Abstract
Purpose
Extramedullary disease (EMD) at diagnosis in patients with acute myeloid leukemia (AML) has been recognized for decades. Reported herein are results from a large study of patients with AML who were treated in consecutive ECOG-ACRIN Cancer Research Group frontline clinical trials in an attempt to define the incidence and clinical implications of EMD.
Methods
Patients with newly diagnosed AML, age 15 years and older, who were treated in 11 clinical trials, were studied to identify EMD, as defined by physical examination, laboratory findings, and imaging results.
Results
Of the 3,522 patients enrolled, 282 were excluded, including patients with acute promyelocytic leukemia, incorrect diagnosis, or no adequate assessment of EMD at baseline. The overall incidence of EMD was 23.7%. The sites involved were: lymph nodes (11.5%), spleen (7.3%), liver (5.3%), skin (4.5%), gingiva (4.4%), and CNS (1.1%). Most patients (65.3%) had only one site of EMD, 20.9% had two sites, 9.5% had three sites, and 3.4% had four sites.
The median overall survival was 1.035 years. In univariable analysis, the presence of any EMD (P = .005), skin involvement (P = .002), spleen (P < .001), and liver (P < .001), but not CNS (P = .34), nodal involvement (P = .94), and gingival hypertrophy (P = .24), was associated with a shorter overall survival. In contrast, in multivariable analysis, adjusted for known prognostic factors such as cytogenetic risk and WBC count, neither the presence of EMD nor the number of specific sites of EMD were independently prognostic.
Conclusion
This large study demonstrates that EMD at any site is common but is not an independent prognostic factor. Treatment decisions for patients with EMD should be made on the basis of recognized AML prognostic factors, irrespective of the presence of EMD.
INTRODUCTION
One of the known manifestations of acute myeloid leukemia (AML) is extramedullary disease (EMD). The overall incidence of EMD reported in the literature is not clearly established, ranging from 2.5%1 to 30%,2 and varies among different types of AML; patients with monocytic AML3 and those with t(8;21)4,5 have a relatively higher incidence. The prognostic impact of EMD is unfavorable in some reports2,5 but not in others.6-8 Reports on the prognosis of specific EMD sites, such as the CNS, are also contradictory.9,10
The current analysis evaluated a large cohort of patients with newly diagnosed AML from 11 consecutive clinical trials conducted by the ECOG-ACRIN Cancer Research Group.11-20 The objectives were to describe the demographic, clinical, and biologic characteristics of patients with newly diagnosed AML with EMD and to evaluate how the presence, extent, and characteristics of EMD may affect response to treatment and outcome.
METHODS
Patient Population
Between 1980 and 2008, 3,522 patients age 15 years and older with untreated AML were enrolled in 11 consecutive, phase II and III, ECOG-ACRIN–led clinical trials.11-20 The treatment protocols, their activation dates, and accrual numbers are summarized in Table 1.
Table 1.
Acute Myeloid Leukemia Protocols Included in the Analysis
| Protocol No. | Clinical Trial Phase | Induction | Consolidation | Maintenance | Activation-Termination Dates | Final Accrual (patients included) |
|---|---|---|---|---|---|---|
| E147911 | III | One or two courses of: dauno 60 mg/m2/d, days 1-3; ARA-C 25 mg/m2 IV push followed by continuous IV 200 mg/m2/d, days 1-5; and PO 6-TG 100 mg/m2 × 2/d, days 1-5 (DAT). | Randomly assigned to either: a) two courses of consolidation therapy followed by maintenance, or b) begin maintenance immediately. A consolidation course consisted of dauno 45 mg/m2, days 1 and 2; ARA-C 100 mg/m2 IV push; and PO 6-TG 100 mg/m2 × 2/d, days 1-5. | PO 6-TG 40 mg/m2 × 2/d, 4 days, followed by SC ARA-C 60 mg/m2 on the fifth day. Duration: 2 years. | Feb 1980 to Apr 1982 | 318 (289) |
| E348015 | III | One or two courses of either: a) full DAT (above), or b) attenuated-dose DAT: dauno 50 mg/m2/d on day 1, SC ARA-C 100 mg/m2 × 2/d, days 1-5 and PO 6-TG 100 mg/m2 × 2/d, days 1-5. | PO 6-TG 40 mg/m2 × 2/d, 4 days, followed by SC ARA-C 60 mg/m2 on the fifth day. Duration: 2 years. | Jul 1981 to Nov 1982 | 45 (39) | |
| E348312 | III | One or two courses of DAT. | Age < 41 years plus HLA-matched sibling: alloBMT. Others were initially randomly assigned to one of three arms: a) observation, b) maintenance, or c) one course of consolidation. After interim analysis, the observation arm was closed. Consolidation therapy: IV ARA-C, 3 g/m2 over 1 hour × 2/d, days 1-6, followed by IV amsacrine, 100 mg/m2/d, days 7-9. | PO 6-TG 40 mg/m2 × 2/d, for 4 days, followed by SC ARA-C 60 mg/m2 on the fifth day. Duration: 2 years. | Mar 1984 to Jan 1988 | 534 (445) |
| PC48613 | II | One or two courses of DAT. | Patients in CR: a) age < 41 years and HLA identical sibling: alloBMT, and b) all others: autoBMT. | Apr 1987 to Apr 1990 | 123 (98) | |
| E348914 | III | One or two courses of idarubicin 12 mg/m2/d, days 1-3; and ARA-C 25 mg/m2 IV push, followed by continuous IV 100 mg/m2/d, days 1-7. | Idarubicin 12 mg/m2/d, days 1 and 2; and ARA-C 25 mg/m2 IV push, followed by continuous IV 100 mg/m2/d, days 1-5. a) Patients with an HLA-matched or single-mismatched family member: alloBMT. All others were randomly assigned to b) autoBMT, or c) a single course of IV ARA-C 3 g/m2 over 3 hours × 2/d, days 1-6. | Feb 1990 to Feb 1995 | 808 (753) | |
| E149016 | III | One or two courses of dauno 60 mg/m2/d, days 1-3; ARA-C 25 mg/m2 IV push followed by continuous IV 100 mg/m2/d, days 1-7; plus GM-CSF v placebo from day 11. | A single course of IV ARA-C 1.5 g/m2 over 1 hour × 2/d, days 1-6, plus GM-CSF v placebo from day 11. | Sep 1990 to Nov 1992 | 124 (115) | |
| E399317 | III | GM-CSF v placebo as priming, ARA-C continuous IV 100 mg/m2/d, days 1-7. Patients were randomly assigned to receive either: a) dauno 45 mg/m2/d, days 1-3; b) mitox 12 mg/m2/d, days 1-3; or c) idarubicin 12 mg/m2/d, days 1-3. | Age < 70 years: IV ARA-C 1.5 g/m2 over 1 hour × 2/d, days 1-6, plus GM-CSF from day 5. Age > 70 years: IV ARA-C 1.5 g/m2 over 1 hour × 2/d, days 1-3, plus GM-CSF from day 5. | Apr 1993 to Feb 1997 | 362 (343) | |
| E499521 | II | Two cycles of: dauno 45 mg/m2/d, days 1-3; ARA-C continuous IV 100 mg/m2/d, days 1-7; and ARA-C 2 g/m2 over 75-90 min × 2/d, days 8-10. | Age < 51 years plus HLA-matched sibling: alloPBSCT. Others: two courses of ARA-C 3 g/m2 over 3 hours × 2/d, days 1, 3, 5, and then autoPBSCT. | Aug 1996 to Feb 1997 | 66 (59) | |
| E399718 | II | Dauno 45 mg/m2/d, days 1-3; ARA-C continuous IV 100 mg/m2/d, days 1-7; and ARA-C 2 g/m2 over 60-90 min x 2/d, days 8-10 plus rhIL-11 and GM-CSF from days 11 to 12. | Two courses of: ARA-C 3g/m2 over 3 hours × 2/d, days 1, 3, 5 plus rhIL-11 and GM-CSF from day 6. | Jun 1998 to Apr 1999 | 36 (35) | |
