Abstract
Purpose
ASCO and the College of American Pathologists (ASCO-CAP) recently recommended further changes to the evaluation of human epidermal growth factor receptor 2 gene (HER2) amplification by fluorescent in situ hybridization (FISH). We retrospectively assessed the impact of these new guidelines by using annotated Breast Cancer International Research Group (BCIRG) -005, BCIRG-006, and BCIRG-007 clinical trials data for which we have detailed outcomes.
Patients and Methods
The HER2 FISH status of BCIRG-005/006/007 patients with breast cancers was re-evaluated according to current ASCO-CAP guidelines, which designates five different groups according to HER2 FISH ratio and average HER2 gene copy number per tumor cell: group 1 (in situ hybridization [ISH]–positive): HER2-to-chromosome 17 centromere ratio ≥ 2.0, average HER2 copies ≥ 4.0; group 2 (ISH-positive): ratio ≥ 2.0, copies < 4.0; group 3 (ISH-positive): ratio < 2.0, copies ≥ 6.0; group 4 (ISH-equivocal): ratio < 2.0, copies ≥ 4.0 and < 6.0; and group 5 (ISH-negative): ratio < 2.0, copies < 4.0. We assessed correlations with HER2 protein, clinical outcomes by disease-free survival (DFS) and overall survival (OS) and benefit from trastuzumab therapy (hazard ratio [HR]).
Results
Among 10,468 patients with breast cancers who were successfully screened for trial entry, 40.8% were in ASCO-CAP ISH group 1, 0.7% in group 2; 0.5% in group 3, 4.1% in group 4, and 53.9% in group 5. Distributions were similar in screened compared with accrued subpopulations. Among accrued patients, FISH group 1 breast cancers were strongly correlated with immunohistochemistry 3+ status (P < .0001), whereas groups 2, 3, 4, and 5 were not; however, groups 2, 4 and, 5 were strongly correlated with immunohistochemistry 0/1+ status (all P < .0001), whereas group 3 was not. Among patients accrued to BCIRG-005, group 4 was not associated with significantly worse DFS or OS compared with group 5. Among patients accrued to BCIRG-006, only group 1 showed a significant benefit from trastuzumab therapy (DFS HR, 0.71; 95% CI, 0.60 to 0.83; P < .0001; OS HR, 0.69; 95% CI, 0.55 to 0.85; P = .0006), whereas group 2 did not.
Conclusion
Our findings support the original categorizations of HER2 by FISH status in BCIRG/Translational Research in Oncology trials.
INTRODUCTION
Amplification and overexpression of the human epidermal growth factor receptor type 2 gene (HER2/ERBB2) is an established therapeutic target in breast and gastric carcinomas.1-5 Because this alteration is found in other carcinomas at varying prevalence,6-8 the alteration may also prove therapeutically useful in some of these cancers. Although not associated with overexpression,9 activating mutations in extracellular and tyrosine kinase domains of HER2/ERBB2 in breast cancer respond to small-molecule inhibitors, such as lapatinib and neratinib, but to date, these findings have been restricted to preclinical model systems.10
As humanized anti-HER2 monoclonal antibodies2-5,11,12 and small-molecule kinase inhibitors13,14 of HER2 are established as effective only in cancers with amplification and overexpression, the US Food and Drug Administration (FDA) has required a companion diagnostic to select patients for these treatments. Because of reported discrepancies in HER2 testing results using HER2 companion diagnostics, ASCO and College of American Pathologists (ASCO-CAP) convened a panel to standardize performance and interpretation of these HER2 diagnostic assays.15,16 This panel was recently reconvened, and new guidelines were once again issued for HER2 test results.17,18 Because these recommendations differ from past ASCO-CAP and FDA recommendations—and given the fact that HER2 status by fluorescent in situ hybridization (FISH) assay was an entry criterion for the Breast Cancer International Research Group (BCIRG)/Translational Research in Oncology (TRIO) clinical trials of trastuzumab and lapatinib in the treatment of breast and gastric cancers, respectively, in the adjuvant and advanced disease settings,4,5,13,14,19-23—we decided to retrospectively re-evaluate our interpretations of the HER2 FISH assays from three BCIRG clinical trials.4,19,24 These trials now have long-term clinical follow-up data available4,19,25 that facilitate determination of whether the new HER2 guidelines for FISH are clinically useful and predictive of known outcomes. In the current study, we compared the original FDA-approved criteria for HER2 gene amplification with current ASCO-CAP guidelines, assessed the number of cases in each guidelines group, and determined whether new ASCO-CAP FISH testing criteria used to define each of the five HER2 FISH groups are correlated with characteristics known to be associated with HER2 gene amplification, such as HER2 protein overexpression, worse clinical outcomes (disease-free survival [DFS] and overall survival [OS]) in the absence of HER2 targeted therapy, and significant improvement in DFS and OS when such patients are treated with HER2-targeted therapy.
PATIENTS AND METHODS
Patients and Clinical Trials
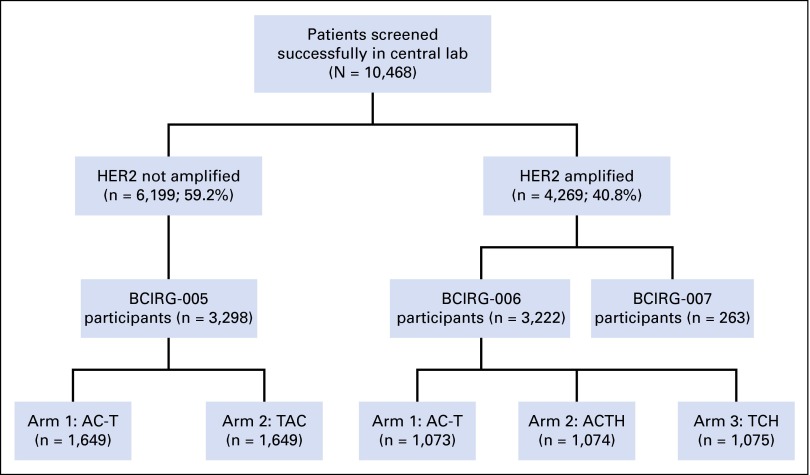
Patients in BCIRG-005/006/007 trials were screened for enrollment in one of two central laboratories by using HER2 gene amplification status determined by FISH as an enrollment criterion4,19,21 (Fig 1). Those patients whose breast cancers were HER2 amplified were eligible for BCIRG-006 or 007, whereas those whose breast cancers were not HER2 amplified were eligible for BCIRG-005 (Fig 1). Criteria for amplified and not amplified that were initially used to screen for entry to these trials are summarized below and in the Data Supplement.
Fig 1.
Specimen accountability on the basis of the CONSORT statement. Breast cancers from patients were evaluated in one of two central laboratories as either human epidermal growth factor receptor 2 gene (HER2) not amplified or HER2 amplified for eligibility to one of three concurrently conducted clinical trials (BCIRG-005, BCIRG-006, AND BCIRG-007). One of the trials, BCIRG-005, required patients whose breast cancers were HER2 not amplified and the other two trials, BCIRG-006 and BCIRG-007, required patients whose breast cancers were HER2 gene amplified as determined with fluorescent in situ hybridization (FISH). Although 10,948 patients were screened in the Breast Cancer International Research Group central laboratories for trial accrual, complete HER2 FISH assay results were available from 10,468 patients for a variety of reasons, including lack of invasive carcinoma in samples submitted, tissue sections that detached from slides during processing, and FISH assay failure as a result of lack of probe hybridization. AC-T, anthracycline, cyclophosphamide, and docetaxel; ACTH, anthracycline, cyclophosphamide, docetaxel, and trastuzumab; TAC, taxotere, docetaxel, and cyclophosphamide. TCH, docetaxel, carboplatin, and trastuzumab.
