Abstract
BACKGROUND
There remain uncertainties about age‐specific effects of breast cancer screening on mortality due to the disease.
METHODS
In 1982, a randomized trial of mammographic screening every 18 months was started in Gothenburg, Sweden. Women between the ages of 39 and 49 years were randomized to an invitation to screening (intervention group; n = 11,792) or to usual care (the control group; n = 14,321). The corresponding numbers for women between the ages of 50 and 59 years were 10,112 and 15,997. Follow‐up data for breast cancer mortality were available up to the end of 2007. Data were analyzed by Poisson regression with conservative variance estimates.
RESULTS
There were 79 breast cancer deaths in the intervention arm and 156 in the control arm, and this meant a significant 30% reduction in breast cancer mortality with the offer of screening (relative risk [RR], 0.70; 95% confidence interval [CI], 0.53‐0.93; P = .01). In women aged 39 to 49 years, there was a significant 40% reduction in breast cancer mortality (RR, 0.60; 95% CI, 0.43‐0.85; P = .003). In the 50‐ to 59‐year age group, there was a nonsignificant 18% breast cancer mortality reduction (RR, 0.82; 95% CI, 0.54‐1.26; P = .4).
CONCLUSIONS
The policy of offering mammographic screening substantially reduces breast cancer mortality and can do so in women younger than 50 years. Cancer 2016;122:1832–5. © 2016 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society.
Keywords: age‐specific results, breast cancer, mammography, randomized trial, screening
Short abstract
In a randomized trial of breast cancer screening, a significant reduction in breast cancer mortality is found with the offer of mammographic screening. The results suggest that screening can be effective in women younger than 50 years.
INTRODUCTION
Randomized trials of breast cancer screening have demonstrated a substantial reduction in breast cancer mortality with the policy of offering regular mammography.1, 2 The crucial mediator of the mortality reduction is the corresponding reduction in advanced‐stage disease.1 Although the mortality reduction has been established for decades, the long‐term effect of the intervention in terms of breast cancer deaths prevented remains a topic of some interest, as does the issue of age‐specific effects of mammography screening.3, 4, 5
The Gothenburg Trial of Mammographic Screening began in 1982 and reported results on breast cancer mortality up to 1996; it indicated a 23% reduction in breast cancer mortality with the offer of screening.6 The results also showed a significant mortality reduction in women younger than 50 years at entry into the trial. In this article, we report updated mortality results for this trial to the end of 2007.
MATERIALS AND METHODS
The design of the trial has been reported elsewhere.6 Briefly, the trial began in 1982, with 21,904 women born from 1923 to 1944 randomized to regular mammography (the intervention group) and with 30,318 randomized to usual care (the control group). These numbers differ slightly from the previous report because of underreporting of the study populations by 254 subjects in the intervention group and by 357 subjects in the control group in the previous article.6 In the original intervention and control cohorts, there were 22,158 women in the intervention cohort and 30,675 women in the control group. Within these cohorts, we identified 254 women in the intervention group and 357 in the control group who had a diagnosis of breast cancer before the initiation of the trial. Because of an oversight, these numbers were subtracted twice from the denominator populations but only once from the numerators, breast cancer cases and deaths, so the latter were reported correctly in the previous publication.
Randomization took place between December 1982 and April 1984. Each year of birth was successively randomized in day‐of‐birth clusters up to November 1983 (years of birth, 1923‐1935), when the software for allocation was augmented to allow for individual randomization, and randomization was by individual thereafter (years of birth, 1936‐1944). We randomized smaller numbers to the intervention than the control because of the limited capacity of the screening service. Each year‐of‐birth cohort was given a randomization ratio to ensure that we did not allocate to screening more women than the service could handle.6 The age range of the women at entry to the trial was 39 to 59 years. The first round attendance at screening was 84%.
Women were offered screening every 18 months. Mammography was the sole screening modality. Two‐view mammography was used in the first screening round. In subsequent rounds, 2‐view mammography was used unless the first‐screen mammogram showed very nondense tissue.6 Mammograms were single‐read for the first 3 rounds and double‐read thereafter.
