Abstract
The ionization of 1,1‐dihydridocyclopentasilane 7 has been found to yield the cyclic polysilanylsilyl cation 8 instead of the expected hydrogen‐substituted silylium ion 6. The silyl cation 8 is stabilized by the formation of an intramolecular Si−H−Si bridge, which also provides the thermodynamic driving force for its formation. In general, the preference for the formation of Si−H−Si bridges can be used to scavenge and identify transient intermediates in the Lewis acid induced rearrangement of polysilanes. The validity of this concept has been demonstrated for one central step in this chemistry, the ring‐contraction reaction of cyclohexasilanes to form silylcyclopentasilanes.
Keywords: cations, density functional calculations, polysilanes, reaction mechanisms, silicon
Introduction
Several well‐characterized examples of silylium ions, tricoordinated silyl cations of the general formula R3Si+, are known.1, 2, 3, 4, 5, 6, 7, 8 Commonly, all of these cations are substituted with three bulky aryl groups to prevent bimolecular reactions with nucleophiles.9 Even the replacement of only one of the aryl groups by a smaller alkyl or even a hydrogen substituent triggers a rich consecutive chemistry.1, 2 For example, in a previous investigation we noticed the surprising formation of the triaryl‐substituted silyl cation 1 from the reaction of dihydridosilane 2 with the trityl cation.10 Cation 1 is formed presumably by the reaction of the incipiently formed cation 3 with the solvent benzene and arenium ion 4 is a likely intermediate (Scheme 1).
Scheme 1.
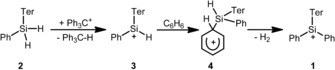
Reaction of dihydridosilane 2 with the trityl cation to give the silylium ion 1 according to ref. 10 (Ter=2,6‐bis(2,4,6‐trimethylphenyl)phenyl).
This observation clearly reveals the principal difficulties in the synthesis of hydrogen‐substituted silylium ions of type 5.11, 12, 13 Nevertheless, the secondary silylium ions 5 are an interesting class of compounds. On the one hand, being isoelectronic with hydridoboranes, they are perfect reagents for hydrosilylation reactions. On the other hand, in principle, secondary silylium ions can be regarded as protonated silylenes, which opens the field for a complementary chemistry based on proton‐transfer reactions to sterically hindered Lewis bases (Scheme 2).
Scheme 2.
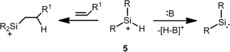
Possible reactivity of secondary silylium ions 5 (:B=sterically hindered Lewis base).
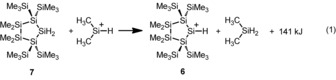
Hence, the above‐mentioned reactivity was our impetus for an investigation into the synthesis of secondary silylium ions of type 5. As a viable target cation, we chose the oligosilanylsilyl cation 6. The results of density functional calculations14, 15 predicted a substantial stabilization of the cation 6 through a combination of favorable α‐ and β‐silyl effects. In detail, according to isodesmic reaction (1), the cyclic cation 6 is more stable than dimethylsilylium, Me2Si+−H, by ΔE=141 kJ mol−1 (at the M06‐2X/6‐311+G(d,p) level of theory). Moreover, in the past, the 1,1,4,4‐tetrakis(trimethylsilyl)tetrasilane‐1,4‐diyl unit proved to be a well‐suited scaffold for the steric protection of reactive main group centers.16, 17, 18, 19
Although the number of applications of silyl cations in synthesis and catalysis has increased in recent years,1, 2, 20, 21 experimental investigations of polysilanyl‐substituted silyl cations, such as cation 6, are rather scarce. This is even more surprising as these cations are believed to be important intermediates in skeletal rearrangement reactions of oligosilanes22 and a deeper understanding of their nature and reactivity could pave the way to a controlled and directed modification of oligo‐ and polysilanes.23, 24, 25, 26, 27, 28, 29, 30 Exploratory investigations by Lambert and Zhang revealed the thermolability of even such small representatives as tris(trimethylsilyl)silylium, (Me3Si)3Si+.31, 32, 33, 34 Subsequently, Sekiguchi and co‐workers demonstrated the tendency of dialkyl‐oligosilyl cations to undergo 1,2‐methyl shifts35 and our group reported recently on the involvement of polysilanylsilyl or germapolysilanylsilyl cations as intermediates in sila‐Wagner–Meerwein rearrangements of polysilanes and germapolysilanes.36, 37 Common to all the studied polysilanyl compounds is their high reactivity, their thermolability, unusual for silyl cations, and their pronounced tendency to undergo skeletal rearrangement reactions. The only examples of polysilanyl cation salts that are stable at room temperature are the highly stabilized representatives of aromatic38, 39 and homoaromatic species40, 41.
Against this background, we describe herein our attempts to prepare the secondary silylium ion 6 and its unexpected rearrangement reaction to give the intramolecular hydrogen‐bridged silyl cation 8 (Scheme 3). As the main driving force for the rearrangement reaction we identified the formation of the Si−H−Si linkage in cation 8.1, 2, 42, 43 In addition, we will demonstrate that the targeted formation of this type of intramolecular Si−H−Si bridge can be used for the stabilization and verification of cationic intermediates in the skeletal rearrangements of oligosilanylsilyl cations.
Scheme 3.
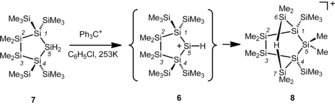
Reaction of dihydridosilane 7 to give the bis‐silylated hydronium ion 8.
