Abstract
Background
Striatal dopamine (DA) has been implicated in alcohol use disorders, but it is still unclear whether or not alcohol can induce dopamine release in social drinkers. Furthermore, no data exist on dopamine responses to alcohol in dependent drinkers. We sought to characterize the DA responses to alcohol intoxication in moderately large samples of social drinkers (SD) and nontreatment-seeking alcoholics (NTS).
Methods
Twenty-four SD and twenty-one NTS received two [11C]raclopride (RAC) PET scans; one at rest, and one during an intravenous alcohol infusion, with a prescribed ascent to a target breath alcohol concentration (BrAC), at which it was then “clamped.” The alcohol clamp was started 5 min after scan start, with a linear increase in BrAC over 15 min to the target of 80 mg%, the legal threshold for intoxication. Target BrAC was maintained for 30 min. Voxel-wise binding potential (BPND) was estimated with MRTM2.
Results
IV EtOH induced significant increases in DA in the right ventral striatum in NTS, but not SD. No decreases in DA were observed in either group.
Conclusions
Alcohol intoxication results in distinct anatomic profiles of DA responses in SD and NTS, suggesting that in NTS, the striatal DA system may process effects of alcohol intoxication differently than in SD.
Keywords: Dopamine, raclopride, alcohol, positron emission tomography, ventral striatum
1. Introduction
There is strong evidence that the neurotransmitter dopamine (DA) plays multiple roles in alcoholism and other addictions (Berridge, 2007; Horvitz, 2000; O'Tousa and Grahame, 2014; Redgrave et al., 1999; Salamone et al., 2005). Despite the wealth of preclinical literature, the functions of DA in human alcohol use disorders (AUD) remain poorly understood. Thus, determining how striatal DA responds to alcohol continues to be pivotal for developing preventative and therapeutic approaches.
Several groups have used positron emission tomography (PET) with the D2/D3 radioligand [11C]raclopride (RAC) to study the effects of alcohol ingestion on striatal DA. Oral alcohol appears to cause modest increases in dopamine in the ventral striatum of healthy subjects (Boileau et al., 2003; Setiawan et al., 2014; Urban et al., 2010), with more notable effects in men (Urban et al., 2010) and in subjects with traits that may increase risk for AUD (Setiawan et al., 2014). However, there are properties of oral alcohol intake that complicate interpretation of these studies. First, the chemosensory (smell, taste) and somatosensory (oral sensations) characteristics of alcohol have powerful Pavlovian associations with intoxication. In rodents, these conditioned cues are believed to mediate the acute increases in striatal DA observed in oral alcohol self-administration studies (Doyon et al., 2005). We have shown in humans that beer flavor provokes DA release in the ventral striatum (Oberlin et al., 2015). Additionally, even with well-controlled dosing, oral ingestion of alcohol results in highly variable rates and concentrations of brain alcohol exposure because of inter-subject differences in stomach pH, volume of stomach contents, age, gender, and first-pass metabolism (Ramchandani et al., 2009). Different brain exposure timecourses, such as those induced by different peak breath alcohol concentrations (BrACs) and/or differing rates of BrAC increase, are likely to induce variation in the timing and magnitude of DA responses across subjects. In turn, variability in DA release profiles can cause unwanted variance in the outcome measure of RAC binding potential (Endres and Carson, 1998; Yoder et al., 2004).
Intravenous (IV) alcohol infusion avoids these potential confounds. The physiologically-based pharmacokinetically (PBPK) model based IV alcohol clamp (O'Connor et al., 2000) precisely controls alcohol infusion rates using PBPK model parameters customized for each individual (Plawecki et al., 2007). This permits control over the timing of alcohol delivery, minimizes experimental variation in the brain's exposure to alcohol across subjects, and allows the maintenance (“clamping”) of a target breath alcohol concentration (BrAC). Our initial RAC PET studies with the PBPK-IV alcohol clamp did not show alcohol-induced DA release at either 60mg% or 80mg% (the latter being the legal definition of intoxication) (Yoder et al., 2007; Yoder et al., 2005). However, these were relatively small samples, and there was variable timing of initiation of alcohol administration, leading to different brain alcohol exposures across subjects. Using similar IV clamping methods, Ramchandani et al. (Ramchandani et al., 2010) found that only social drinkers with the minor (and statistically rare) G allele of the functional μ-opioid receptor polymorphism (OPRM1 A118G) had measurable IV alcohol-induced DA release. In contrast, there were no apparent effects in subjects homozygous for the major 118A allele. More recently, Aalto et al. reported striatal DA release from a bolus IV alcohol infusion in a small group of social drinkers (Aalto et al., 2015), although imaging during this non-PBPK paradigm may have captured both ascending and descending limbs of brain alcohol exposure. Taken together, the PBPK-IV clamp data seem to suggest that IV alcohol may not produce a robust DA response in social drinkers; however, Type II error cannot be ruled out, given the sample sizes of all three PBPK-based infusion studies.
Although evidence suggests that alcoholics have functional alteration of the DA system (Martinez et al., 2005; Narendran et al., 2014; Volkow et al., 2007), no research has used an alcohol challenge to induce DA release in dependent drinkers. Prior work examined abstinent alcoholics during treatment and/or withdrawal, and utilized psychostimulants to probe DA function.
Thus, to better understand how the DA system responds to alcohol both in subjects with and without alcohol use disorders, we studied nontreatment-seeking alcoholics (NTS, n = 21) and social drinkers (SD, n = 24) with a behaviorally relevant stimulus: alcohol intoxication. We hypothesized that alcohol challenge would result in DA release in both SD and NTS.