BCIRG-006 trial (n = 3,222) is a randomized, three-arm study of adjuvant chemotherapy with or without trastuzumab in patients with HER2-amplified stage I to III breast cancer who were accrued between April 2001 and March 2004.4 Therapy in the control arm was adjuvant anthracycline, cyclophosphamide, and docetaxel (AC-T) with or without hormonal therapy depending on tumor estrogen receptor and progesterone receptor status at site investigator discretion. Therapy in the two experimental arms involved trastuzumab (H) with patients randomly assigned to either standard AC-T adjuvant chemotherapy or nonanthracycline chemotherapy with docetaxel and a platinum salt, again, with or without hormonal therapy depending on tumor estrogen receptor and progesterone receptor status. This trial demonstrated significant improvement in DFS for both trastuzumab-containing treatment arms compared with control AC-T adjuvant chemotherapy alone. Outcomes are summarized in the Data Supplement and reported elsewhere.4,26
BCIRG-005 clinical trial (n = 3,298) is a randomized study of concurrent (taxotere, adriamycin, and cyclophosphamide) or sequential (AC-T) adjuvant anthracycline-containing chemotherapy in patients with HER2-normal (nonamplified) stage II and III breast cancer who were accrued from August 2000 to February 2003. This trial demonstrated that sequential and combination regimens that incorporated three drugs were equally efficacious but differed significantly in toxicity profile. Clinical outcomes are summarized in the Data Supplement, and trial details are reported elsewhere.19,25
BCIRG-007 trial (n = 263), a randomized phase III trial of docetaxel and trastuzumab compared with docetaxel, carboplatin, and trastuzumab in women with HER2-amplified metastatic breast cancer,24 was screened for HER2 status by FISH concurrently with BCIRG-005 and BCIRG-006. Data for HER2 gene amplification and expression are included in the current study; however, outcome information is not included as this trial had no control, nontrastuzumab treatment arm (Data Supplement).
Laboratory Methods
HER2 gene amplification status was determined by using FISH as described in the Data Supplement. Patients whose breast cancers were HER2 amplified—HER2-to-chromosome 17 centromere (CEP17) FISH ratio ≥ 2.0—without regard to the average HER2 gene copy number as approved by the FDA met an eligibility criterion for BCIRG-006 and BCIRG-007, whereas those whose breast cancers were HER2 nonamplified by FDA-approved criteria met the eligibility criterion for BCIRG-005 (Fig 1). HER2 protein expression was evaluated in a blinded fashion by using the HercepTest (DAKO, Carpinteria, CA) immunohistochemical (IHC) assay (Data Supplement); however, only FISH was used for enrollment.
Breast cancers screened for enrollment into these BCIRG/TRIO trials were simultaneously screened for all three clinical trials: BCIRG-005, BCIRG-006, and BCIRG-007. As personnel in central laboratories had no knowledge of which cases were potential participants for any of the studies, all screened cases were handled in the same fashion without any distinction related to trial design. As previously described,21 only 5% of these specimens had prior assessment for HER2 status by FISH in local laboratories, whereas approximately 60% had been previously assessed by some HER2 IHC assay. Because of a relatively high false-positive rate (22%) among outside IHC3+ cases, outside IHC assays were not considered sufficiently accurate for accrual to or exclusion from any of the trials.21 For current comparisons of FISH to IHC, these cases were all analyzed in the same fashion as they were initially processed, that is, without reference to their potential to be included in any particular trial. We consider this the most appropriate way to avoid introducing bias into the comparison of HER2 gene amplification by FISH with HER2 protein expression by IHC.
Interpretation of FISH Assays According to ASCO-CAP Guidelines
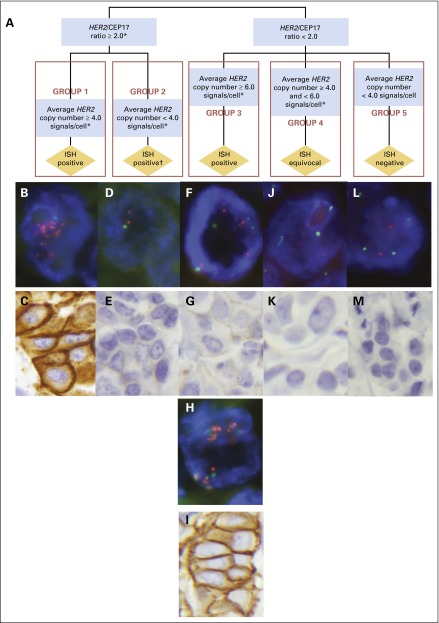
We re-evaluated HER2 status of all samples for the current study by using FISH according to the new ASCO-CAP guidelines, which separates in situ hybridization (ISH) into five groups (Fig 2). Three of these groups identify breast cancers that are ISH positive, one ISH equivocal, and one ISH negative. Breast cancers with HER2-to-CEP17 ratios of ≥ 2.0 are divided in two groups, one with an average HER2 gene copy number of ≥ 4.0/tumor cell (our group 1) and one with an average HER2 gene copy number of < 4.0/tumor cell (our group 2). Breast cancers with HER2-to-CEP17 ratios of < 2.0 are divided into three additional groups: one with average HER2 gene copy number of ≥ 6.0/tumor cell (our group 3), which is also classified as ISH positive; another with average HER2 gene copy number of ≥ 4.0 but < 6.0/tumor cell (our group 4), which is classified as ISH equivocal; and one with breast cancers that contain an average HER2 gene copy number of < 4.0/tumor cell (our group 5), which is classified as ISH negative. According to the newly proposed ASCO-CAP guidelines17,18 breast cancers in groups 1, 2, and 3 are interpreted as ISH positive, group 4 as ISH equivocal, and group 5 as ISH negative (Fig 2).
Fig 2.
Schematic diagram of the ASCO and College of American Pathologists (ASCO-CAP) algorithm for human epidermal growth factor receptor 2 (HER2) testing by fluorescent in situ hybridization (FISH) as published by the ASCO-CAP guidelines committee,17,18 modified here by introduction of the numbers 1 to 5 to identify the various ASCO-CAP FISH groups categorized, followed by FISH and immunohistochemistry (IHC) photomicrographs of representative cases from each of the five groups. (A) Breast cancers with HER2-to-chromosome 17 centromere (CEP17) ratios ≥ 2.0 are divided in two groups, one with an average HER2 gene copy number per tumor cell ≥ 4.0 (in situ hybridization [ISH] positive; our group 1) and one with an average HER2 gene copy number per tumor cell < 4.0 (ISH positive; our group 2). Breast cancers with HER2-to-CEP17 ratios < 2.0 are separated into three additional groups: one with average HER2 gene copy number per tumor cell ≥ 6.0 (ISH positive; our group 3), another with average HER2 gene copy number per tumor cell ≥ 4.0 but < 6.0 (ISH equivocal; our group 4), and one with breast cancers that contained an average HER2 gene copy number per tumor cell < 4.0 (ISH negative; our group 5). Therefore, according to the ASCO-CAP guidelines17,18 breast cancers in groups 1, 2, and 3 are interpreted as ISH positive, group 4 as ISH equivocal, and group 5 as ISH negative. (B-M) ASCO-CAP guidelines algorithm ISH groups compared with observed HER2 gene amplification status by FISH and HER2 protein expression status by IHC staining using the DAKO HercepTest IHC assay. ASCO-CAP guidelines algorithm identification of subdivisions by HER2 FISH ratios and average HER2 gene copy number into group 1 is categorized as ISH positive, with results as illustrated in panels B (FISH) and C (IHC); group 2 is also categorized as ISH positive, but with our contradictory results as illustrated in panels D (FISH) and E (IHC); group 3 is categorized as ISH positive, but with mixed results as illustrated in panels F (FISH), G (IHC), H (FISH), and I (IHC); group 4 is