Abstract
Background
Delayed initiation of antiretroviral therapy (ART) in eligible patients is a concern in resource-limited countries.
Methods
We analyzed data on HIV-positive patients ≥15 years enrolled at 41 ICAP-supported health care facilities in Rwanda, 2005–2010, to determine time to ART initiation among patients eligible at enrollment compared with those ineligible or of indeterminate eligibility who become eligible during follow-up. ART eligibility was based on CD4+ cell count (CD4+) and WHO staging; patients lacking CD4+ and WHO stage were considered indeterminate. Cumulative incidence of reaching ART eligibility and to ART initiation after eligibility was generated using competing risk estimators.
Results
A total of 31,033 ART-naive adults were enrolled; 64.2% were female. At enrollment, 10,158 (32.7%) patients were ART eligible, 13,372 (43.1%) were ineligible for ART, and 7503 (24.2%) patients were indeterminate. Among patients retained in care pre-ART eligibility, 17.9% [95% confidence interval (CI): 17.2 to 18.6] of ineligible and 22.8% (95% CI: 21.7 to 23.8) of indeterminate patients at enrollment reached ART eligibility within 12 months. Cumulative incidence of ART initiation within 3 months for patients eligible at enrollment was 77.2% (95% CI: 76.4 to 78.0) compared with 67.9% (95% CI: 66.4 to 69.3) for ineligible and 63.8% (95% CI: 61.9 to 65.8) for patients with indeterminate eligibility at enrollment (P < 0.05). Over the study period, there was more rapid ART initiation for patients who became ART eligible.
Conclusions
We found higher rates of ART initiation within 3 months among patients who were ART eligible at enrollment compared with those who reached eligibility during follow-up. From 2006 to 2011, earlier initiation of ART after eligibility was observed likely reflecting improved program quality.
Keywords: antiretroviral therapy, Rwanda, eligibility for treatment, CD4 cell count
BACKGROUND
Timely initiation of antiretroviral therapy (ART) for patients with HIV disease is critical for achieving optimal treatment outcomes. Late start of ART, after reaching advanced clinical and/or immunological disease status, is associated with increased risk and severity of opportunistic infections, as well as higher mortality.1–4 In 2010, the WHO updated its guidelines to recommend starting ART at CD4+ ≤350 cells per cubic millimeter, and in 2013, raised the recommended eligibility threshold to CD4+ ≤500 cells per cubic millimeter.5,6
In 2012, 9.7 million people in low- and middle-income countries were on treatment representing 65% of the global targets.7 Up to half of all patients in resource limited setting (RLS) enroll in care when they are at advanced stage of disease, often below the CD4+ cell count (CD4+) threshold for ART initiation and require ART upon enrollment in care.2,8,9 However, a substantial proportion of patients eligible for ART at enrollment do not have timely treatment initiation (defined variously as within 2 weeks to 6 months),10–13 and a significant proportion of such patients are lost to follow-up (LTF) or die before starting ART.9,13–15
In addition to rapid initiation of those immediately eligible for treatment, another important priority for HIV programs is retaining patients not eligible for treatment at enrollment to provide clinical and laboratory monitoring and to ensure receipt of appropriate intervention packages.16 Retention of patients before ART eligibility and initiation has been challenging, with studies demonstrating that healthier patients at earlier stages of HIV disease are more likely to be LTF compared with those who are ART eligible at enrollment.8,17,18 A recent meta-analysis of data on retention of patients not eligible for treatment at enrollment found that more than half were LTF before reaching eligibility or initiating ART.9 Loss of patients who are not eligible for treatment will result in many of these patients returning to care later with advanced disease leading to suboptimal treatment outcomes and death and may also result in missed opportunities for prevention interventions.3,4,19,20
The Rwanda National HIV Care and Treatment Program has achieved significant success in scaling up ART with 94% of eligible patients receiving treatment in 2012.7 Rwanda was also one of the first countries in sub-Saharan Africa to adopt a higher CD4+ threshold for ART eligibility, instituting ART initiation at CD4+ ≤350 cells per cubic millimeter in July of 2007.21 In this analysis, we examined time to ART eligibility among adult patients (≥15 years of age) and time to ART initiation among eligible patients receiving care at health facilities in Rwanda from 2005 to 2010.
METHODS
Ethics Statement
The data for this analysis are from the Identifying Optimal Models of HIV Care in Africa study, which uses de-identified routinely collected patient-level health information from electronic databases at participating health facilities. All data are de-identified at health facilities, and investigators have no access to identifiable patient information. The Optimal Models study was reviewed and approved by the Rwandan National Ethics Committee (RNEC), Columbia University Medical Center Institutional Review Board (IRB), US Centers for Disease Control and Prevention (CDC), and the US Office of the Global AIDS Coordinator (OGAC).
A longitudinal analysis was performed using routinely collected data on HIV-infected adults (≥15 years) who enrolled at 41 health facilities in Kigali and the western province of Rwanda between January 2005 and September 2010. Health facilities received support from ICAP at Columbia University, which worked with the Government of Rwanda to provide technical support to HIV prevention, care, and treatment services with funding from the President’s Emergency Plan for AIDS Relief (PEPFAR) through the US CDC. Patient-level data for the Optimal Models Study were collected by clinicians at participating health facilities during routine medical care. Information on deaths was collected from facility records. Trained data clerks at facilities entered medical records into on-site electronic databases, and biannual data quality assessments were conducted to review completeness and accuracy.