Results and Discussion
The reaction of cyclo‐oligosilane 7 with trityl tetrakis(pentafluorophenyl)borate, [Ph3C][B(C6F5)4] (9), in benzene under ambient conditions resulted in the formation of a mixture of products, as indicated by numerous signals of variable intensity in the 29Si{1H} INEPT NMR spectrum. However, a set of five 29Si NMR signals (δ 29Si=95.5, −4.6 −14.9, −31.2, and −126.3 ppm) dominated. To prevent side‐reactions due to the reactivity demonstrated by other secondary silylium ions (Scheme 1), we changed the solvent to chlorobenzene. Chlorobenzene is known to coordinate to silylium ions through the chlorine atom, which excludes arenium‐ion‐like reactivity.1 After changing the solvent to chlorobenzene and lowering the temperature (T=−20 °C), the same reaction gave a set of NMR spectra that indicated the selective formation of a single compound (see Figure 1). The polysilanylsilyl cation formed was identified by five 29Si NMR signals with chemical shifts very close to those obtained in benzene (δ(29Si)=95.3, −4.6, −15.2, −31.7, and −127.7 ppm). In the 1H–29Si HMQC spectrum, the most deshielded resonance at δ(29Si)=95.3 ppm shows a cross‐peak with a broad 1H NMR signal at δ(1H)=1.10 ppm (1 H; Figure 1). In the proton‐coupled 29Si INEPT NMR spectrum, this resonance appears as a doublet of multiplets with a coupling constant of J(SiH)=46 Hz. The deshielded 29Si NMR resonance and the size of the Si−H coupling constant (J) are characteristic of Si−H−Si linkages in silicon cations.43 In addition, the high‐field signal at δ(29Si)=−127.7 ppm indicates the presence of tetrasilyl‐substituted silicon atoms.44 These distinctive 29Si NMR spectroscopic features clearly rule out the targeted formation of the hydrogen‐substituted silyl cation 6. The additional 29Si NMR resonances at δ(29Si)=−15.2 and −31.7 ppm could be assigned to SiMe2 groups by their characteristic NMR chemical shifts and by their correlation with 1H NMR signals with a relative intensity of six hydrogen atoms. Additional NMR spectroscopic analysis of the silicon cation formed finally revealed the bicyclic structure 8 with the bridging Me2Si−H−SiMe2 unit as the most prominent structural feature.
Figure 1.
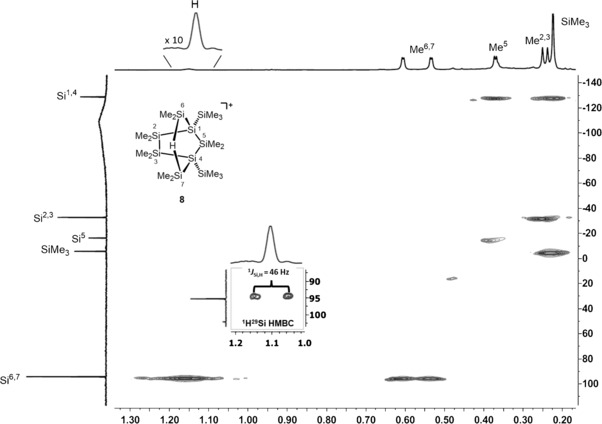
1H–29Si HMQC NMR spectrum of cation 8 (499.87/99.31 MHz, 253 K, C6H5Cl).
Further support for the identification of cation 8 came from the close agreement between calculated 29Si NMR chemical shifts (GIAO/M06 L/6‐311G(2d,p)) for a molecular structure optimized by DFT calculations at the M06‐2X/6‐311+G(d,p) level of theory (see Figure 2) and the experimental data (see Table 1).14, 15, 45 In particular, the calculated 29Si NMR chemical shifts and 1 J(SiH) coupling constants of the characteristic symmetric SiMe2−H−SiMe2 unit (δ(29Si)calcd=99.5 ppm; 1 J(SiH)calcd=44 Hz) agree convincingly with the experimental parameters obtained in chlorobenzene (δ(29Si)exp=95.3 ppm; 1 J(SiH)exp=46 Hz). Even though solutions of polysilanylsilyl cation 8 with different weakly coordinating anions, such as [B(C6F5)4]−, [B12Cl12]2−, or [Al{OC(CF3)4}4]−, in several arenes or halogenated arenes were prepared, no single crystals suitable for X‐ray crystal analysis were obtained.
Figure 2.
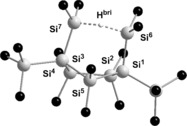
Calculated molecular structure of cation 8 (M06‐2X/6‐311+G(d,p)). Only the hydrogen atom bridging Si6 and Si7 is shown, all other hydrogen atoms have been omitted for clarity. Selected atom distances [pm] and bond angles [°]: Si6−Si7=317.8, Si6−H=163.6, Si7−H=163.9, Si6−H−Si7=151.8.
Table 1.
Experimental and calculated (italic) NMR parameters for polysilanyl cations 8 and 19. Calculated data for the secondary silylium ion 6 and the hydrogen‐bridged cation 18 are provided for comparison.
