Abstract
Background
In the fine needle aspiration cytology (FNAC) for tumors of the breast, evaluation is frequently difficult because of the thick‐layered cell clusters and blood inclusion. Such problems may be resolved by the returned cell block method, but its use has not spread worldwide. Here, we examined the application of the returned cell block method to cases involving difficulty in the evaluation of FNAC to diagnose tumors of the breast.
Methods
In Juntendo University Nerima Hospital, there were 22 cases which were difficult to diagnose by Papanicolaou stain only, and they underwent additional examination using the returned cell block method (cell block from a Papanicolaou staining smear on a glass slide). The usefulness of the returned cell block method in these cases was examined.
Results
Among the 22 cases, a correct diagnosis was facilitated in 20 cases using the returned cell block method. In 16 of the 20 cases, the difficulty in FNAC was because of thick‐layered cell clusters (12 cases) and blood inclusion (four cases). Among the 12 cases with difficulty because of the thick‐layered cell clusters, 10 cases (83%) comprised intraductal papilloma (six cases) and intraductal papillary carcinoma (four cases). Papilloma and papillary carcinoma were correctly diagnosed by the addition of histological images and immunostaining of myoepithelial cells using the returned cell block method.
Conclusion
The application of the returned cell block method is useful for precise evaluation of the cytological diagnosis of tumors of the breast, especially papillary lesions. Diagn. Cytopathol. 2016;44:505–511. © 2016 The Authors Diagnostic Cytopathology Published by Wiley Periodicals, Inc.
Keywords: fine needle aspiration cytology, tumors of the breast, returned cell block method, intraductal papilloma, intraductal papillary carcinoma
Fine needle aspiration cytology (FNAC) has been extensively used for many years for the diagnosis of breast lesions, but its use has recently been reduced in many screening programs because of its controversial rates of inadequacy and suboptimal accuracy in inexperienced hands, especially in Western countries.1 On the other hand, some studies have confirmed the value of cytomorphology as a breast cancer risk predictor.2 Hatada et al. reported that a combination of ultrasound‐guided (US)‐core needle biopsy (CNB) and US‐FNAC can markedly improve the preoperative diagnosis of breast cancer patients, although US‐CNB is more useful for the evaluation of breast lesions than US‐FNAC.3 This reduced accuracy of FNAC compared with CNB for breast lesions is because of thick‐layered cell clusters and blood inclusion in FNAC samples.4 Also, the application of immunostains to CNB has increased the accuracy of diagnosing breast lesions.
The returned cell block method, consisting of making a cell block from a Papanicolaou staining smear on a glass slide, cutting sections of the block, and staining with conventional and immunostains, was firstly reported by Itho et al. in 1989.5 This method is useful for resolving the problem of the thick‐layered cell clusters and blood inclusion in FNAC, and can facilitate immunostains to increase the accuracy of diagnosis, but it has not spread worldwide. Here, we examined the application of the returned cell block method to cases involving difficulty in the evaluation of FNAC to diagnose tumors of the breast.
Materials and Methods
There were 2,739 cases of FNAC examination for tumors of the breast from August 1, 2005 to July 30, 2013 in Juntendo University Nerima Hospital. Although most cases could be diagnosed by Papanicolau stain only, there were 22 cases which were difficult to diagnose solely with this method, and they underwent additional examination using the returned cell block method. The method requires time, so its application was limited to selected cases. In the present study, FNAC diagnosis by Papanicolau stain only, diagnosis using the returned cell block method, and pathological diagnosis by excisional biopsy were compared among the 22 cases. Diagnosis using the returned cell block method and pathological diagnosis by excisional biopsy were based on the newly published WHO classification of tumors of the breast.6
The processes of the returned cell block method were as follows:
(1) Mark the site of the targeted cell clusters with water‐based ink or a glass pen on the cover glass (Fig. 1A), and mark from the reverse side of the glass slide according to the marking on the cover glass (Fig. 1B). (2) Remove the cover glass by dipping in xylene solution overnight and completely dissolve the mounting medium on the glass slide by dipping in xylene solution for 30–60 min after the removal of the cover glass. (3) Peel off the targeted cell clusters with a surgical knife and pick them up with a pipette or tweezers under a microscope (Figs. 2A and B). (4) Move the cell clusters into the metal mold (Fig. 3A), pour paraffin into the mold while being careful to avoid the loss of the small cell clusters (Fig. 3B), and then produce a paraffin block. (5) Cut the paraffin block into 4 μm‐thick slices (Fig. 4), and conduct HE stain, special stains, and immunostaining of the cut sections. In addition, the processes of dewaxing and hydrating the cut sections through a graded series of alcohol to water lead to the disappearance of Papanicolaou stain and effective use of HE stain, special stains, and immunostaining.