| E399919 | III | One or two courses of dauno 45 mg/m2/d, days 1-3; ARA-C continuous IV 100 mg/m2/d, days 1-7; and zosuquidar v placebo. | 1) ARA-C 1.5 g/m2 over 1 hour, days 1-6: age < 70 years, × 2/d; age > 70 years, × 1/d. 2) A course identical to the induction regimen, including either zosuquidar or placebo. | Jul 2002 to Sep 2005 | 449 (421) | |
| E190020 | III | Dauno 45 v 90 mg/m2/d, days 1-3; ARA-C continuous IV 100 mg/m2/d, days 1-7. | Unfavorable/intermediate risk cytogenetic profile or a WBC count > 100,000/μL at diagnosis plus HLA-matched sibling: alloHSCT. All others: 1) two courses of ARA-C 3 g/m2 over 3 hours × 2/d, days 1, 3, and 5; 2) random assignment: GO 6 mg/m2 v no GO; and 3) autoHSCT. | Dec 2002 to Nov 2008 | 657 (644) |
Abbreviations: allo, allogeneic; ARA-C, cytarabine; auto, autologous; BMT, bone marrow transplantation; CR, complete remission; d, day; DAT, daunorubicin plus ARA-C plus 6-TG; dauno, daunorubicin; GM-CSF, granulocyte-macrophage colony-stimulating factor; GO, gemtuzumab ozogamicin; HSCT, hematopoietic stem-cell transplantation; IV, intravenous; mitox, mitoxantrone; PBSCT, peripheral blood stem-cell transplantation; PO, orally; rhIL-11, recombinant human interleukin 11; SC, subcutaneous; 6-TG, thioguanine.
Patients were excluded from the current analysis if they had a diagnosis other than AML, had no documented EMD ascertainment at baseline, withdrew from the study before treatment had begun, or had no survival follow up. Patients with acute promyelocytic leukemia were included in the older AML studies but are excluded from the analysis as a result of the unique behavior and treatment of this disease. Each protocol was approved by an institutional review board. All patients signed a written informed consent.
Cytogenetic Risk Classification
Cytogenetic risk was classified as favorable, intermediate, unfavorable, or undetermined after central review by the ECOG-ACRIN Cytogenetics Committee, according to the definitions established by the Southwest Oncology Group and ECOG-ACRIN.22 The favorable risk category included patients with inv(16)/t(16;16)/del(16q) or t(8;21), with or without other chromosomal abnormalities. The intermediate risk category included patients characterized by +8; −Y; +6; del(9q); del(12p), or normal karyotype. The unfavorable risk category was defined by the presence of one or more of −5/del(5q); −7/del(7q); inv(3q)/t(3;3); deletion 20q or 21q; translocation involving 11q23, t(6;9); t(9;22); deletion 17p; or complex karyotype, defined as three or more chromosomal abnormalities. Minimal cytogenetic information was available for patients enrolled in the following earlier protocols: E1479, E1490, E3480, E3483, or PC486.
Flow Cytometry
The diagnosis of AML was confirmed by multiparameter flow cytometry for patients in protocols E3489, E1490, E3993, E3997, E4995, PC486, E3999, and E1900. Three adhesion molecules (CD11a, CD11b, and CD56) were evaluated for their association with EMD.
EMD Assessment and Treatment
In all 11 trials, bone marrow (BM) leukemic involvement was an eligibility criterion; thus patients with an isolated extramedullary myeloid sarcoma without BM involvement are not included. The presence of EMD was recorded at baseline. EMD in these studies was defined clinically by physical examination and radiology without necessarily requiring a biopsy. A lumbar puncture (LP) was mandatory (five trials), recommended for patients with high blast count (two trials) or if CNS signs or symptoms were present (three trials).
The treatment of CNS involvement was on the basis of intrathecal methotrexate in seven trials, high-dose cytarabine in two trials where this was part of the induction protocol, or physician’s choice.
Individual EMD sites were first evaluated as a whole group, then separately by site and finally, by classifying them as three organ-based subgroups: hematopoietic (lymph nodes [LNs], spleen, or liver), nonhematopoietic (skin or gingiva with or without EMD in hematopoietic sites), and rare areas of involvement (CNS, bone, lung, or myeloid sarcoma with or without EMD in previous sites).
Hematopoietic Stem-Cell Transplantation
Among the 11 studies, hematopoietic stem-cell transplantation (HSCT) was part of the treatment regimen in five (E3483, E3489, PC486, E4995, and E1900), and patients were classified according to the type of transplantation received. In most cases, HSCT was done as part of protocol treatment. However, if a patient’s record had a definitive comment indicating that the patient received HSCT off-protocol, this information was applied. All other patients were classified as no transplantation.
Statistical Analysis
Descriptive statistics were used to characterize patients and their disease. A t test was used to explore potential differences in continuously parameterized disease and patient characteristics between patients with and without EMD. χ2 tests or Fisher’s exact tests were used to test for differences in categorical features. A two-sided P value of .05 was considered statistically significant for these tests.
Univariable analyses of potential prognostic factors were done. The method of Kaplan and Meier was used to estimate median overall survival (OS) within each prognostic category. Differences were assessed using a one-sided log-rank test. Cox proportional hazards models were used to examine the effect of one-unit increases in continuous variables on OS.
Multivariable models were built using backward selection. First, factors (or groups of factors) significant at the .10 level in univariable analyses were included in the model. Factors were dropped one at a time by comparing nested models using the Schwarz-Bayesian criterion and −2 log-likelihood. The final model is the one that minimized these criteria. To minimize the effect of missing data, indicator variables for missing values were included.
RESULTS
Incidence and Sites
Of the 3,522 enrolled patients, 282 were excluded, because of diagnosis of acute promyelocytic leukemia (n = 168) or leukemia other than AML (n = 29), no EMD evaluation at baseline (n = 41), retrospective central review ineligibility (n = 24), or no survival data (n = 20). The overall incidence of EMD was 23.7% (769 of 3,240 patients). The involved sites were LNs, 11.5% (374 patients); spleen, 7.3% (238); liver, 5.3% (173); skin, 4.5% (146); gingiva, 4.4% (143); CNS, 1.1% (36); peripheral nervous system and myeloid sarcoma, 0.2% (8) each; and other sites, 1.1% (35). Most patients with EMD (n = 502, 65.3%) had only one site of EMD, 161 (20.9%) had two sites, 73 (9.5%) had three sites, 26 (3.4%) had four sites, and seven (0.9%) had five or six sites.