For analyses, patients were categorized into 3 groups (eligible, ineligible, and indeterminate) indicating eligibility for ART based on available clinical and immunological characteristics at the time of enrollment in HIV care. ART eligibility was defined using the Rwanda national guidelines that were in place at the date of enrollment for each patient (Table 1). Rwandan national guidelines changed in mid-2007 increasing the eligibility threshold from CD4+ 200 to 350 cells per cubic millimeter. To account for implementation of the guidelines change, for the analysis, the change was lagged to January 1, 2008.
TABLE 1.
Rwandan National ART Guidelines and Eligibility Group Definitions
| 2003–2007 | 2008–2010* | |
|---|---|---|
| Rwandan ART eligibility guidelines | WHO stage 4 regardless of CD4+ | WHO stage 4 regardless of CD4+ |
| CD4+ ≤200 | CD4+ ≤350 regardless of WHO stage | |
| CD4+ 200–350 and WHO stage 3 | ||
| Eligibility groups based on enrollment data | Eligible: see above Ineligible | Eligible: see above Ineligible |
| CD4+ >200 and WHO stage 1 or 2 | CD4+ >350 and WHO stage 1–3 | |
| CD4+ >350 and WHO stage 1–3 | ||
| Indeterminate | Indeterminate | |
| CD4+ 200–350 and missing WHO stage | CD4+ >350 and missing WHO stage | |
| WHO stage 1–3 and missing CD4+ | WHO stage 1–3 and missing CD4+ | |
| Missing both CD4+ and WHO stage | Missing both CD4+ and WHO stage |
Change in Rwanda national guidelines occurred in July 2007, lagged to January 1, 2008, for analysis purposes.
Data from follow-up visits after enrollment and before ART initiation were examined to identify when patients who were ineligible or indeterminate at enrollment reached ART eligibility using CD4+ and/or WHO stage and prevailing guidelines at subsequent visits (Table 1). Time from enrollment to reaching ART eligibility was calculated using the enrollment date and the visit date at which patients were identified as being ART eligible. Patients who were eligible for ART at enrollment and those who reached eligibility during follow-up were examined to assess the time from eligibility to ART initiation. Time from eligibility to ART initiation was calculated using the enrollment date and the ART initiation date for patients who were eligible for ART at enrollment in care, for patients who were ineligible or indeterminate at enrollment in care, the visit date when patients were identified as eligible for ART during follow-up was used. Incidence of LTF and death was estimated for 2 time periods: the period between enrollment and reaching ART eligibility (for patients ineligible and indeterminate at enrollment) and the period between ART eligibility and initiating ART (for patients who enrolled eligible and those who reached eligibility during follow-up). LTF for the analysis was defined as not attending a clinical care visit within 12 months (guidelines called for CD4 monitoring every 6 months before ART initiation); deaths were ascertained from patient charts.
Cumulative incidence of reaching ART eligibility and of initiating ART after reaching eligibility was calculated using competing risk estimators. For the outcome of reaching ART eligibility, death and starting ART before reaching ART eligibility were treated as competing risks; for the outcome of ART initiation after reaching eligibility, only death was treated as a competing risk. Cumulative incidence of initiating ART after reaching ART eligibility was compared across the 3 groups defined by ART eligibility status at enrollment in care (ie, eligible, ineligible, and indeterminate for ART). Cumulative incidence of ART initiation was also compared by calendar year (2005–2010) (for these analyses, ineligible and indeterminate groups were combined). Competing risks regression was applied to estimate subdistributional hazard ratios (SHRs) for the association between patient- and facility-level factors and ART initiation. Multivariable models were adjusted for age, gender, point of entry into care, CD4+, and WHO stage at ART eligibility, facility type, and setting (urban/rural). Median CD4+ at enrollment and at ART initiation was compared across eligibility groups and over time using Kruskal–Wallis tests and Spearman correlation coefficients. Age and CD4 were modeled as categorical variables in statistical models. All statistical analyses were performed using SAS 9.3 (SAS, Cary, NC) and Stata 12.1 (StataCorp LP, College Station, TX).
RESULTS
A total of 31,033 HIV-infected ART-naive patients ≥ 15 years from 41 health facilities in Rwanda were included. The median age at enrollment was 34.2 years [interquartile range (IQR): 27.8–41.8], and the majority of patients were female (64.2%) (Table 2). Most patients (62.1%) were enrolled through voluntary counseling and testing (VCT) services. Roughly half of the patients (55.4%) were enrolled at health facilities in urban areas. At enrollment, 80.8% of the patients had WHO disease stage information recorded, 3 quarters (75.4%) were stage I or II, and only 2.3% were in stage IV. Among patients with enrollment CD4+ (85.2%), more than half (54.6%) had CD4+ >350 cells per cubic millimeter and 23.7% had CD4+ <200 cells per cubic millimeter (Table 2). Based on enrollment CD4+ and WHO stage, 10,158 (32.7%) patients were ART eligible, 13,372 (43.1%) were ineligible for ART, and 7503 (24.2%) patients had insufficient information to determine eligibility for ART and were considered indeterminate (see Supplemental Figure 1, http://links.lww.com/QAI/A592).
TABLE 2.