categorized as ISH equivocal, but with contradictory results as illustrated in panels J (FISH) and K (IHC); and group 5 is categorized as ISH negative, with confirmatory results as illustrated in panels L (FISH) and M (IHC). (B) ASCO-CAP group 1 breast cancer with HER2 gene amplification by FISH, consistent with the ASCO-CAP guidelines designation of ISH positive (and Breast Cancer International Research Group [BCIRG] designation of HER2 amplified). Average HER2 gene copy number for this case was 16.85 copies per tumor cell, and the CEP17 copy number per cell was 2.28 with a HER2-to-CEP17 FISH ratio of 7.38. HER2 signals are sufficiently numerous and are not captured in a single plain of focus in this photomicrograph so that some appear out of focus. Computer enhancement was not used for any image (BCIRG01661, original photomicrograph at 1,000×). (C) ASCO-CAP group 1 breast cancer case with HER2 protein overexpression, IHC3+ by the HercepTest IHC assay (BCIRG01661, original magnification, ×400). (D) ASCO-CAP group 2 breast cancer. Average HER2 gene copy number for this breast cancer was 3.75 copies per tumor cell, with a CEP17 copy number of 1.80 per cell and a HER2-to-CEP17 FISH ratio of 2.08. This breast cancer was evaluated in the BCIRG/Translational Research in Oncology (TRIO) central laboratory as HER2 not amplified by FISH, which contradicted the ASCO-CAP guidelines designation of ISH positive, and the patient was accrued to the BCIRG-005 trial. Of 52 patients whose breast cancers were in this group, three were accrued to BCIRG-005 and 46 were accrued to BCIRG-006 (BCIRG02899, original magnification, ×1,000). (E) ASCO-CAP group 2 breast cancer, corresponding to the breast cancer in panel D, with HER2 protein expression determined as IHC0 with HercepTest IHC assay, which contradicted the ASCO-CAP guidelines designation of ISH positive (BCIRG02899, original magnification, ×400). (F) ASCO-CAP group 3 breast cancer. One of our group 3N cases was reported to have a lack of HER2 gene amplification by FISH in the BCIRG/TRIO central laboratory, contrary to the current ASCO-CAP guidelines designation of ISH positive. Average HER2 gene copy number for this breast cancer was 7.35 copies per tumor cell, average CEP17 copy number was 4.20 per cell, and, therefore, there was a HER2-to-CEP17 FISH ratio of 1.75 (BCIRG04086, original magnification, ×1,000). (G) ASCO-CAP group 3 breast cancer. Our Group3N, with low HER2 protein expression by IHC (IHC0/1+), reported previously as HER2 not amplified, contrary to the current ASCO-CAP guidelines designation of ISH positive (BCIRG04086, original magnification, ×400). (H) ASCO-CAP group 3 breast cancer, one of the BCIRG group 3A cases, with an average HER2 gene copy number of 27.50 per tumor cell, an average CEP17 copy number of 20.67 per tumor cell, and, therefore, a HER2 FISH ratio of only 1.33. Please note that the HER2 gene signals (orange) and CEP17 signals (green) are aggregated together in a limited geographic area of the nucleus, making assessment of individual signals challenging without the aid of single band-pass filters (Data Supplement Figure S1). This breast cancer was reported as HER2 amplified in the BCIRG/TRIO central laboratory, and the patient was accrued to BCIRG-006. This case is consistent with the ASCO-CAP guidelines designation of ISH positive (BCIRG00575, original magnification, ×1,000). (I) ASCO-CAP group 3 breast cancer, the same group3A in panel H, with HER2 protein overexpression by IHC (IHC3+ by HercepTest), consistent with the ASCO-CAP guidelines designation of ISH positive (BCIRG00575, original magnification, ×400; Data Supplement Figure S1E). (J) ASCO-CAP group 4 breast cancer, referred to by the current ASCO-CAP guidelines as ISH equivocal. BCIRG/TRIO central laboratory reported the case as HER2 not amplified by FISH, with an average HER2 gene copy number of 4.22 per tumor cell, an average CEP17 copy number of 2.23 per tumor cell, and, therefore, an HER2-to-CEP17 FISH ratio of 1.89. The patient was randomly assigned to BCIRG-005 (BCIRG01911, original magnification, ×1,000). (K) ASCO-CAP group 4 breast cancer, as in panel J, with low HER2 protein expression by HercepTest (IHC0; BCIRG01911, original magnification, ×400). (L) ASCO-CAP group 5 breast cancer, consistent with the guidelines designation of ISH negative, which was reported by the BCIRG/TRIO central laboratory as HER2 not amplified by FISH. The case had an average HER2 gene copy number of 1.35 per tumor cell, with 1.50 CEP17 copies per cell and an HER2-to-CEP17 ratio of 0.90 (BCIRG04095, original magnification, ×1,000). (M) ASCO-CAP group 5 breast cancer, see panel L, with low HER2 protein expression by IHC with HercepTest (IHC0), consistent the ASCO-CAP guidelines designation of ISH negative (BCIRG04095, original magnification, ×400). This figure has been modified with permission from Figure 3 of the previously published article by Wolff et al.17 Copyright 2013 American Society of Clinical Oncology.
Statistical Methods
Standard statistical methods (Data Supplement) were used to assess significance for associations between ASCO-CAP FISH groups and HER2 protein expression (Friedman tests and χ2 tests) and clinical outcomes (log-rank tests) in BCIRG-00519,25 and BCIRG-006.4,26 Hazard ratios (HRs) were estimated by using Cox proportional hazards regression models.
RESULTS
To determine what proportion of breast cancers are in each ASCO-CAP category, we re-examined our HER2 FISH assessments from the BCIRG clinical trials conducted from 2000 to 2004—BCIRG-005, BCIRG-006, and BCIRG-007—and reclassified all screened cases into five groups according to the new ASCO-CAP guidelines (Table 1 and Fig 2).
Table 1.
HER2 FISH Assay Results From BCIRG Clinical Trials According to ASCO-CAP Guidelines Categories
| HER2 FISH Groups of Breast Cancers Screened for Patient Enrollment Onto BCIRG Trials, 2000-2004 | ||
|---|---|---|
| ASCO-CAP FISH Group | Description of HER2 FISH Category | No. of Cases (%) |
| 1 | Ratio ≥ 2.0, HER2 average ≥ 4.0 | 4,269 (40.8) |
| 2 | Ratio ≥ 2.0, HER2 average < 4.0 | 71 (0.7) |
| 3 | Ratio < 2.0, HER2 average ≥ 6.0 | 55 (0.5) |
| 4 | Ratio < 2.0, HER2 average ≥ 4.0, < 6.0 | 432 (4.1) |
| 5 | Ratio < 2.0, HER2 average < 4.0 | 5,641 (53.9) |
| Total* | 10,468* (100.0) | |
| HER2 FISH Assay Groups for Patients Randomly Assigned to a BCIRG Trial | ||
| 1 | Ratio ≥ 2.0, HER2 average ≥ 4.0 | 3,321 (49.9) |
| 2 | Ratio ≥ 2.0, HER2 average < 4.0 | 52 (0.8) |
| 3 | Ratio < 2.0, HER2 average ≥ 6.0 | 16 (0.2) |
| 4 | Ratio < 2.0, HER2 average ≥ 4.0, < 6.0 | 183 (2.8) |
| 5 | Ratio < 2.0, HER2 average < 4.0 | 3,079 (46.3) |
| Total | 6,651† | |
| HER2 FISH Assay Groups Among Patients Randomly Assigned to a Trial and With HER2 IHC‡ Assay Results Available | ||
| 1 | Ratio ≥ 2.0, HER2 average ≥ 4.0 | 2,040 (47.1) |
| 2 | Ratio ≥ 2.0, HER2 average < 4.0 | 35 (0.8) |
| 3 | Ratio < 2.0, HER2 average ≥ 6.0 | 9§ (0.2) |
| 4 | Ratio < 2.0, HER2 average ≥ 4.0, < 6.0 | 134 (3.1) |
| 5 | Ratio < 2.0, HER2 average < 4.0 | 2,113 (48.8) |
| Total | 4,331 (100) | |
Abbreviations: BCIRG, Breast Cancer International Research Group; CAP, College of American Pathologists; FISH, fluorescent in situ hybridization; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry.