EMD Assessment
There were several versions of case report forms in use over the time interval, and the diagnosis method was characterized in four different ways. In general, multiple methods of diagnosis were not captured. Among 308 extramedullary sites identified for studies E1479 and E3480, 89.6% were identified by physical examination, 2.6% by biopsy, and 7.8% by other, which included x-ray, scan, or chemical means. For no other studies was there a distinction between physical examination and imaging as a basis for diagnosis. There were 92 distinct sites noted on study E3999, 94.6% by being clinically involved and 5.4% by being pathologically involved. For study E1900, no details about the method of diagnosis were captured. For all other studies, among 735 distinct sites, 92.9% were diagnosed clinically and 7.1% pathologically. Thus, the vast majority (> 90%) were diagnosed by physical examination rather than by biopsy, but the role of scans is unclear (Appendix Table A1, online only).
Characteristics of Patients With EMD
Patients with EMD, compared with those without EMD, were younger (median age, 45.7 v 52.9 years; P < .001) and males (57.9% v 52%; P = .006). They had a poorer performance status (PS; 76.4% with Eastern Cooperative Oncology Group PS 0-1 v 86%; P < .001) and higher WBC count (median, 41.6/μL v 10.2/μL; P < .001). The median percentage of blasts in the BM and in the peripheral blood was higher in the EMD group. Other characteristics did not differ significantly by EMD status (Tables 2 and 3).
Table 2.
Patient Characteristics: Categorical Factors
| Factor | Patients Without Extramedullary Disease (n = 2,472) | Patients With Extramedullary Disease (n = 769) | Total (n = 3,240) | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Sex, P = .005 | ||||||
| Male | 1,284 | 52.0 | 445 | 57.9 | 1,729 | 53.4 |
| Female | 1,185 | 48.0 | 324 | 42.1 | 1,509 | 46.6 |
| Unknown | 2 | — | 2 | |||
| Race/ethnicity, P = .27 | ||||||
| White | 2,193 | 89.5 | 679 | 89.3 | 2,872 | 89.4 |
| Hispanic | 69 | 2.8 | 17 | 2.2 | 86 | 2.7 |
| Black | 143 | 5.8 | 50 | 6.6 | 193 | 6.0 |
| Asian | 20 | 0.8 | 2 | 0.3 | 22 | 0.7 |
| Other | 26 | 1.1 | 12 | 1.6 | 38 | 1.2 |
| Unknown | 20 | 9 | 29 | |||
| ECOG performance status, P < .001 | ||||||
| 0 | 914 | 37.3 | 198 | 25.9 | 1,112 | 34.6 |
| 1 | 1,194 | 48.7 | 387 | 50.5 | 1,581 | 49.2 |
| 2 | 265 | 10.8 | 128 | 16.7 | 393 | 12.2 |
| 3 | 67 | 2.7 | 40 | 5.2 | 107 | 3.3 |
| 4 | 11 | 0.5 | 13 | 1.7 | 24 | 0.8 |
| Unknown | 20 | 3 | 23 | |||
| FAB class, P < .001 | ||||||
| M0 | 56 | 2.3 | 7 | 0.9 | 63 | 2.0 |
| M1 | 489 | 19.9 | 116 | 15.1 | 605 | 18.8 |
| M2 | 688 | 28.0 | 127 | 16.6 | 815 | 25.3 |
| M4 | 639 | 26.0 | 299 | 39.0 | 938 | 29.1 |
| M5 | 166 | 6.8 | 122 | 15.9 | 288 | 8.9 |
| M6 | 100 | 4.1 | 12 | 1.6 | 112 | 3.5 |
| M7 | 12 | 0.5 | — | — | 12 | 0.4 |
| RAEB-T | 12 | 0.5 | 3 | 0.4 | 15 | 0.5 |
| AML, NOS | 129 | 5.2 | 45 | 5.9 | 174 | 5.4 |
| Other | 169 | 6.9 | 35 | 4.6 | 204 | 6.3 |
| Unknown | 11 | 3 | 14 | |||
| Cytogenetics, P = .61 | ||||||
| Favorable | 174 | 12.9 | 26 | 10.7 | 200 | 12.5 |
| Intermediate | 492 | 36.3 | 84 | 34.4 | 576 | 36.1 |
| Unfavorable | 316 | 23.3 | 59 | 24.2 | 375 | 23.5 |
| Undetermined | 372 | 27.5 | 75 | 30.7 | 447 | 28.0 |
| Unknown | 1,117 | 525 | 1,642 | |||
| Response to induction, P = .77 | ||||||
| CR | 1,472 | 60.0 | 451 | 59.0 | 1,923 | 59.7 |
| PR | 3 | 0.1 | 1 | 0.1 | 4 | 0.1 |
| SD | 687 | 28.0 | 227 | 29.7 | 914 | 28.4 |
| PD | 131 | 5.3 | 34 | 4.4 | 165 | 5.1 |
| Unevaluable | 161 | 6.6 | 52 | 6.8 | 213 | 6.6 |
| Unknown | 17 | 4 | 21 | |||
| Underwent transplantation, P = .07 | ||||||
| No | 1,614 | 82.1 | 538 | 85.3 | 2,152 | 82.8 |
| Yes | 353 | 18.0 | 93 | 14.7 | 446 | 17.2 |
| Unknown | 504 | 138 | 642 | |||
Abbreviations: AML, acute myelogenous leukemia; CR, complete remission; ECOG, Eastern Cooperative Oncology Group; FAB, French-American-British; NOS, not otherwise specified; NR, not reached or not estimable; OS, overall survival; PD, progressive disease; PR, partial response; RAEB-T, refractory anemia with excessive blasts in transformation; SD, stable disease.
Table 3.
