Summary
C-type lectin receptors sense a diversity of endogenous and exogenous ligands that may trigger differential responses. Here, we have found that human and mouse Mincle bind to a ligand released by Leishmania, a eukaryote parasite that evades an effective immune response. Mincle-deficient mice had milder dermal pathology and a tenth of the parasite burden compared to wild-type mice after Leishmania major intradermal ear infection. Mincle deficiency enhanced adaptive immunity against the parasite, correlating with increased activation, migration and priming by Mincle-deficient dendritic cells (DCs). Leishmania triggered a Mincle-dependent inhibitory axis characterized by SHP1 coupling to the FcRγ chain. Selective loss of SHP1 in CD11c+ cells phenocopies enhanced adaptive immunity to Leishmania. In conclusion, Leishmania shifts Mincle to an inhibitory ITAM (ITAMi) configuration that impairs DC activation. Thus, ITAMi can be exploited for immune evasion by a pathogen and may represent a paradigm for ITAM-coupled receptors sensing self and non-self.
Introduction
C-type lectin receptors (CLRs) are equipped with the C-type lectin domain, a versatile structure for binding diverse ligands that allows sensing of self and non-self (Dambuza and Brown, 2015; Sancho and Reis e Sousa, 2012). Eukaryote parasites, such as Leishmania, are detected by CLRs, Toll-like receptors, and opsonizing antibodies via Fc receptors, which trigger a combination of activating and inhibitory pathways (Lefèvre et al., 2013; Woelbing et al., 2006). Mice infected intradermally with Leishmania major develop lesions similar to those seen in patients with localized cutaneous leishmaniasis (Belkaid et al., 2000). L. major is a poor inducer of dendritic cell (DC) activation and inhibits migration of DCs to draining lymph nodes (dLNs) (Ng et al., 2008; Ribeiro-Gomes et al., 2012), although DCs do eventually migrate and promote T helper 1 (Th1) cell immunity and macrophage microbicidal activity (Leon et al., 2007). The mechanisms by which Leishmania initially blunts DC activation and T cell priming remain ill-defined. It has been argued that they may involve uptake of apoptotic infected neutrophils by DCs (Ribeiro-Gomes et al., 2012) or direct DC contact with parasite products (Srivastav et al., 2012). However, the receptor(s) mediating L. major-induced DC suppression have not been identified.
Mincle (macrophage-inducible C-type lectin, also known as Clec4e or Clecsf9) (Matsumoto et al., 1999) is weakly expressed in myeloid cells, including DCs, and is induced upon their activation in a macrophage C-type lectin (MCL, Clec4d, Clecsf8)-dependent fashion (Miyake et al., 2013; Yamasaki et al., 2008). Mincle was identified as an FcRγ chain-coupled CLR for endogenous SAP-130 exposed and released by dead cells (Yamasaki et al., 2008) but also recognizes glycolipids on the cell walls of bacteria and fungi, including trehalose-6, 6-dimycolate (TDM) and its synthetic analogue trehalose-6, 6-dibehenate (TDB) (Ishikawa et al., 2009; Ishikawa et al., 2013; Schoenen et al., 2010; Wells et al., 2008; Yamasaki et al., 2009). Binding of these ligands to Mincle triggers phosphorylation of immunoreceptor tyrosine-based activation motif (ITAM) tyrosine residues in the FcRγ chain by Src-family kinases, followed by the recruitment and activation of the kinase Syk, which is facilitated by the phosphatase SHP2 as a scaffold (Deng et al., 2015). Subsequently, Syk generates an activating signal mediated by the protein CARD9 that boosts immunity to infections and inflammation in response to bacterial adjuvants (Ishikawa et al., 2009; Schoenen et al., 2010; Shenderov et al., 2013; Sousa et al., 2011; Yamasaki et al., 2009). Classically considered an activating CLR, Mincle has recently been associated with dampening of immunity (Seifert et al., 2016; Wevers et al., 2014; Wuthrich et al., 2015), acting by repressing IL12-p35 transcription through a Syk-Akt-PKB-dependent pathway in response to Fonsecaea (Wevers et al., 2014).
Here, we have found that loss of Mincle resulted in reduced parasitemia and enhanced immunity to L. major, correlating with stronger DC activation, priming and migration to dLN. Leishmania released a soluble proteinaceous Mincle ligand and induced a Mincle-dependent inhibitory axis. This inhibitory axis involved transient Syk activation that mediated coupling of SHP1 to FcRγ chain and dampened DC activation. Recruitment of SHP1 to the ITAM and mediating inhibitory signaling toward heterologous receptors (inhibitory ITAM, ITAMi) have been described for Fc receptors binding monomeric immunoglobulins (Aloulou et al., 2012; Ben Mkaddem et al., 2014; Hamerman et al., 2009; Pasquier et al., 2005), but not downstream of pattern recognition receptors. Our results reveal the relevance of the ITAMi pathway activated via Mincle after detection of a pathogen and as a mechanism of immune evasion by L. major. This ligand-dependent dual sensing and activation of the ITAM domain may be a paradigm for other ITAM-coupled receptors that have to deal with diverse exogenous and endogenous ligands.
Results
Leishmania releases a soluble proteinaceous ligand for Mincle
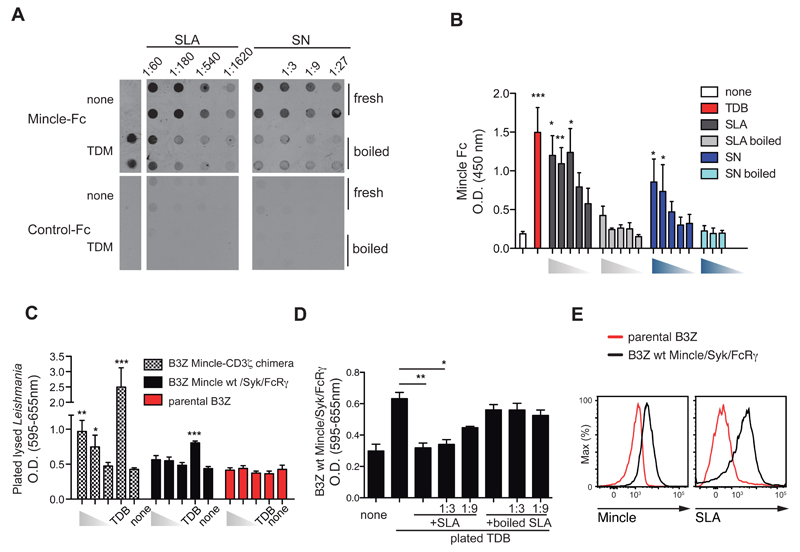
While screening for pathogens expressing Mincle ligands by dot blot, we found that the human Mincle ectodomain-Fc chimera (Mincle-Fc) specifically bound soluble Leishmania major extracts from freeze-thawed promastigotes (Figure 1A, left). Mincle-Fc also bound to blotted supernatants from L. major promastigotes kept for 3h at 37ºC to favor secretion (Figure 1A, right) and detected plated soluble Leishmania antigen (SLA) or supernatants (SN) by ELISA (Figure 1B); in contrast, control-Fc or macrophage C-type lectin (MCL)-Fc did not bind to blotted or plated Leishmania extracts (Figure S1A). Loss of binding upon boiling of the parasite preparations indicated that the ligand is heat-sensitive (Figure 1A, B). Treatment of plated Leishmania extract with sodium periodate, which oxidizes glycans, did not affect binding of Mincle-Fc to the Leishmania extract, but did inhibit the trehalose-dependent binding to TDM (Figure S1B).