2. Materials and Methods
2.1. Subjects
Procedures were approved by the Indiana University Institutional Review Board in accordance with the Belmont Report. Written informed consent was obtained after confirmation that breath alcohol concentration (BrAC) was 0 mg% and study procedures were explained. Twenty-four social drinkers (SD) and twenty-one nontreatment-seeking alcoholics (NTS) completed procedures with viable datasets. Subsets of baseline RAC data have been published (Albrecht et al., 2013; Yoder et al., 2011a; Yoder et al., 2012). General inclusion criteria were: men and women 21-55 years of age and the ability to read, understand, and complete all procedures in English. Subjects underwent the Semi-Structured Assessment for the Genetics of Alcoholism (Bucholz et al., 1994) to confirm the presence or absence of alcohol use disorders (AUD). NTS met DSM-IV criteria for alcohol dependence, had neither received treatment within the past year, nor were actively seeking treatment. Exclusion criteria were: history or presence of any psychiatric, neurological, or other medical disorder and/or current use of psychotropic or other medications that could influence study outcome, contraindications for safety during magnetic resonance imaging (MRI), positive urine pregnancy test, testing positive for illicit/illegal substance use via urine toxicology screen (results were evaluated within context of reported use, e.g., recent outpatient surgical procedures). One NTS subject met criteria for marijuana abuse as determined by the Structured Clinical Diagnostic Interview for DSM-IV disorders I module for substance use disorders. Nicotine dependence was permitted. Inclusion of subjects who self-reported occasional marijuana use was determined on a case-by-case basis by the PI (KKY).
Other characterization included: medical history and demographic questionnaires, the 90-day Timeline Follow-Back for alcohol use (TLFB, (Sobell et al., 1986)), an adaptation of the TFLB for marijuana use, Alcohol Dependency Scale (ADS; (Skinner and Allen, 1982)), Fagerstrom Smoking Questionnaire (Pomerleau et al., 1994), Edinburgh Handedness Inventory (Oldfield, 1971), and an internally-developed substance use questionnaire.
2.2. Intravenous Alcohol Infusion
Individualized alcohol infusion profiles were precomputed via the PBPK model. Input parameters included age, height, weight, and gender (O'Connor et al., 2000). Two profiles were calculated, such that IV infusions of alcohol (6% vol/vol) would achieve target BrACs of 60 mg% (“morning infusion” ∼4-6 hours before PET; see below) and 80 mg% (during the second PET scan).
The PBPK-modeled IV alcohol procedure results in intoxication similar to that of oral alcohol ingestion (Ramchandani et al., 2004). However, as the infusion itself and the rapid rise of BrAC can be novel, and given that novelty can induce DA release (De Leonibus et al., 2006; Rebec et al., 1997), we had subjects undergo a brief IV alcohol infusion prior to PET imaging. Subjects (n = 42) received an IV infusion the morning of the PET study (see 2.3). A 60 mg% target for the morning infusion insured sufficient time (e.g., ∼4-6 hours) for BrAC to return to 0 mg% prior to baseline PET. Two SD and 1 NTS received the morning infusion on a separate day (i.e., PET was acquired at a later date).
2.3. Study Day Procedures
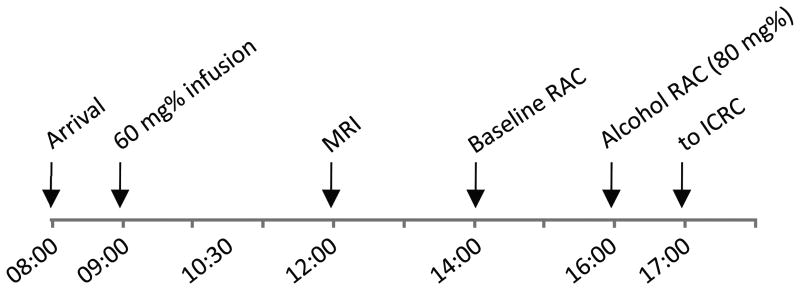
Figure 1 presents a typical study-day timeline. To prevent unwanted effects of craving or abstinence, subjects were not required to remain abstinent from alcohol, nicotine, or caffeine prior to the study. Subjects were not permitted to smoke cigarettes or have caffeinated drinks during the study day. Upon arrival, BrAC measurements were taken to ensure BrAC = 0 mg%. An IV catheter was placed in an antecubital vein. Blood samples were acquired for OPRM1 genotyping (see Supplementary Materials). Subjects were given a full breakfast, and then underwent an IV alcohol infusion to a target of 60 mg% (see 2.2). The first 21 subjects to participate received a “ramp” to target BrAC over 15 min, and were “clamped” for 30 min. The remaining 24 subjects (14 SD, 10 NTS) received only the 15 min ramp to target of 60 mg% (elimination of the clamp permitted scheduling flexibility, as subjects would return to 0 mg% more quickly before imaging). Subjects subsequently received a structural MRI scan, lunch, and two RAC scans in the afternoon (see below). After completing the alcohol RAC PET scan, subjects were escorted back to the CRC where they remained until BrAC was <20mg%.
Figure 1.
Typical study day timeline. RAC = [11C]raclopride PET scan; ICRC = Indiana Clinical Research Center.