Characteristics at Enrollment for HIV-Infected Adult Patients (≥15 Years) Enrolled in Care at 41 ICAP-Supported Health Facilities in Rwanda 2005–2010 (N = 31,033)
| Adult Patients | Total
|
Eligible
|
Ineligible
|
Indeterminate
|
||||
|---|---|---|---|---|---|---|---|---|
| N = 31,033 | 100.0% | N = 10,158 | 32.7% | N = 13,372 | 43.1% | N = 7503 | 24.2% | |
| Age, yrs: median (IQR) | 34.2 (27.8–41.8) | 36.6 (30.4–43.7) | 32.8 (26.7–40.5) | 33.3 (27.0–40.8) | ||||
| Age category, yrs | ||||||||
| 15–20 | 931 | 3.0 | 191 | 1.9 | 491 | 3.7 | 249 | 3.3 |
| 21–30 | 9494 | 30.6 | 2214 | 21.8 | 4736 | 35.4 | 2544 | 33.9 |
| 31–40 | 11,205 | 36.1 | 3946 | 38.9 | 4613 | 34.5 | 2646 | 35.3 |
| 41–50 | 6623 | 21.3 | 2662 | 26.2 | 2464 | 18.4 | 1497 | 20.0 |
| 51–60 | 2139 | 6.9 | 883 | 8.7 | 824 | 6.2 | 432 | 5.8 |
| >60 | 641 | 2.1 | 262 | 2.6 | 244 | 1.8 | 135 | 1.8 |
| Sex | ||||||||
| Male | 11,107 | 35.8 | 4358 | 42.9 | 4214 | 31.5 | 2535 | 33.8 |
| Female | 19,926 | 64.2 | 5800 | 57.1 | 9158 | 68.5 | 4968 | 66.2 |
| Marital status | ||||||||
| Missing | 7857 | 25.3 | 2255 | 22.2 | 3467 | 25.9 | 2135 | 28.5 |
| Single | 3602 | 15.5 | 1216 | 15.4 | 1528 | 15.4 | 858 | 16.0 |
| Married/in union | 13,701 | 59.1 | 4391 | 55.6 | 6136 | 62.0 | 3174 | 59.1 |
| Widowed | 4625 | 20 | 1848 | 23.4 | 1712 | 17.3 | 1065 | 19.8 |
| Divorced or separated | 1248 | 5.4 | 448 | 5.7 | 529 | 5.3 | 271 | 5.1 |
| Point of entry into care | ||||||||
| VCT | 19,267 | 62.1 | 6493 | 63.9 | 8222 | 61.5 | 4552 | 60.7 |
| PMTCT | 6332 | 20.4 | 1363 | 13.4 | 3254 | 24.3 | 1715 | 22.9 |
| Inpatient/TB/HIV | 1679 | 5.4 | 925 | 9.1 | 452 | 3.4 | 302 | 4.0 |
| Outpatient | 1395 | 4.5 | 648 | 6.4 | 541 | 4.1 | 206 | 2.8 |
| Other/unknown | 2360 | 7.6 | 729 | 7.2 | 903 | 6.8 | 728 | 9.7 |
| WHO stage | ||||||||
| Missing | 5945 | 19.2 | 1562 | 15.4 | — | — | 4383 | 58.4 |
| Stage I | 11,555 | 46.1 | 2280 | 26.5 | 7830 | 58.6 | 1445 | 46.3 |
| Stage II | 7344 | 29.3 | 2324 | 27.0 | 4150 | 31.0 | 870 | 27.9 |
| Stage III | 5624 | 22.4 | 3427 | 39.9 | 1392 | 10.4 | 805 | 22.4 |
| Stage IV | 565 | 2.3 | 565 | 6.6 | — | — | — | — |
| CD4+ cell count, median (IQR) | 385 (208–605) | 167 (91–251) | 539 (408–730) | 557 (417–745) | ||||
| Missing | 4583 | 14.8 | 86 | 0.9 | — | — | 4493 | 59.9 |
| <100 | 2782 | 10.5 | 2782 | 27.4 | — | — | — | — |
| 100–199 | 3498 | 13.2 | 3498 | 34.4 | — | — | — | — |
| 200–350 | 5738 | 21.7 | 3711 | 36.5 | 1683 | 12.6 | 344 | 4.6 |
| 350+ | 14,432 | 54.6 | 81 | 0.8 | 11,689 | 87.4 | 2666 | 35.5 |
| Calendar year of enrollment into care | ||||||||
| 2005 | 4980 | 16.1 | 1503 | 14.8 | 2130 | 16.0 | 1347 | 18.0 |
| 2006 | 6423 | 20.7 | 1764 | 17.4 | 2791 | 21.0 | 1868 | 25.0 |
| 2007 | 6290 | 20.3 | 1979 | 19.5 | 2602 | 19.5 | 1709 | 22.8 |
| 2008 | 5435 | 17.5 | 1998 | 19.7 | 2197 | 16.4 | 1240 | 16.5 |
| 2009 | 4892 | 15.8 | 1819 | 17.9 | 2145 | 16.0 | 928 | 12.4 |
| 2010 | 3013 | 9.7 | 1095 | 10.8 | 1507 | 11.3 | 411 | 5.5 |
| Setting | ||||||||
| Urban city | 17,197 | 55.4 | 5857 | 57.7 | 7207 | 53.9 | 4133 | 55.1 |
| Rural | 13,836 | 44.6 | 4301 | 42.3 | 6165 | 46.1 | 3370 | 44.9 |
| Facility type | ||||||||
| Primary | 9870 | 31.8 | 2958 | 29.1 | 4013 | 30.0 | 2899 | 38.6 |
| Secondary | 14,304 | 46.1 | 4794 | 47.2 | 6117 | 45.7 | 3393 | 45.2 |
| Others | 6859 | 22.1 | 2406 | 23.7 | 3242 | 24.2 | 1211 | 16.1 |
Period Between Enrollment in Care and Reaching ART Eligibility
The cumulative incidence of LTF at 12 months after enrollment for patients ineligible for ART at enrollment was 10.1% [95% confidence interval (CI): 9.5 to 10.6] and 13.8% (95% CI: 13.0 to 14.6) for those indeterminate at enrollment (Table 3). At 12 months after enrollment, the cumulative incidence of mortality was 1.2% (95% CI: 1.0 to 1.4) for ineligible patients and 3.2% (95% CI: 2.8 to 3.6) for those who were indeterminate at enrollment. Among patients retained in care, 17.9% (95% CI: 17.2 to 18.6) of ineligible and 22.8% (95% CI: 21.7 to 23.8) of indeterminate patients reached eligibility for ART within 12 months of enrollment (Table 3).