Figure 1. Leishmania releases a soluble ligand for Mincle.
(A) Dot blots for Mincle-Fc (top) or control-Fc (bottom) with membranes spotted with fresh or boiled soluble Leishmania extracts (SLA) (left) or supernatants (SN) (right) from the indicated dilutions of stationary cultured parasites. Culture medium (none) or TDM were used as controls. (B) ELISA with Mincle-Fc of different doses of SLA or supernatants (fresh or boiled) from L. major promastigotes and controls (none or TDB). (C) NFAT reporter activity in response to 106, 105 or 104 of plated lysed-Leishmania or TDB in B3Z cells expressing human Mincle-CD3ζ chimera, WT mouse Mincle receptor co-expressing Syk and FcRγ, or the parental cells. (D) NFAT reporter activity in B3Z cells expressing WT mouse Mincle receptor, FcRγ, and Syk and exposed to plated TDB in the presence of the indicated dilutions of fresh or boiled SLA. (E) Staining with anti-Mincle (left) and fluorochrome-labeled SLA on control and Mincle-expressing. (A, E) Data are from one representative experiment of four (A) or three (E) performed. (B, C, D) Bars show arithmetic mean + SEM corresponding to three independent experiments. * p < 0.05; ** p < 0.01; *** p < 0.001 (one way ANOVA with Bonferroni post-hoc test).
To determine whether the ligand bound cellular Mincle, B3Z NFAT reporter cells (Karttunen et al., 1992) were transduced with a chimera comprising the extracellular human Mincle and intracellular CD3ζ, or alternatively with the wild type (WT) mouse Mincle receptor co-transduced with the FcRγ chain and Syk. The CD3ζ chimera responds to any multimeric ligand, whereas WT Mincle requires the Syk kinase transduction pathway to activate an NFAT reporter (Sancho et al., 2009). Plated Leishmania lysates triggered the Mincle-CD3ζ reporter, but not the WT Mincle-FcRγ-Syk or the parental cell line (Figure 1C). SLA did not trigger the Mincle-CD3ζ chimera or the WT Mincle (not shown), suggesting a low valency of the soluble ligand. In contrast, SLA blocked the triggering of WT Mincle or CD3ζ chimera by plated TDB in a dose-dependent and heat-sensitive manner (Figure 1D and not shown). SLA-mediated blockade did not affect the triggering of Mincle by plated 1B6 anti-Mincle antibody, indicating specificity for a TDB-Mincle binding site (Figure S1C). In addition, fluorochrome-labeled SLA bound to Mincle-expressing B3Z cells, but not to the parental cell line (Figure 1E and not shown).
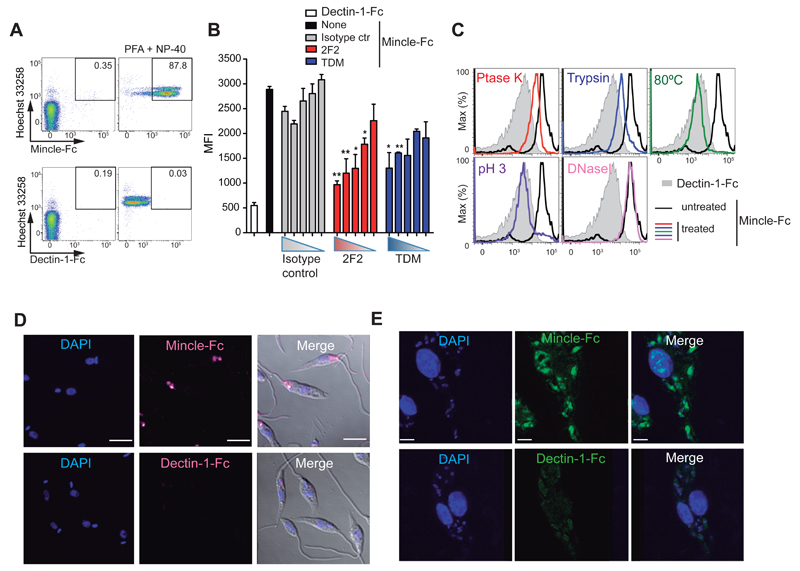
Mincle-Fc also stained fixed and permeabilized L. major promastigotes, whereas Dectin-1-Fc did not (Figure 2A and S2A). Binding of Mincle-Fc to fixed and permeabilized L. major was specifically inhibited by preincubation of the ectodomain with 2F2 anti-Mincle or with soluble TDM (Figure 2B and S2B), but not with 1B6 anti-Mincle (not shown). Moreover, treatment of fixed and permeabilized Leishmania promastigotes with proteinase K, trypsin, heat, or low pH, but not DNaseI, inhibited labeling by Mincle-Fc chimera, suggesting a proteinaceous nature of the ligand (Figure 2C). Notably, other Leishmania species were also specifically stained by Mincle-Fc (Figure S2C).
Figure 2. The Leishmania ligand for Mincle is proteinaceous and present at all parasite stages.
