Hemmen JLV, Sejnowski TJ, editors. 23 Problems in Systems Neuroscience. New York (NY): Oxford University Press; pp 44–64. [Google Scholar]
- Eichenbaum H, Lipton PA. 2008. Towards a functional organization of the medial temporal lobe memory system: Role of the parahippocampal and medial entorhinal cortical areas. Hippocampus 18:1314–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. 2002. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Zacks JM, Raichle ME. 2006. Coherent spontaneous activity accounts for trial‐to‐trial variability in human evoked brain responses. Nat Neurosci 9:23–25. [DOI] [PubMed] [Google Scholar]
- Frisk V, Milner B. 1990. The role of the left hippocampal region in the acquisition and retention of story content. Neuropsychologia 28:349–359. [DOI] [PubMed] [Google Scholar]
- Fyhn M, Molden S, Witter MP, Moser EI, Moser MB. 2004. Spatial representation in the entorhinal cortex. Science 305:1258–1264. [DOI] [PubMed] [Google Scholar]
- Grasby PM, Frith CD, Friston KJ, Frackowiak RSJ, Dolan RJ. 1993. Activation of the human hippocampal‐formation during auditory‐verbal long‐term‐memory function. Neurosci Lett 163:185–188. [DOI] [PubMed] [Google Scholar]
- Gron G, Wunderlich AP, Spitzer M, Tomczak R, Riepe MW. 2000. Brain activation during human navigation: Gender‐different neural networks as substrate of performance. Nat Neurosci 3:404–408. [DOI] [PubMed] [Google Scholar]
- Hargreaves EL, Rao G, Lee I, Knierim JJ. 2005. Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science 308:1792–1794. [DOI] [PubMed] [Google Scholar]
- Hartley T, Maguire EA, Spiers HJ, Burgess N. 2003. The well‐worn route and the path less traveled: Distinct neural bases of route following and wayfinding in humans. Neuron 37:877–888. [DOI] [PubMed] [Google Scholar]
- Henriksen EJ, Colgin LL, Barnes CA, Witter MP, Moser M‐B, Moser EI. 2010. Spatial representation along the proximodistal axis of CA1. Neuron 68:127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RNA. 2003. Analysis of fMRI time series: Linear time‐invariant models, event‐related fMRI and optimal experiment design In: Frackowiak R, Frith C, Dolan R, Price C, editors. Human Brain Function. London: Elsevier; pp 793–822. [Google Scholar]
- Iaria G, Petrides M, Dagher A, Pike B, Bohbot VD. 2003. Cognitive strategies dependent on the hippocampus and caudate nucleus in human navigation: variability and change with practice. J Neurosci 23:5945–5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igloi K, Doeller CF, Berthoz A, Rondi‐Reig L, Burgess N. 2010. Lateralized human hippocampal activity predicts navigation based on sequence or place memory. Proc Natl Acad Sci USA 107:14466–14471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn MC, Bingman VP. 2004. Lateralization of spatial learning in the avian hippocampal formation. Behav Neurosci 118:333–344. [DOI] [PubMed] [Google Scholar]
- Kawakami R, Shinohara Y, Kato Y, Sugiyama H, Shigemoto R, Ito I. 2003. Asymmetrical allocation of NMDA receptor epsilon 2 subunits in hippocampal circuitry. Science 300:990–994. [DOI] [PubMed] [Google Scholar]
- Kjelstrup KB, Solstad T, Brun VH, Hafting T, Leutgeb S, Witter MP, Moser EI, Moser M‐B. 2008. Finite scale of spatial representation in the hippocampus. Science 321:140–143. [DOI] [PubMed] [Google Scholar]
- Klur S, Muller C, de Vasconcelos AP, Ballard T, Lopez J, Galani R, Certa U, Cassel JC. 2009. Hippocampal‐dependent spatial memory functions might be lateralized in rats: An Approach combining gene expression profiling and reversible inactivation. Hippocampus 19:800–816. [DOI] [PubMed] [Google Scholar]