Although 10,948 patients were screened in the BCIRG central laboratories for trial accrual, complete HER2 FISH assay results were not available from 480 cases for a variety of reasons, including lack of invasive carcinoma in samples submitted, tissue sections that detached from slides during processing, and FISH assay failure as a result of lack of probe hybridization.
Although 3,298 patients enrolled in BCIRG-005, 3,222 enrolled in BCIRG-006, and 263 enrolled in BCIRG-007 study for a total of 6,783 patients, data were available for 6,676, with 24 missing either average HER2 copy number or the ratio, and one randomly assigned patient did not enroll.
HER2 IHC assay results using the HercepTest.
The distribution by ASCO-CAP ISH group among the 10,468 patients whose breast cancers were successfully screened for enrollment into the three BCIRG/TRIO trials demonstrates that 40.8% were in group 1, 0.7% in group 2, 0.5% in group 3, 4.1% in group 4, and 53.9% in group 5 (Table 1 and Fig 3). A similar distribution was observed among randomly assigned patients whose cancers had FISH assay results available for analysis (Table 1) as well as those randomly assigned whose breast cancers were also evaluated by the HercepTest for HER2 protein expression (Table 1).
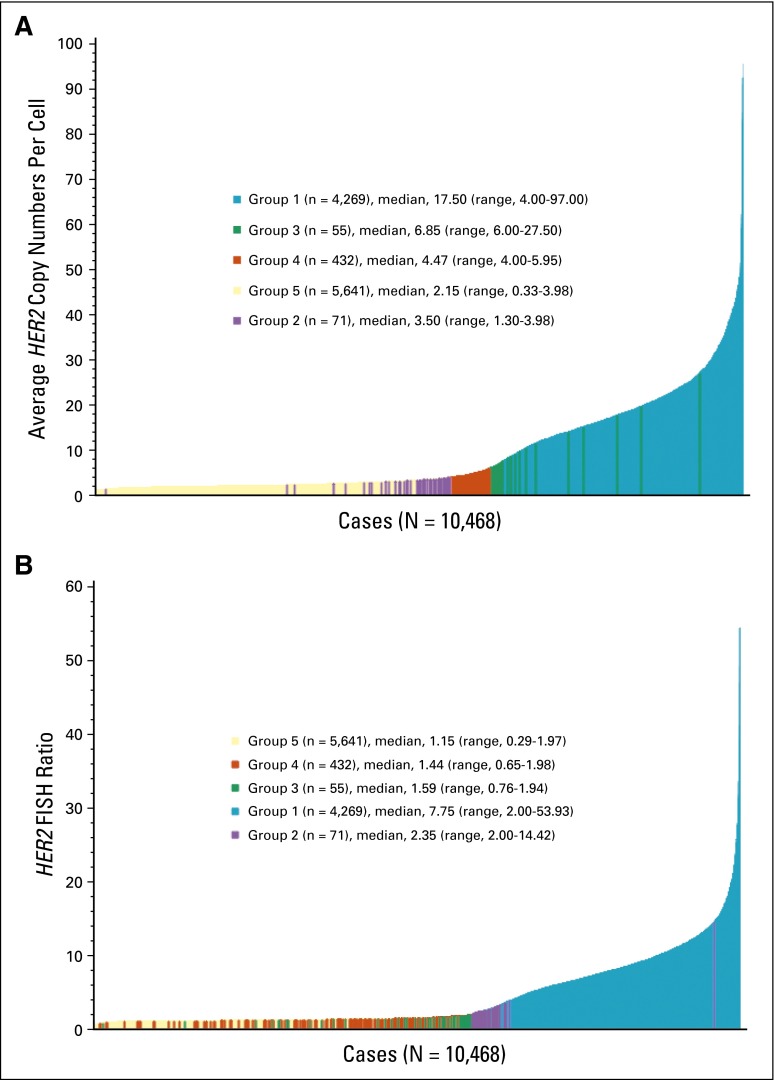
Fig 3.
Distribution of average human epidermal growth factor receptor 2 gene (HER2) copy number and HER2 FISH ratios among breast cancers successfully screened for enrollment into Breast Cancer International Research Group trials from 2000 to 2004. (A) Plot of average HER2 gene copy number per tumor cell nucleus from lowest to highest, with cases identified according to the ASCO and College of American Pathologists (ASCO-CAP) guidelines as groups 1 (blue), 2 (purple), 3 (green), 4 (orange), and 5 (yellow; N = 10,468. (B) Plot of HER2 FISH ratios from lowest to highest, as in panel A, with identification of ASCO-CAP groups 1 (blue), 2 (purple), 3 (green), 4 (red), and 5 (yellow; N = 10,468).
As expected, there was a significant association between increasing HER2 FISH ratios and increasing IHC scores among those breast cancers for which both an HER2 FISH assessment and an HER2 protein expression assessment by HercepTest IHC assay were available (P < .0001; Table 2). Similarly, an association was also observed between increasing average HER2 gene copy number per tumor cell and increasing IHC scores (P < .0001; Table 2). Assessment of HER2 gene amplification status typically involves an evaluation of both average HER2 gene copy number per tumor cell and HER2-to-CEP17 ratio. The new ASCO-CAP guidelines have formalized this evaluation to create five different groups (Table 1 and Fig 2), which we evaluated by group to determine if HER2 protein—either low expression or overexpression—is associated with each ASCO-CAP FISH group (Table 2).
Table 2.
Comparison of HER2 FISH Ratios and Average HER2 Gene Copy Numbers With HER2 Protein Expression by HercepTest IHC Scores in BCIRG Clinical Trials
| Overall Comparison of HER2 FISH Ratios and HER2 Copy Numbers With HER2 Protein by HercepTest IHC Scores | ||||||||
|---|---|---|---|---|---|---|---|---|
| PathVysion HER2 FISH Assay | HercepTest IHC Score* | Total | P† | ASCO-CAP FISH Group | ||||
| HER2 FISH Ratio | Average HER2 Copy Number per Cell | 0 | 1+ | 2+ | 3+ | |||
| < 2.0 | — | 2,098 (93.0%) | 137 (6.1%) | 19 (0.8%) | 3 (0.1%) | 2,257 (100%) | < .0001† | NA |
| 2.00-5.0 | — | 170 (35.4%) | 95 (19.8%) | 104 (21.6%) | 111 (23.1%) | 480 (100%) | NA | |
| 5.01-10.0 | — | 64 (6.7%) | 112 (11.7%) | 288 (30.1%) | 493 (51.5%) | 957 (100%) | NA | |
| > 10.0 | — | 30 (4.7%) | 65 (10.2%) | 181 (28.4%) | 361 (56.7%) | 637 (100%) | NA | |
| Total‡ | — | 2,362 | 409 | 592 | 968 | 4,331‡ | ||
| — | < 4.0 | 2,017 (93.6%) | 122 (5.7%) | 14 (0.6%) | 1 (0.05%) | 2,154 (100%) | < .0001† | NA |
| — | 4.01-6.0 | 166 (72.2%) | 44 (19.1%) | 17 (7.4%) | 3 (1.3%) | 230 (100%) | NA | |
| — | 6.01-8.0 | 48 (47.5%) | 23 (22.8%) | 19 (18.8%) | 11 (10.9%) | 101 (100%) | NA | |
| — | 8.01-10.0 | 25 (20.3%) | 27 (22.0%) | 34 (27.6%) | 37 (30.1%) | 123 (100%) | NA | |
| — | > 10.0 | 107 (6.2%) | 193 (11.2%) | 510 (29.5%) | 916 (53.1%) | 1,726 (100%) | NA | |
| Total‡ | 2,363 | 409 | 594 | 968 | 4,334‡ | |||
| Comparison of HER2 FISH Ratios and Copy Numbers With HER2 Protein by HercepTest Scores According to ASCO-CAP Groupings | ||||||||
| < 2.0 | < 4.0 | 1,988 (94.1%) | 114 (5.4%) | 10 (0.5%) | 1 (0.05%) | 2,113 (100%) | < .0001§ | Group 5 |
| ≥ 4.0-5.99 | 105 (78.4%) | 21 (15.7%) | 7 (5.2%) | 1 (0.7%) | 134 (100%) | < .0001§ | Group 4 | |
| ≥ 6.0 | 5 (55.6%) | 2 (22.2%) | 1 (11.1%) | 1 (11.1%) | 9 (100%) | .3881§ | Group 3 | |
| Total | 2,098 (93.0%) | 137 (6.1%) | 18 (0.8%) | 3 (0.1%) | 2,256 (100%) | < .0001§ | Groups 3-5 | |
| ≥ 2.0 | < 4.0 | 24 (68.6%) | 8 (22.9%) | 3 (8.6%) | 0 (0%) | 35 (100%) | < .0007‖ | Group 2 |
| ≥ 4.0-5.99 | 65 (65.7%) | 22 (22.2%) | 10 (10.1%) | 2 (2.0%) | 99 (100%) | < .0001‖ | Group 1 | |
| ≥ 6.0 | 175 (9.0%) | 242 (12.5%) | 561 (28.9%) | 963 (49.6%) | 1,941 (100%) | < .0001¶ | Group 1 | |
| Total | 264 (12.7%) | 272 (13.1%) | 574 (27.7%) | 965 (46.5%) | 2,075 (100%) | < .0001# | ||
| Total | 2,362 | 409 | 592 | 968 | 4,331 | |||
NOTE. Data from the BCIRG-007 trial comparing FISH with IHC are included in Table 2 but not in Table 3 of outcomes, because BCIRG-007 lacks a nontrastuzumab control arm.