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compd | δ [ppm] (1 J(SiH) [Hz]) | ||||||||
| δ Si(Si1) | δ Si(Si2) | δ Si(Si3) | δ Si(Si4) | δ Si(Si5) | δ Si(Si6) | δ Si(Si7) | δ Si(SiMe3) | δ H(Hbr) | |
| 8 [a] | −127.7 | −31.7 | −31.7 | −127.7 | −15.2 | 95.3 (46) | 95.3 (46) | −4.6 | 1.10 (46) |
| 8 [b] | −128.8 | −32.9 | −32.9 | −128.8 | −16.4 | 94.1 (45) | 94.1 (45) | −5.9 | |
| 8 [c] | −126.2 | −31.2 | −31.2 | −126.2 | −14.9 | 95.5 (46) | 95.5 (46) | −4.6 | 1.01 (46) |
| 8 [d] | −135.1 | −25.9 | −25.9 | −135.1 | −5.7 | 99.5 (44)[e] | 99.5 (44) | −2.0 | 1.81 |
| 6 [e] | −43.3 | −24.9 | −24.9 | −43.3 | 594.5 | 2.8 | |||
| 19 [f] | −107.5 | −35.3 | −33.0 | 64.1 (43) | −33.4 | 78.1 (39) | −0.7 −4.2 | −1.07 (40) | |
| 19 [d] | −120.8 | −32.4 | −34.3 | 61.2 (37)[e] | −34.7 | 66.7 (39)[e] | 0.7 −2.3 | −0.14 | |
| 18 [d] | 52.2 | −19.8 | −32.9 | 52.2 | −19.8 | −32.9 | 1.0 | −1.75 | |
[a] At 253 K in C6D5Cl. [b] At 258 K in o‐C6H4Cl2 with [D6]acetone capillary. [c] At 303 K in C6D6. [d] Calculated at M06 L/6‐311G(2d,p)//M06‐2X/6‐311+G(d,p). [e] Calculated at the B3LYP/IGLOIII//M06‐2X/6‐311+G(d,p) level. [f] At 233 K in C6D5Cl.
The isomerization reaction of silylium ion 6 to give the hydrogen‐bridged cation 8 was further investigated by means of DFT calculations.14, 15 Although the secondary silylium ion 6 was found to be a minimum, the hydrogen‐bridged cation 8 was predicted to be significantly more stable (by ΔE==−67 kJ mol−1, Figure 3). The formation of the Si−H−Si linkage is the main thermodynamic driving force for this reaction as its formation from its non‐bridged isomer 10 e was calculated to be exothermic by ΔE==−77 kJ mol−1. In addition, we identified a possible reaction channel connecting both cations (Figure 3). Along this reaction path we located five intermediate silyl cations, 10 a–e, which are interconverted by 1,3‐methyl shifts (Figure 3). These interconversions proceed via four‐membered cyclic transition states. Transfer of the methyl group may occur in an antara‐ or suprafacial manner with regard the migrating methyl group. The antarafacial transition states are very high in energy, with energy barriers of ΔE=98–113 kJ mol−1. In contrast, a possible reaction channel connecting the cations 6 and 8 in which the methyl groups shift in a suprafacial way reveals barriers of only ΔE=25–33 kJ mol−1 (Figure 3, see the Supporting Information for the predicted structures of the transition states and intermediates). Such 1,3‐methyl shifts are common in silyl cation chemistry. Seminal contributions by Eaborn et al. disclosed the occurrence of such 1,3‐methyl shifts during the solvolysis of tris(trimethylsilyl)methylsilyl halides.46
Figure 3.
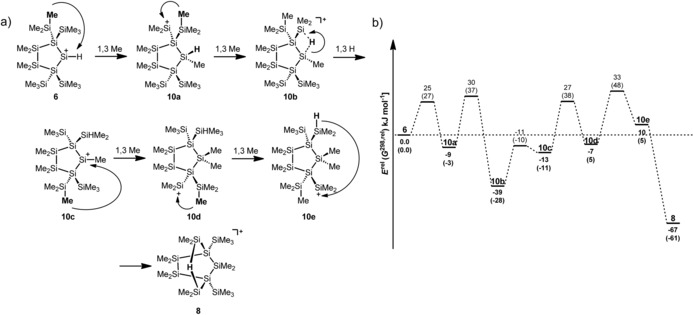
a) Suggested reaction path for the isomerization reaction of the secondary silylium ion 6 to give the bicyclic bis‐silylhydronium ion 8. b) Calculated reaction coordinate for this reaction (energies E rel and free enthalpies G 298 rel (in kJ mol−1) at 298 K and 0.1013 MPa are computed relative to compound 6 at the M06‐2X/6‐311+G(d,p) level of theory).
Related 1,3‐methyl migrations are important steps in skeletal rearrangement processes of polysilanyl cations and in inter‐ and intramolecular substituent exchange reactions in silylium ions.7, 8, 36, 37 Recently, we disclosed a similar rearrangement of the closely related silyl cation 11.42
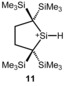
In general, the potential energy surface along the reaction coordinate is predicted by the calculations to be rather shallow with only small activation barriers for the subsequent methyl shifts (Figure 3). The rate‐determining step is the exothermic formation of cation 8. This step involves an overall energy barrier of ΔE=33 kJ mol−1, which is in qualitative agreement with the fast formation of cation 8 at temperatures as low as T=−20 °C. These experiments are interesting in their own right as they clearly demonstrate the high reactivity of secondary silylium ions, which prevents their detection, isolation, and characterization even under carefully controlled reaction conditions. They also indicate the high mobility of alkyl groups in polysilanyl cations. In addition, a more general implication of this chemistry emerges. There is a clear preference for polysilanyl cations to form Si−H−Si bridges, which stabilize these systems significantly, allow their spectroscopic characterization, and in some cases even their isolation. For example, silyl cation 10 e, a conformer of cation 8 that does not contain the Si−H−Si unit, is markedly less stable than cation 8 by ΔE=77 kJ mol−1.
This fact suggests that the lifetime of fleeting intermediates in this type of rearrangement can be extended significantly by using appropriately designed starting materials, which allow the formation of Si−H−Si linkages in an auto‐scavenge reaction. These intermediates then can be verified and identified spectroscopically. Such experiments would provide important insights into the actual rearrangement mechanism.