Figure 1.
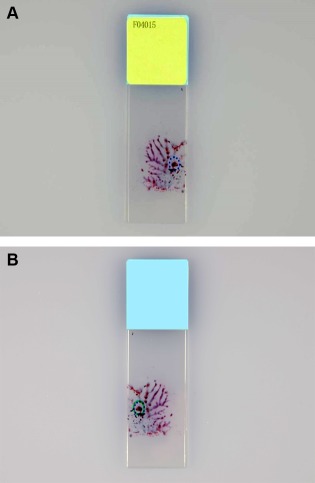
Returned cell block method: Marking of the target cell cluster on the glass slide. (A) Marking the site of the target cell cluster on the cover glass; (B) Marking from the reverse side of the glass slide according to the marking on the cover glass. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 2.
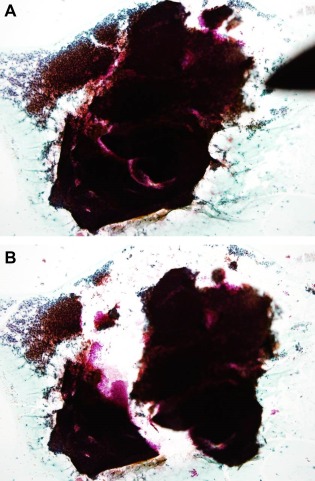
Returned cell block method: Identify the target cell cluster. (A) Peel off the target cell cluster with a surgical knife; (B) The target cell cluster is peeled off. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 3.
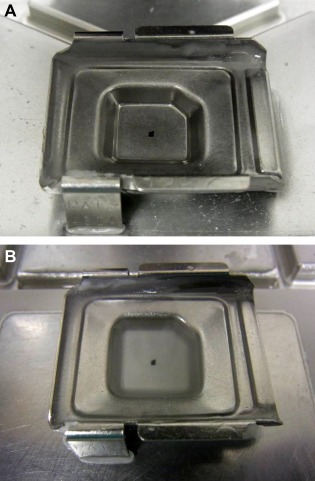
Returned cell block method: Moving the cell cluster to the metal mold and embedding in paraffin. (A) The cell cluster being moved to the metal mold; (B) Pouring paraffin into the metal mold containing the cell cluster. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 4.

Returned cell block method: Cut the paraffin block containing the cell cluster at 4 μm thick. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Immunostainings of p63 (DAKO, Tokyo, Japan), alpha‐smooth muscle actin (SMA: DAKO, Tokyo, Japan), and E‐cadherin (DAKO, Tokyo Japan) in sections were performed with the LSAB method using XT Benchmark (Ventana, Yokohama, Japan). The antibodies were used at a dilution of 1:100. Specimens of p63 and E‐cadherin were treated by incubating in EDTA buffer at 100°C for 60 min. After washing in 0.01 mol/L PBS, endogenous peroxidase activity was blocked by treating for 4 min with 3% aqueous hydrogen peroxidase. Visualization was performed with DAB (Dako Japan, Kyoto, Japan).
The diagnosis using FNAC and the returned cell block method was made by two cytotechnologists (S.A. and Y.A) and two pathologists (K.O. and T.M). The pathological diagnosis of excisional biopsy was made by two pathologists (K.O. and T.M.).
The current study was approved by the Research Ethics Committee of Juntendo University Nerima Hospital.