Abbreviations: BCIRG, Breast Cancer International Research Group; CAP, College of American Pathologists; FISH, fluorescent in situ hybridization; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; NA, not applicable.
HercepTest scores were not available for 2,336 cases.
P value of Friedman test for increasing FISH ratio with increasing IHC.
The HER2 FISH ratio was not available for three cases.
P value of χ2 test for association between an HER2 FISH ratio < 2.0 and a lack of HER2 overexpression (ie, IHC0 and IHC1+).
P value of χ2 test for association between an HER2 FISH ratio ≥ 2.0, with either an average HER2 gene copy number < 4.0, or ≥ 4.0 but < 6.00, and a lack of HER2 overexpression (IHC0 and IHC1+).
P value of χ2 test for association between an HER2 FISH ratio ≥ 2.,0 with an average HER2 gene copy number ≥ 6.0, and HER2 overexpression (IHC3+).
P value of χ2 test for association between an HER2 FISH ratio ≥ 2.0 (without regard to average HER2 gene copy number/tumor cell nucleus) and HER2 protein overexpression (IHC3+).
HER2 Protein Expression by IHC in Each ASCO-CAP FISH Group
We determined whether HER2 ISH-positive breast cancers, categorized by the new ASCO-CAP guidelines as groups 1, 2, and 3, were correlated with HER2 protein overexpression or, alternatively, low expression. As described in the Data Supplement, we found that only breast cancers in group 1 (FISH ratio ≥ 2.0, average HER2 copy number/cell ≥ 4.0) were significantly associated with HER2 overexpression (IHC3+), with 75% of these showing either IHC2+ (28%) or IHC3+ (47.3%) immunostaining (P < .0001; Table 2).
In contrast, breast cancers from group 2 (FISH ratio ≥ 2.0, average HER2 copy number/cell < 4.0) were associated with low HER2 expression, not overexpression (P = .007), as > 90% showed either IHC0 or IHC1+ immunostaining (Table 2), whereas breast cancers in group 3 (FISH ratio < 2.0, average HER2 copy number/cell ≥ 6.0) were not significantly (P = .3881) associated with either overexpression or low expression. Breast cancers in ASCO-CAP ISH groups 4 and 5—ISH equivocal and ISH negative, respectively—were also significantly associated with low HER2 expression (both P < .0001; Table 2).
Breast cancers of group 3 (FISH ratio < 2.0, average HER2 copy number/cell ≥ 6.0) were composed of two different groups of breast cancers, a substantial majority (76%) of which were associated with low HER2 expression, whereas a minority (Data Supplement, Table S1 and Fig S2) showed HER2 overexpression.
Clinical Outcomes by ASCO-CAP ISH Groups
Because HER2 amplification is a known adverse prognostic marker for shorter DFS and OS and predictive of improved outcomes with trastuzumab therapy, we used these outcomes to determine whether ASCO-CAP FISH groups were associated with particular end points, as expected for either HER2-positive disease or HER2-negative disease. Because the natural history of HER2 gene amplification and overexpression in patients with breast cancer is associated with worse DFS and OS in the absence of HER2-targeted therapy8,27,28 and with significantly improved DFS and OS with HER2-targeted therapy,2-5,20,29,30 we have used these clinical outcomes to support the assignment of the various FISH groups as either amplified or not amplified as summarized below.
ASCO-CAP group 1 (ISH positive), HER2-to-CEP17 ratio ≥ 2.0 and average HER2 copy number ≥ 4.0 per tumor cell.
As expected, those patients whose breast cancers were HER2 amplified, with HER2 FISH ratios of ≥ 2.0 and average HER2 copy number of ≥ 4.0, had improved DFS and OS when treated with trastuzumab compared with those treated with conventional (AC-T) chemotherapy alone (n = 3,109; DFS: HR, 0.71; 95% CI, 0.60 to 0.83; P < .0001; and OS: HR, 0.69; 95% CI, 0.55 to 0.85; P = .0006; Tables 3 and 4).
Table 3.
Comparison of HER2 Ratio and Average HER2 Gene Copy Number and ASCO-CAP Groupings With Clinical Outcomes in BCIRG-005
| HER2 FISH (HER2/CEP17) Ratio | HER2 Copies per Cell | No. of Subjects | DFS, No. of Events | OS, No. of Events | DFS HR (95% CI) and P for Log-Rank Test* | OS HR (95% CI) and P for Log-Rank Test* | ASCO-CAP FISH Group |
|---|---|---|---|---|---|---|---|
| < 2.0 | < 4.0 | 3,079 | 971 | 606 | 1.0 (reference) | 1.0 (reference) | Group 5 |
| 4.01-6.0 | 176 | 51 | 30 | 0.923 (0.697 to 1.224) | 0.878 (0.609 to 1.267) | Group 4 | |
| P = .5795 | P = .4872 | ||||||
| ≥ 6 | 11 | 6 | 4 | 2.502 (1.121 to 5.583) | 2.351 (0.879 to 6.284) | Group 3 | |
| P = .0252 | P = .0885 |
NOTE. The hazard ratios are for each ASCO group compared with ASCO Group 5 taken as the reference. There were too few patients accrued to BCIRG-005 with a HER2 FISH ratio ≥ 2.0 for analysis of DFS or OS.
Abbreviations: BCIRG, Breast Cancer International Research Group; CAP, College of American Pathologists; DFS, disease-free survival; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; OS, overall survival.
Group 5 (reference) compared with each other group in BCIRG-005 (HER2 not amplified breast cancers).
Table 4.
Comparison of HER2 Ratio and Average HER2 Gene Copy Number and ASCO-CAP Groupings With Clinical Outcomes in BCIRG-006
| HER2 FISH (HER2/CEP17) Ratio | HER2 Copies per Cell | No. of Subjects | DFS Control, Events/No. of Subjects | DFS Trastzumab, No. of Events/Subjects | DFS, HR (95% CI)* | DFS P for Log-Rank Test* | OS Control | OS Trastzumab | OS, HR (95% CI)* | OS P for Log-Rank Test* | ASCO-CAP FISH Group |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ≥ 2.0 | < 4.0 | 46 | 4/18 | 6/28 | 1.10 (0.31 to 3.89) | .8860 | 2/18 | 4/28 | 3.15 (0.35 to 28.63) | .2839 | Group 2 |
| ≥ 4 | 3,109 | 251/1,031 | 391/2,078 | 0.71 (0.60 to 0.83) | < .0001 | 138/1,031 | 202/2,078 | 0.69 (0.55 to 0.85) | .0006 | Group 1 | |
| Total | 3,155 |
NOTE. The HRs are for trastuzumab treatment arms compared with control chemotherapy-only arm. There were too few patients (n = 5) accrued to BCRIG-006 with a HER2 FISH ratio < 2.0 and ≥ 6.0 average HER2 gene copy number/tumor cell for analysis of the HR.