Patient Characteristics at Baseline: Continuous Factors
| Factor | No Extramedullary Disease | Extramedullary Disease | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | No. | Mean | Median | SD | No. | Mean | Median | SD | No. | Mean | Median | SD | |
| Age (years) | < .001 | 2,469 | 51.4 | 52.9 | 15.9 | 769 | 46.4 | 45.7 | 16.6 | 3,238 | 50.2 | 51.1 | 16.2 |
| Hemoglobin (g/dL) | .001 | 2,448 | 9.2 | 9.1 | 2.1 | 754 | 9.5 | 9.4 | 2.3 | 3,202 | 9.2 | 9.2 | 2.1 |
| Platelets | .90* | 2,449 | 80.4 | 54.0 | 92.1 | 764 | 79.0 | 53.5 | 95.0 | 3,213 | 80.0 | 54.0 | 92.8 |
| WBC | < .001* | 2,459 | 25.2 | 8.6 | 39.4 | 765 | 52.4 | 31.7 | 65.1 | 3,224 | 31.6 | 12.0 | 48.2 |
| Blasts, marrow (%) | < .001 | 2,287 | 61.2 | 63.0 | 24.9 | 699 | 69.6 | 76.0 | 24.4 | 2,986 | 63.1 | 68.0 | 25.0 |
| Blasts, PB (%) | < .001 | 2,357 | 34.6 | 26.0 | 31.1 | 739 | 44.6 | 44.0 | 32.9 | 3,096 | 37.0 | 30.0 | 31.8 |
| CD11a | .04 | 992 | 63.2 | 79.0 | 35.7 | 108 | 70.5 | 86.5 | 32.1 | 1,100 | 63.9 | 80.0 | 35.4 |
| CD11b | < .001 | 1,175 | 24.8 | 9.0 | 32.0 | 133 | 38.2 | 29.0 | 36.0 | 1,308 | 26.1 | 10.0 | 32.6 |
| CD56 | .46 | 980 | 13.3 | 0.0 | 28.0 | 108 | 15.5 | 0.5 | 29.8 | 1,088 | 13.5 | 0.0 | 28.2 |
Abbreviations: PB, peripheral blood; SD, standard deviation.
t test done on log-transformed values.
FAB Category and EMD
The proportion of patients classified as French-American-British (FAB) M4 and M5 was higher among those with EMD (39% and 15.9%, respectively) compared with others (26% and 6.8%, respectively). In every EMD subgroup, the most common FAB category was M4 (approximately 40% of the patients in every subgroup, compared with 26% of the non-EMD patients). Among 815 patients with FAB M2, 15.6% had EMD and among the 98 patients with recorded t(8;21), only 10.2% had EMD.
Responses
The complete remission (CR) rate for all patients was 59.7% and was similar for patients with or without EMD (59% and 60%, respectively). The CR rate was similar for patients with individual EMD sites except for those with splenic and gingival involvement, who had a nonsignificant lower CR rate compared with the whole cohort (P = .06 and .08, respectively).
Survival
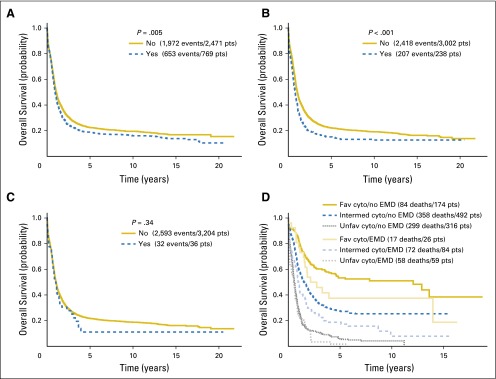
The median OS was 1.035 years. There were 2,625 deaths among the 3,240 patients included in the analysis. In univariable analysis (Table 4), EMD was associated with a shorter OS (P = .005; Fig 1A). Among individual EMD sites, the analysis revealed that skin (P = .002), spleen (P < .001; Fig 1B), and liver (P < .001), but not CNS (P = .34; Fig 1C), nodal involvement (P = .94), and gingival hypertrophy (P = .24), were associated with shorter OS.
Table 4.
Univariate Analyses: Overall Survival
| Categorical Variable | No. | No. of Deaths | Median OS (months) | 95% CI (months) | Log-Rank P |
|---|---|---|---|---|---|
| EMD | |||||
| No | 2,471 | 1,972 | 12.7 | 12.1 to 13.7 | .005 |
| Yes | 769 | 653 | 11.3 | 10.4 to 12.8 | |
| EMD category* | |||||
| Hematopoietic | 398 | 329 | 10.9 | 9.8 to 12.9 | .01 |
| Nonhematopoietic | 208 | 183 | 11.2 | 9.4 to 12.9 | |
| Rare | 164 | 141 | 12.4 | 10.3 to 14.8 | |
| No. of EMD sites | |||||
| 0 | 2,471 | 1,972 | 12.7 | 12.1 to 13.7 | .002 |
| 1 | 502 | 420 | 12.2 | 10.6 to 13.5 | |
| 2-5 | 267 | 233 | 10.2 | 9.2 to 12.0 | |
| CNS involvement | |||||
| No | 3,204 | 2,593 | 12.4 | 11.9 to 13.1 | .34 |
| Yes | 36 | 32 | 10.5 | 7.2 to 17.7 | |
| Liver involvement | |||||
| No | 3,067 | 2,470 | 12.6 | 12.0 to 13.3 | < .001 |
| Yes | 173 | 155 | 9.7 | 8.1 to 11.9 | |
| Splenic involvement | |||||
| No | 3,002 | 2,418 | 12.7 | 12.1 to 13.4 | < .001 |
| Yes | 238 | 207 | 9.5 | 7.8 to 11.2 | |
| Nodal involvement | |||||
| No | 2,866 | 2,317 | 12.5 | 11.9 to 13.2 | .94 |
| Yes | 374 | 308 | 11.3 | 10.2 to 13.5 | |
| Skin involvement | |||||
| No | 3,094 | 2,496 | 12.6 | 12.0 to 13.4 | .002 |
| Yes | 146 | 129 | 10.1 | 8.1 to 12.3 | |
| Gingival involvement | |||||
| No | 3,097 | 2,500 | 12.4 | 11.9 to 13.1 | .24 |
| Yes | 143 | 125 | 12.0 | 10.5 to 16.4 | |
| Lung involvement | |||||
| No | 3,217 | 2,605 | 12.4 | 11.9 to 13.1 | .34 |
| Yes | 23 | 20 | 9.2 | 4.4 to 16.0 | |
| Bone involvement | |||||
| No | 3,232 | 2,619 | 12.4 | 11.8 to 13.1 | .92 |
| Yes | 8 | 6 | 12.8 | 0.3 to NR | |
| Myeloid sarcoma | |||||
| No | 3,232 | 2,618 | 12.4 | 11.8 to 13.1 | .93 |
| Yes | 8 | 7 | 14.1 | 8.4 to 26.2 | |
| Other EMD | |||||
| No | 3,205 | 2,595 | 12.4 | 11.9 to 13.1 | .29 |
| Yes | 35 | 30 | 10.5 | 7.2 to 14.7 | |
| Performance status | |||||
| 0 | 1,112 | 841 | 16.3 | 14.6 to 18.3 | < .001 |
| 1 | 1,581 | 1,297 | 12.0 | 11.0 to 12.8 | |
| 2-4 | 524 | 466 | 8.1 | 7.1 to 9.3 | |
| Sex | |||||
| Male | 1,729 | 1,441 | 11.7 | 10.8 to 12.4 | < .001 |
| Female | 1,509 | 1,182 | 13.4 | 12.5 to 14.5 | |
| Race/ethnicity | |||||
| White | 2,872 | 2,335 | 12.4 | 11.7 to 13.1 | .17 |
| Hispanic | 86 | 64 | 12.9 | 8.9 to 20.6 | |
| Black | 193 | 161 | 11.4 | 10.2 to 14.2 | |
| Asian | 22 | 14 | 28.2 | 12.2 to NR | |
| Other | 38 | 29 | 13.8 | 8.7 to 29.8 | |
| FAB class (log-rank test for each class v all others) | |||||
| M0 | 63 | 59 | 10.7 | 8.6 to 15.3 | .03 |
| M1 | 605 | 480 | 12.3 | 11.1 to 14.0 | .44 |