To avoid confounds of nicotine withdrawal and/or cigarette craving on D2/D3 receptor availability (Brody et al., 2006; Volkow et al., 2006; Wong et al., 2006), transdermal nicotine patches (TNP) were placed on cigarette smokers (SD, n = 12; NTS, n = 18) shortly after arrival; dose was based on self-report of cigarettes smoked/day. TNPs effectively minimize craving in cigarette smokers during studies of similar length (Yoder et al., 2012). Test-retest variability of RAC is stable in subjects wearing TNP (Yoder et al., 2011a).
Subjective ratings were acquired every 5- 10 min during the morning infusion and both RAC scans. Questions were presented on a computer monitor; subjects responded along a visual analog scale (VAS) with a mouse. Items included four questions “How high (intoxicated, stimulated, sedated) do you feel right now?” that were anchored by 0 (“not at all”) and 100 (“the most ever”). Area under the curve (AUC) was calculated with the trapezoidal rule for VAS data collected during PET.
2.4. Imaging
An MP-RAGE structural MRI was acquired in a Siemens 3T Magnetom Trio-Tim (Siemens, Erlangen, Germany).
Subjects received two [11C]raclopride (RAC) scans in the afternoon: a resting baseline acquisition, followed ∼2 hours later by a scan with an IV alcohol infusion (EtOH; Figure 1). BrAC in all subjects was 0 mg% prior to baseline scanning. RAC synthesis was completed as described previously (Fei et al., 2004). RAC PET scans were acquired in a Siemens EXACT HR+ (3D mode; septa retracted). A 10-min transmission scan was obtained for attenuation correction. PET scans were initiated with the IV infusion of 514 ± 56 MBq RAC over 1.5 min, with dynamic acquisition for 50 min. IV EtOH began 5 min after the second scan start. BrAC was raised to the 80mg% target over 15 min, and was maintained there for 30 min.
PET scans were acquired on the same day to minimize within-subject variance and subject attrition; therefore, a fixed scan order was needed to preclude residual effects of alcohol on a subsequent baseline scan. This invariant order is similar to the bolus-infusion method in PET studies, in which baseline is established prior to tracer displacement with a drug. A resting baseline was chosen over a placebo infusion, as placebo conditions can also increase striatal dopamine (de la Fuente-Fernandez et al., 2001; Kaasinen et al., 2004), which could result in underestimation of any bona fide increase in DA levels, or overestimation of decreases in DA (Yoder et al., 2011b). Similarly, to minimize effects of uncertainty on baseline RAC (Yoder et al., 2008), subjects were made explicitly aware of what would happen during each scan. With this design, subjects are thus closer to a behavioral state that would exist during naturalistic drinking.
2.5. Image Processing
Image processing is similar to that described previously (Yoder et al., 2012). Briefly, using SPM5 (http://www.fil.ion.ucl.ac.uk/spm/), dynamic PET data were co-registered to the MP-RAGE and spatially normalized to Montreal Neurological Institute (MNI) space via the transformation matrix obtained for the structural MRI volume. Final voxel size was isotropic 2 mm/side.
Cerebellar regions of interest (ROIs) were created for each subject, and time-activity curves for this ROI were generated with the MarsBaR toolbox (http://marsbar.sourceforge.net/).
2.6. Parametric Image Generation and Analysis
D2/D3 receptor availability was indexed with BPND, the binding potential of [11C]raclopride (Innis et al., 2007)). Voxel-wise BPND estimates were obtained with the multilinear reference tissue method with a common reference region efflux rate to facilitate robust performance on noisy voxel data (Ichise et al., 2003). Parametric BPND images were smoothed with an isotropic 4mm Full Width at Half Maximum Gaussian kernel. In three subjects, there was uncorrectable head motion near the end of scanning, so either 30 min (2 SD) or 45 min (1 NTS) of data were utilized to generate the BPND images for both scan conditions. This does not result in major decrements in BPND values, and does not corrupt the validity of the within-subject analysis approach (Yoder et al., 2009).
Smoothed parametric images were entered into a 2 scan (rest, IV alcohol) x group (SD, NTS) full factorial model in SPM5. Model contrasts tested for main effects of alcohol (p < 0.005, uncorrected, cluster extent threshold k = 20). Within the SPM models, the search area was restricted to a bilateral binary striatal mask created by averaging a subset of SD and NTS resting scan parametric images, including all voxels with BPND values > 0.5. Functional clusters from the main effect contrast were defined as ROIs; average BPND from both scans were extracted using MarsBaR. Change in RAC availability was calculated as [ΔBPND = ((BPND-rest-BPND-alcohol)/BPND-rest)*100%]. One-sample t-tests determined whether extracted ΔBPND values were different from zero.
2.7. Other Statistical Analyses
Chi-squared and independent t-tests were utilized for categorical and continuous variables, respectively. One-way ANOVA tested for differences in RAC parameters, with Group, Scan, and Group*Scan as factors. Repeated-measures, two-way ANOVA was used to determine effects of Time, Group, and Time*Group on subjective ratings. Correlations were tested with Pearson's correlation. Significance was set at p < 0.05 (two-tailed). Data are mean ± s.d. unless otherwise specified.
3. Results
3.1. Subjects
Subject characteristics are presented in Table 1.
Table 1.