TABLE 3.
Cumulative Incidence of Reaching ART Eligibility, LTF, Mortality and ART Initiation by Enrollment Eligibility Group Among Adult Patients Enrolled in Care at 41 Rwandan Health Facilities (N = 31,033)
| Enrollment Eligibility Groups
|
|||
|---|---|---|---|
| Eligible | Ineligible | Indeterminate | |
| Period between enrollment and reaching ART eligibility | |||
| LTF: 6 mo | N/A | 7.2% (6.8 to 7.7) | 11.7% (10.9 to 12.4) |
| LTF: 12 mo | N/A | 10.1% (9.5 to 10.6) | 13.8% (13.0 to 14.6) |
| Mortality: 6 mo | N/A | 0.6% (0.5 to 0.8) | 2.2% (1.9 to 2.6) |
| Mortality: 12 mo | N/A | 1.2% (1.0 to 1.4) | 3.2% (2.8 to 3.6) |
| Reached ART eligibility: 6 mo | N/A | 8.0% (7.6 to 8.5) | 12.9% (12.1 to 13.7) |
| Reached ART eligibility: 12 mo | N/A | 17.9% (17.2 to 18.6) | 22.8% (21.7 to 23.8) |
| Period between ART eligibility and ART initiation | |||
| LTF: 6 mo | 2.7% (2.4 to 3.1) | 1.5% (1.2 to 1.9) | 1.5% (1.1 to 2.1) |
| LTF: 12 mo | 3.1% (2.7 to 3.4) | 2.1% (1.7 to 2.6) | 2.2% (1.6 to 2.8) |
| Mortality: 6 mo | 2.8% (2.5 to 3.2) | 0.6% (0.4 to 1.0) | 1.3% (0.9 to 1.8) |
| Mortality: 12 mo | 3.3% (2.9 to 3.6) | 0.7% (0.5 to 1.0) | 1.5% (1.0 to 2.1) |
| Initiated ART: 1 mo (30 days) | 53.0% (52.0 to 54.0) | 41.5% (40.0 to 43.0) | 40.8% (38.8 to 42.7) |
| Initiated ART: 3 months (90 days) | 77.2% (76.4 to 78.0) | 67.9% (66.4 to 69.3) | 63.8% (61.9 to 65.8) |
| Initiated ART: 6 months (180 days) | 83.2% (82.4 to 83.9) | 77.2% (75.8 to 78.5) | 74.0% (72.0 to 75.7) |
| Initiated ART: 12 months (365 days) | 87.6% (86.9 to 88.2) | 85.5% (84.3 to 86.5) | 83.7% (82.0 to 85.1) |
All values are represented as cumulative incidence (95% CI).
Period Between ART Eligibility and ART Initiation
Cumulative incidence of LTF and mortality in the period between ART eligibility and ART initiation are also shown in Table 3. In patients who were eligible for ART at enrollment, LTF and mortality at 6 months after enrollment but before ART initiation were 2.7% (95% CI: 2.4 to 3.1) and 2.8% (95% CI: 2.5 to 3.2), respectively. Among patients who were ineligible at enrollment, 1.5% (95% CI: 1.2 to 1.9) were LTF and 0.6% (95% CI: 0.4 to 1.0) had died 6 months after reaching ART eligibility but before starting ART (Table 3). Cumulative incidence of LTF and morality 6 months after reaching ART eligibility in patients who were indeterminate at enrollment were 1.5% (95% CI: 1.1 to 2.1) and 1.3% (95% CI: 0.9 to 1.8), respectively (Table 3).
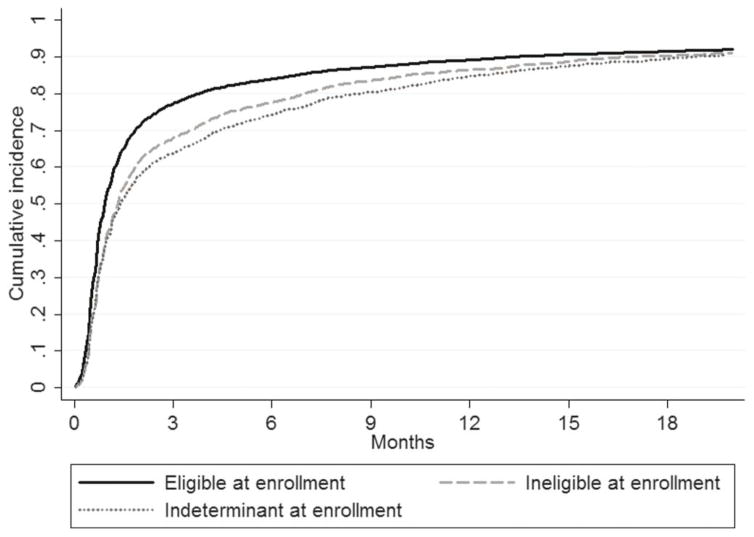
Overall, more than 80% of patients who were eligible at enrollment or reached eligibility during follow-up initiated ART within 12 months (Fig. 1). Among patients who were eligible for treatment at enrollment, 53.0% (95% CI: 52.0 to 54.0) started ART within 1 month of enrollment and 77.2% (95% CI: 76.4 to 78.0) by 3 months after enrollment (Table 3). For patients who were ineligible at enrollment (and reached ART eligibility), 41.5% (95% CI: 40.0 to 43.0) and 67.9% (95% CI: 66.4 to 69.3) initiated ART within 1 and 3 months of reaching ART eligibility, respectively. For patients who were indeterminate at enrollment, 40.8% (95% CI: 38.8 to 42.7) and 63.8% (95% CI: 61.9 to 65.8) started treatment within 1 and 3 months of reaching ART eligibility, respectively (Table 3).