This concept has been demonstrated for the rearrangements of cyclohexasilanes 12 to yield silylcyclopentasilanes 13 (Scheme 4).26, 28, 29, 30 This reaction is a prototype for ring‐contraction reactions mediated by strong Lewis acids, which are important tools for the skeletal modification of polysilanes. The reaction is thought to proceed via cyclohexasilanyl cations 14, which undergo ring contraction to cyclopentasilanylsilyl cations 15, followed by trapping by a nucleophile to give neutral cyclopentasilane derivatives 13.29 Although we found that the reaction of 12 a to 13 a is also catalyzed by 1 mol % of trityl tetrakis(pentafluorophenyl)borate (9) in dichloromethane or benzene as solvent (Scheme 4), we were unable to detect cationic intermediates in stoichiometric reactions with trityl borate 9 under ambient conditions at T=−20 °C. Subsequently, we studied the reaction of hydrogen‐substituted cyclohexasilane 16, which smoothly generated the cation even at low temperatures. The 29Si NMR spectra of the reaction of compound 16 with trityl borate 9 in toluene recorded at T=−40 °C suggest the generation of cationic species. The number of 29Si NMR signals and their NMR chemical shifts indicate the formation of toluene complexes of rearranged silylcyclopentasilanyl cations as the main products in this reaction. Significant formation of side‐ or decomposition products prevented, however, a reliable structural assignment (see the Supporting Information).
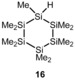
Scheme 4.
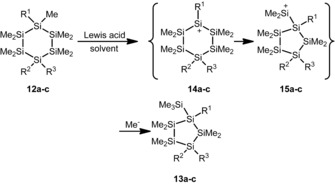
Lewis acid‐catalyzed rearrangement of cyclohexasilanes 12 to give silylcyclopentasilanes 13 (a: R1=R2=R3=Me; b: R1=R2=SiMe3, R3=Me; c: R1=R2=R3=SiMe3). Reaction conditions for 12 a: Lewis acid: Ph3C+, solvent: CD2Cl2 or C6D6; for 12 b: Lewis acid: AlCl3, solvent: cyclohexane; for 12 c: Lewis acid: AlCl3, solvent: cyclohexane).14
Finally, the use of 1,4‐dihydridocyclohexasilane 17 as the starting material for cation generation provided deeper insights into the rearrangement of the cyclohexasilanes (Scheme 5). The ionization of compound 17 was easily accomplished by removal of the first hydride with trityl tetrakis(pentafluorophenyl)borate (9) in chlorobenzene at low temperatures. Based on the previously suggested mechanism (Scheme 4), the formation of two different cations, the symmetric cyclohexasilanyl cation 18 and the cyclopentasilanylsilyl cation 19, could be expected as a result of the auto‐scavenge reaction by the remote Si−H functionality (Scheme 5).
Scheme 5.
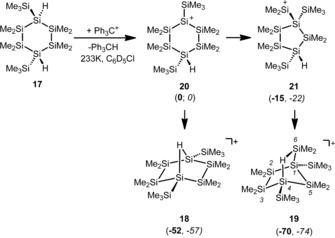
Reaction of 1,4‐dihydridocyclohexasilane 17 with the trityl cation and the possible formation of hydrido‐bridged cations 18 and 19 in auto‐scavenge reactions. Calculated energy differences (ΔE, bold) and free enthalpy differences at 298 K (ΔG 298, italics) for cations 18–21 relative to cation 20 are given in parentheses (in kJ mol−1, calculated at the M06‐2X/6‐311+G(d,p) level).
The 29Si NMR spectra recorded at T=−40 °C clearly show the predominant formation of one cationic compound. The number and intensity of the 29Si NMR signals in the inverse‐gated 29Si{1H} NMR spectrum indicate that this compound contains seven different silicon atoms. Two low‐field doublets at δ(29Si)=78.1 and 64.1 ppm with characteristic small J coupling constants of 1 J(SiH)=39 and 43 Hz show the formation of a cation with an unsymmetrically substituted Si−H−Si bridge (see Figure 4 and Table 1). In addition, the 1H–29Si HMQC NMR spectrum shows a correlation between both low‐field resonances with a broad 1H NMR signal at an unusually high field for Si−H and Si−H−Si groups (δ(1H)=−1.07 ppm).15 Analysis of the signal multiplicity resulting from long‐range Si−H couplings and of the δ(29Si)‐NMR chemical shifts of the remaining signals suggest the structure of cyclopentasilanylsilyl cation 19 as the predominantly formed cation. This assignment is supported by the close agreement between calculated 29Si NMR chemical shifts (GIAO/M06 L/6‐311G(2d,p)) and the experimental data (see Table 1).47 Solutions of 19[B(C6F5)4] in chlorobenzene are marginally stable at temperatures around T=−40 °C. After several hours and at higher temperatures, significant decomposition occurs.
Figure 4.
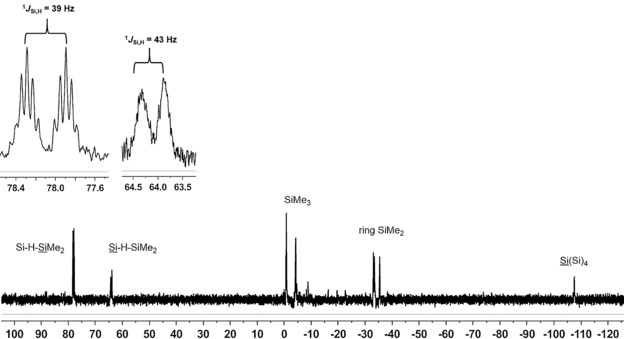
29Si INEPT NMR spectrum (99.31 MHz, 233 K, C6D5Cl) of cyclopentasilanylsilyl cation 19 obtained from the reaction of cyclohexasilane 17 with trityl tetrakis(pentafluorophenyl)borate 9.