| M2 | 815 | 631 | 14.5 | 12.9 to 15.8 | < .001 |
| M4 | 938 | 760 | 12.8 | 11.6 to 14.1 | .50 |
| M5 | 288 | 241 | 10.7 | 9.8 to 12.5 | .06 |
| M6 | 112 | 98 | 9.2 | 7.7 to 10.2 | .009 |
| M7 | 12 | 8 | 14.2 | 2.9 to NR | .42 |
| Other | 393 | 335 | 11.4 | 9.9 to 13.2 | .01 |
| Cytogenetic risk category | |||||
| Favorable | 200 | 101 | 94.2 | 42.2 to 167.6 | < .001 |
| Intermediate | 576 | 430 | 16.9 | 14.3 to 20.8 | |
| Unfavorable | 375 | 357 | 6.8 | 6.1 to 8.0 | |
| Undetermined | 447 | 376 | 11.0 | 9.9 to 12.9 | |
| Received transplantation | |||||
| No | 2,152 | 1,906 | 9.4 | 8.8 to 10.0 | < .001 |
| Yes | 446 | 259 | 40.6 | 33.2 to 65.0 | |
| Response to induction | |||||
| CR | 1,923 | 1,393 | 22.3 | 20.7 to 24.0 | < .001 |
| PR | 4 | 4 | 4.1 | 3.3 to NR | |
| SD | 914 | 845 | 6.0 | 5.3 to 6.5 | |
| PD | 165 | 164 | 3.1 | 2.3 to 4.6 | |
| Unevaluable | 213 | 200 | 2.2 | 1.3 to 5.2 |
NOTE. The No. of extramedullary sites was a continuous variable. Total No., 3,240; No. of deaths, 2,625; hazard ratio, 1.095; 95% CI, 1.043 to 1.148; Wald P < .001.
Abbreviations: CR, complete remission; EMD, extramedullary disease; FAB, French-American-British; NR, not reached or not estimable; OS, overall survival; PD, progressive disease; PR, partial response; SD, stable disease.
EMD category: none = no site of EMD disease; hematopoietic = EMD in nodes, liver, spleen only; nonhematopoietic = EMD in skin or gingiva with or without EMD in hematopoietic sites; rare = CNS, bone, lung, or myeloid sarcoma with or without EMD in previous sites.
Fig 1.
Differences in overall survival by (A) presence of extramedullary disease, (B) presence of splenic involvement, (C) presence of CNS involvement, and (D) combination of cytogenetic risk and presence of extramedullary involvement. P values are from the log-rank test. Cyto, cytogenetic; EMD, extramedullary disease; fav, favorable; intermed, intermediate; pts, patients; unfav, unfavorable.
A greater number of EMD-involved sites, both as a categorical variable (1 v ≥ 2) and as a continuous variable, was negatively associated with OS (P = .002 and P < .001, respectively). Each additional site of EMD conferred a 9.5% increase in the risk of death. Among EMD subgroups (hematopoietic, nonhematopoietic, and rare), the rare areas of involvement had an OS advantage compared with the other two subgroups (median OS of 12.4 v 11.2 and 10.9 months, respectively; P = .01).
Parameters associated with longer OS were good PS, female sex, M2 FAB category, favorable cytogenetic risk group, undergoing HSCT, achieving CR postinduction, younger age, later year of registration, lower WBC count at diagnosis, higher platelet count, and low percentage of blasts in the BM.
A multivariable model (Table 5) was constructed to examine the effect of EMD on OS after adjusting for known prognostic factors. Earlier year of registration, older age, high WBC count, low platelet count, worse PS (compared with Eastern Cooperative Oncology Group PS 0), high cytogenetic risk status, and not achieving a CR were associated with shorter OS. Neither the presence of EMD, the number of extramedullary sites, nor any EMD-specific site, including the CNS, contributed prognostic significance to the multivariable models.
Table 5.
Multivariable Model
| Factor | Parameter Estimate | Standard Error | P | Hazard Ratio | 95% HR Confidence Limits |
|---|---|---|---|---|---|
| Presence of extramedullary disease | −0.038 | 0.090 | .671 | 0.963 | 0.807 to 1.148 |
| No. of extramedullary sites | 0.277 | 0.187 | .138 | 1.320 | 0.915 to 1.903 |
| Year registered to study | −0.022 | 0.003 | .000 | 0.978 | 0.973 to 0.983 |
| Age | 0.023 | 0.001 | .000 | 1.024 | 1.021 to 1.026 |
| Log WBC | 0.068 | 0.015 | .000 | 1.070 | 1.040 to 1.102 |
| Log platelets | −0.059 | 0.023 | .010 | 0.943 | 0.902 to 0.986 |
| Hemoglobin | −0.022 | 0.010 | .024 | 0.978 | 0.960 to 0.997 |
| PS 1* | 0.128 | 0.045 | .005 | 1.136 | 1.039 to 1.242 |
| PS 2-4* | 0.193 | 0.062 | .002 | 1.213 | 1.075 to 1.368 |
| Male | 0.067 | 0.040 | .094 | 1.070 | 0.988 to 1.158 |
| Extramedullary site: gingiva | −0.118 | 0.211 | .576 | 0.888 | 0.587 to 1.345 |
| Extramedullary site: nodes | −0.237 | 0.199 | .235 | 0.789 | 0.534 to 1.166 |
| Extramedullary site: spleen | −0.157 | 0.201 | .436 | 0.855 | 0.576 to 1.268 |
| Extramedullary site: liver | −0.204 | 0.203 | .314 | 0.815 | 0.548 to 1.213 |
| CNS involvement | −0.331 | 0.286 | .247 | 0.718 | 0.410 to 1.257 |
| Extramedullary site: skin | −0.098 | 0.213 | .648 | 0.907 | 0.597 to 1.378 |
| Extramedullary site: lung | −0.087 | 0.303 | .774 | 0.917 | 0.507 to 1.659 |
| Extramedullary site: bone | −0.277 | 0.448 | .537 | 0.758 | 0.315 to 1.826 |
| Myeloid sarcoma | −0.433 | 0.426 | .309 | 0.648 | 0.281 to 1.494 |
| FAB intermediate risk* | 0.024 | 0.049 | .619 | 1.025 | 0.931 to 1.128 |
| FAB high risk* | 0.185 | 0.089 | .038 | 1.203 | 1.010 to 1.433 |
| FAB other* | 0.087 | 0.069 | .207 | 1.091 | 0.953 to 1.248 |
| Achieved CR to induction | −1.132 | 0.042 | .000 | 0.322 | 0.297 to 0.350 |
Abbreviations: CR, complete remission; FAB, French-American-British; PS, performance status.
Reference categories are PS 0, favorable risk cytogenetics.