Subject demographics and characteristics.
| SD | NTS | p | |
|---|---|---|---|
| N | 24 | 21 | n.s. |
| Age | 33.9 ± 8.5 | 36.6 ± 8.5 | n.s. |
| gender | 6F | 3F | n.s. |
| race | 8AA | 10AA | n.s. |
| ethnicity | 1HL | 0HL | N/A |
| education (years) | 14.1 ± 2.3 | 12.9 ± 1.5 | < 0.05 |
| handedness | 24R | 20R, 1A | N/A |
| OPRM1 A118G* | 118AA: 16 118AG: 5 118GG: 1 |
118AA: 17 118AG: 2 118GG: 0 |
|
| drinks/dd | 2.9 ± 1.3 | 9.1 ± 3.0 | < 1x10-10 |
| drinks/week | 4.4 ± 3.1 | 40.6 ± 20.3 | < 1x10-10 |
| ADS | 4.0 ± 2.9 | 12.9 ± 6.2 | < 1x10-06 |
| cigarette smokers | 12 | 18 | < 0.02 |
| Fagerstrom (smokers only) | 3.85 ± 1.46 | 3.78 ± 2.18 | n.s. |
AA = African American, HL = Hispanic Latino, R = Right handed, A = Ambidextrous.
Genotyping data available for 22 SD and 19 NTS.
3.2. [11C]Raclopride Parameters
Radioactivity and mass dose injected are presented in Table 2. There were no significant effects of Group, Scan, or Group*Scan on either parameter.
Table 2.
[11C]raclopride injection parameters by group and scan.
| SD | NTS | |
|---|---|---|
| MBq | ||
| Rest | 529 ± 55.5 | 503 ± 59.2 |
| EtOH | 522 ± 51.8 | 507 ± 59.2 |
| nmol/kg | ||
| Rest | 0.13 ± 0.06 | 0.15 ± 0.07 |
| EtOH | 0.12 ± 0.05 | 0.13 ± 0.05 |
3.3. Breath Alcohol Concentration
Table 3 presents the post-infusion BrAC measurements for both the “morning infusion” and the RAC scan infusion. There were no significant group differences for either measurement.
Table 3.
Post-infusion breath alcohol concentrations (BrAC).
| SD | NTS | p | |
|---|---|---|---|
| Morning infusion (target: 60 mg%) | 59.9 ± 10.1 | 56.0 ± 9.0 | n.s. |
| PET infusion (target: 80 mg%) | 72.7 ± 9.7 | 69.9 ± 9.8 | n.s. |
3.4. Subjective Responses to IV Alcohol during RAC PET
Figure 2 illustrates the time course of subjective ratings of “High” for SD and NTS during the alcohol RAC scan. The time course of the subjective perceptions followed the timing of the IV alcohol infusion (see also 2.4). There was a significant effect of Time, but no effect of Group or Time*Group. Least significant difference post-hoc testing revealed that ratings at t = 5, 10, 15, and 20 minutes were different from all other time points (p < 0.02). Ratings at t = 30, 40, and 50 min were not different from one another. Effects were similar for “Intoxicated”, “Sedated”, and “Stimulated,” (data not shown); these four ratings are highly intercorrelated (data not shown).
Figure 2.
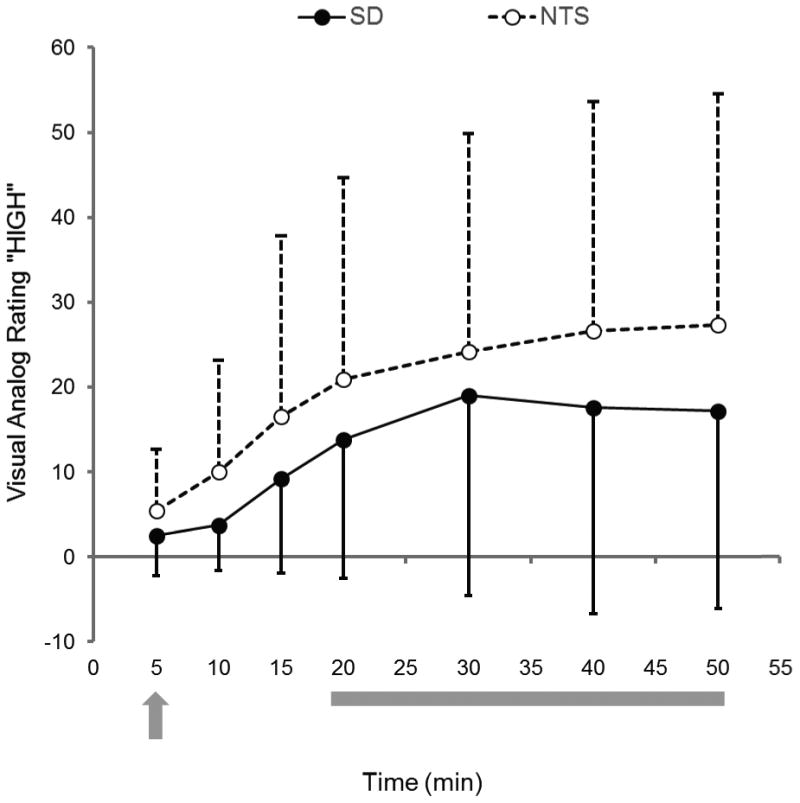
Subjective ratings for “high” during the alcohol RAC PET scan. Left y-axis, visual analog ratings for “high” for SD (filled circles, black line) and NTS (open circles, dotted line). Data are mean ± s.d. Data were not available for one NTS subject. Alcohol infusion was started 5 minutes after the RAC injection (t = 0; gray arrow). The BrAC target for the 30 minute IV alcohol clamp (gray bar) was 80 mg%. See text for details.