It is particularly noteworthy that no characteristic 29Si NMR signals due to the formation of the symmetric cation 18 were detected (see Table 1 for the theoretical prediction). The absence of significant amounts of cation 18 in the reaction mixture is interesting, because the results of calculations suggest that its formation from the primarily formed cyclohexasilanyl cation 20 is exothermic by ΔE=−52 kJ mol−1 (Scheme 5). Evidently, the ring contraction reaction of cation 20 to give the five‐membered ring cation 21 is faster than the formation of the Si−H−Si linkage to form the symmetric cation 18. In contrast, the lifetime of compound 21 is longer, which allows for the formation of the unsymmetrical Si−H−Si‐bridged cation 19, which is the most stable isomer in the series of cations 18–21. As a consequence, the predominant formation of cation 19 indicates that cation 20 is either not an intermediate in this reaction and the ring contraction to form cation 21 occurs simultaneously with ionization or, at most, it is a high‐lying intermediate with a very low barrier to rearrangement to give cyclopentasilanylsilyl cation 21. This example shows that the formation of cations with an intramolecular Si−H−Si linkage can be detected straightforwardly by NMR spectroscopy and that this feature can be applied in auto‐scavenge reactions to reveal important details of otherwise complex reaction sequences in cationic rearrangement reactions of oligo‐ and polysilanes.
Conclusion
Our attempts to synthesize the hydrogen‐substituted polysilanyl silylium ion 6 by hydride transfer reaction from the corresponding cyclic dihydrido‐oligosilane 7 resulted in the selective and clean formation of the rearranged bis‐silylated hydronium ion 8. This result demonstrates the strong tendency of polysilanyl cations to undergo skeletal rearrangement reactions and indicates the superior thermodynamic stability of polysilanylsilyl cations stabilized by the formation of a Si−H−Si linkage. In addition, our findings suggest that the lifetime of fleeting intermediates in skeletal rearrangement reactions of polysilanylsilyl cations can be extended significantly by using appropriately designed starting materials, which allow the targeted formation of Si−H−Si bridges in an auto‐scavenge reaction. This concept has been successfully demonstrated for one of the key steps in the Lewis acid catalyzed rearrangement reactions of poly‐ and oligosilanes, the cyclohexasilane/silylcyclopentasilane ring‐contraction reaction. By using the appropriately substituted dihydridocyclosilane 17 as the starting material, we identified solely the product 19 of the auto‐scavenge reaction of the cyclopentasilanylsilyl cation 21. This result provides strong evidence for a synchronous ionization/rearrangement reaction pathway without passing through a cyclohexasilanyl cation intermediate. We are currently investigating the scope of the targeted formation of cations featuring a Si−H−Si bridge for mechanistic studies in Lewis acid initiated skeletal rearrangement reactions of poly‐ and oligosilanes.
Experimental Section
General
All manipulations of air‐ and moisture‐sensitive compounds were carried out under an argon or nitrogen atmosphere using Schlenk techniques or in a standard glovebox (Braun Unilab). NMR spectra were recorded on Bruker Avance 500, Bruker Avance III 500, and Varian Inova 300 spectrometers. 1H NMR spectra were calibrated against the residual proton signal of the solvent as internal reference ([D5]chlorobenzene: δ(1H) (C6D4HCl)=7.14 ppm; [D6]acetone: δ(1H) ((CD3)(CD2H)CO)=2.05 ppm) and 13C NMR spectra by using the central line of the solvent signal ([D5]chlorobenzene: δ(13C) (C6D5Cl)=134.2 ppm; [D6]acetone: δ(13C) ((CD3)2CO)=29.8 ppm). 29Si{1H} NMR spectra were calibrated against an external standard (Me2SiHCl: δ(29Si) =11.1 ppm versus tetramethylsilane (TMS)). The inverse‐gated 29Si{1H} NMR spectra were recorded with a relaxation delay D1 of 10 s. Based on our experience, at T=−20 or −40 °C this delay is long enough to allow a reliable integration of the peaks. The 29Si{1H} INEPT spectra were recorded with the following delays: D3=D4=0.0056 s for cation 8 and D3=D4=0.0061 s for cation 19.
Triphenylmethyl tetrakis(pentafluorophenyl) borate ([Ph3C][B(C6F5)4], 9) was used as hydride abstraction reagent and prepared according to a modified literature procedure.48, 49 The silyl cations were obtained as salts of the tetrakis(pentafluorophenyl)borate. The NMR characterization data for the anion are given below and not repeated for each cation preparation reaction. Negligible differences were found for the NMR chemical shifts and coupling constants of the anion depending on the solvent or temperature. Data for [B(C6F5)4]−: 13C{1H} NMR (125.71 MHz, 243.1 K, C6D5Cl): δ=148.9 (d, 1 J C,F=239 Hz, CFortho, [B(C6F5)4]−), 138.8 (d, 1 J C,F=235 Hz, CFpara, [B(C6F5)4]−), 136.9 (d, 1 J C,F=233 Hz, CFmeta, [B(C6F5)4]−), 123.7–125.6 ppm (m, Cipso, [B(C6F5)4]−); 19F{1H} NMR (470.28 MHz, 243.1 K, C6D5Cl): δ=−131.9 (br s, 2 F; CFortho, [B(C6F5)4]−), −161.9 (br s, 1 F; CFpara, [B(C6F5)4]−), −165.8 ppm (br s, 2 F; CFmeta, [B(C6F5)4]−); 11B{1H} NMR (160.38 MHz, 243.1 K, C6D5Cl): δ=−16.8 ppm ([B(C6F5)4]−).