EMD Throughout the Years of Registration
The year of registration was found to be a significant statistical variable in most of the different multivariable models that were performed (P = .043 to < .001). Among patients registered before 2002, the presence of EMD did not affect OS. Patients enrolled before 1990 had median survival of approximately 12.5 months and it was 12.8 months among those enrolled between 1990 and 1999, regardless of EMD status. Among patients enrolled after 2002, survival continued to improve over time among patients without EMD (median survival, 14.1 months; P < .001), but not among patients with EMD (median survival, 8.3 months; P = .353). The negative impact of EMD on outcome was seen predominantly among older patients with EMD enrolled in E3999, but not with younger patients enrolled in E1900 during the same time period.
EMD and Cytogenetics
Cytogenetic data were available in 49% of the study cohort. Of these, 200 patients had known favorable cytogenetics, and EMD was present in only 26 patients (13%). No significant difference in OS was found between the groups, but the numbers are too small for a definitive assessment (Fig 1D). The incidence of EMD among those patients with other than favorable cytogenetics was 15.1%, similar to that in patients with favorable cytogenetics. Although both the presence of EMD and other than favorable cytogenetic risk were significant predictors of poor survival (by pairwise survival estimates and hazard ratios) compared with no-EMD and favorable cytogenetic risk, respectively, the interaction of these factors was not significant (P = .61).
EMD and Adhesion Molecules
There was a higher incidence of CD11b-positive AML among patients with EMD (29%) than patients without EMD (9%; P < .001). The percentage of CD11a and CD56-positive blasts was comparable between the two groups.
EMD and HSCT
Data about transplantation, in studies where this was not part of the protocol, are limited. The percentage of patients who underwent a transplantation was slightly lower among the EMD group compared with the others (14.7% v 18%, respectively; P = .07). The median OS of the 446 patients who underwent HSCT was 40.6 months, compared with 9.4 months of the 2,152 patients who did not (P < .001). Nevertheless, undergoing transplantation was not found to be a significant variable in a multivariable analysis.
DISCUSSION
On the basis of data from 11 consecutive ECOG-ACRIN clinical trials, the presence of EMD at diagnosis does not have independent prognostic value. This observation is clinically meaningful, given that the incidence of EMD in adults with newly diagnosed AML was approximately 24%. The rate of EMD reported in the literature has a broad range related to definitions and method of evaluation. Some define EMD as organ involvement, not including liver, spleen, and LNs.6 Using the terms myeloid sarcoma or chloroma, some imply a discrete mass of myeloblasts, not including cases of organ infiltration.1
We and others2 used clinical assessment of EMD without requiring a biopsy, whereas others established the diagnosis of EMD only if pathologically confirmed.1,23 LNs, spleen, and gingiva are likely to be considered as EMD sites by physical examination, but usually would not be biopsied and therefore not be considered as pathologically proven EMD. With more sensitive diagnostic tools, such as positron emission tomography (PET), the rate of presumed EMD at diagnosis is likely to be even higher.24 For example, in 26 patients with newly diagnosed AML, Cribe et al25 demonstrated that 18F-labeled fluorodeoxyglucose PET testing doubled the rate of EMD, from 31% by clinical examination to 65%. It is possible that many patients with AML have clusters of leukemic cells in different organs, in addition to the blood and marrow, and the only question is the resolution of the test used for assessment. However, it is neither ethical nor practical to biopsy every suspected EMD site, and it would be prohibitively costly to perform PET-computed tomography for every patient with AML. Therefore, despite its limitations, clinical evaluation remains the main assessment method for EMD in routine practice.
To the best of our knowledge, this is the largest study of EMD with information on the distribution of EMD among different sites. LNs and spleen were the most common sites reported, with incidences of 11.5% and 7.3%, respectively, followed by liver (5.3%), skin (4.5%), and gingiva (4.4%), whereas CNS involvement was observed in only 1.1%. The low incidence of CNS involvement is the only variable that is consistent in early studies24,26 as well as contemporary studies.27 Rozovski et al9 recently reported a 3.3% incidence of CNS involvement among 1,412 patients with newly diagnosed AML who did not undergo a routine LP compared with 19% of 42 patients who underwent a routine LP. We did not observe such a difference; therefore, the rarity of the reported CNS involvement supports not performing a routine LP in patients with AML unless neurologically indicated.
The number of involved sites is usually not reported in the literature. In our study, 35% of patients with EMD had more than one involved site; 9.5% had three sites and some patients even had five and six involved sites. The relatively high rate of multiple-site EMD involvement suggests that the development of EMD is an intrinsic feature of the leukemic cells and depends on factors such as the expression of cell surface adhesion molecules.2,28 In our cohort, the median percentage of CD11b-positive blasts was significantly higher among the patients with EMD but, in contrast to the report by Chang et al,2 CD56 was not.
In the multivariable model, earlier year of registration, older age, high WBC count, low platelet count, poor PS, high cytogenetic risk status, and not achieving a CR were associated with a shorter OS. However, EMD as a group, as well as every individual EMD site, had no independent effect on prognosis. It is possible that individual sites of EMD are in fact associated with poorer prognosis; however, these patients also have other unfavorable prognostic factors, such as high WBC count and unfavorable cytogenetics, whereas EMD has no independent prognostic effect. Indeed, patients with EMD, in this series and in others,2,29,30 had higher WBC counts at diagnosis. The similar outcome of patients with CNS involvement to others may also be explained in a different way. CNS is the only EMD site that mandates a specific therapeutic approach, intrathecal methotrexate and/or high-dose cytarabine, which may overcome the potential negative effect of CNS involvement.
The median age of the patients was 51.1 years. Patients with EMD, compared with those without EMD, were younger (median age, 45.7 v 52.9 years; P < .001). There was a statistically significant decline in the incidence of extramedullary disease with increasing age, and a statistically significant decline in survival with increasing age among patients both with and without EMD. Nevertheless, the interaction of age-by-EMD status was not statistically significant, so the effect of age on survival was similar in both groups (Appendix Table A2, online only).
We classified the patients with EMD into three subgroups: hematopoietic organs (lymph nodes, spleen, or liver), nonhematopoietic organs (skin or gingiva with or without EMD in hematopoietic sites), and rare areas of involvement (CNS, bone, lung, or myeloid sarcoma with or without EMD in previous sites). The rationale for this division was that every subgroup might develop in a different context. The first subgroup is probably influenced by the time from first symptoms to diagnosis and treatment of AML. The second depends on specific subtypes of AML, mainly those with a monocytic component.3 The third subgroup is composed of rare sites and the context of development is unknown. Interestingly, the third group had better survival compared with the two others.
By stratifying patients with available cytogenetic data (approximately 50% of the cohort) into favorable and other than favorable cytogenetics, we thought that the effect of EMD on prognosis might appear significant, particularly in the patients with favorable cytogenetics. Indeed, the median OS among the favorable cytogenetic group was 32.9 versus 94.2 months, with and without EMD, respectively (Fig 1D). However, the number of patients was small and the difference was not significant (P = .145). Nevertheless, the question of the effect of EMD among patients with favorable cytogenetics needs to be studied in larger cohorts.