3.5. Dopamine Responses to IV Alcohol
Figure 3 shows the main effects of alcohol ([BPND- BL > BPND-EtOH]; indicative of increases in dopamine; p < 0.005, uncorrected; k = 20). Across all subjects, alcohol increased DA in the right ventral striatum (VST, cluster size k = 48; peak voxel at [10 6 -10], Z = 3.56, punc < 0.0001) and in the right piriform cortex (cluster size k = 63; peak voxel [30 6 -16]; Z = 3.87, punc < 0.0001). The extracted ΔBPND values for each cluster are listed in Table 4.
Figure 3.
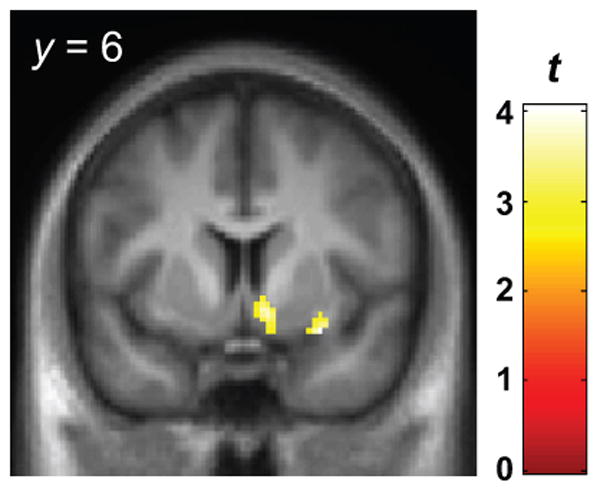
Voxel-wise results for main effect of alcohol across all subjects, indicating where IV ethanol increased dopamine relative to the resting baseline [BPND-rest > BPND-alcohol]. See text for details. Display height, p < 0.005, uncorrected; cluster extent threshold k = 20 voxels. Color bar indicates the t-statistic. See text for details. The magnitudes of ΔBPND from each cluster, by group, are presented in Table 4.
Table 4.
ΔBPND values for the clusters extracted from the main effects of alcohol contrast.
| SD | NTS | |||||
|---|---|---|---|---|---|---|
| BPND-rest | BPND-alcohol | ΔBPND | BPND-rest | BPND-alcohol | ΔBPND | |
| Right Ventral Striatum | 1.42 ± 0.17 | 1.34 ± 0.21 | 5.2 ± 13.0 | 1.47 ± 0.20 | 1.33 ± 0.25 | 9.0 ± 13.6† |
| Right Piriform Cortex | 0.73 ± 0.21 | 0.62 ± 0.22 | 13.9 ± 19.2‡ | 0.74 ± 0.31 | 0.65 ± 0.17 | 4.5 ± 30.6 |
significantly different from zero, p = 0.006
significantly different from zero, p = 0.002
In the right VST cluster, ΔBPND was significantly different from zero in NTS, but not SD. SD had a significant increase in DA in a region approximating the right piriform area.
Alcohol did not result in any decreases in striatal DA in either SD or NTS.
The results remained significant without the NTS subject with comorbid marijuana abuse (data not shown). This subject's ΔBPND value from the VST cluster was within 1 standard deviation of the group mean; ΔBPND from the piriform region was within 2 s.d. of the group mean.
3.6. DA Responses and Subjective Ratings
Extracted BPND data from the right VST and piriform area clusters were assessed for relationships with subjective rating AUC. There were no correlations between BPND in either region and AUC for subjective ratings of “High”, “Intoxicated”, “Sedated”, and “Stimulated.” When groups were tested separately, there were no correlations between subjective ratings and BPND in NTS. In SD, there were modest correlations between BPND and “High” and “Intoxicated” (r = 0.36 and 0.31, respectively, p < 0.05). However, visual analysis of the plot revealed that the significance was likely driven by outliers (data not shown).
3.7. OPRM1 A118G Polymorphism
As the OPRM1 A118G polymorphism may modulate the DA response to IV alcohol (Ramchandani et al., 2010), we verified this genotype in our subjects (Table 1; for methods, see Supplementary Material). Genotyping data were unavailable for 2 SD and 2 NTS. The low sample frequency of the G allele precluded analyses by genotype.
4. Discussion
This study examined the effect of alcohol intoxication on striatal DA responses in moderately large samples of SD and NTS. IV alcohol infusion with PBPK modeling (O'Connor et al., 2000; Ramchandani et al., 1999) was employed to precisely control the timing of brain alcohol exposure across all subjects, and to avoid the confound of conditioned chemosensory cues on VST DA (Doyon et al., 2003; Oberlin et al., 2013). The primary finding was that SD and NTS subjects exhibited different magnitudes of VST DA release to an IV alcohol infusion targeted to a BrAC of 80mg%. Specifically, NTS subjects had statistically significant increases in DA in right ventral striatum, whereas SD did not. No decreases in DA were observed in either group. To our knowledge, this is the first study to report the effects of alcohol intoxication on the striatal DA system in dependent, current drinkers.