FIGURE 1.
Cumulative incidence of ART initiation after date of ART eligibility among Rwandan adult patients who were eligible at enrollment and those who were ineligible or indeterminate at enrollment and who reached eligibility (N = 17,926).
In the analysis of ART initiation rates over time, patients who were ART eligibile at enrollment in care or reached ART initiation from 2008 to 2010 had significantly faster ART initiation compared with those who enrolled in care between 2005 and 2007 (P < 0.0001) (see Supplemental Figure 3, http://links.lww.com/QAI/A592). ART initiation within 3 months of enrollment among patients who were eligible at enrollment increased from 75.5% (95% CI: 73.2 to 77.7) in 2005 to 84.6% (95% CI: 84.7) in 2010. Among patients who were ineligible or indeterminate at enrollment (combined) and reached ART eligibility during follow-up, cumulative incidence of ART initiation within 3 months of reaching eligibility increased from 52.3% (95% CI: 44.6 to 61.2) to 81.5% (95% CI: 78.53 to 84.0) from 2005 to 2010 (see Supplemental Figure 4, http://links.lww.com/QAI/A592).
Median CD4+ at enrollment in care increased in all groups from 2005 to 2010 (see Supplemental Figure 5, http://links.lww.com/QAI/A592) and over time the proportion of all enrolled patients in care with CD4+ <200 cells per cubic millimeter at enrollment declined from 23.1% in 2005 to 17.2% in 2010 (data not shown). Among patients who started ART, regardless of eligibility status at enrollment, median CD4+ at ART initiation increased over time: among patients eligible for ART at enrollment, the median CD4+ at ART initiation increased from 128 in 2005 to 216 cells per cubic millimeter in 2010 (P < 0.0001); for patients who were ineligible or indeterminate at enrollment (combined) and started ART after reaching eligibility, median CD4+ at ART initiation increased from 208 in 2005 to 314 cells per cubic millimeter in 2010 (P < 0.0001) (see Supplemental Figure 5, http://links.lww.com/QAI/A592). The median CD4+ at ART initiation in the eligible at enrollment group remained lower than for the ineligible and indeterminate groups across all years.
In multivariable modeling of predictors of ART initiation for all patients who enrolled eligible or reached ART eligibility during follow-up, women and younger patients were significantly less likely to start ART overall [female- vs. male-adjusted SHR (aSHR): 0.8, 95% CI: 0.8 to 0.9; 15–20 vs. 31–40 years aSHR: 0.8, 95% CI: 0.8 to 0.9; and 21–30 vs. 31–40 years aSHR: 0.9, 95% CI: 0.8 to 0.9] (Table 4). Enrolling in care through inpatient wards was associated with lower incidence of ART initiation compared with patients enrolled through VCT (inpatient vs. VCT aSHR: 0.8, 95% CI: 0.7 to 0.9). Patients with CD4+ <200 cells per cubic millimeter were more likely to start ART compared with patients with CD4+ >350 cells per cubic millimeter (CD4+ < 100 cells/mm3 vs. >350 aSHR 2.8, 95% CI: 1.7 to 4.5; CD 100–199 cells/mm3 vs. >350 aSHR 2.8, 95% CI: 1.7 to 4.4).
TABLE 4.
Predictors of Initiating ART Among Adult Rwandan Patients Eligible at Enrollment and Among Those Reaching Eligibility During Follow-up (Ineligible and Indeterminate at Enrollment) (N = 17,926)
| Adult Patients | Univariate
|
Multivariate
|
|---|---|---|
| Subdistributional Hazard Ratio (95% CI) | Subdistributional Hazard Ratio (95% CI) | |
| Female | 0.9 (0.9 to 1.0) | 0.8 (0.8 to 0.9) |
| Age at ART Initiation, yrs | ||
| 15–20 | 0.9 (0.8 to 1.0) | 0.8 (0.7 to 0.9) |
| 21–30 | 0.9 (0.9 to 0.9) | 0.9 (0.8 to 0.9) |
| 31–40 | 1 (reference) | 1 (reference) |
| 41–50 | 1.0 (1.0 to 1.1) | 1.1 (1.0 to 1.1) |
| 51–60 | 1.1 (1.0 to 1.2) | 1.2 (1.1 to 1.3) |
| >60 | 1.1 (1.0 to 1.2) | 1.1 (1.0 to 1.3) |
| Point of entry into care | ||
| VCT | 1 (reference) | 1 (reference) |
| PMTCT | 1 (0.9 to 1.1) | 1.0 (1.0 to 1.1) |
| Inpatient/TB/HIV | 08 (0.7 to 1.0) | 0.8 (0.6 to 0.9) |
| Outpatient | 1.0 (0.9 to 1.1) | 0.8 (0.8 to 1.0) |
| Other/unknown | 0.8 (0.6 to 0.9) | 0.9 (0.8 to 1.0) |
| Marital status | ||
| Single | 0.9 (0.8 to 1.0) | 0.9 (0.9 to 1.00) |
| Married/in union | 1 (reference) | 1 (reference) |
| Divorced/separate | 0.9 (0.8 to 1.0) | 0.9 (0.8 to 1.0) |
| Widowed | 1.0 (1.0 to 1.1) | 1.0 (0.9 to 1.0) |
| Missing | 0.8 (0.7 to 0.9) | 0.8 (0.7 to 1.0) |
| WHO stage when reaching ART eligibility | ||
| Stage I | 1 (reference) | 1 (reference) |
| Stage II | 1.1 (1.1 to 1.2) | 1.1 (1.0 to 1.2) |
| Stage III | 1.0 (0.9 to 1.2) | 1.1 (1.0 to 1.1) |
| Stage IV | 0.8 (06 to 0.9) | 1.3 (0.9 to 2.0) |
| Missing | — | — |
| CD4+ cell count when reaching ART eligibility | ||
| <100 | 1.99 (1.5 to 2.7) | 3.0 (1.9 to 4.8) |
| 100–199 | 2.00 (1.5 to 2.7) | 3.0 (1.9 to 4.8) |
| 200–350 | 1.71 (1.2 to 2.4) | 2.1 (1.3 to 3.3) |
| >350 | 1 (reference) | 1 (reference) |
| Missing | — | — |
| Calendar year reaching ART eligibility | ||