Results
By the returned cell block method, we could easily recognize the morphology of the tumor cell clusters. In thick cell layered clusters, it was difficult to judge the atypia of tumor cells and lack of myoepithelial cells (Fig. 5A). However, the sections with H‐E stain from the returned cell block improved the recognition of tumor cell clusters, and we could easily recognize the structure of cell clusters and atypia of the tumor cells (Fig. 5B).
Figure 5.
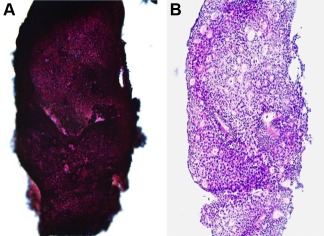
Comparison of cell cluster images between (A) Papanicolau stain in an FNAC sample and (B) HE stain in a returned cell block. The thickness of the cell cluster in FNAC (A) was resolved by the returned cell block method (B), and the returned cell block method facilitated effective recognition of the cell cluster structure and precise evaluation of cellular atypia. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Among the 22 cases, correct diagnosis was made in 20 cases by the returned cell block method, but in two cases an accurate diagnosis was not possible, even with the returned cell block method, because there were few tumor cells. We summarized FNAC diagnosis by Papanicolau stain only, diagnosis using the returned cell block method, reasons for the application of the returned cell block method, and pathological diagnosis by excisional biopsy in the 20 cases (Table 1).
Table 1.
FNAC Diagnosis by Papanicolau Stain Only, Diagnosis Using the returned Cell Block Method, and Pathological Diagnosis by Excisional Biopsy in 20 Cases with the Precise Evaluation of Returned Cell Blocks
| Case | Cytological diagnosis | Pathological diagnosis by excisional biopsy | |
|---|---|---|---|
| FNAC | Returned cell block (Reason for application) | ||
| 1 | Papillary tumor | IDP (Thick) | IDP |
| 2 | Papillary tumor | IDP (Thick) | IDP |
| 3 | Papillary tumor | IDP (Thick) | NP |
| 4 | Papillary tumor | IDP (Thick) | NP |
| 5 | Papillary tumor | IDP (Thick) | NP |
| 6 | Papillary tumor | IDP (Thick) | NP |
| 7 | Papillary tumor | IPC (Thick) | IPC |
| 8 | Papillary tumor | IPC (Thick) | IPC |
| 9 | Papillary tumor | IPC (Thick) | IPC |
| 10 | Papillary tumor | IPC (Thick) | IPC |
| 11 | Suspicious of malignancy | IC of NST (Thick) | IC of NST |
| 12 | Suspicious of malignancy | DCIS (Thick) | DCIS |
| 13 | Suspicious of malignancy | IC of NST (Blood) | IC of NST |
| 14 | Suspicious of malignancy | IC of NST (Blood) | IC of NST |
| 15 | Suspicious of malignancy | IC of NST (Blood) | IC of NST |
| 16 | Suspicious of malignancy | DCIS (Blood) | DCIS |
| 17 | Carcinoma | IC of NST (confirmationa) | DCIS |
| 18 | Carcinoma | IC of NST (confirmationa) | IC of NST |
| 19 | Carcinoma | ILC (confirmationa) | ILC |
| 20 | Carcinoma | MC (confirmation of mucin) | MC |
FNAC: Fine needle aspiration cytology, IDP: Intraductal papilloma, IPC: Intraductal papillary carcinoma, IC of NST: Invasive carcinoma of no special type, DCIS: Ductal carcinoma in situ, ILC: Invasive lobular carcinoma, MC: Mucinous carcinoma, Thick: Thick‐layered cell clusters, Blood: Blood inclusion, NP: Not performed.
Confirmation of IC of NST or ILC.