Cation preparation
Synthesis of hydrogen‐bridged bis‐silyl borate 8[B(C6F5)4] from dihydridocyclopentasilane 7 in chlorobenzene: Dihydridocyclopentasilane 7 (87 mg, 1 equiv, 0.18 mmol) and trityl tetrakis(pentafluorophenyl)borate (9; 0.162 g, 1 equiv, 0.18 mmol) were evacuated in different Schlenk tubes for 1 h and then each dissolved in chlorobenzene or [D5]chlorobenzene (0.5 mL). The solution of trityl tetrakis(pentafluoro)phenyl borate (9) was cooled to T=−20 °C in an EtOH/N2 bath. The silane was added through a Teflon tube and the reaction mixture was stirred at T=−20 °C for 1.5 h. The solution was then transferred into an NMR tube and stored at T=−60 °C overnight. The NMR spectra were recorded at T=−20 °C. Data for cation 8: 1H NMR (499.87 MHz, 253.0 K, C6H5Cl, [D6]acetone lock): δ=1.10 (m, 1 J Si,H=46, 3 J H,H=2.3 Hz, 1 H; Si−H−Si), 0.56 (d, 3 J H,H=2.3 Hz, 6 H; (H 3C)MeSi6,7), 0.48 (d, 3 J H,H=2.3 Hz, 6 H; Me(H 3C)Si6,7), 0.32 (2 s, 2×3 H; Si5(CH 3)2), 0.20 (s, 6 H; Si2,3Me(CH 3)), 0.19 (s, 6 H; Si2,3(CH 3)Me), 0.17 ppm (s, 18 H; Si(CH 3)3); 13C{1H} NMR (125.71 MHz, 253.0 K, C6H5Cl, [D6]acetone lock): δ=1.8 (Si(CH3)3), 1.8 ((H3 C)MeSi6,7), 1.1 (Me(H3 C)Si6,7), −0.9 (Me(H3 C)Si5), −1.0 ((H3 C)MeSi5), −2.6 (Si2,3Me(CH3)), −3.5 ppm (Si2,3(CH 3)Me); 29Si{1H} NMR (99.31 MHz, 253 K, C6H5Cl, [D6]acetone lock): δ=94.2 (Si6,7), −5.7 (2×SiMe3), −16.3 (Si5), −32.8 (Si2,3), −128.8 ppm (Si1,4); 29Si INEPT NMR (99.31 MHz, 253 K, C6H5Cl, [D6]acetone lock): δ=94.2 (dsept., 1 J Si,H=46, 2 J Si,H=6 Hz, Si6,7), −5.7 (m, 2×SiMe3), −16.3 (sept., 2 J Si,H=6 Hz, Si5), −32.8 (m, Si2,3), −128.8 ppm (s, Si1, 4); 29Si{1H} NMR (99.31 MHz, 253 K, C6D5Cl): δ=95.3 (Si6,7), −4.6 (2×SiMe3), −15.2 (Si5), −31.7 (Si2,3), −127.7 ppm (Si1,4).
Synthesis of hydrogen‐bridged bis‐silyl borate 8[B(C6F5)4] from dihydridocyclopentasilane 7 in o ‐dichlorobenzene: Dihydridocyclopentasilane 7 (50 mg, 1 equiv, 0.10 mmol) and trityl tetrakis(pentafluorophenyl)borate (9; 92 mg, 1 equiv, 0.10 mmol) were evacuated in different Schlenk tubes for 1 h and then each dissolved in o‐dichlorobenzene (1 mL). The solution of trityl tetrakis(pentafluoro)phenyl borate (9) was cooled to T=−15 °C in an EtOH/N2 bath. The silane was added through a Teflon tube and the reaction mixture stirred at T=−15 °C for 2 h. The solution was then transferred into an NMR tube and stored at T=−25 °C overnight. The NMR spectra were recorded at T=−15 °C with an external [D6]acetone lock. Data for cation 8: 29Si{1H} NMR (99.31 MHz, 253 K, C6H4Cl2, [D6]acetone lock): δ=94.1 (Si6,7), −5.9 (2×SiMe3), −16.4 (Si5), −32.9 (Si2,3), −128.8 ppm (Si1,4); 29Si INEPT NMR (99.31 MHz, 253 K, C6H4Cl2, [D6]acetone lock): δ=94.1 (dsept., 1 J Si,H=46, 2 J Si,H=6 Hz, Si6,7), −5.9 (m, 2×SiMe3), −16.4 (m, Si5), −32.9 (m, Si2,3), −128.8 ppm (s, Si1,4).