| 2005 | 1 (reference) | 1 (reference) |
| 2006 | 1.0 (0.9 to 1.3) | 0.9 (0.8 to 1.1) |
| 2007 | 0.9 (0.7 to 1.0) | 0.8 (0.7 to 1.0) |
| 2008 | 1.27 (1.1 to 1.4) | 1.2 (1.0 to 1.4) |
| 2009 | 1.34 (1.2 to 1.5) | 1.3 (1.1 to 1.5) |
| 2010 | 1.34 (1.2 to 1.5) | 1.3 (1.1 to 1.6) |
| Enrollment ART eligibility | ||
| Eligible | 1.2 (1.1 to 1.3) | — |
| Ineligible | 1 (reference) | — |
| Indeterminate | 1.0 (0.9 to 1.0) | — |
| Primary facility vs. others | 1.0 (0.9 to 1.2) | 0.9 (0.8 to 1.1) |
| Rural vs. urban/semiurban | 1.2 (1.1 to 1.4) | 1.2 (1.0 to 1.3) |
DISCUSSION
In this large cohort of adults with HIV in Rwanda, a high proportion of patients eligible for ART at enrollment and patients who reached eligibility during follow-up initiated treatment. Among those meeting ART eligibility criteria at enrollment, 77.2% started ART within 3 months (90 days) and 83.2% had started treatment within 6 months. In contrast, for patients who were ineligible at enrollment and reached ART eligibility, 67.8% started treatment within 3 months of reaching eligibility and 77.2% initiated within 6 months. Eighty percent of all patients who enrolled eligible or reached eligibility started ART within 12 months. Although rates of ART initiation were relatively high for all years, from 2005 to 2010 there was a significant increase in the proportion of patients started on ART within 1 and 3 months. A trend over time was also observed for higher CD4+ at ART initiation among patients, although CD4+ at ART initiation for patients who were eligible for treatment at enrollment remained low.
The findings from this study differ from those reported from other African countries. Systematic reviews of cohort data from 12 countries in sub-Saharan Africa examining ART initiation among eligible patients have found that only between 63%8 and 68%10 of eligible patients started treatment, with median time from reaching eligibility to ART initiation ranging from 22 to 208 days.15,22–24 Data from a North American cohort of over 9000 patients enrolled in care between 2001 and 2009 showed that only 57% started ART within 6 months of reaching eligibility.12 In a previous analysis of patients from 8 health facilities in Rwanda, Kayigamba et al11 found that among 81 patients eligible for treatment only 56% initiated ART within 90 days. The previous report from Rwanda showing lower proportions of patients initiating treatment focused on a smaller number of patients (482) from 8 health facilities over an observation period of only 3 months, which could account for the differences between our findings.
Patients in this cohort from Rwanda had relatively high CD4+ at enrollment in comparison with other African cohorts. Across 29 treatment programs in sub-Saharan Africa, Mugglin et al8 found median CD4+ at enrollment ranging from 154 (IQR: 57–302) to 274 cells per cubic millimeter (IQR: 139–435). In a separate analysis of sub-Saharan African HIV treatment programs, the proportion of patients eligible for ART at enrollment was 41% (CD4+ ≤200 cells/mm3) and 57% (CD4+ ≤350 cells/mm3).9 In contrast, median CD4+ at enrollment was 385 cells per cubic millimeter overall in our cohort, and more than half of the patients were not eligible for treatment at enrollment in care based on national guidelines. The high proportion of patients at earlier stages of HIV disease at enrollment into care suggests good availability of HIV testing in Rwanda and successful efforts to reach and enroll asymptomatic individuals into care after HIV diagnosis. It should also be noted, however, that despite the overall good health status of this cohort, almost a third of patients were eligible for ART at the time of enrollment in HIV care, 60% of whom had CD4+ <200 cells per cubic millimeter. Late enrollment in HIV care remains a significant challenge for many treatment programs. A decrease in late enrollment in care over time was observed in this Rwandan cohort; from 2005 to 2010, there was a reduction in the proportion of patients enrolling in care with CD4+ <200 cells per cubic millimeter from 23.1% to 17.2%.
Although the cumulative incidence of ART initiation was similar at 12 months among the 3 groups defined by ART eligibility status at enrollment in care, patients who were eligible for ART at enrollment were found to have higher rates of ART initiation at 1 and 3 months compared with patients who were ineligible or indeterminate at enrollment. It is possible that this finding reflects prioritization of sicker patients for ART initiation as patients who were eligible for ART at enrollment had significantly lower CD4+ at ART initiation and may have been rapidly started on treatment as a result. Indeed, across all enrollment eligibility groups, patients with lower CD4+ at ART eligibility had higher rates of starting treatment, which further suggest that sicker patients were prioritized for ART initiation.