In conclusion, this large study demonstrates that EMD is more common than previously reported and frequently occurs in multiple sites, although CNS involvement is rare. Perhaps surprisingly, EMD at presentation does not have independent prognostic significance. Importantly, the presence of EMD should not affect the choice of postremission therapy.
Acknowledgment
We thank Martin M. Oken, MD, and Peter A. Cassileth, MD (deceased), for their significant contributions to this manuscript.
Appendix
Table A1.
Extramedullary Disease Assessment
| Site | E1479, E3480 (n = 328) | E3999 (n = 422) | E1900 (n = 644) | Others (n = 1,847) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | PE | BX | Other | No. | C | P | No. | Inv | No. | C | P | |
| CNS | 324 | 7 | 0 | 3 | — | — | — | 644 | 6 | — | — | — |
| Peripheral nervous system | 327 | 6 | 0 | 2 | — | — | — | 644 | 0 | — | — | — |
| Leukemic meningitis | — | — | — | — | 397 | 0 | 0 | — | — | 1,399 | 5 | 11 |
| Liver | 326 | 41 | 0 | 11 | 416 | 9 | 0 | — | — | 1,748 | 112 | 0 |
| Spleen | 326 | 56 | 0 | 4 | 412 | 23 | 0 | 644 | 3 | 1,745 | 151 | 0 |
| Nodes | 325 | 68 | 4 | 0 | — | — | — | — | — | — | — | — |
| Mediastinal nodes/mass | — | — | — | — | 398 | 5 | 0 | 644 | 2 | 1,740 | 14 | 0 |
| Peripheral nodes | — | — | — | — | 415 | 15 | 1 | — | — | 1,796 | 247 | 10 |
| Gingival hypertrophy* | 322 | 55 | 411 | 17 | 0 | 642 | 32 | — | — | — | ||
| Cranial nerve palsy | — | — | — | — | 411 | 0 | 0 | — | — | — | ||
| Skin | 325 | 28 | 2 | 0 | 416 | 11 | 2 | 643 | 7 | 1,810 | 75 | 21 |
| Other | 285 | 15 | 2 | 4 | 367 | 7 | 2 | 622 | 15 | 1,199 | 79 | 10 |
NOTE. Dashes indicate terms not appearing on forms.
Abbreviations: BX, biopsy; C, clinically positive or involved; Inv, involved; No., number of responses to the question on the form; Other, x-ray, scan, or chemical; P, pathologically positive or involved; PE, physical examination.
For E1479 and E3480, gingival hypertrophy appeared as a symptom and not as a site of extramedullary disease; shown is the number of patients with symptoms present. For other studies in particular, comments were reviewed to elicit details about extramedullary disease coded as other; these were recoded in the appropriate category where possible.
Table A2.
Extramedullary Disease by Age
| Variable | Age Category (years) | |||
|---|---|---|---|---|
| < 37 | 37-50 | 51-63 | > 63 | |
| Patients | 804 | 816 | 809 | 809 |
| Patients with EMD | 267 | 195 | 160 | 147 |
| Percent | 33.2 | 23.9 | 19.8 | 18.2 |
| Median survival, no EMD (months) | 22.6 | 16.7 | 11.9 | 7.7 |
| IQR (months) | 9.9 to NR | 7.5 to 157.0 | 5.0 to 32.3 | 1.9 to 19.1 |
| Median survival, EMD (months) | 16.2 | 13.2 | 10.5 | 4.9 |
| IQR (months) | 7.9 to 159.8 | 7.7 to 38.5 | 2.8 to 21.1 | 1.0 to 10.8 |
Abbreviations: EMD, extramedullary disease; IQR, interquartile range; NR, not reached.
Footnotes
Written on behalf of the ECOG-ACRIN Cancer Research Group.
Supported by Grants No. CA180820, CA21115, CA180794, CA23318, CA66636, CA17145, CA49883, CA73590, CA13650, CA15488, CA14548, CA14958, CA180791, CA189859, CA180853, CA180790, and CA180795 from the National Cancer Institute of the National Institutes of Health.
Presented as an oral abstract at the American Society of Hematology annual meeting, New Orleans, LA, December 7-10, 2013.
The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Chezi Ganzel, Dan Douer, Jacob M. Rowe, Elisabeth M. Paietta, Martin S. Tallman
Collection and assembly of data: Judith Manola, Elisabeth M. Paietta, Ju-Whei Lee
Data analysis and interpretation: Chezi Ganzel, Judith Manola, Jacob M. Rowe, Hugo F. Fernandez, Elisabeth M. Paietta, Mark R. Litzow, Selina M. Luger, Hillard M. Lazarus, Larry D. Cripe, Martin S. Tallman
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Extramedullary Disease in Adult Acute Myeloid Leukemia Is Common but Lacks Independent Significance: Analysis of Patients in ECOG-ACRIN Cancer Research Group Trials, 1980-2008
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Chezi Ganzel
No relationship to disclose
Judith Manola
No relationship to disclose
Dan Douer
Consulting or Advisory Role: Pfizer, Amgen, Sigma-Tau/Baxalta, Gilead Sciences
Speakers’ Bureau: Jazz Pharmaceuticals
Research Funding: Incyte, Gilead Sciences, Bristol-Myers Squibb
Jacob M. Rowe
No relationship to disclose
Hugo F. Fernandez
Consulting or Advisory Role: Chimerix
Speakers’ Bureau: Sanofi
Elisabeth M. Paietta
No relationship to disclose
Mark R. Litzow
No relationship to disclose
Ju-Whei Lee
No relationship to disclose
Selina M. Luger
No relationship to disclose
Hillard M. Lazarus
No relationship to disclose
Larry D. Cripe
No relationship to disclose
Martin S. Tallman
No relationship to disclose
REFERENCES
- 1.Muss HB, Moloney WC. Chloroma and other myeloblastic tumors. Blood. 1973;42:721–728. [PubMed] [Google Scholar]
- 2.Chang H, Brandwein J, Yi QL, et al. Extramedullary infiltrates of AML are associated with CD56 expression, 11q23 abnormalities and inferior clinical outcome. Leuk Res. 2004;28:1007–1011. doi: 10.1016/j.leukres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Tallman MS, Kim HT, Paietta E, et al. Acute monocytic leukemia (French-American-British classification M5) does not have a worse prognosis than other subtypes of acute myeloid leukemia: A report from the Eastern Cooperative Oncology Group. J Clin Oncol. 2004;22:1276–1286. doi: 10.1200/JCO.2004.08.060. [DOI] [PubMed] [Google Scholar]
- 4.Tallman MS, Hakimian D, Shaw JM, et al. Granulocytic sarcoma is associated with the 8;21 translocation in acute myeloid leukemia. J Clin Oncol. 1993;11:690–697. doi: 10.1200/JCO.1993.11.4.690. [DOI] [PubMed] [Google Scholar]
- 5.Byrd JC, Weiss RB, Arthur DC, et al. Extramedullary leukemia adversely affects hematologic complete remission rate and overall survival in patients with t(8;21)(q22;q22): Results from Cancer and Leukemia Group B 8461. J Clin Oncol. 1997;15:466–475. doi: 10.1200/JCO.1997.15.2.466. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi R, Tawa A, Hanada R, et al. Extramedullary infiltration at diagnosis and prognosis in children with acute myelogenous leukemia. Pediatr Blood Cancer. 2007;48:393–398. doi: 10.1002/pbc.20824. [DOI] [PubMed] [Google Scholar]