Synthesis of hydrogen‐bridged bis‐silyl borate 19[B(C6F5)4] from 1,4‐dihydridocyclohexasilane 17: Trityl tetrakis(pentafluorophenyl)borate (9; 92 mg, 1 equiv, 0.10 mmol) was evacuated in an NMR tube and cooled to T=−80 °C. A solution of 1,4‐dihydridocyclohexasilane 17 (44 mg, 1 equiv, 0.10 mmol) in [D5]chlorobenzene (0.7 mL) was added to the cold trityl borate through a syringe. The addition was performed slowly enough that the solution froze before it reached the trityl borate. The NMR tube was carefully warmed until the solvent melted and the silane 17 slowly reached the trityl borate 9. At that point, the NMR tube, which was wrapped in a gauze bandage soaked in T=−70 °C cold ethanol, was quickly shaken with a vortex mixer and then quickly transferred to the NMR spectrometer precooled to T=−40 °C. Data for cation 19: 1H NMR (499.87 MHz, 233.0 K, C6D5Cl): δ=0.62 (Si6(CH 3)2), 0.50 (Si3(CH 3)2), 0.32 (Si5(CH 3)2), 0.23 (Si2(CH 3)2), 0.20 (Si4Si(CH 3)3), 0.17 (Si1Si(CH 3)3), −1.07 ppm (br, 1 J Si,H=40 Hz, Si−H−Si); 13C{1H} NMR (125.71 MHz, 233.0 K, C6D5Cl): δ=1.8 (SiMe3), 0.2 (SiMe3), −0.9 (SiMe2), −1.6 (SiMe2), −2.4 (SiMe2), −2.5 (SiMe2), −2.7 (SiMe2), −3.4 (SiMe2), −5.0 ppm (SiMe2); 29Si{1H} NMR (99.31 MHz, 233 K, C6D5Cl): δ=78.1 (Si6), 64.1 (Si4), −0.7 (Si4‐SiMe3), −4.2 (Si1‐SiMe3), −33.0 (Si3), −33.4 (Si5), −35.3 (Si2), −107.5 ppm (Si1); 29Si INEPT NMR (99.31 MHz, 233 K, C6D5Cl): δ=78.1 (dsept., 1 J Si,H=39, 3 J Si,H=5 Hz, Si6), 64.1 (d, 1 J Si,H=43 Hz, Si4), −0.7 (oct., 3 J Si,H=7 Hz, (Si4‐SiMe3)), −4.2 (m, Si1‐SiMe3), −33.0 (m, Si3), −33.4 (m, Si5), −35.3 (m, Si2), −107.5 ppm (m, Si1).
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) and the Fonds zur Förderung wissenschaftlicher Forschung (FWF) through the European Research Area (ERA) program (Mu1440/8‐1 and FWF‐I00669) and by the Carl von Ossietzky University Oldenburg. The simulations were performed at the HPC Cluster HERO (High‐End Computing Resource Oldenburg), located at the University of Oldenburg (Germany) and funded by the DFG through its Major Research Instrumentation Programme (INST 184/108‐1 FUGG) and the Ministry of Science and Culture (MWK) of the Lower Saxony State.
L. Albers, J. Baumgartner, C. Marschner, T. Müller, Chem. Eur. J. 2016, 22, 7970.
Contributor Information
Dr. Judith Baumgartner, Email: baumgartner@tugraz.at.
Prof. Christoph Marschner, Email: christoph.marschner@tugraz.at.
Prof. Thomas Müller, Email: thomas.mueller@uni-oldenburg.de.
References
- 1. Müller T. in Structure and Bonding, Vol. 155 (Ed.: Scheschkewitz), Springer, Heidelberg, 2014, pp. 107. [Google Scholar]
- 2. Müller T. in Science of Synthesis Knowledge Updates, Vol. 3 (Ed.: M. Oestreich) Thieme, Stuttgart, 2013, pp. 1. [Google Scholar]
- 3. Lambert J. B., Zhao Y., Angew. Chem. Int. Ed. Engl. 1997, 36, 400; [Google Scholar]; Angew. Chem. 1997, 109, 389. [Google Scholar]
- 4. Müller T., Zhao Y., Lambert J. B., Organometallics 1998, 17, 278. [Google Scholar]
- 5. Lambert J. B., Zhao Y., Wu H., Tse W. C., Kuhlmann B., J. Am. Chem. Soc. 1999, 121, 5001. [Google Scholar]
- 6. Kim K.-C., Reed C. A., Elliott D. W., Mueller L. J., Tham F., Lin L., Lambert J. B., Science 2002, 297, 825. [DOI] [PubMed] [Google Scholar]
- 7. Schäfer A., Reißmann M., Schäfer A., Saak W., Haase D., Müller T., Angew. Chem. Int. Ed. 2011, 50, 12636; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 12845. [Google Scholar]
- 8. Schäfer A., Reißmann M., Jung S., Schäfer A., Saak W., Brendler E., Müller T., Organometallics 2013, 32, 4713. [Google Scholar]
- 9.The only example of a characterized diarylalkylsilylium ion is bis(2,4,6-triisopropyl)phenylethyl silylium, see ref. [8].
- 10. Gerdes C., Saak W., Haase D., Müller T., J. Am. Chem. Soc. 2013, 135, 10353. [DOI] [PubMed] [Google Scholar]
- 11. Müller T., Jutzi P., Kühler T., Organometallics 2001, 20, 5619. [Google Scholar]
- 12. Jutzi P., Bunte E.-A., Angew. Chem. Int. Ed. Engl. 1992, 31, 1605; [Google Scholar]; Angew. Chem. 1992, 104, 1636. [Google Scholar]
- 13.To the best of our knowledge, the only report on an experimental detection of a secondary silylium ion is that of argon-tagged dimethylsilylium in the gas phase: DeBlase A. F., Scerba M. T., Lectka T., Johnson M. A., Chem. Phys. Lett. 2013, 568, 9–13. [Google Scholar]
- 14.All calculations were performed by using the Gaussian 09 program, Gaussian 09, Revision B.01, M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox, Gaussian, Inc., Wallingford CT, 2009.
- 15.For details, see the Supporting Information.