Our analysis showed that over time higher proportions of patients were initiated on ART within 1 and 3 months from date of eligibility. This faster ART initiation may be related to the change in Rwanda’s national ART eligibility guidelines that were introduced in July 2007.21 Starting at that time, all adults with CD4+ ≤350 cells per cubic millimeter were considered eligible for ART regardless of WHO stage, whereas previously patients with CD4+ 200–350 cells per cubic millimeter were eligible only if they were at WHO stage III. The simplification of guidelines may have facilitated identification of those who were ART eligible resulting in more timely treatment initiation. It is also possible that the improvement in time to ART initiation may be the result of the maturation of Rwanda’s National ART Program and improved quality of service delivery over time. Although this analysis was not designed to identify the factors that contributed to improvements in time to ART initiation, our data are further evidence of the strength of the Rwandan National ART program and may provide support for the beneficial impact of simplified treatment eligibility guidelines.
As noted, our analysis included a large number of patients who were not eligible for ART at enrollment, which allowed us to examine pre-ART patient outcomes in the period before and after ART eligibility. There are few reports of outcomes for patients in RLS in the time before they reach ART eligibility. Findings from South Africa indicate that only 43% of patients who were ineligible for treatment at enrollment were retained in pre-ART care at 12 months.25 In our cohort, less than 14% of patients who were ineligible or indeterminate at enrollment were LTF in the period before they reached ART eligibility. These low rates of pre-ART LTF are consistent with previous analyses from Rwanda showing good patient retention.26 We found even lower rates of LTF and mortality in the period after ART eligibility but before ART initiation, which is likely a result of the high proportions of patients who were initiated within 6 months of reaching eligibility for treatment. Our findings among eligible patients contrast those from other African countries, which have shown higher rates of LTF and mortality among patients in need of ART. Recent data from Uganda showed that 21.3% of eligible patients who did not initiate ART were LTF at 12 months and cumulative incidence of death was 7.7%.27 In Nigeria, 39% of patients who did not initiate treatment by 90 days after enrollment were LTF by 12 months and 7% died.13
The strengths of this analysis include the large and representative cohort; the 31,033 HIV-infected ART-naive adults included in this analysis represent 24% of all adult patients enrolled in care in Rwanda between 2005 and 2010. Patients in the analysis came from 41 different health facilities ranging in size from primary health clinics to large district hospitals and were located in both rural and urban areas. The use of routinely collected data from HIV care and treatment programs is both an asset and limitation of this analysis. Although highly representative of actual care in Rwanda, the data do not include variables of interest, such as viral load and patient demographic characteristics that might be important predictors of ART initiation, such as distance of residence from health facility. In addition, missing values for determining ART eligibility at enrollment were another limitation with 24.2% of patients being grouped as indeterminate for ART eligibility. Although the indeterminate group appeared very similar to the ineligible group in terms of patient characteristics, they are likely a mix of both ineligible and eligible patients, as indicated by the more rapid time to ART eligibility in the indeterminate group (compared with the ineligible group). Furthermore, we do not know the outcomes of patients who transferred or were LTF, and it is likely that we have underestimated mortality as a result of under-ascertainment of deaths.
Overall, we found high rates of ART initiation among patients who were eligible for treatment at enrollment and among those who reached eligibility during follow-up in this large cohort of adult patients from Rwanda. Loss to follow-up and mortality before ART initiation were low. We also observed an improvement in time to ART initiation from 2005 to 2010, which may reflect changes to national ART eligibility guidelines and the accumulation of experience and improvements in service delivery within the Rwandan National ART Program. Late enrollment in care remains a challenge, with one-third of patients enrolling in care eligible for treatment. The results from this large cohort in Rwanda are further evidence that HIV care and treatment programs can achieve high rates of retention and ensure that patients who are in need of treatment receive it.
Supplementary Material
Acknowledgments
Supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention under the terms of Cooperative Agreement Nos. 5U62PS223540 and 5U2GPS001537. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of PEPFAR or the Centers for Disease Control and Prevention.
The authors thank the patients and staff at participating health centers and the ICAP-Rwanda team. They also acknowledge the Rwanda Biomedical Center and Rwandan Ministry Health for their guidance and support. In addition, the authors thank the US Centers for Disease Control and Prevention for technical support and funding.
Footnotes
Presented at the International Conference on AIDS and Sexually Transmitted Infections in Africa (ICASA), December 7–11, 2013, Cape Town, South Africa.
The authors have no conflicts of interest to disclose.
C.A.T., C.W., W.M.E.-S., and E.J.A. contributed to design and analytic approach; C.W. and C.A.T. conducted analyses; all authors contributed to manuscript writing.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.jaids.com).