In 16 of the 20 cases, the difficulty of FNAC was because of the thick‐layered cell clusters (12 cases) and blood inclusion (four cases). These difficulties were resolved by the returned cell block method, facilitating a correct diagnosis (Table 1). Among the 12 cases with difficulty because of thick‐layered cell clusters, 10 cases (83%) comprised intraductal papilloma (six cases) and intraductal papillary carcinoma (four cases). In the 10 cases, the diagnosis with FNAC by Papanicolau stain only was papillary tumor, but papilloma or papillary carcinoma was correctly diagnosed by the addition of histological images and immunostaining of myoepithelial cells using the returned cell block method (Table 1; Figs. 6 and 7).
Figure 6.
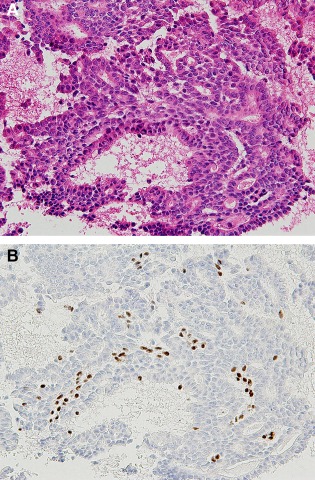
Intraductal papilloma diagnosed by a returned cell block (Case 2). (A) Note the papillary arrangement of epithelial cells with mild cellular atypia admixed with myoepithelial cells (HE stain, ×200); (B) The myoepithelial cells are confirmed by p63 immunostaining (×200). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 7.
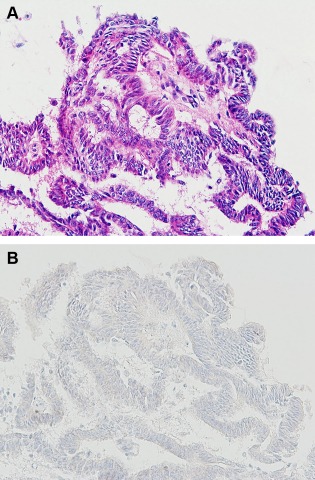
Intraductal papillary carcinoma diagnosed by a returned cell block (Case 7). (A) Note the papillary arrangement of epithelial cells with marked cellular atypia (HE stain, ×200); (B) The lack of myoepithelial cells is confirmed by p63 immunostaining (×200). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In three cases, carcinoma was diagnosed based on the findings of FNAC only, but differentiation between invasive carcinoma of no special type (NST) and invasive lobular carcinoma was very difficult; however, immunostaining of E‐cadherin with the returned cell block method facilitated the correct diagnosis of invasive carcinoma of NST (two cases) and invasive lobular carcinoma (one case) (Fig. 8). In one case, special stains for the confirmation of mucin in the returned cell block led to the diagnosis of mucinous carcinoma.
Figure 8.
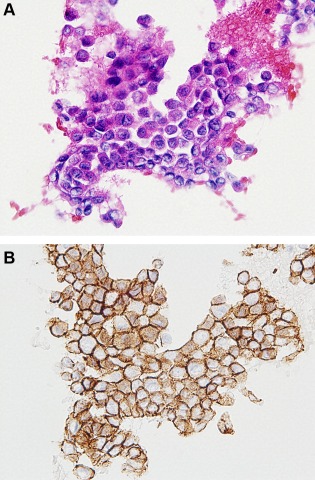
Invasive carcinoma of no specific type diagnosed by a returned cell block using E‐cadherin immunostaining (Case 18). (A) Note that the cluster consisted of small epithelial cells with marked cellular atypia (HE stain, ×400); (B) E‐cadherin immunoreactivity of the epithelial cells facilitated a diagnosis of invasive carcinoma of no special type (E‐cadherin immunostaining, ×400). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In addition, two cases of intraductal papilloma, four cases of intraducal papillary carcinoma, five cases of invasive carcinoma of NST, two cases of ductal carcinoma in situ, one case of invasive lobular carcinoma, and one case of mucinous carcinoma, which were diagnosed by the returned cell block method, showed the same results as on pathological diagnosis by excisional biopsy (Table 1). In addition, the results of immunostaining between the returned cell block and excisional biopsy were the same. In one case with a diagnosis of invasive carcinoma of NST by the returned cell block method, the pathological diagnosis was corrected to ductal carcinoma in situ (Table 1). In four cases with a diagnosis of intraductal papilloma by the returned cell block method, pathological examination was not performed (Table 1).