After 5 rounds of screening in the 39‐ to 49‐year age group and during the fourth round in the 50‐ to 59‐year age group, the control group was invited to a single round of screening. All breast cancers in both groups diagnosed between randomization and the completion of the control group screen (including those screen‐detected during this period but histologically confirmed within 30 days thereafter) were included for follow‐up for mortality. The primary endpoint was mortality from these breast cancers, with mortality data available up to December 31, 2007, the end date chosen by the Swedish Overview Group, which was responsible for follow‐up data. This gave a median of 24 years' follow‐up. Cancers were ascertained from the National Cancer Registry, which was augmented by local medical records. Causes of death were taken from the Swedish Cause of Death Registry. The analysis of breast cancer mortality data was performed via Poisson regression with a conservative variance estimate to take account of the cluster randomization7, 8; this yielded relative risks (RRs), 95% confidence intervals (CIs), and likelihood ratio chi‐square tests for effect. Our primary mortality analysis included 9 breast cancer deaths (2 in the intervention arm and 7 in the control arm) that the treating physician had not reported to the cancer registry; therefore, these patients did not appear in the registry's records, even though they had breast cancer. We performed a secondary analysis that excluded these cases.
The trial was approved by the ethics committee of Sahlgrenska University Hospital (Gothenburg, Sweden).
RESULTS
Table 1 shows the study population by age and trial arm along with the corresponding numbers of invasive breast cancers in the trial and breast cancer deaths (no breast cancer deaths occurred in the in situ cases). There was a significant 30% reduction in breast cancer mortality in the intervention arm (RR, 0.70; 95% CI, 0.53‐0.93; P = .01). Figure 1 shows the cumulative mortality in the 2 trial arms over time. Although there was no statistically significant heterogeneity by age, the mortality reduction in the intervention arm was significant (40%) for the 39‐ to 49‐year age group (RR, 0.60; 95% CI, 0.43‐0.85; P = .003) and nonsignificant (18%) for the 50‐ to 59‐year age group (RR, 0.82; 95% CI, 0.54‐1.26; P = .4).
Table 1.
Study Population, Invasive Breast Cancers Diagnosed up to the End of the Control Group Screen, and Deaths From These Breast Cancers
| Trial Arm | Age at Randomization, y | Women Randomized, No. | Invasive Breast Cancers, No. | Breast Cancer Deaths, No. |
|---|---|---|---|---|
| Intervention | 39–49 | 11,792 | 125 | 33 |
| 50–59 | 10,112 | 147 | 46 | |
| 39–59 | 21,904 | 272 | 79 | |
| Control | 39–49 | 14,321 | 183 | 68 |
| 50–59 | 15,997 | 234 | 88 | |
| 39–59 | 30,318 | 417 | 156 |
Figure 1.
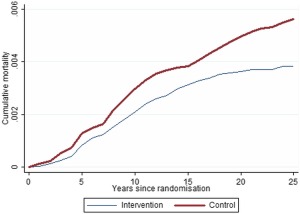
Cumulative breast cancer mortality rates over time in the intervention and control arms.
In the secondary analysis, which removed the 9 breast cancer deaths for cases that were not reported to the cancer registry, there was a similar significant reduction in breast cancer mortality (RR, 0.72; 95% CI, 0.54‐0.94; P = .01). The result for the 39‐ to 49‐year age group was a significant reduction of 40% as before (RR, 0.60; 95% CI, 0.53‐0.83; P = .002), and for the 50‐ to 59‐year age group, the reduction was nonsignificant at 14% (RR, 0.86; 95% CI, 0.56‐1.29; P = .5).
Table 2 shows breast cancer cases and deaths by detection mode. In the intervention arm, the majority of cases were screen‐detected, but the majority of breast cancer deaths were in the symptomatic cancer groups (interval and non‐attender cancers); the crude fatality rate was particularly high for the nonattenders (48%). Only 17% of the breast cancers (54 of 316) but 33% of the breast cancer deaths (26 of 79) occurred in the nonattenders.
Table 2.
Breast Cancer Cases and Deaths by Detection Mode
| Trial Arm | Detection Mode | Breast Cancers (Invasive and In Situ), No. | Breast Cancer Deaths, No. (%) |
|---|---|---|---|
| Intervention | Prevalent screen | 73 | 10 (14) |
| Incident screen | 132 | 25 (19) | |
| Interval cancer | 57 | 18 (32) | |
| Nonattender | 54 | 26 (48) | |
| Control | Symptomatic before screen | 326 | 138 (42) |
| Prevalent screen | 137 | 18 (13) |
Table 3 shows cancers by the invasive/in situ status and by lymph node status for invasive cases; they are stratified by trial arm and age group. There was a nonsignificant reduction in the incidence of node‐positive cases in the intervention arm (RR, 0.80; 95% CI, 0.61‐1.05; P = .1). The reduction in the incidence rate of node‐positive cancers was significant in the 39‐ to 49‐year age group (RR, 0.65; 95% CI, 0.43‐0.97; P = .03) but not in the 50‐ to 59‐year group (RR, 0.98; 95% CI, 0.67‐1.43; P = .9).