(A) Mincle-Fc and Dectin-1-Fc staining with Hoechst 33258 counterstaining in live L. major promastigotes or paraformaldehyde (PF)-fixed parasites permeabilized with NP-40. (B) Mean fluorescence intensity in fixed and permeabilized L. major promastigotes stained with Dectin-1-Fc (Control-Fc) or Mincle-Fc preincubated with titrated dilutions of anti-Mincle (clone 2F2), isotype control antibody (mouse IgM), or TDM (10 µg/ml starting dose, 3 fold dilution). Bars show arithmetic mean + SEM corresponding to three independent experiments. * p < 0.05; ** p < 0.01; *** p < 0.001 (one-way ANOVA with Bonferroni post-hoc test). (C) Fixed and permeabilized L. major promastigotes were subjected to the indicated treatments (colors) or untreated (black) and stained with Mincle-Fc chimera. Gray histograms show Dectin-1-Fc staining. (D, E) Confocal images of Mincle-Fc and Dectin-1-Fc staining in fixed and permeabilized Leishmania promastigotes (D) and bone-marrow-derived macrophages preincubated with promastigotes (E). Nuclei are counterstained with DAPI. Scale bar: 5 μm. (A, C-E) Plots and images are from single representative experiments of three performed.
Confocal analysis of Mincle-Fc staining in fixed and permeabilized L. major promastigotes revealed an intracellular granular pattern, including the flagellar pocket close to the kinetoplast, a unique site for exocytosis (Figure 2D). Mincle-Fc also stained the parasitophorous vacuole containing L. major amastigotes after uptake of the parasite by cultured macrophages (Figure 2E), alongside the staining of the endogenous nuclear ligand for Mincle (Yamasaki et al., 2008). Dectin-1 Fc did not stain fixed and permeabilized promastigotes or amastigotes (Figure 2D and E) but did label endocytosed zymosan (Figure S2D). Thus, Leishmania produced a proteinaceous ligand(s) for Mincle that was detected in all tested Leishmania species and was present at both the promastigote and amastigote stages.
Mincle is expressed during Leishmania infection
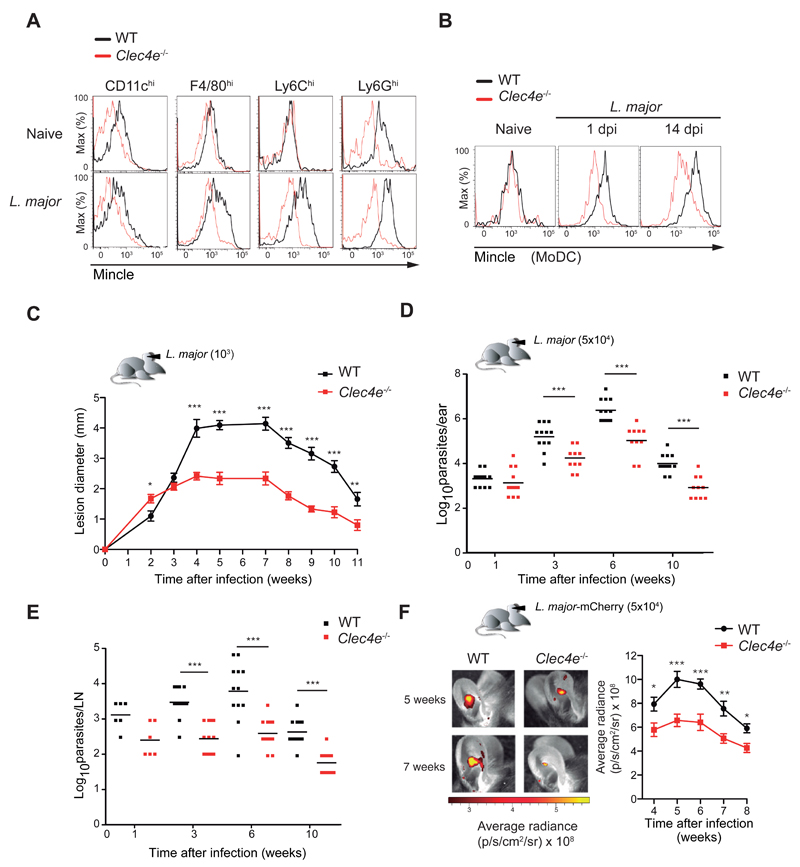
The typical route of Leishmania infection is a skin bite by a parasite-inoculated sandfly. We therefore analyzed Mincle expression in dermal cell types of WT and Mincle-deficient (Clec4e-/-) mice after L. major infection. The pinnae of both ears were inoculated by intradermal (i.d.) injection of 1000 L. major metacyclic promastigotes, and ear infiltrates were analyzed 24 hours later and compared with dermis taken from the ears of uninfected mice. Mincle expression by myeloid cells was modest in unchallenged dermis (Figure 3A and S3A) but was upregulated upon L. major infection in tissue macrophages, neutrophils, and monocyte-derived DCs (MoDCs) infiltrating the infection site (Figure 3A and B) and was maintained throughout the course of infection (Figure 3B). Mincle staining of myeloid cells was also observed in human skin samples and serial spleen sections from patients infected with Leishmania infantum (Figure S3B and C).
Figure 3. Mincle deficiency increases resistance to cutaneous leishmaniasis.
Flow cytometry analysis of Mincle expression in the indicated cell subsets isolated from ears of naive or L. major-infected WT or Clec4e-/- mice 1d p.i. MoDC, monocyte-derived DC. Histograms show representative data from three independent experiments (n=9). (C) Time profiles of lesion diameter in the ear pinnae of WT and Clec4e-/- mice infected i.d. with 1000 L. major parasites. Data arithmetic means ± SEM from a representative experiment (n=16) of three performed. (D, E) Parasite load in the ear (D) and dLNs (E) of WT and Clec4e-/- mice at the indicated times after i.d. infection in the ear with 5 x 104 L. major parasites. Squares show individual data and horizontal bars show arithmetic means from a representative experiment of three performed. (F) Left: In vivo imaging of mouse ears at the indicated times after i.d. inoculation with 5 x 104 mCherry+ L. major metacyclic promastigotes. Right: Progression of fluorescence signal (pixel/second/cm2/sr) expressed as arithmetic mean ± SEM (n=6). (C-F) * p < 0.05; ** p < 0.01; *** p < 0.001 (Student’s t test at each time point).