Discussion
Concerning the evaluation of FNAC, the application of immunostaining of a smear increases the accuracy. The cell transfer method involves immunostaining of a smear on a glass slide, peeling off the thin‐layered cell clusters, moving them to another glass slide, and immunostaining of the moved cell clusters.7, 8 On the other hand, in the returned cell block method, we can obtain multiple glass slides from tissue samples by making a cell block,9 and this method yields data from both conventional stains and immunostainings of the same cell block. Also, the returned cell block method resolves the problems of the thick cell layer and blood inclusion. Although almost all cases can be diagnosed by Papanicolau stain only, it is sometimes difficult in mammary lesions to make a diagnosis by FNAC because of thick‐layered cell clusters and blood inclusion. Furthermore, the diagnosis of mammary papillary lesions by FNSC has occasionally been difficult. In such cases, the returned cell block method is very useful for not only reducing the layers of thick cell clusters and blood, but also obtaining multiple glass slides for immunohistochemical staining.
The most important new finding in the current study is that the returned cell block method is useful for differentiation between intraductal papilloma and intraductal papillary carcinoma. FNAC in the cases of papillary tumor revealed that the tumor cell clusters tended to be thick, and so the returned cell block method for papillary tumors was particularly useful. Simsir et al. reported that a papillary pattern can be seen in a variety of mammary lesions and careful cytological evaluation allows further classification of most of these lesions into true papillary or nonpapillary proliferations and, of further clinical significance, into benign or atypical categories.10 In intraductal papilloma, there are mixed epithelial and myoepithelial cells, with a haphazard cellular architecture, and the epithelial nuclei are normochromatic.11 In intraductal papillary carcinoma, the epithelial cells are arranged in a more rigid architecture, with hyperchromatic nuclei and, within the lesions, myoepithelial cells are present in a reduced number or absent. Thus, the differentiation between these diseases is made based on the histological appearances plus the evaluation of immunohistochemical stains for markers of myoepithelial cells, such as p63, SMA, and calponin.12, 13, 14, 15 The returned cell block method could help cytopathologists recognize and make diagnoses of papillary lesions by combination with immunocytochemistry of myoepithelial markers such as p63 and SMA. In this study, all 10 cases of papillary tumor diagnosed by FNAC were correctly diagnosed as intraductal papilloma (six cases) and intraductal papillary carcinoma (four cases) by the application of the returned cell block method.
As for additional findings in the current study, the returned cell block method provides data useful for differentiation between invasive carcinoma of NST and invasive lobular carcinoma by E‐cadherin immunostaining.16 The differentiation between invasive carcinoma and invasive lobular carcinoma based on FNAC using the returned cell block method is very important for partial resection of the mamma, because invasive lobular carcinoma occasionally spreads over an area wider than that shown on imaging examinations.4
There were concerns over staining properties in immunocytochemistry because FNAC samples were fixed using alcohol. However, staining properties of p63, SMA, and E‐cadherin by the returned cell block were not poor. Ikeda et al. examined immunocytochemical studies with alcohol‐fixed cytocentrifuged preparations for diagnosis in routine effusion cytology.17 They revealed that such studies with alcohol‐fixed cytocentrifuged preparations showed good sensitivity, and the results obtained were correlated with those of immunocytochemical studies using formalin‐fixed, paraffin‐embedded cell blocks. Thus, we considered that alcohol fixation did not influence the immunohistochemical results regarding staining properties.
In conclusion, the current study indicated that the application of the returned cell block method is useful for precise evaluation of the cytological diagnosis of tumors of the breast, especially intraductal papilloma and intraductal papillary carcinoma.
Acknowledgment
The authors thank Mr. D. Mrozek (Medical English Service, Kyoto, Japan) for organizing the English revision of this article.
Conflicts of interest: The authors declare that there are no conflicts of interest.