Abbreviations: BCIRG, Breast Cancer International Research Group; CAP, College of American Pathologists; DFS, disease-free survival; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; OS, overall survival.
Trastuzumab-containing treatment arms compared with control (chemotherapy alone) treatment arm.
ASCO-CAP group 2 (ISH positive), HER2-to-CEP17 ratio ≥ 2.0 and average HER2 copy number < 4.0.
Among patients who were randomly assigned to BCIRG-006 trial of adjuvant trastuzumab whose breast cancers had an HER2 FISH ratio of ≥ 2.0 but average HER2 copy number of < 4.0/tumor cell, there was no apparent benefit from trastuzumab therapy, either in terms of DFS (n = 46; HR, 1.10; 95% CI, 0.31 to 3.89; P = .886) or OS (HR, 3.15; 95% CI, 0.35 to 28.63; P = .284; Tables 3 and 4).
ASCO-CAP group 3 (ISH positive), HER2-to-CEP17 ratio < 2.0 and average HER2 copy number ≥ 6.0.
Overall, patients with breast cancer in this FISH group who were accrued to BCIRG-005 had a worse DFS (HR, 2.50; P = .0252) and OS (HR, 2.35; P = .0885; Tables 3 and 4) than did the comparator group, group 5. However, during central laboratory FISH screening, patients whose breast cancers had HER2 ratios of < 2.0 and average HER2 copy numbers of ≥ 6.0/tumor cell were considered to consist of a minority of HER2-amplified breast cancers within a majority pool of HER2-nonamplified breast cancers. These cases were distinguished from one another by additional analyses21,26,31,32 (Data Supplement). Most patients in this HER2 FISH group were accrued to BCIRG-005 as not amplified, whereas few were accrued to BCIRG-006 through protocol amendment as amplified. This approach with separation into two subgroups is supported by HER2 IHC assay results (Data Supplement). Although we had divided group 3 breast cancers into two different subgroups—one eligible for BCIRG-005 with an average of 7.43 HER2 genes/tumor cell, and the other eligible for BCIRG-006 with an average of 16.38 HER2 genes/tumor cell—we considered the small numbers insufficient for definitive evaluation of this group in either BCIRG-005 or BCIRG-006.
ASCO-CAP group 4 (ISH equivocal), HER2-to-CEP17 ratio < 2.0 and average HER2 copy number ≥ 4.0 and < 6.0/tumor cell.
Because patients with breast cancers that had a ratio of < 2.0 were considered HER2 not amplified, these patients were accrued to the BCIRG-005 trial of sequential (AC-T) or concurrent (taxotere, adriamycin, and cyclophosphamide) chemotherapy.19 Outcomes among these 176 patients did not differ significantly from outcomes in group 5 (DFS: HR, 0.923; 95% CI, 0.70 to 1.22; P = 0.58; and OS: HR, 0.88; 95% CI, 0.61 to 1.27; P = 0.49; Tables 3 and 4).
ASCO-CAP group 5 (ISH negative), HER2-to-CEP17 ratio < 2.0 and average HER2 copy number < 4.0/tumor cell.
HER2 status by FISH for these patients with breast cancer was considered HER2 not amplified or ISH negative and served as the baseline comparison group for DFS and OS in the BCIRG-005 trial.
DISCUSSION
The most recent ASCO-CAP guidelines have again redefined HER2 gene amplification as determined by ISH in a fashion that is different from prior definitions, particularly the FDA-approved package inserts for HER2 FISH companion diagnostic assays,33,34 which includes criteria used for BCIRG/TRIO clinical trials,4,19,21,22,27 as well as prior 2007 ASCO-CAP guidelines.15,16 Originally, HER2 gene amplification was assessed by Southern blot using hybridization of a radiolabeled HER2 gene probe compared with hybridization of a probe for a control gene, for example, arginase (ARG1),28 myeloperoxidase (MPO),8,35 or TP53,36 as an internal control for amplification. A ratio between HER2 and control signals ≥ 2.0 was evaluated as amplification. Subsequently, gene amplification was assessed by FISH using either CEP1727,37 or another gene on the same chromosome32 as an internal control, again with a ratio of ≥ 2.0 being considered as evidence for HER2 amplification. Therefore, similar strategies have been used over a 30-year period to assess breast cancers as either amplified or not amplified. These criteria were used for enrollment in all major trials of trastuzumab,2-5 lapatinib,13,14 and, more recently, pertuzumab11 and trastuzumab emtansine,12 which demonstrated a clinical benefit for HER2-targeted therapies.
ASCO-CAP guidelines changed the HER2-to-CEP17 ratio used for amplification from ≥ 2.0 to ≥ 2.2 in 2007,15,16 then changed the ratio back to ≥ 2.0 in 201317 and 201418 with the addition of formalized categories using average HER2 copy numbers per tumor cell. Because these new criteria for amplification by ISH are likely to select somewhat different patient populations for HER2-targeted therapies, we retrospectively re-evaluated these issues with breast cancers that had annotated long-term clinical outcomes from our clinical trials.
Because HER2 amplification is accepted as directly associated with protein overexpression,8,22,38 a worse DFS and OS in the absence of HER2-targeted therapy,27,28 and with improved outcomes with HER2-targeted therapy,2-5,13 we used these as criteria for assessment of each newly defined ASCO-CAP group (Table 5). In these analyses, most patients experienced no change in HER2 amplification status as determined by FISH, as ASCO-CAP groups 1 and 5 represent the vast majority of patients (approximately 95%) and because the status as amplified (group 1) and not amplified (group 5) is not changed by the new guidelines (Table 5). Although we find only a small minority of patients (approximately 5%) are affected by the new ASCO-CAP guidelines changes, our findings contradict the designations of these guidelines for groups 2, 3, and 4. Groups 2 and 4, designated ISH positive and ISH equivocal, respectively, by the ASCO-CAP guidelines, seem to be HER2 not amplified on the basis of associations with a lack of protein overexpression (groups 2 and 4), a lack of response to trastuzumab treatment (group 2), and similar prognosis as group 5 for patients (group 4) treated with chemotherapy alone (Table 5). Overall, we observe approximately 99.3% agreement with initial FDA-approved guidelines and 94.7% agreement with current ASCO-CAP guidelines (Table 5). The 4.6% differential is related to the only two groups, groups 3 and 4, introduced by ASCO-CAP that lead to different assessments of HER2 status compared with FDA criteria. Finally, our observations indicate group 2, which represents 0.7% of breast cancers, is misclassified by both the FDA and ASCO-CAP guidelines as amplified and ISH positive (Table 5).
Table 5.