Table 3.
Cancers by Age, Trial Arm, Invasive/In Situ Status, and Node Status
| Trial Arm | Age at Randomization, y | In Situ Cancers, No. | Node‐Negative Invasive Cancers, No. | Node‐Positive Invasive Cancers, No. | Node Status Not Known/Nodes Not Examined, No. |
|---|---|---|---|---|---|
| Intervention | 39–49 | 24 | 76 | 39 | 10 |
| 50–59 | 20 | 84 | 46 | 17 | |
| 39–59 | 44 | 160 | 85 | 27 | |
| Control | 39–49 | 13 | 96 | 73 | 14 |
| 50–59 | 33 | 132 | 74 | 28 | |
| 39–59 | 46 | 228 | 147 | 42 |
DISCUSSION
The Gothenburg mammographic screening trial now joins the Swedish Two‐County Trial, the Canadian National Breast Screening Study, and the UK Age Trial in reporting extended follow‐up.9, 10, 11 Results from the Swedish Two‐County Trial suggest that follow‐up of 25 years is sufficient for measuring the relative and absolute effects of the policy of mammographic screening on breast cancer mortality.9 The updated results of the Gothenburg trial are similar to those published previously,6 but with the larger numbers of deaths with increased follow‐up, the estimates have greater precision, and the mortality reduction in the intervention arm is now unequivocally statistically significant. Overall, the relative reduction in breast cancer mortality was 30%, and this is consistent with the 20% reduction in the incidence of node‐positive disease.
If one converts the relative reduction in breast cancer mortality to an absolute reduction, there was 1 breast cancer death prevented per 541 women screened for 4 to 6 years. This is consistent with the figure calculated by the UK independent review (180 needed to be screened for 20 years to prevent 1 breast cancer death).2
The overall result is similar to the result of the Swedish Two‐County Trial,9 which showed a greater benefit than other breast screening trials.1, 2 We suggest that the strong effect on breast cancer mortality is due partly to the use of mammography quality standards similar to those of the Swedish Two‐County Trial, partly to the short screening interval of 18 months, and possibly partly to the 4 to 5 rounds of screening, which allowed the full mortality benefit to be observed.
As in our previous results, the reduction in mortality was stronger in the 39‐ to 49‐year age group. This is unusual and, as noted previously, may be partly due to the short screening interval combined with the relatively large number of screening episodes per person.6 The results are consistent with the effect of screening on the incidence of node‐positive disease (Table 3). They are also consistent with those of a major cohort evaluation of screening in this age group.12 The UK Age Trial found a smaller effect of screening in this age group that was partly due to lower participation rates and partly due to the fact that the screening in the UK Age Trial had only a transient effect on mortality from aggressive, grade 3 breast cancers.11 In the current study, on the other hand, there was a substantial effect on deaths due to grade 3 breast cancers in women younger than 50 years. There were 12 deaths due to grade 3 cancers in the intervention arm in the 39‐ to 49‐year age group, whereas there were 24 deaths in the control arm (RR, 0.61; 95% CI, 0.31‐1.21; P = .2).
The lesser effect on breast cancer mortality in the 50‐ to 59‐year age group was primarily due to an absence of an effect on women aged 50 to 54 years.6 There was no reduction in breast cancer mortality in the 50‐ to 54‐year age group and a 30% reduction in the 55‐ to 59‐year age group (data not shown). The reason for this is not clear, but a similar phenomenon has been observed in other studies.13, 14, 15 It may be that starting screening at this perimenopausal time of life when the breast tissue is changing rapidly is a greater challenge for radiology. This, together with the short screening period of 4.8 years in this age group,6 may be responsible for the lack of an effect in women aged 50 to 54 years.
Although it has been shown that the design feature of a closure screen for the control group is conservative,16 concern has been expressed that this might lead to an overestimation of benefit.17 If we remove deaths due to breast cancers diagnosed at the closure screen in the control group and those diagnosed contemporaneously in the intervention group, we have 71 breast cancer deaths in the intervention group and 137 in the control group (RR, 0.72; 95% CI, 0.53‐0.96; P = .03).
In conclusion, updated results of the Gothenburg trial continue to indicate a substantial and significant reduction in breast cancer mortality. The results suggest that mammography screening can successfully reduce breast cancer mortality in women younger than 50 years.