References
- 1.Strategies for Management of Antiretroviral Therapy Study. Emery S, Neuhaus JA, Phillips AN, et al. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008;197:1133–1144. doi: 10.1086/586713. [DOI] [PubMed] [Google Scholar]
- 2.Lawn SD, Harries AD, Anglaret X, et al. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.When To Start Consortium. Sterne JA, May M, Costagliola D, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–1363. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Severe P, Juste MA, Ambroise A, et al. Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med. 2010;363:257–265. doi: 10.1056/NEJMoa0910370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. Rapid Advice: Antiretroviral Therapy for HIV Infection in Adults and Adolescents. Geneva, Switzerland: HIV/AIDS, World Health Organization; 2009. [Google Scholar]
- 6.WHO. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Geneva, Switzerland: HIV/AIDS, World Health Organization; 2013. [PubMed] [Google Scholar]
- 7.WHO. Global Update on HIV Treatment 2013: Results, Impact and Opportunities. Geneva, Switzerland: WHO; 2013. [Google Scholar]
- 8.Mugglin C, Estill J, Wandeler G, et al. Loss to programme between HIV diagnosis and initiation of antiretroviral therapy in sub-Saharan Africa: systematic review and meta-analysis. Trop Med Int Health. 2012;17:1509–1520. doi: 10.1111/j.1365-3156.2012.03089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kranzer K, Govindasamy D, Ford N, et al. Quantifying and addressing losses along the continuum of care for people living with HIV infection in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2012;15:17383. doi: 10.7448/IAS.15.2.17383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med. 2011;8:e1001056. doi: 10.1371/journal.pmed.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kayigamba FR, Bakker MI, Fikse H, et al. Patient enrolment into HIV care and treatment within 90 days of HIV diagnosis in eight Rwandan health facilities: a review of facility-based registers. PLoS One. 2012;7:e36792. doi: 10.1371/journal.pone.0036792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanna DB, Buchacz K, Gebo KA, et al. Trends and disparities in antiretroviral therapy initiation and virologic suppression among newly treatment-eligible HIV-infected individuals in North America, 2001–2009. Clin Infect Dis. 2013;56:1174–1182. doi: 10.1093/cid/cit003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aliyu MH, Blevins M, Parrish DD, et al. Risk factors for delayed initiation of combination antiretroviral therapy in rural North Central Nigeria. J Acquir Immune Defic Syndr. 2013;65:e41–9. doi: 10.1097/QAI.0b013e31829ceaec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geng EH, Glidden DV, Bangsberg DR, et al. A causal framework for understanding the effect of losses to follow-up on epidemiologic analyses in clinic-based cohorts: the case of HIV-infected patients on antiretroviral therapy in Africa. Am J Epidemiol. 2012;175:1080–1087. doi: 10.1093/aje/kwr444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGrath N, Glynn JR, Saul J, et al. What happens to ART-eligible patients who do not start ART? Dropout between screening and ART initiation: a cohort study in Karonga, Malawi. BMC Public Health. 2010;10:601. doi: 10.1186/1471-2458-10-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO. Essential Prevention and Care Interventions for Adults and Adolescents Living With HIV in Resource-Limited Settings. Geneva, Swizerland: WHO; 2008. [Google Scholar]
- 17.Lessells RJ, Mutevedzi PC, Cooke GS, et al. Retention in HIV care for individuals not yet eligible for antiretroviral therapy: rural KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr. 2011;56:e79–86. doi: 10.1097/QAI.0b013e3182075ae2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mugisha V, Teasdale CA, Wang C, et al. Determinants of mortality and loss to follow-up among adults enrolled in HIV care services in Rwanda. PLoS One. 2014;9:e85774. doi: 10.1371/journal.pone.0085774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher JD, Cornman DH, Osborn CY, et al. Clinician-initiated HIV risk reduction intervention for HIV-positive persons: Formative Research, Acceptability, and Fidelity of the Options Project. J Acquir Immune Defic Syndr. 2004;37:S78–S87. doi: 10.1097/01.qai.0000140605.51640.5c. [DOI] [PubMed] [Google Scholar]
- 20.Rose CD, Courtenay-Quirk C, Knight K, et al. HIV intervention for providers study: a randomized controlled trial of a clinician-delivered HIV risk-reduction intervention for HIV-positive people. J Acquir Immune Defic Syndr. 2010;55:572–581. doi: 10.1097/QAI.0b013e3181ee4c62. [DOI] [PubMed] [Google Scholar]
- 21.Rwanda MoH. Guide de Prise en Charge de Personnes Infectees par le HIV au Rwanda. Kigali, Rwanda: Ministry of Health, Rwanda; 2007. [Google Scholar]
- 22.Lawn SD, Myer L, Harling G, et al. Determinants of mortality and nondeath losses from an antiretroviral treatment service in South Africa: implications for program evaluation. Clin Infect Dis. 2006;43:770–776. doi: 10.1086/507095. [DOI] [PubMed] [Google Scholar]
- 23.Murphy RA, Sunpath H, Taha B, et al. Low uptake of antiretroviral therapy after admission with human immunodeficiency virus and tuberculosis in KwaZulu-Natal, South Africa. Int J Tuberc Lung Dis. 2010;14:903–908. [PMC free article] [PubMed] [Google Scholar]
- 24.Bassett IV, Wang B, Chetty S, et al. Loss to care and death before antiretroviral therapy in Durban, South Africa. J Acquir Immune Defic Syndr. 2009;51:135–139. doi: 10.1097/qai.0b013e3181a44ef2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clouse K, Pettifor AE, Maskew M, et al. Patient retention from HIV diagnosis through one year on antiretroviral therapy at a primary healthcare clinic in Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2012;62:e39–46. doi: 10.1097/QAI.0b013e318273ac48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rich ML, Miller AC, Niyigena P, et al. Excellent clinical outcomes and high retention in care among adults in a community-based HIV treatment program in rural Rwanda. J Acquir Immune Defic Syndr. 2012;59:e35–42. doi: 10.1097/QAI.0b013e31824476c4. [DOI] [PubMed] [Google Scholar]
- 27.Namusobya J, Semitala FC, Amanyire G, et al. High retention in care among HIV-infected patients entering care with CD4 levels >350 cells/μL under routine program conditions in Uganda. Clin Infect Dis. 2013;57:1343–1350. doi: 10.1093/cid/cit490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.