- 7. Oestgaard LSG, Sengeloev H, Holm MS, et al: Extramedullary leukemia and myeloid sarcoma in AML: Results from a population-based registry study of 2261 patients. 53rd American Society of Hematology Annual Meeting and Exposition, December 10-13, 2011. [Google Scholar]
- 8.Tsimberidou AM, Kantarjian HM, Wen S, et al. Myeloid sarcoma is associated with superior event-free survival and overall survival compared with acute myeloid leukemia. Cancer. 2008;113:1370–1378. doi: 10.1002/cncr.23691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rozovski U, Ohanian M, Ravandi F, et al. Incidence of and risk factors for involvement of the central nervous system in acute myeloid leukemia. Leuk Lymphoma. 2015;56:1392–1397. doi: 10.3109/10428194.2014.953148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng CL, Li CC, Hou HA, et al. Risk factors and clinical outcomes of acute myeloid leukaemia with central nervous system involvement in adults. BMC Cancer. 2015;15:344. doi: 10.1186/s12885-015-1376-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cassileth PA, Begg CB, Bennett JM, et al. A randomized study of the efficacy of consolidation therapy in adult acute nonlymphocytic leukemia. Blood. 1984;63:843–847. [PubMed] [Google Scholar]
- 12.Cassileth PA, Lynch E, Hines JD, et al. Varying intensity of postremission therapy in acute myeloid leukemia. Blood. 1992;79:1924–1930. [PubMed] [Google Scholar]
- 13.Cassileth PA, Andersen J, Lazarus HM, et al. Autologous bone marrow transplant in acute myeloid leukemia in first remission. J Clin Oncol. 1993;11:314–319. doi: 10.1200/JCO.1993.11.2.314. [DOI] [PubMed] [Google Scholar]
- 14.Cassileth PA, Harrington DP, Appelbaum FR, et al. Chemotherapy compared with autologous or allogeneic bone marrow transplantation in the management of acute myeloid leukemia in first remission. N Engl J Med. 1998;339:1649–1656. doi: 10.1056/NEJM199812033392301. [DOI] [PubMed] [Google Scholar]
- 15.Kahn SB, Begg CB, Mazza JJ, et al. Full dose versus attenuated dose daunorubicin, cytosine arabinoside, and 6-thioguanine in the treatment of acute nonlymphocytic leukemia in the elderly. J Clin Oncol. 1984;2:865–870. doi: 10.1200/JCO.1984.2.8.865. [DOI] [PubMed] [Google Scholar]
- 16.Rowe JM, Andersen JW, Mazza JJ, et al. A randomized placebo-controlled phase III study of granulocyte-macrophage colony-stimulating factor in adult patients (> 55 to 70 years of age) with acute myelogenous leukemia: A study of the Eastern Cooperative Oncology Group (E1490) Blood. 1995;86:457–462. [PubMed] [Google Scholar]
- 17.Rowe JM, Neuberg D, Friedenberg W, et al. A phase 3 study of three induction regimens and of priming with GM-CSF in older adults with acute myeloid leukemia: A trial by the Eastern Cooperative Oncology Group. Blood. 2004;103:479–485. doi: 10.1182/blood-2003-05-1686. [DOI] [PubMed] [Google Scholar]
- 18.Cripe LD, Rader K, Tallman MS, et al. Phase II trial of subcutaneous recombinant human interleukin 11 with subcutaneous recombinant human granulocyte-macrophage colony stimulating factor in patients with acute myeloid leukemia (AML) receiving high-dose cytarabine during induction: ECOG 3997. Leuk Res. 2006;30:823–827. doi: 10.1016/j.leukres.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Cripe LD, Uno H, Paietta EM, et al. Zosuquidar, a novel modulator of P-glycoprotein, does not improve the outcome of older patients with newly diagnosed acute myeloid leukemia: A randomized, placebo-controlled trial of the Eastern Cooperative Oncology Group 3999. Blood. 2010;116:4077–4085. doi: 10.1182/blood-2010-04-277269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361:1249–1259. doi: 10.1056/NEJMoa0904544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cassileth PA, Lee SJ, Litzow MR, et al. Intensified induction chemotherapy in adult acute myeloid leukemia followed by high-dose chemotherapy and autologous peripheral blood stem cell transplantation: An Eastern Cooperative Oncology Group trial (E4995) Leuk Lymphoma. 2005;46:55–61. doi: 10.1080/10428190412331283288. [DOI] [PubMed] [Google Scholar]
- 22.Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: A Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96:4075–4083. [PubMed] [Google Scholar]
- 23.Pileri SA, Ascani S, Cox MC, et al. Myeloid sarcoma: Clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia. 2007;21:340–350. doi: 10.1038/sj.leu.2404491. [DOI] [PubMed] [Google Scholar]
- 24.Dekker AW, Elderson A, Punt K, et al. Meningeal involvement in patients with acute nonlymphocytic leukemia. Incidence, management, and predictive factors. Cancer. 1985;56:2078–2082. doi: 10.1002/1097-0142(19851015)56:8<2078::aid-cncr2820560832>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 25.Cribe AS, Steenhof M, Marcher CW, et al. Extramedullary disease in patients with acute myeloid leukemia assessed by 18F-FDG PET. Eur J Haematol. 2013;90:273–278. doi: 10.1111/ejh.12085. [DOI] [PubMed] [Google Scholar]
- 26.Stewart DJ, Keating MJ, McCredie KB, et al. Natural history of central nervous system acute leukemia in adults. Cancer. 1981;47:184–196. doi: 10.1002/1097-0142(19810101)47:1<184::aid-cncr2820470130>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 27.Schlenk RF, Döhner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–1918. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 28.Byrd JC, Edenfield WJ, Shields DJ, et al. Extramedullary myeloid cell tumors in acute nonlymphocytic leukemia: A clinical review. J Clin Oncol. 1995;13:1800–1816. doi: 10.1200/JCO.1995.13.7.1800. [DOI] [PubMed] [Google Scholar]
- 29.Bisschop MM, Révész T, Bierings M, et al. Extramedullary infiltrates at diagnosis have no prognostic significance in children with acute myeloid leukaemia. Leukemia. 2001;15:46–49. doi: 10.1038/sj.leu.2401971. [DOI] [PubMed] [Google Scholar]
- 30.Hiçsönmez G, Cetin M, Tuncer AM, et al. Children with acute myeloblastic leukemia presenting with extramedullary infiltration: The effects of high-dose steroid treatment. Leuk Res. 2004;28:25–34. doi: 10.1016/s0145-2126(03)00159-0. [DOI] [PubMed] [Google Scholar]