Even with a large sample, and uniformity in timing and character of brain alcohol exposure, we did not find that intravenously administered alcohol induced significant changes in VST DA in social drinkers. The only other published PBPK-modeled IV alcohol infusion protocol to examine DA release with PET was Ramchandani et al. (2010); our social-drinking sample most closely corresponds to their OPRM1 118AA group. There was no apparent effect on IV alcohol on VST DA in the Ramchandani et al. 118AA sample, which is consistent with data in the present study. Aalto et al. (2015) reported alcohol-induced DA release in 9 social drinkers using a bolus-infusion RAC PET protocol. The bolus-infusion approach, as in our design, measures baseline and challenge conditions successively within the same session, and thus avoids between-day variance. However, some other features may not make their study a good comparison to the present work. The ethanol concentration they used was above 6.0% v/v (7.6%), which, in our experience, can cause significant endothelial irritation. Additionally, their range of ethanol exposure was quite large (90-160 mg%), and the non-PBPK infusion is likely to have included both the rising and descending limbs of the BrAC curve. Finally, subjects in the Aalto study were naïve to the alcohol infusion experience, increasing the likelihood of novelty effects on DA release (De Leonibus et al., 2006; Rebec et al., 1997).
The observed increase in DA levels in the ventral striatum of NTS with IV alcohol infusion may seem intuitively obvious, given the VST's oft-cited role in “reward”. However, we did not observe any IV alcohol-induced DA release in the SD subjects, who presumably drink alcohol on occasion for its reinforcing properties. This discrepant result raises two interesting points. First, it suggests that the VST may be sensitized in alcoholics. Given that the VST codes for stimulus valence (Bischoff-Grethe et al., 2009; Mattfeld et al., 2011; Seymour et al., 2007), an attractive interpretation is that IV alcohol intoxication has higher reinforcement value in NTS than in SD. Second, several studies have found that abstinent/detoxified alcoholics have lower psychostimulant-induced increases in DA compared to healthy controls (Martinez et al., 2005; Narendran et al., 2014; Volkow et al., 2007), which is typically taken as evidence for a hypofunctional DA system in chronic alcoholism. However, this phenomenon may be unique to stimulants such as methylphenidate and amphetamine, which have direct and powerful effects on dopamine concentration at the levels of the nerve terminal and synapse. Indeed, Spreckelmeyer et al. (Spreckelmeyer et al., 2011) found that opioids, which elicit a very modest effect on DA release, did not induce measurable differences in opioid-induced DA release between detoxified alcoholics and controls. This, coupled with our present data, indicate psychostimulants may not be an appropriate proxy for characterizing the nature of dopaminergic function in alcoholism. The contextually relevant stimulus of alcohol intoxication may constitute a better tool for understanding how the DA system functions in the development and maintenance of AUD.
It is not clear how to interpret the IV alcohol-induced increase in DA in the piriform cortex in SD. The signal-to-noise properties of RAC make it unsuitable for hypothesis testing in regions with modest levels of D2/D3 receptors (i.e., outside the striatum). Although this region was apparently included in our mask (2.6), the result was unexpected. Of note, there was large variance in BPND in this region (Table 4).
Although this is a relatively large PET study, it still may be underpowered in SD because of the presumably smaller effect size. Thus, the data could contain type II errors in the VST of the SD sample. However, it is worth noting the results are consistent with our prior work (Yoder et al., 2005) and results from Ramchandani et al. (2010) in SD with the most common OPRM1 genotype. Another limitation is that we cannot distinguish between a predisposing difference in VST DA function and long-term effects of chronic drinking, a problem common to all neuroimaging studies of addiction. The prospective longitudinal studies required to answer this question are not possible, as research radiotracers cannot be administered to minors. Finally, this study did not employ a placebo condition as a baseline comparator. As noted (see 2.4), placebos can induce DA release, and the uncertainty of subject expectations that may accompany placebo designs can confound baseline RAC BP. We thus opted to implement a resting baseline and expected alcohol paradigm, the latter of which more closely models expectations during naturalistic drinking.
5. Conclusions
We used a PBPK-modeled IV alcohol clamp technique to examine the effects of alcohol intoxication on striatal DA release in SD and NTS. We observed increases in right VST DA in NTS, but not SD. To our knowledge, this is the first report of the effects of alcohol on DA in currently drinking alcohol-dependent subjects. The results suggest that the DA system may process alcohol intoxication differently in NTS than in SD. The data may reflect relative group differences in perceived valence, reinforcement, or expectancies related to alcohol intoxication, hypotheses that require further investigation.