Comparison of FISH Groups, FDA Guidelines Status, and ASCO-CAP Guidelines Status, and Associations With Outcomes in BCIRG Clinical Trials
| FISH | Group | Frequency, %* | FDA Status† | ASCO-CAP Guidelines | HER2 Protein Expression | Prognosis (BCIRG-005 trial) | Response to HER2-Targeted Therapy (BCIRG-006) | BCIRG/TRIO Study Conclusion | |
|---|---|---|---|---|---|---|---|---|---|
| Ratio | Average HER2 per Tumor Cell | ||||||||
| ≥ 2.0 | ≥ 4.0 | 1 | 40.8 | Amplified | ISH positive | HER2 overexpression (P < .0001; IHC3+) | Not included in trial | Significantly improved outcomes | HER2 amplified |
| ≥ 2.0 | < 4.0 | 2 | 0.7 | Amplified | ISH positive | HER2 low expression (P < .0001; IHC0/1+) | Not included in trial | No significant benefit | HER2 not amplified |
| < 2.0 | ≥ 6.0 | 3 | 0.5 | Not amplified | ISH positive | Combination of HER2 low and overexpression | Indeterminate mixed category | Indeterminate, mixed category | Mixed HER2 not amplified and amplified, on the basis of expression |
| < 2.0 | ≥ 4.0, < 6.0 | 4 | 4.1 | Not amplified | ISH equivocal | HER2 low expression (P < .0001; IHC0/1+) | Not associated with worse outcomes | Not included in trial | HER2 not amplified |
| < 2.0 | < 4.0 | 5 | 53.9 | Not amplified | ISH negative | HER2 low expression (P < .0001; IHC0/1+) | Not associated with worse outcomes | Not included in trial | HER2 not amplified |
Abbreviations: BCIRG, Breast Cancer International Research Group; CAP, College of American Pathologists; FDA, US Food and Drug Administration; FISH, fluorescent in situ hybridization; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; ISH, in situ hybridization; TRIO, Translational Research in Oncology.
Frequencies are based on screened population in Table 1.
Although cancers in group 3 are designated as ISH positive by ASCO-CAP guidelines, our results suggest that this group is a mixture of HER2 not amplified and amplified breast cancers, with the majority being not amplified on the basis of criteria described previously.21,31,32 These categorizations are supported by associations with HER2 expression (Data Supplement Table S1), as well as similar findings from a clinical consultation practice where breast cancers with high average HER2 gene copy number per cell are associated with HER2 protein overexpression (IHC2+ and IHC3+), whereas those with lower average HER2 copy number per cell are associated with low HER2 protein expression by IHC.26 Nevertheless, we did not have a sufficient number of cases in these subgroups in the BCIRG trials to separately evaluate their association with clinical outcomes.
Because the HER2-not amplified BCIRG-005 trial completed accrual in February 2003 and the HER2-amplified BCIRG-006 trial continued accrual until March 2004 with local laboratory IHC prescreening for approximately 60% of breast cancers submitted to the central laboratories, the prevalence of HER2 amplification in the screened population increased from 26% while both trials were in the accrual stage21 to 40% when BCIRG-006 completed accrual a year later. Nevertheless, the distribution of cases in groups 2, 3, and 4 are similar to those in our consultation practice where the ASCO-CAP group 1, or HER2 amplification, rate is 18%.26
There are now nearly three decades of accumulated experience and published data studying this alteration in human breast cancers. Although guidelines are helpful, diagnostic judgment and long-term outcome data remain important in the evaluation of testing criteria.
Supplementary Material
Acknowledgment
We thank the patients who enrolled in the trials and gave their consent as well as the Translational Research in Oncology (previously known as Breast Cancer International Research Group) Investigators who recruited them at each of the clinical sites and staff at those sites who supported the trial: Angela Santiago (University of Southern California [USC] central laboratory), Roberta Guzman (USC central laboratory), Armen Gasparyan (USC central laboratory), Jian-Yuan Zhou, MD (USC central laboratory), Rooba Wardeh, MD (USC central laboratory), Yong-Tian (Brandon) Li, MD (USC central laboratory), Hedvika Novotny (Basel, Switzerland), Sandra Schneider (Basel, Switzerland), and Rosemarie Chaffard (Basel, Switzerland) for technical assistance; Sandra Swain, MD, chair of the data and safety monitoring committee; and all steering committee and translational research committee members.
Footnotes
Supported in part by grants from the Breast Cancer Research Foundation (M.F.P.), California Breast Cancer Research Program (M.F.P.), Tower Cancer Research Foundation (Jessica M. Berman Senior Investigator Award), a gift from Dr. Richard Blach (M.F.P.), and Entertainment Industry Foundation (M.F.P. and D.J.S.) as well as an endowed chair, the Harold E. Lee Chair for Cancer Research (M.F.P.). The project was also supported in part by Grant No. P30CA014089 from the National Cancer Institute. Support for these trials was provided to the Breast Cancer International Research Group (BCIRG), now Translational Research in Oncology. The sponsor and major funder of the BCIRG-006 trial was Sanofi, with additional support provided by Genentech. Trastuzumab was provided by Genentech free of charge for study patients in the United States and was purchased by Sanofi for all study patients in other countries. Docetaxel was provided by Sanofi for all study patients. The BCIRG-005 trial was sponsored by Sanofi. The BCIRG-007 trial was sponsored by F. Hoffman-La Roche Ltd and Genentech.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT00021255 and NCT00312208.
AUTHOR CONTRIBUTIONS
Conception and design: Michael F. Press, Dennis J. Slamon
Financial support: Michael F. Press
Administrative support: Michael F. Press, Valerie Bee, Ivonne Villalobos, Mary-Ann Lindsay
Provision of study materials or patients: Michael F. Press, Guido Sauter, Wolfgang Eiermann, Nicholas Robert, Tadeusz Pienkowski, John Crown, Miguel Martin, Vicente Valero, John R. Mackey, Martina Mirlacher, Dennis J. Slamon
Collection and assembly of data: Michael F. Press, Guido Sauter, Wolfgang Eiermann, Nicholas Robert, Tadeusz Pienkowski, John Crown, Miguel Martin, Vicente Valero, John R. Mackey, Valerie Bee, Yanling Ma, Ivonne Villalobos, Martina Mirlacher, Mary-Ann Lindsay, Dennis J. Slamon
Data analysis and interpretation: Michael F. Press, Guido Sauter, Marc Buyse, Hélène Fourmanoir, Emmanuel Quinaux, Denice D. Tsao-Wei, Ivonne Villalobos, Anaamika Campeau, Dennis J. Slamon
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
HER2 Gene Amplification Testing by Fluorescent In Situ Hybridization (FISH): Comparison of the ASCO-College of American Pathologists Guidelines With FISH Scores Used for Enrollment in Breast Cancer International Research Group Clinical Trials
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Michael F. Press
Honoraria: Biocartis, DAKO, Halozyme, Puma Biotechnology, Cepheid, Ventana Medical Systems
Consulting or Advisory Role: Biocartis, Halozyme, Puma Biotechnology, Cepheid, Ventana Medical Systems
Research Funding: Cepheid (Inst)
Guido Sauter
No relationship to disclose
Marc Buyse
Employment: International Drug Development Institute
Stock or Other Ownership: International Drug Development Institute
Hélène Fourmanoir
Employment: Business and Decision Life Sciences
Emmanuel Quinaux
No relationship to disclose
Denice D. Tsao-Wei
No relationship to disclose
Wolfgang Eiermann
Consulting or Advisory Role: Roche
Speakers' Bureau: Roche
Travel, Accommodations, Expenses: Roche
Nicholas Robert
Consulting or Advisory Role: New Century Health
Research Funding: Sideout Foundation
Other Relationship: Paradigm Dx
Tadeusz Pienkowski
Honoraria: Roche, AstraZeneca
Research Funding: Roche, Pfizer
Travel, Accommodations, Expenses: Roche, Novartis
John Crown
Honoraria: Eisai, Pfizer
Research Funding: Eisai (Inst)
Miguel Martin
Consulting or Advisory Role: Genentech, Novartis, Amgen, Pfizer, Eli Lilly