- Knierim JJ, Lee I, Hargreaves EL. 2006. Hippocampal place cells: Parallel input streams, subregional processing, and implications for episodic memory. Hippocampus 16:755–764. [DOI] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. 2007. Match mismatch processes underlie human hippocampal responses to associative novelty. J Neurosci 27:8517–8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehn H, Steffenach HA, van Strien NM, Veltman DJ, Witter MP, Haberg AK. 2009. A specific role of the human hippocampus in recall of temporal sequences. J Neurosci 29:3475–3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, Moser MB. 2005. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science 309:619–623. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Frith CD. 2003. Lateral asymmetry in the hippocampal response to the remoteness of autobiographical memories. J Neurosci 23:5302–5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Burgess N, Donnett JG, Frackowiak RSJ, Frith CD, O'Keefe J. 1998. Knowing where and getting there: A human navigation network. Science 280:921–924. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD. 2000. Navigation‐related structural change in the hippocampi of taxi drivers. Proc Nat Acad Sci USA 97:4398–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Mummery CJ. 1999. Differential modulation of a common memory retrieval network revealed by positron emission tomography. Hippocampus 9:54–61. [DOI] [PubMed] [Google Scholar]
- Milner B. 1971. Interhemispheric differences in the localization of psychological processes in man. Br Med Bull 27:272–277. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. 1982. Place navigation impaired in rats with hippocampal lesions. Nature 297:681–683. [DOI] [PubMed] [Google Scholar]
- Moser E, Moser MB, Andersen P. 1993. Spatial‐learning impairment parallels the magnitude of dorsal hippocampal‐lesions, but is hardly present following ventral lesions. J Neurosci 13:3916–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J. 1976. Place units in the hippocampus of the freely moving rat. Exp Neurol 51:78–109. [DOI] [PubMed] [Google Scholar]
- Olman CA, Inati S, Heeger DJ. 2007. The effect of large veins on spatial localization with GE BOLD at 3 T: Displacement, not blurring. Neuroimage 34:1126–1135. [DOI] [PubMed] [Google Scholar]
- Pestilli F, Carrasco M, Heeger DJ, Gardner JL. 2011. Attentional enhancement via selection and pooling of early sensory responses in human visual cortex. Neuron 72:832–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, Lupien S, Evans AC. 2000. Volumetry of hippocampus and amygdala with high‐resolution MRI and three‐dimensional analysis software: Minimizing the discrepancies between laboratories. Cereb Cortex 10:433–442. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Vilberg KL. 2013. Brain networks underlying episodic memory retrieval. Curr Opin Neurobiol 23:255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendan HE, Searl MM, Melrose RJ, Stern CE. 2003. An fMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron 37:1013–1025. [DOI] [PubMed] [Google Scholar]
- Shallice T, Fletcher P, Frith CD, Grasby P, Frackowiak RSJ, Dolan RJ. 1994. Brain‐regions associated with acquisition and retrieval of verbal episodic memory. Nature 368:633–635. [DOI] [PubMed] [Google Scholar]
- Shinohara Y, Hosoya A, Yamasaki N, Ahmed H, Hattori S, Eguchi M, Yamaguchi S, Miyakawa T, Hirase H, Shigemoto R. 2012. Right‐hemispheric dominance of spatial memory in split‐brain mice. Hippocampus 22:117–121. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Yacoub E, Chaimow D, Logothetis NK, Ugurbil K. 2007. Spatio‐temporal point‐spread function of fMRI signal in human gray matter at 7 Tesla. Neuroimage 35:539–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiers HJ, Burgess N, Hartley T, Vargha‐Khadem F, O'Keefe J. 2001a. Bilateral hippocampal pathology impairs topographical and episodic memory but not visual pattern matching. Hippocampus 11:715–725. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Burgess N, Maguire EA, Baxendale SA, Hartley T, Thompson PJ, O'Keefe J. 2001b. Unilateral temporal lobectomy patients show lateralized topographical and episodic memory deficits in a virtual town. Brain 124:2476–2489. [DOI] [PubMed] [Google Scholar]
- Stern CE, Corkin S, Gonzalez RG, Guimaraes AR, Baker JR, Jennings PJ, Carr CA, Sugiura RM, Vedantham V, Rosen BR. 1996. The hippocampal formation participates in novel picture encoding: Evidence from functional magnetic resonance imaging. Proc Natl Acad Sci USA 93:8660–8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, Moser EI. 2014. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci 15:655–669. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. 1998. Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science 281:1188–1191. [DOI] [PubMed] [Google Scholar]