These results were presented in part at the 103rd Annual Meeting of the US‐Canadian Academy of Pathology, San Diego, March 2014
References
- 1. Kocjan G, Bourgain C, Fassina A, et al. The role of breast FNAC in diagnosis and clinical management: a survey of current practice. Cytopathology 2008;19:271–278. [DOI] [PubMed] [Google Scholar]
- 2. Masood S. Expanded role of cytopathology in breast cancer diagnosis, therapy and research: The impact of fine needle aspiration biopsy and imprint cytology. Breast J 2012;1:1–2. [DOI] [PubMed] [Google Scholar]
- 3. Hatada T, Ishii H, Ichii S, et al. Diagnostic value of ultrasound‐guided fine‐needle aspiration biopsy, core‐needle biopsy, and evaluation of combined use in the diagnosis of breast lesions. J Am Coll Surg 2000;190:299–303. [DOI] [PubMed] [Google Scholar]
- 4. Masood S. Beast. In Sidawy MK, Ali SZ. editors. Fine needle aspiration cytology. Philadelphia: Elsevier Inc; 2007. p 127–158. [Google Scholar]
- 5. Itoh O, Katoh H, Murase H, et al. A study of the returned cell block method with a cell cluster picked out from a Papanicolaou smear sample. J Jpn Soc Clin Cytol 1989;28:842–847. [Google Scholar]
- 6. WHO classification of tumours of the breast. Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ, editors, Lyon; IARC Press; 2012. 240 p. [Google Scholar]
- 7. Brown GG, Tao LC. Restoration of broken cytology slides and creation of multiple slides from a single smear preparation. Acta Cytol 1992;36:259–263. [PubMed] [Google Scholar]
- 8. Sherman ME, Jimenex‐Joseph D, Gangi MD, et al. Immunostaining of small cytologic specimens: Facilitation with cell transfer. Acta Cytol 1994;38:18–22. [PubMed] [Google Scholar]
- 9. Azami S, Aoki Y. Cytology: Returned cell block method. Med Tech 2013;41:617–621. [Google Scholar]
- 10. Simsir A, Waisman J, Thorner K, et al. Mammary lesions diagnosed as “papillary” by aspiration biopsy: 70 cases with follow‐up. Cancer Cytopathol 2003;99:156–65. [DOI] [PubMed] [Google Scholar]
- 11. O'Malley F, Visscher D, MacGrogan, Tan PH, Ichihara S. Intraductal papillary lesions In: Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ, editors. WHO classification of tumours of the breast. Lyon: IARC Press; 2012. p 99–109. [Google Scholar]
- 12. Moriya T, Kasajima A, Ishida K, et al. New trends of immunohistochemistry for making differential diagnosis of breast lesions. Med Mol Morphol 2006;39:8–13. [DOI] [PubMed] [Google Scholar]
- 13. Troxell ML, Masek M, Sibley RK. Immunohistochemical staining of papillary breast lesions. Appl Immunohistochem Mol Morphol 2007;15:145–153. [DOI] [PubMed] [Google Scholar]
- 14. Tse GM, Tan PH, Lui PC, et al. The role of immunohistochemistry for smooth‐muscle actine, p63, CD10 and cytokeratin 14 in the differential diagnosis of papillary lesions of the breast. J Clin Pathol 2007;60:315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Collins LC, Schnitt SJ. Papillary lesions of the breast: selected diagnostic and management issues. Histopathology 2008;52:20–29. [DOI] [PubMed] [Google Scholar]
- 16. Gamallo C, Palacios J, Suarez A, et al. Correlation of E‐cadherin expression with differentiation grade and histological type in breast carcinoma. Am J Pathol 1993;142:987–993. [PMC free article] [PubMed] [Google Scholar]
- 17. Ikeda K, Tate G, Suzuki T, et al. Comparison of immunocytochemical sensitivity between formalin‐fixed and alcohol‐fixed specimens reveals the diagnostic value of alcohol‐fixed cytocentrifuged preparations in malignant effusion cytology. Am J Clin Pathol 2011;136:934–942. [DOI] [PubMed] [Google Scholar]