Research Funding: Novartis (Inst)
Vicente Valero
Consulting or Advisory Role: Genentech
Research Funding: Genentech (Inst)
John R. Mackey
Stock or Other Ownership: Pacylex Pharmaceuticals
Honoraria: Eli Lilly, Roche, Pfizer
Patents, Royalties, Other Intellectual Property: Pacylex Pharmaceuticals
Valerie Bee
No relationship to disclose
Yanling Ma
No relationship to disclose
Ivonne Villalobos
Honoraria: Biocartis (I), Halozyme (I), Puma Biotechnology (I), Cepheid (I), DAKO (I), Ventana Medical Systems (I)
Consulting or Advisory Role: Biocartis (I), DAKO (I), Halozyme (I), Puma Biotechnology (I), Cepheid (I), Ventana Medical Systems (I)
Research Funding: Novartis (Inst), Eli Lilly (Inst), Cepheid (Inst)
Anaamika Campeau
Employment: Kaiser Permanente (I), Aetna (I), Lakeside Medical Group (I)
Travel, Accommodations, Expenses: Aetna (I)
Martina Mirlacher
No relationship to disclose
Mary-Ann Lindsay
Research Funding: Translational Research in Oncology
Dennis J. Slamon
Leadership: Biomarin
Stock or Other Ownership: Pfizer
Honoraria: Novartis
Consulting or Advisory Role: Novartis, Eli Lilly
Research Funding: Pfizer, Novartis
Travel, Accommodations, Expenses: Biomarin, Pfizer, Novartis
REFERENCES
- 1.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 2.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 3.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 4.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 6.Press MF, Pike MC, Hung G, et al. Amplification and overexpression of HER-2/neu in carcinomas of the salivary gland: Correlation with poor prognosis. Cancer Res. 1994;54:5675–5682. [PubMed] [Google Scholar]
- 7.Saffari B, Jones LA, el-Naggar A, et al. Amplification and overexpression of HER-2/neu (c-erbB2) in endometrial cancers: Correlation with overall survival. Cancer Res. 1995;55:5693–5698. [PubMed] [Google Scholar]
- 8.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 9.Wen W, Chen W, Xiao N, et al. Mutations in the kinase domain of the HER2/ERBB2 gene identified in a wide variety of human cancers. J Mol Diagn. 2015;17:487–495. doi: 10.1016/j.jmoldx.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bose R, Kavuri SM, Searleman AC, et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013;3:224–237. doi: 10.1158/2159-8290.CD-12-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baselga J, Cortés J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 14.Press MF, Finn RS, Cameron D, et al. HER-2 gene amplification, HER-2 and epidermal growth factor receptor mRNA and protein expression, and lapatinib efficacy in women with metastatic breast cancer. Clin Cancer Res. 2008;14:7861–7870. doi: 10.1158/1078-0432.CCR-08-1056. [DOI] [PubMed] [Google Scholar]
- 15.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 16.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131:18–43. doi: 10.5858/2007-131-18-ASOCCO. [DOI] [PubMed] [Google Scholar]
- 17.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 18.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med. 2014;138:241–256. doi: 10.5858/arpa.2013-0953-SA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eiermann W, Pienkowski T, Crown J, et al. Phase III study of doxorubicin/cyclophosphamide with concomitant versus sequential docetaxel as adjuvant treatment in patients with human epidermal growth factor receptor 2-normal, node-positive breast cancer: BCIRG-005 trial. J Clin Oncol. 2011;29:3877–3884. doi: 10.1200/JCO.2010.28.5437. [DOI] [PubMed] [Google Scholar]
- 20.Mass RD, Press MF, Anderson S, et al. Evaluation of clinical outcomes according to HER2 detection by fluorescence in situ hybridization in women with metastatic breast cancer treated with trastuzumab. Clin Breast Cancer. 2005;6:240–246. doi: 10.3816/CBC.2005.n.026. [DOI] [PubMed] [Google Scholar]
- 21.Press MF, Sauter G, Bernstein L, et al. Diagnostic evaluation of HER-2 as a molecular target: An assessment of accuracy and reproducibility of laboratory testing in large, prospective, randomized clinical trials. Clin Cancer Res. 2005;11:6598–6607. doi: 10.1158/1078-0432.CCR-05-0636. [DOI] [PubMed] [Google Scholar]
- 22.Sauter G, Lee J, Bartlett JM, et al. Guidelines for human epidermal growth factor receptor 2 testing: Biologic and methodologic considerations. J Clin Oncol. 2009;27:1323–1333. doi: 10.1200/JCO.2007.14.8197. [DOI] [PubMed] [Google Scholar]
- 23.Hecht JR, Bang YJ, Qin SK, et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC--A randomized phase III trial. J Clin Oncol. 2016;34:443–451. doi: 10.1200/JCO.2015.62.6598. [DOI] [PubMed] [Google Scholar]
- 24.Valero V, Forbes J, Pegram MD, et al. Multicenter phase III randomized trial comparing docetaxel and trastuzumab with docetaxel, carboplatin, and trastuzumab as first-line chemotherapy for patients with HER2-gene-amplified metastatic breast cancer (BCIRG 007 study): Two highly active therapeutic regimens. J Clin Oncol. 2011;29:149–156. doi: 10.1200/JCO.2010.28.6450. [DOI] [PubMed] [Google Scholar]
- 25.Mackey JR, Pienkowski T, Crown J, et al. Long-term outcomes after adjuvant treatment of sequential versus combination docetaxel with doxorubicin and cyclophosphamide in node-positive breast cancer: BCIRG-005 randomized trial. Ann Oncol. 2016;27:1041–1047. doi: 10.1093/annonc/mdw098. [DOI] [PubMed] [Google Scholar]
- 26. doi: 10.5858/arpa.2016-0009-OA. Press MF, Villalobos IE, Santiago A, et al: Assessing the new American Society of Clinical Oncology/College of American Pathologists guidelines for HER2 testing by fluorescence in situ hybridization: Experience of an academic consultation practice. Arch Pathol Lab Med 10.5858/arpa.2016-0009-OA [Epub ahead of print on April 15, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Press MF, Bernstein L, Thomas PA, et al. HER-2/neu gene amplification characterized by fluorescence in situ hybridization: Poor prognosis in node-negative breast carcinomas. J Clin Oncol. 1997;15:2894–2904. doi: 10.1200/JCO.1997.15.8.2894. [DOI] [PubMed] [Google Scholar]
- 28.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 29.Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 30.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 31.Press MF. How is Her-2/neu status established when Her-2/neu and chromosome 17 centromere are both amplified? Am J Clin Pathol. 2006;126:673–674. doi: 10.1309/GM16-C018-06EF-URX7. [DOI] [PubMed] [Google Scholar]
- 32.Troxell ML, Bangs CD, Lawce HJ, et al. Evaluation of Her-2/neu status in carcinomas with amplified chromosome 17 centromere locus. Am J Clin Pathol. 2006;126:709–716. doi: 10.1309/9EYM-6VE5-8F2Y-CD9F. [DOI] [PubMed] [Google Scholar]
- 33.Abbott Laboratories PathVysion HER-2 DNA probe kit. https://www.abbottmolecular.com/en-us/staticAssets/pdfs/us/pathvysion-watermark-pl-rev-6.pdf.
- 34.DAKO HER2 IQFISH pharmDx. http://www.dako.com/download.pdf?objectid=122737002.
- 35.Slamon DJ, Clark GM. In reply: Amplification c-erbB-2 and aggressive human breast tumors? Science. 1988;240:1796–1798. doi: 10.1126/science.240.4860.1796. [DOI] [PubMed] [Google Scholar]
- 36.Clark GM, McGuire WL. Follow-up study of HER-2/neu amplification in primary breast cancer. Cancer Res. 1991;51:944–948. [PubMed] [Google Scholar]
- 37.Press MF, Slamon DJ, Flom KJ, et al. Evaluation of HER-2/neu gene amplification and overexpression: Comparison of frequently used assay methods in a molecularly characterized cohort of breast cancer specimens. J Clin Oncol. 2002;20:3095–3105. doi: 10.1200/JCO.2002.09.094. [DOI] [PubMed] [Google Scholar]
- 38.Pauletti G, Godolphin W, Press MF, et al. Detection and quantitation of HER-2/neu gene amplification in human breast cancer archival material using fluorescence in situ hybridization. Oncogene. 1996;13:63–72. [PubMed] [Google Scholar]
- 39.Park JM, Yang X, Park JJ, et al. Assessment of novel anti-p185HER-2 monoclonal antibodies for internalization-dependent therapies. Hybridoma. 1999;18:487–495. doi: 10.1089/hyb.1999.18.487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.