Supplementary Material
References
- Aalto S, Ingman K, Alakurtti K, Kaasinen V, Virkkala J, Nagren K, Rinne JO, Scheinin H. Intravenous ethanol increases dopamine release in the ventral striatum in humans: PET study using bolus-plus-infusion administration of [(11)C]raclopride. J Cereb Blood Flow Metab. 2015;35:424–431. doi: 10.1038/jcbfm.2014.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht DS, Kareken DA, Yoder KK. Effects of smoking on D/D striatal receptor availability in alcoholics and social drinkers. Brain Imaging Behav. 2013 doi: 10.1007/s11682-013-9233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Bischoff-Grethe A, Hazeltine E, Bergren L, Ivry RB, Grafton ST. The influence of feedback valence in associative learning. Neuroimage. 2009;44:243–251. doi: 10.1016/j.neuroimage.2008.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, Tremblay RE, Dagher A. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49:226–231. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Scheibal D, Hahn E, Shiraga S, Zamora-Paja E, Farahi J, Saxena S, London ED, McCracken JT. Gene variants of brain dopamine pathways and smoking-induced dopamine release in the ventral caudate/nucleus accumbens. Arch Gen Psychiatry. 2006;63:808–816. doi: 10.1001/archpsyc.63.7.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Fernandez R, Ruth TJ, Sossi V, Schulzer M, Calne DB, Stoessl AJ. Expectation and dopamine release: mechanism of the placebo effect in Parkinson's disease. Science. 2001;293:1164–1166. doi: 10.1126/science.1060937. [DOI] [PubMed] [Google Scholar]
- De Leonibus E, Verheij MM, Mele A, Cools A. Distinct kinds of novelty processing differentially increase extracellular dopamine in different brain regions. Eur J Neurosci. 2006;23:1332–1340. doi: 10.1111/j.1460-9568.2006.04658.x. [DOI] [PubMed] [Google Scholar]
- Doyon WM, Anders SK, Ramachandra VS, Czachowski CL, Gonzales RA. Effect of operant self-administration of 10% ethanol plus 10% sucrose on dopamine and ethanol concentrations in the nucleus accumbens. J Neurochem. 2005;93:1469–1481. doi: 10.1111/j.1471-4159.2005.03137.x. [DOI] [PubMed] [Google Scholar]
- Doyon WM, Ramachandra V, Samson HH, Czachowski CL, Gonzales RA. Accumbal dopamine concentration during operant self-administration of a sucrose or a novel sucrose with ethanol solution. Alcohol. 2004;34:261–271. doi: 10.1016/j.alcohol.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Doyon WM, York JL, Diaz LM, Samson HH, Czachowski CL, Gonzales RA. Dopamine activity in the nucleus accumbens during consummatory phases of oral ethanol self-administration. Alcohol Clin Exp Res. 2003;27:1573–1582. doi: 10.1097/01.ALC.0000089959.66222.B8. [DOI] [PubMed] [Google Scholar]
- Endres CJ, Carson RE. Assessment of dynamic neurotransmitter changes with bolus or infusion delivery of neuroreceptor ligands. J Cereb Blood Flow Metab. 1998;18:1196–1210. doi: 10.1097/00004647-199811000-00006. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, Suhara T, Suzuki K, Innis RB, Carson RE. Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab. 2003;23:1096–1112. doi: 10.1097/01.WCB.0000085441.37552.CA. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Aalto S, Nagren K, Rinne JO. Expectation of caffeine induces dopaminergic responses in humans. Eur J Neurosci. 2004;19:2352–2356. doi: 10.1111/j.1460-9568.2004.03310.x. [DOI] [PubMed] [Google Scholar]
- Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, Kegeles L, Talbot P, Evans S, Krystal J, Laruelle M, Abi-Dargham A. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry. 2005;58:779–786. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Mattfeld AT, Gluck MA, Stark CE. Functional specialization within the striatum along both the dorsal/ventral and anterior/posterior axes during associative learning via reward and punishment. Learning and Memory. 2011;18:703–711. doi: 10.1101/lm.022889.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendran R, Mason NS, Paris J, Himes ML, Douaihy AB, Frankle WG. Decreased prefrontal cortical dopamine transmission in alcoholism. Am J Psychiatry. 2014;171:881–888. doi: 10.1176/appi.ajp.2014.13121581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor S, Ramchandani VA, Li TK. PBPK modeling as a basis for achieving a steady BrAC of 60 +/- 5 mg% within ten minutes. Alcohol Clin Exp Res. 2000;24:426–427. [PubMed] [Google Scholar]
- O'Tousa D, Grahame N. Habit formation: implications for alcoholism research. Alcohol. 2014;48:327–335. doi: 10.1016/j.alcohol.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Dzemidzic M, Tran SM, Soeurt CM, Albrecht DS, Yoder KK, Kareken DA. Beer flavor provokes striatal dopamine release in male drinkers: mediation by family history of alcoholism. Neuropsychopharmacology. 2013;38:1617–1624. doi: 10.1038/npp.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Dzemidzic M, Tran SM, Soeurt CM, O'Connor SJ, Yoder KK, Kareken DA. Beer self-administration provokes lateralized nucleus accumbens dopamine release in male heavy drinkers. Psychopharmacology (Berl) 2015;232:861–870. doi: 10.1007/s00213-014-3720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Plawecki MH, Decarlo R, Ramchandani VA, O'Connor S. Improved Transformation of Morphometric Measurements for a Priori Parameter Estimation in a Physiologically-Based Pharmacokinetic Model of Ethanol. Biomedical Signal Processing and Control. 2007;2:97–110. doi: 10.1016/j.bspc.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau CS, Carton SM, Lutzke ML, Flessland KA, Pomerleau OF. Reliability of the Fagerstrom Tolerance Questionnaire and the Fagerstrom Test for Nicotine Dependence. Addict Behav. 1994;19:33–39. doi: 10.1016/0306-4603(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Morzorati S, Flury L, Blekher T, Foroud T, Li TK, S Oc. Comparison of subjective responses to alcohol when administered using different routes of administration that result in similar breath alcohol levels. Alcohol Clin Exp Res. 2004;28:147A. [Google Scholar]