Mincle deficiency increases resistance to cutaneous leishmaniasis
To determine the contribution of Mincle to the immune response against L. major, we monitored cutaneous disease during an 11-week period after ear inoculation with 1000 L. major metacyclic promastigotes in WT or Clec4e-/- mice. In the first 2 weeks after infection, the inflammatory pathology in Clec4e-/- mice was similar to or greater than that in WT mice, but the response subsequently plateaued and there was no development of dermal lesions (Figure 3C). A similar pathology was provoked with inoculation of 5 x 104 parasites (Figure S3D and E), the dose subsequently used to induce a robust adaptive response in the challenge region. Since the third week of infection, parasite loads in the ears and dLNs of Clec4e-/- mice were 90% lower than those of their WT counterparts (Figure 3D and E). Real-time tracking of i.d. ear infection with L. major mCherry confirmed better control of infection was in Clec4e-/- mice, with significantly lower parasite load at all times analyzed (Figure 3F). Mincle-deficient mice thus controlled the infection earlier and more effectively than WT mice, leading to reduced pathology.
Mincle deficiency strengthens the adaptive response to L. major
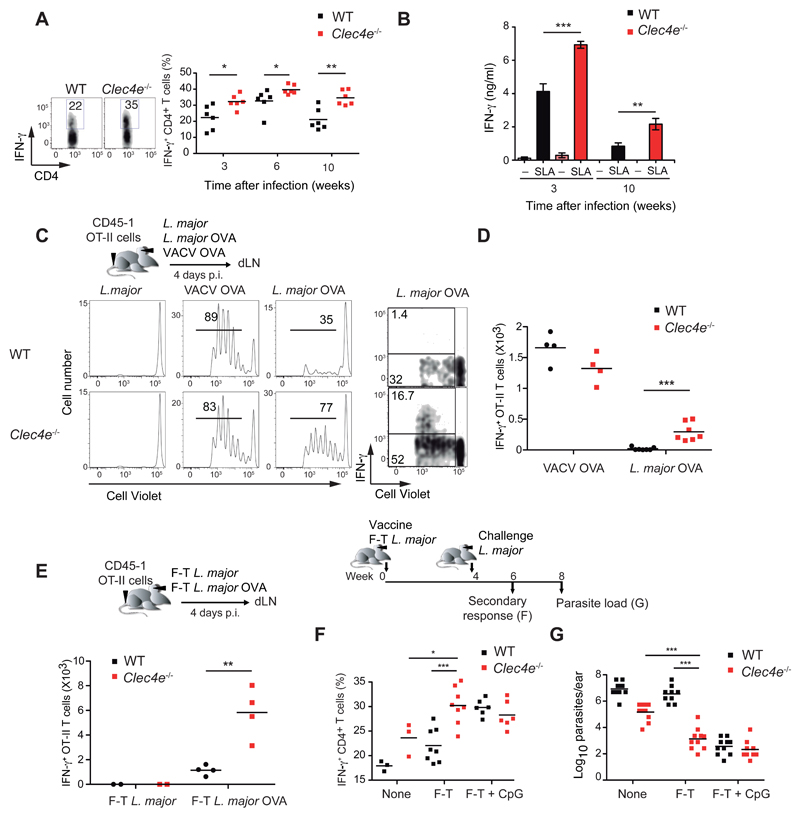
Polyclonal effector CD4+ T cells producing IFN-γ (but not CD8+ T cells) were significantly more abundant in the ears of infected Clec4e-/- mice at 3, 6 and 10 weeks p.i. (Figure 4A and Figure S4A). CD4+ T cells present in dLNs from infected Clec4e-/- mice showed augmented production of IFN-γ, but not IL-10, in response to SLA (Figure 4B and Figure S4B). The strong Th1 effector CD4+ T cell response also correlated with higher anti-Leishmania IgG2a but not IgG1 antibodies in Clec4e-/- mice (Figure S4C).
Figure 4. Increased adaptive response and enhanced CD4+ T cell priming during L. major infection in Mincle-deficient mice.
(A-B) WT and Clec4e-/- mice were infected i.d. in the ear with 5 x 104 L. major parasites. (A) IFN-γ production in CD4+ T cells in response to polyclonal restimulation of ear infiltrates at the indicated times. Left: representative plots 3 weeks p.i. Right: individual data and arithmetic means. (B) IFN-γ in supernatants from dLN cells extracted at the indicated times and restimulated with SLA. Data are arithmetic means + SEM (n=6) of one representative experiment of three performed. (C-D) WT and Clec4e-/- mice were transferred with CD45.1+ OTII OVA-specific T cells labeled with Cell Violet and infected i.d. in the ear with 5 x 104 particles of either L. major, L. major expressing OVA (L. major-OVA), or recombinant vaccinia virus expressing OVA (VACV-OVA). (C) Left: Representative histograms showing Cell Violet dilution in OTII cells in dLNs, 4 days p.i. Right: Representative plots of Cell Violet dilution and IFN-γ production following ex vivo restimulation with OVA peptide. (D) Quantification of IFN-γ+ OT-II absolute numbers in the dLNs. (E) Mice were vaccinated in the ear with 1 x 105 freeze-thawed (F-T) parasites and transferred with OTII as in (C). Quantification of OTII cells that were IFN-γ+ in the dLNs upon ex vivo restimulation with OVA peptide. (F-G) WT and Clec4e-/- mice were vaccinated i.d. in the ear with F-T L. major and challenged with live parasites in the same site 4 weeks later. (F) IFN-γ production in CD4+ effector T cells in the ear upon restimulation as in (A), assessed 2 weeks p.i. (G) Parasite load in the infected ears was evaluated 4 weeks p.i. (A, D, E, F, G) individual data and arithmetic mean of a representative experiment of three performed. * p < 0.05; ** p < 0.01; *** p < 0.001: (A-E) Student’s t test; (F, G) one way ANOVA with Bonferroni post-hoc test.
To investigate the mechanism of the enhanced adaptive response to L. major in the absence of Mincle, we analyzed early CD4+ T cell priming. As described (Pagán et al., 2013; Ribeiro-Gomes et al., 2012), infection with L. major expressing the model antigen ovalbumin (OVA) induced poor priming of OVA-specific CD4+ T cells (Figure 4C). Priming was boosted in Clec4e-/- mice, with enhanced CD4+ T cell proliferation in vivo and IFN-γ production upon OVA restimulation ex vivo (Figure 4C, D, and Figure S4D). The specificity of the Mincle-dependent decrease in CD4+ T cell priming for L. major was confirmed by identical effector responses in WT and Clec4e-/- mice upon infection with OVA-expressing vaccinia virus (Figure 4C, D, and Figure S4D). These data show that Leishmania targets Mincle to decrease priming of a CD4+ Th1 cell-type response against the parasite.