FUNDING SUPPORT
The Gothenburg trial was funded by the City of Gothenburg board of health care.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosure.
Nils Bjurstam was the principal investigator, initiated and supervised the study, and contributed to the informatics, analysis, interpretation, and drafting of the manuscript. Lena Björneld was responsible for the day‐to‐day management and conduct of the study and contributed to the informatics, analysis, interpretation, and drafting of the manuscript. Stephen W. Duffy was responsible for the statistical analysis and contributed to the analysis, interpretation, and drafting of the manuscript.
We thank Nick Day and Laszlo Tabar for their invaluable guidance at the time of setting up the trial. We thank all the professionals involved in the trial and the subjects who participated. Special thanks are due to the Swedish Overview Group and, in particular, Lennarth Nyström and Jan Frisell (among others) for their essential contributions to the informatics and follow‐up of the Gothenburg trial.
REFERENCES
- 1. Tabar L, Yen AMF, Wu WYY, et al. Insights from the breast cancer screening trials: how screening affects the natural history of breast cancer and implications for evaluating service screening programs. Breast J. 2015;21:13‐20. [DOI] [PubMed] [Google Scholar]
- 2. Independent UK Panel on Breast Cancer Screening . The benefits and harms of breast cancer screening: an independent review. Lancet. 2012;380:1778‐1786. [DOI] [PubMed] [Google Scholar]
- 3. Oeffinger KC, Fontham ETH, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599‐1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Duffy SW, Chen THH, Smith RA, et al. Real and artificial controversies in breast cancer screening. Breast Cancer Manage. 2013;2:519‐528. [Google Scholar]
- 5. Gotzsche PC, Jorgensen KJ. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2013;6:CD001877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bjurstam N, Björneld L, Warwick J, et al. The Gothenburg mammographic screening trial. Cancer. 2003;97:2387‐2396. [DOI] [PubMed] [Google Scholar]
- 7. Huber PJ. The behavior of maximum likelihood estimates under nonstandard conditions. Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability, Volume 1: Statistics, 221‐233. Berkeley, CA:University of California Press; 1967. http://projecteuclid.org/euclid.bsmsp/1200512988.
- 8. White H. A heteroskedasticity‐consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817‐830. [Google Scholar]
- 9. Tabar L, Vitak B, Chen THH, et al. Swedish Two‐County Trial: impact of mammographic screening on breast cancer mortality during three decades. Radiology. 2011;260:658‐663. [DOI] [PubMed] [Google Scholar]
- 10. Miller AB, Wall C, Baines CJ, Sun P, To T, Narod SA. Twenty‐five year follow‐up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: randomised screening trial. BMJ. 2014;348:g366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moss SM, Wale C, Smith R, Evans A, Cuckle H, Duffy SW. Effect of mammographic screening from age 40 years on breast cancer mortality in the UK Age Trial at 17 years' follow‐up: a randomised controlled trial. Lancet Oncol. 2015;16:1123‐1132. [DOI] [PubMed] [Google Scholar]
- 12. Hellqvist BN, Duffy SW, Abdsaleh S, et al. Effectiveness of population‐based service screening with mammography for women ages 40–49 years: evaluation of the Swedish Mammography Screening in Young Women (SCRY) cohort. Cancer. 2011;117:714‐722. [DOI] [PubMed] [Google Scholar]
- 13. Tabar L, Fagerberg G, Chen HH, Duffy SW, Gad A. Screening for breast cancer in women aged under 50: mode of detection, incidence, fatality and histology. J Med Screen. 1995;2:94‐98. [DOI] [PubMed] [Google Scholar]
- 14. Alexander FE, Anderson TJ, Brown HK, et al. Fourteen years of follow‐up from the Edinburgh randomised trial of breast‐cancer screening. Lancet. 1999;353:1903‐1908. [DOI] [PubMed] [Google Scholar]
- 15. UK Trial of Early Detection of Breast Cancer Group . Sixteen‐year mortality from breast cancer in the UK Trial of Early Detection of Breast Cancer. Lancet. 1999;353:1909‐1914. [PubMed] [Google Scholar]
- 16. Duffy SW, Smith RA. A note on the design of screening trials. J Med Screen. 2015;22:65‐68. [DOI] [PubMed] [Google Scholar]
- 17. Autier P, Boniol M, Smans M, Sullivan R, Boyle P. Statistical analyses in Swedish randomised trials on mammography screening and in other randomised trials on cancer screening: a systematic review. J R Soc Med. 2015;108:440‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]