- Ramchandani VA, O'Connor S, Blekher T, Kareken D, Morzorati S, Nurnberger J, Jr, Li TK. A preliminary study of acute responses to clamped alcohol concentration and family history of alcoholism. Alcohol Clin Exp Res. 1999;23:1320–1330. [PubMed] [Google Scholar]
- Ramchandani VA, Plawecki M, Li TK, O'Connor S. Intravenous ethanol infusions can mimic the time course of breath alcohol concentrations following oral alcohol administration in healthy volunteers. Alcohol Clin Exp Res. 2009;33:938–944. doi: 10.1111/j.1530-0277.2009.00906.x. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Umhau J, Pavon FJ, Ruiz-Velasco V, Margas W, Sun H, Damadzic R, Eskay R, Schoor M, Thorsell A, Schwandt ML, Sommer WH, George DT, Parsons LH, Herscovitch P, Hommer D, Heilig M. A genetic determinant of the striatal dopamine response to alcohol in men. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebec GV, Christensen JR, Guerra C, Bardo MT. Regional and temporal differences in real-time dopamine efflux in the nucleus accumbens during free-choice novelty. Brain Res. 1997;776:61–67. doi: 10.1016/s0006-8993(97)01004-4. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K. Is the short-latency dopamine response too short to signal reward error? Trends Neurosci. 1999;22:146–151. doi: 10.1016/s0166-2236(98)01373-3. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote SM, Weber SM. Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine. Curr Opin Pharmacol. 2005;5:34–41. doi: 10.1016/j.coph.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Setiawan E, Pihl RO, Dagher A, Schlagintweit H, Casey KF, Benkelfat C, Leyton M. Differential striatal dopamine responses following oral alcohol in individuals at varying risk for dependence. Alcohol Clin Exp Res. 2014;38:126–134. doi: 10.1111/acer.12218. [DOI] [PubMed] [Google Scholar]
- Seymour B, Daw N, Dayan P, Singer T, Dolan R. Differential encoding of losses and gains in the human striatum. J Neurosci. 2007;27:4826–4831. doi: 10.1523/JNEUROSCI.0400-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. J Abnorm Psychol. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Sobell MB, Sobell LC, Klajner F, Pavan D, Basian E. The reliability of a timeline method for assessing normal drinker college students' recent drinking history: utility for alcohol research. Addict Behav. 1986;11:149–161. doi: 10.1016/0306-4603(86)90040-7. [DOI] [PubMed] [Google Scholar]
- Spreckelmeyer KN, Paulzen M, Raptis M, Baltus T, Schaffrath S, Van Waesberghe J, Zalewski MM, Rosch F, Vernaleken I, Schafer WM, Grunder G. Opiate-induced dopamine release is modulated by severity of alcohol dependence: an [(18)F]fallypride positron emission tomography study. Biol Psychiatry. 2011;70:770–776. doi: 10.1016/j.biopsych.2011.05.035. [DOI] [PubMed] [Google Scholar]
- Urban NB, Kegeles LS, Slifstein M, Xu X, Martinez D, Sakr E, Castillo F, Moadel T, O'Malley SS, Krystal JH, Abi-Dargham A. Sex Differences in Striatal Dopamine Release in Young Adults After Oral Alcohol Challenge: A Positron Emission Tomography Imaging Study With [(11)C]Raclopride. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, Ma Y, Pradhan K, Wong C. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27:12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A, Brasic JR, Kimes AS, Maris MA, Kumar A, Contoreggi C, Links J, Ernst M, Rousset O, Zukin S, Grace AA, Lee JS, Rohde C, Jasinski DR, Gjedde A, London ED. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology. 2006;31:2716–2727. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
- Yoder KK, Albrecht DS, Kareken DA, Federici LM, Perry KM, Patton EA, Zheng QH, Mock BH, O'Connor S, Herring CM. Test-retest variability of [(11) C]raclopride-binding potential in nontreatment-seeking alcoholics. Synapse. 2011a;65:553–561. doi: 10.1002/syn.20874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KK, Albrecht DS, Kareken DA, Federici LM, Perry KM, Patton EA, Zheng QH, Mock BH, O'Connor SJ, Herring CM. Reliability of striatal [(11)C]raclopride binding in smokers wearing transdermal nicotine patches. Eur J Nucl Med Mol Imaging. 2012;39:220–225. doi: 10.1007/s00259-011-1965-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KK, Constantinescu CC, Kareken DA, Normandin MD, Cheng TE, O'Connor SJ, Morris ED. Heterogeneous effects of alcohol on dopamine release in the striatum: a PET study. Alcohol Clin Exp Res. 2007;31:965–973. doi: 10.1111/j.1530-0277.2007.00390.x. [DOI] [PubMed] [Google Scholar]
- Yoder KK, Kareken DA, Morris ED. What were they thinking? Cognitive states may influence [(11)C]raclopride binding potential in the striatum. Neurosci Lett. 2008;430:38–42. doi: 10.1016/j.neulet.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KK, Kareken DA, Morris ED. Assessing Dopaminergic Neurotransmission with PET: Basic Theory and Applications in Alcohol Research. Current Medical Imaging Reviews. 2011b;7:118–124. [Google Scholar]
- Yoder KK, Kareken DA, Seyoum RA, O'Connor S J, Wang C, Zheng QH, Mock B, Morris ED. Dopamine D(2) receptor availability is associated with subjective responses to alcohol. Alcohol Clin Exp Res. 2005;29:965–970. doi: 10.1097/01.alc.0000171041.32716.42. [DOI] [PubMed] [Google Scholar]
- Yoder KK, Morris ED, Constantinescu CC, Cheng TE, Normandin MD, O'Connor SJ, Kareken DA. When what you see isn't what you get: alcohol cues, alcohol administration, prediction error, and human striatal dopamine. Alcohol Clin Exp Res. 2009;33:139–149. doi: 10.1111/j.1530-0277.2008.00821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KK, Wang C, Morris ED. Change in binding potential as a quantitative index of neurotransmitter release is highly sensitive to relative timing and kinetics of the tracer and the endogenous ligand. J Nucl Med. 2004;45:903–911. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.