To determine the relevance of enhanced priming in a context of vaccination, we transferred OVA-specific CD4+ T cells i.v. and subsequently injected 1 x 105 freeze-thawed L. major-OVA i.d. into the ear. Injection of dead parasites into Clec4e-/- mice resulted in increased numbers of OVA-specific CD4+ T cells producing IFN-γ upon restimulation ex vivo (Figure 4E and Figure S4E). We next analyzed whether Mincle deficiency also strengthens the function of the memory CD4+ T cell compartment. Vaccination with freeze-thawed Leishmania followed by L. major rechallenge 4 weeks later induced IFN-γ+ CD4+ effector T cells in the ear of Clec4e-/- but not WT mice (Figure 4F), thus generating a protective response with reduced parasitemia (Figure 4G). This Mincle-dependent vaccination deficiency using freeze-thawed Leishmania extracts in WT mice could be reverted by the use of CpG as adjuvant (Figure 4F and G), consistent with published findings (Walker et al., 1999). These results indicated that upon sensing Leishmania, Mincle inhibited the generation of effector and memory CD4+ T cells and impaired the adaptive response to L. major.
Mincle absence increases DC activation and migration to dLNs after L. major infection
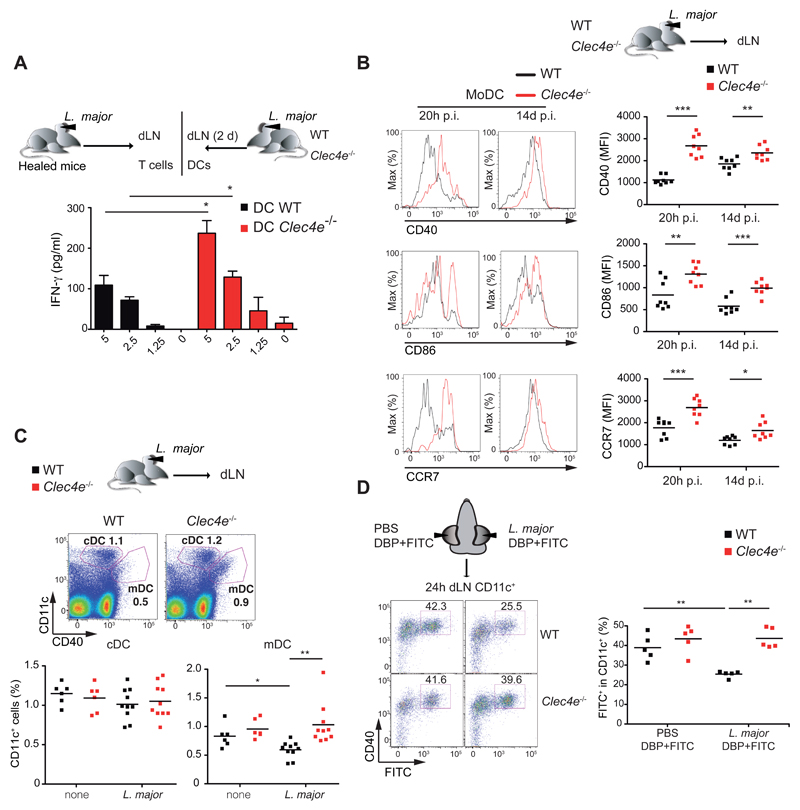
Given the increased adaptive response, we next investigated whether Mincle-deficient DCs had an enhanced ability to prime anti-L. major responses. DCs extracted from dLNs of Clec4e-/- mice were better than WT at restimulating L. major-specific CD4+ T cells obtained from healed WT mice (Figure 5A). Early after L. major infection, CD40 expression on DCs in the ear was significantly upregulated in Clec4e-/- mice compared with WT mice (Figure S5A). Moreover, MoDCs infiltrating the dermis of Mincle-deficient mice also showed upregulation of the activation markers CD40 and CD86 and the chemokine receptor CCR7 at 20h and 14d after infection (Figure 5B).
Figure 5. Enhanced DC activation and migration to dLNs after L. major infection in Mincle-deficient mice.
(A) IFN-γ in supernatants of T cells from healed L. major-infected mice after co-culture for 3 d with CD11c+ cells recovered from the dLNs of WT and Clec4e-/- mice 2 d p.i. Data are arithmetic means + SEM from two independent experiments (n=6). (B) Left panels: Representative histograms of CD40, CD86 and CCR7 staining in MoDCs (CD11b+Ly6C+CD11c+MHCII+ gated cells) from ears of infected mice. Right panels: Mean fluorescence intensity (MFI) of CD40, CD86 and CCR7 expression on MoDCs. (C) Representative dot plots (Upper) and frequencies (Lower) of CD11c- and CD40-positive cells in dLNs from uninfected mice or 24 h after L. major infection in the ear. (D) Ears of mice inoculated with PBS in the left ear and L. major parasites (105) in the right ear were FITC painted (see Methods) and dLNs were harvested 24h later. Left : Representative plots of dLN cells gated for CD11c and stained with anti-CD40 and FITC. Right: frequencies of FITC+ CD11c+ dLN cells. (B-D) Individual data and arithmetic means corresponding to a representative experiment of two (B) or three (C, D) performed. (A-D) * p < 0.05; ** p < 0.01; *** p < 0.001 (Student’s t test).
In addition, L. major infection decreased the numbers of migratory DCs in a Mincle-dependent manner (Figure 5C). The effect of Mincle on the capacity of dermal DCs to migrate to the dLNs was further investigated in FITC skin sensitization assays. L. major infection inhibited migration of FITC+ CD11c+ DCs to dLNs in WT mice but not in Clec4e-/- mice (Figure 5D). Mincle-dependent inhibition of DC migration was maintained two weeks after infection (Figure S5B). These results suggest that Leishmania sensing by Mincle impaired DC activation in the infection site and subsequently limited their capacity to migrate to dLNs, contributing to the reduced priming to L. major in the presence of Mincle.