- 16. Arp H., Baumgartner J., Marschner C., Müller T., J. Am. Chem. Soc. 2011, 133, 5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arp H., Baumgartner J., Marschner C., Zark P., Müller T., J. Am. Chem. Soc. 2012, 134, 6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hlina J., Baumgartner J., Marschner C., Albers L., Müller T., Organometallics 2013, 32, 3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Review: Marschner C., Eur. J. Inorg. Chem. 2015, 3805. [Google Scholar]
- 20. Klare H. F. T., Oestreich M., Dalton Trans. 2010, 39, 9176. [DOI] [PubMed] [Google Scholar]
- 21. Stahl T., Klare H. F. T., Oestreich M., ACS Catal. 2013, 3, 1578. [Google Scholar]
- 22. Marschner C. in Structure and Bonding, Vol. 155 (Ed.: Scheschkewitz), Springer, Heidelberg, 2014,pp. 163. [Google Scholar]
- 23. Ishikawa M., Kumada M., J. Chem. Soc. D 1969, 567b. [Google Scholar]
- 24. Ishikawa M., Kumada M., J. Chem. Soc. D 1970, 157a. [Google Scholar]
- 25. Ishikawa M., Iyoda J., Ikeda H., Kotake K., Hashimoto T., Kumada M., J. Am. Chem. Soc. 1981, 103, 4845. [Google Scholar]
- 26. Ishikawa M., Watanabe M., Iyoda J., Ikeda H., Kumada M., Organometallics 1982, 1, 317. [Google Scholar]
- 27. Blinka T. A., West R., Organometallics 1986, 5, 128. [Google Scholar]
- 28. Fischer J., Baumgartner J., Marschner C., Science 2005, 310, 825. [DOI] [PubMed] [Google Scholar]
- 29. Wagner H., Wallner A., Fischer J., Flock M., Baumgartner J., Marschner C., Organometallics 2007, 26, 6704. [Google Scholar]
- 30. Fischer R., Baumgartner J., Kickelbick G., Hassler K., Marschner C., Monatsh. Chem. 2006, 137, 613. [Google Scholar]
- 31. Lambert J. B., Zhang S., J. Chem. Soc. Chem. Commun. 1993, 383. [Google Scholar]
- 32. Lambert J. B., Zhang S., Ciro S. M., Organometallics 1994, 13, 2430. [Google Scholar]
- 33. Ottosson C.-H., Cremer D., Organometallics 1996, 15, 5495. [Google Scholar]
- 34. Ottosson C.-H., Szabó K. J., Cremer D., Organometallics 1997, 16, 2377. [Google Scholar]
- 35. Nakamoto M., Fukawa T., Sekiguchi A., Chem. Lett. 2004, 33, 38. [DOI] [PubMed] [Google Scholar]
- 36. Wagner H., Baumgartner J., Müller T., Marschner C., J. Am. Chem. Soc. 2009, 131, 5022. [DOI] [PubMed] [Google Scholar]
- 37. Albers L., Meshgi M. A., Baumgartner J., Marschner C., Müller T., Organometallics 2015, 34, 3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ichinohe M., Igarashi M., Sanuki K., Sekiguchi A., J. Am. Chem. Soc. 2005, 127, 9978. [DOI] [PubMed] [Google Scholar]
- 39. Igarashi M., Ichinohe M., Sekiguchi A., J. Am. Chem. Soc. 2007, 129, 12660. [DOI] [PubMed] [Google Scholar]
- 40. Sekiguchi A., Matsuno T., Ichinohe M., J. Am. Chem. Soc. 2000, 122, 11250. [Google Scholar]
- 41. Inoue S., Ichinohe M., Yamaguchi T., Sekiguchi A., Organometallics 2008, 27, 6056. [Google Scholar]
- 42. Schäfer A., Reißmann M., Schäfer A., Schmidtmann M., Müller T., Chem. Eur. J. 2014, 20, 9381. [DOI] [PubMed] [Google Scholar]
- 43.Previous examples of compounds with Si−H−Si 2e–3c bonds have been found, for intramolecular variants, see:
- 43a. Müller T., Angew. Chem. Int. Ed. 2001, 40, 3033–3036; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2001, 113, 3123–3126; [Google Scholar]
- 43b. Panisch R., Bolte M., Müller T., J. Am. Chem. Soc. 2006, 128, 9676–9682; [DOI] [PubMed] [Google Scholar]
- 43c. Khalimon A. Y., Lin Z. H., Simionescu R., Vyboishchikov S. F., Nikonov G. I., Angew. Chem. Int. Ed. 2007, 46, 4530–4533; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2007, 119, 4614–4617; [Google Scholar]
- 43d. Sekiguchi A., Murakami Y., Fukaya N., Kabe Y., Chem. Lett. 2004, 33, 530–531; [Google Scholar]
- 43e. Kordts N., Borner C., Panisch R., Saak W., Müller T., Organometallics 2014, 33, 1492–1498; for intermolecular variants, see: [Google Scholar]
- 43f. Hoffmann S. P., Kato T., Tham F. S., Reed C. A., Chem. Commun. 2006, 767–769; [DOI] [PubMed] [Google Scholar]
- 43g. Nava M., Reed C. A., Organometallics 2011, 30, 4798–4800; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43h. Connelly S. J., Kaminsky W., Heinekey D. M., Organometallics 2013, 32, 7478–7481. [Google Scholar]
- 44. Krempner C., Polymers 2012, 4, 408. [Google Scholar]
- 45.The five calculated 29Si NMR chemical shifts of cation 8 correlate linearly with the experimental data measured in chlorobenzene: δ(29Si)calc=(1.044±0.038) ppm, δ(29Si)exp=(0.735±3.109) ppm; R 2=0.991.
- 46. Eaborn C., Lickiss P. D., Najim S. T., Stanczyk W. A., J. Chem. Soc. Chem. Commun. 1987, 1461. [Google Scholar]
- 47.The seven calculated 29Si NMR chemical shifts of cation 19 correlate linearly with the experimental data measured in chlorobenzene: Κ(29Si)calc=(1.006±0.052) ppm, δ(29Si)exp=(-2.744±3.114) ppm; R 2 = 0.984.
- 48. Massey A. G., Park A. J., J. Organomet. Chem. 1964, 2, 245. [Google Scholar]
- 49. Chien J. C. W., Tsai W. M., Rausch M. D., J. Am. Chem. Soc. 1991, 113, 8570. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