L. major promotes a Mincle- and SHP1-dependent inhibitory axis in DCs
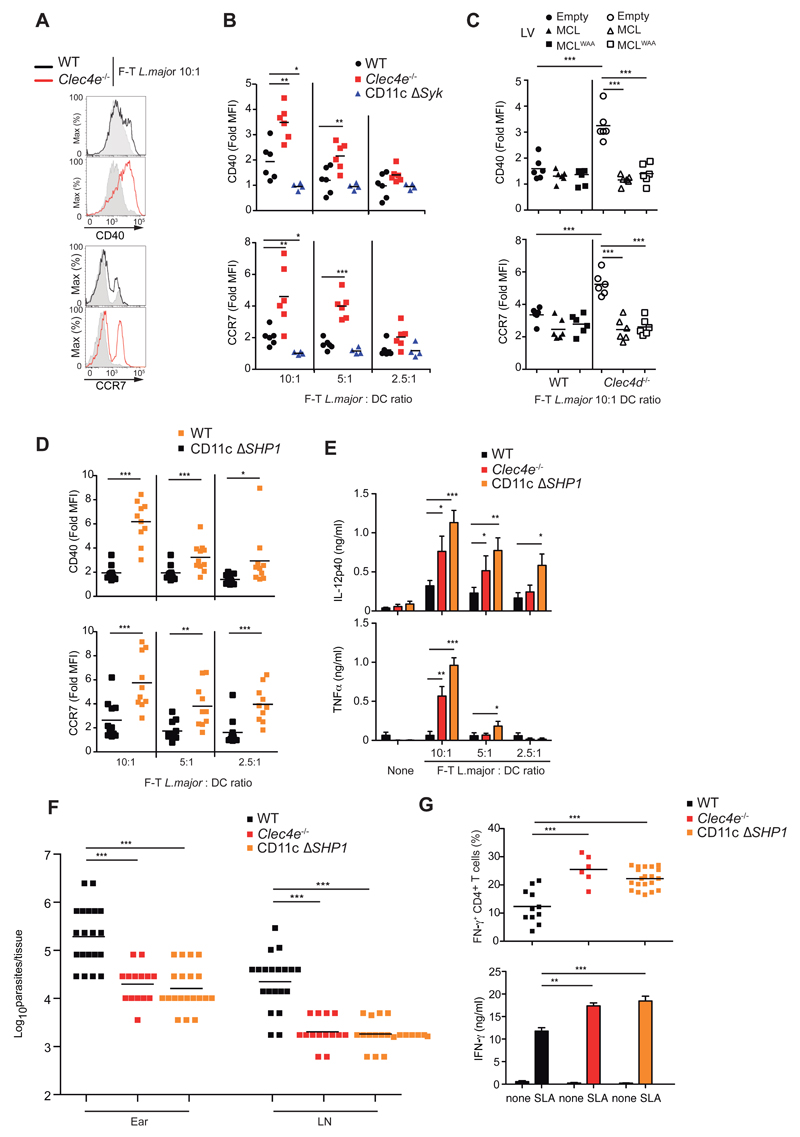
To test whether increased DC activation in the absence of Mincle was intrinsic, we generated GMCSF bone-marrow-derived cells akin to DCs (GM-DCs) from WT and Clec4e-/- mice (Figure S6A). Stimulation with freeze-thawed L. major induced increased expression of CD40, CD86 and CCR7 in Mincle-deficient CD11c+ GM-DCs (Figure 6A and 6B, and S6B and S6C), suggesting an intrinsic effect. As Syk is downstream Mincle (Yamasaki et al., 2008), we tested the absence of Syk in the CD11c compartment (CD11cΔSyk) (Iborra et al., 2012). GM-DCs from CD11cΔSyk mice showed impaired activation by freeze-thawed L. major (Figure 6B and S6C), suggesting the possible existence of an unidentified activating Syk-coupled DC receptor for L. major (Lefèvre et al., 2013).
Figure 6. Mincle and SHP1 inhibit DC activation by freeze-thawed L. major.
(A) Histogram overlays for CD40 and CCR7 in CD11c+ GM-DCs from WT and Clec4e-/- mice left untreated (gray histograms) or treated with freeze-thawed (F-T) L. major. Data are representative of three independent experiments (n=6). (B-D) Fold induction of MFI for CD40 and CCR7 upon F-T L. major treatment of (B) GM-DCs obtained from WT, Clec4e-/-, and CD11cΔSyk mice; (C) WT and Clec4d-/- GM-DCs transduced with empty vector, MCL, or MCLWAA; and (D) GM-DCs from WT and CD11cΔSHP1 mice. (E) IL-12p40 and TNFα in culture supernatants 20h after exposure of WT, Clec4e-/-, and CD11cΔSHP1 GM-DCs to different doses of F-T L. major. Data are arithmetic means + SEM of three independent experiments. (F-G) WT, Clec4e-/-, and CD11cΔSHP1 mice inoculated with 5 x 104 L. major parasites i.d. in the ear were sacrificed 3 weeks after infection. (F) Parasite load in the infected ear and dLNs. (G) Top: intracellular IFN-γ in CD4+ T cells after polyclonal restimulation of ear infiltrates. Bottom: IFN-γ in supernatants after SLA restimulation of 2 x 106 dLN cells. Data are arithmetic means+ SEM of three independent experiments. (B, D, F, G) Individual data and arithmetic mean corresponding to one representative experiment of three performed; (C) Pooled data from three independent experiments (n=6). (B-G) * p < 0.05; ** p < 0.01; *** p < 0.001 (B, E-G) one way ANOVA with Bonferroni post-hoc test; (C, D) Student’s t test.
MCL and Mincle are mutually regulated and act as heterodimers for binding to TDM (Kerscher et al., 2016; Lobato-Pascual et al., 2013; Miyake et al., 2015; Miyake et al., 2013). Consistent with these reports, GM-DCs derived from Clec4d-/- mice lacked expression of not only MCL but also Mincle (Figure S6D). Mincle expression was rescued by transduction with WT MCL or MCLWAA (Figure S6D), which contains a mutation in calcium-binding motif of the C-type lectin domain (Miyake et al., 2015). The impaired expression of Mincle and MCL in Clec4d-/- mice resulted in increased activation of DCs exposed to freeze-thawed L. major (Figure 6C). Reexpression of Mincle mediated by transduction of both MCL or MCLWAA correlated with impaired DC activation by L. major (Figure 6C), suggesting that regulation of Mincle expression by MCL contributes to responses to L. major.
Infection with Fonsecaea triggers Akt-dependent repression of IL12p35 transcription (Wevers et al., 2014). In contrast, freeze-thawed L. major did not induce Akt activation in WT mice (Figure S6E). We hypothesized that DC activation by L. major might be antagonized by Mincle through the recruitment of SHP1 in an inhibitory ITAM (ITAMi) configuration (Aloulou et al., 2012; Ben Mkaddem et al., 2014; Hamerman et al., 2009; Pasquier et al., 2005). Consistent with this notion, treatment with the SHP1/2 phosphatase inhibitor NSC-87877 increased DC activation by L. major (Figure S6F), contrasting with the absence of an effect with the Akt inhibitor VIII. Notably, NSC-87877 did not further activate Mincle-deficient DCs in response to the parasite (Figure S6G), suggesting that Mincle and phosphatase activity act in the same pathway. Supporting this conclusion, the enhanced freeze-thawed L. major-mediated activation seen in Mincle-deficient mice was phenocopied in GM-DCs from mice lacking SHP1 in the CD11c compartment (CD11cΔSHP1) (Abram et al., 2013) (Figure 6D and S6H). freeze-thawed L. major-induced cytokine production was also higher in GM-DCs lacking Mincle or SHP1 (Figure 6E). Moreover, like Clec4e-/- mice tested in parallel, CD11cΔSHP1 mice displayed lower ear and LN parasitemia in response to L. major infection (Figure 6F) and showed increased adaptive immunity (Figure 6G). Thus, our results suggested that Mincle inhibited DC activation through SHP1.
L. major shifts Mincle to an inhibitory ITAM configuration that suppresses heterologous receptors
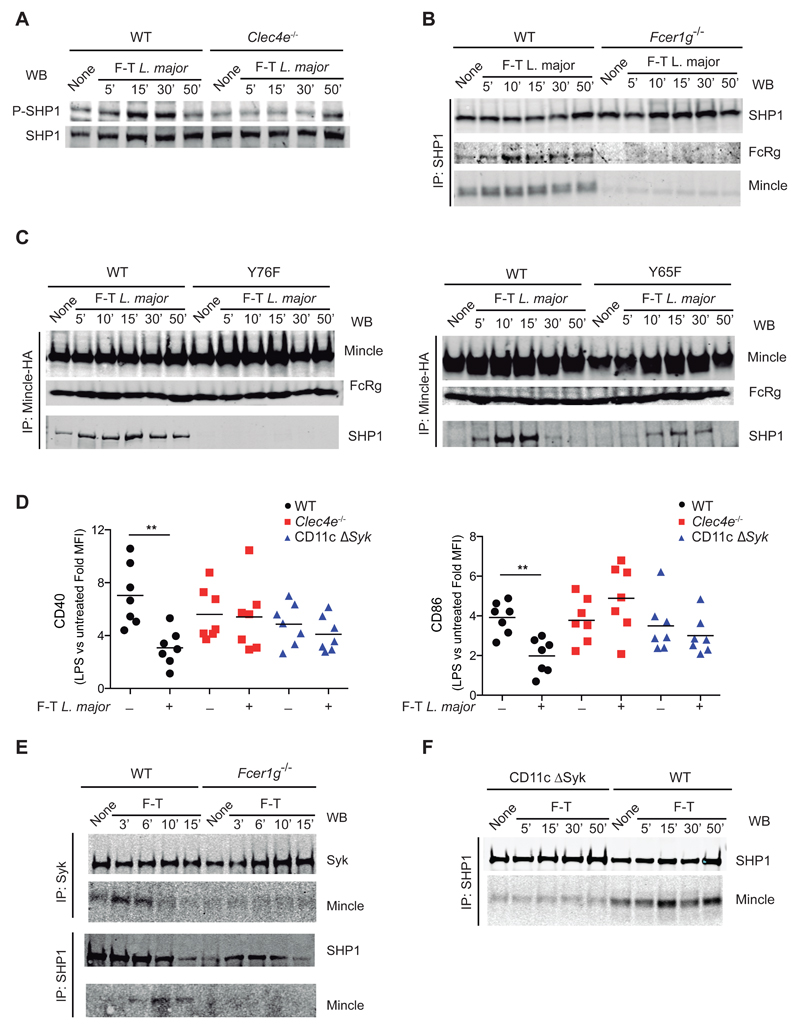
Participation of Mincle and SHP1 in the same axis was further supported by Mincle-dependent phosphorylation of SHP1 (but not SHP2) in freeze-thawed L. major-stimulated GM-DCs (Figure 7A and Figure S7A and B). Pull-down of SHP1 in WT or FcRγ-chain-deficient GM-DCs revealed specific FcRγ-dependent association of SHP1 with Mincle (Figure 7B). Notably, treatment of GM-DCs with plated TDB induced FcRγ-dependent association of Mincle with Syk, but not with SHP1 (Figure S7C). Moreover, pull-down of Mincle from B3Z transfectants expressing tyrosine mutants in the FcRγ ITAM domain demonstrated that the membrane-distal tyrosine 76 was crucial for association of Mincle-FcRγ with SHP1, whereas tyrosine 65 was at least partially dispensable (Figure 7C), consistent with the ITAMi configuration (Ben Mkaddem et al., 2014).
Figure 7. L. major promotes a Mincle/FcRγ/SHP1 axis that impairs DC activation.
(A) Western blot (WB) for P-SHP1 and total SHP1 in WT and Clec4e-/- GM-DCs lysed at the indicated times of stimulation with F-T L. major. (B) SHP1 immunoprecipitation and WB for SHP1, FcRγ and Mincle in WT and Fcer1g-/- GM-DCs lysed at the indicated times of stimulation with F-T L. major. (C) Mincle immunoprecipitation in B3Z cells transduced with mouse Mincle (Clec4e), Syk, and either WT FcRγ chain or the Y65F (left) or Y76F mutants (right). WB for Mincle, FcRγ and SHP1. (D) Fold induction of MFI for CD40 and CCR7 upon LPS stimulation (200 ng/ml) of F-T L. major-pretreated for 30 min (+) or non-pretreated (-) GM-DCs from WT, Clec4e-/- and CD11cΔSyk mice. ** p < 0.01; Student’s t test comparing F-T L. major pretreatment and no pretreatment within each genotype. (E) Syk (upper blots) and SHP1 (lower blots) immunoprecipitation from F-T L. major-treated WT and Fcer1g-/- GM-DCs and WB for Syk and Mincle (upper) or SHP1 and Mincle (lower). (F) SHP1 immunoprecipitation from F-T L. major-treated WT and CD11cΔSyk GM-DCs and WB for SHP1 and Mincle. (A-F) Western Blots are from single representative experiments of at least three performed.
We next tested the effect of the L. major-induced Mincle-dependent inhibitory axis on GM-DC activation promoted by LPS. freeze-thawed L. major dampened LPS-induced activation in GM-DCs and this inhibition was dependent on Mincle and Syk (Figure 7D). The ITAMi configuration is dependent on transient activation of Syk (Ben Mkaddem et al., 2014). We found that Syk transiently associated with Mincle in GM-DCs stimulated with freeze-thawed L. major in a manner dependent on the FcRγ chain (Figure 7E). Notably, CD11cΔSyk DCs showed impaired SHP1 recruitment to Mincle (Figure 7F). These results suggest that L. major shifts Mincle to an ITAMi configuration that suppresses heterologous activating receptors, dampening DC activation and thus impairing the induction of adaptive immune responses.
Discussion
Parasites that depend on an invertebrate vector for cyclical transmission have evolved mechanisms to delay or prevent sterilizing immunity in vertebrate hosts, thereby prolonging parasite availability to the vector (Yazdanbakhsh and Sacks, 2010). Leishmania parasites replicate silently in the skin for several weeks after inoculation (Belkaid et al., 2000), suggesting that they might actively dampen DC recognition or activation (Srivastav et al., 2012) and establish a functional immune privilege in the skin (Peters and Sacks, 2006). In this study, we have found that L. major parasites release a soluble ligand that binds Mincle, triggering an ITAMi signaling pathway that suppresses DC activation by heterologous activating receptors concomitantly sensing L. major. Mincle deficiency thus favored stronger DC activation in response to L. major infection, manifested in higher expression of costimulatory molecules, migration to dLNs, and priming of a Th1 cell response to parasite antigens. Increased Th1 cell-type immunity correlated with reduced parasite load and pathology in Mincle-deficient mice. These results reveal how the ITAMi pathway can be targeted by a pathogen as a mechanism to evade immune surveillance, and illustrate a SHP1-based inhibitory pathway in an ITAM-coupled CLR.
Mincle is a FcRγ-Syk-coupled CLR (Kerscher et al., 2013; Sancho and Reis e Sousa, 2012, 2013) with a well-established role in inducing inflammation and host immunity in response to glycolipid ligands in the cell wall of bacteria and fungi (Ishikawa et al., 2009; Ishikawa et al., 2013; Schoenen et al., 2010; Shenderov et al., 2013; Sousa et al., 2011; Wells et al., 2008; Yamasaki et al., 2009). However, recent reports point to an additional, negative role for Mincle in the control of immunity (Seifert et al., 2016; Wevers et al., 2014; Wuthrich et al., 2015). Mincle detection of Fonsecaea involves an Akt-dependent pathway that selectively impairs IL12p35 transcription (Wevers et al., 2014). Therefore, the finding that Mincle sensing of Leishmania induces global DC inhibition through a SHP1-dependent and Akt-independent pathway was highly unexpected.
Engagement of FcRγ chain-coupled receptors by low-affinity or avidity ligands may cause hypophosphorylation of ITAM domains and result in recruitment of SHP1, a configuration termed inhibitory ITAM (ITAMi) (Aloulou et al., 2012; Ben Mkaddem et al., 2014; Hamerman et al., 2009; Pasquier et al., 2005). Our results provide an example of a functional ITAMi coupled to a pattern recognition receptor and support the potential physiological relevance of this signaling module (Aloulou et al., 2012; Blank et al., 2009; Pasquier et al., 2005). Transient Syk activation is required for the ITAMi configuration (Ben Mkaddem et al., 2014). We found transient Syk association with Mincle following freeze-thawed L. major stimulation and we showed that Syk is indeed required for SHP1 recruitment to Mincle. However, we found that the overall response of CD11cΔSyk DCs to freeze-thawed L. major was impaired, likely because Syk was required for intracellular signaling pathways by other pattern recognition receptors that mediate activating signals to the parasite. Consistent with the ITAMi configuration, SHP1 associated through the membrane distal tyrosine 76 (Ben Mkaddem et al., 2014), which was crucial for association of Mincle-FcRγ to SHP1. Notably, soluble Leishmania extract inhibited DC activation upon LPS challenge in a Mincle-dependent manner, showing the potential of this FcRγ/SHP1 axis to interfere with diverse activating pathways through heterologous receptors, all these features defining the ITAMi pathway.
Given that the Leishmania ligand was soluble, avidity for Mincle could be reduced (Iborra and Sancho, 2015). In contrast to Leishmania ligand, we did not find SHP1 associated with Mincle when GM-DCs were treated with plated TDB. Together with Mincle, the CLR MCL binds to and is essential for TDM adjuvant potential (Furukawa et al., 2013; Miyake et al., 2013). Mincle, MCL and FcRγ form a heteromeric complex that facilitates signaling (Lobato-Pascual et al., 2013). In addition, MCL and Mincle mutually regulate their expression (Kerscher et al., 2016; Miyake et al., 2015; Miyake et al., 2013). Here, we have found that control of Mincle expression by MCL is required for dampening DC activation in response to L. major. Our results do not support a direct role for MCL in the recognition of L. major by Mincle, since MCL-Fc did not bind to Leishmania extract and its inhibitory effect on DCs was maintained with a MCLWAA mutant in the lectin domain that allows Mincle expression (Miyake et al., 2015), although the possibility that MCL could contribute directly cannot be completely ruled out. Our data show that the L. major ligand triggers SHP-1 phosphorylation via Mincle in B3Z cells in the absence of MCL. It is therefore feasible that the signal triggered by binding of L. major ligand to Mincle (in homo or heteromeric configuration) could be weaker than that triggered by the binding of TDM-coated structures to the Mincle-MCL heteromer, and this weaker signaling may favor the ITAMi configuration.
The presence of a ligand for Mincle may contribute to the low effectiveness of candidate vaccines based on whole killed Leishmania or attenuated parasites (Duthie et al., 2012). Our results indicate that blocking Mincle or SHP1 during a vaccination setting may improve vaccine efficiency by allowing Th1 responses to be induced. Moreover, our findings suggest that Mincle can couple to an activating ITAM or to an ITAMi configuration depending on the nature of the ligand, an idea that could apply to other ITAM-coupled CLRs with a diverse ligand range or that can heterodimerize with multiple receptors (Iborra and Sancho, 2015).
Experimental Procedures
Mice
Mouse colonies were bred at the CNIC under specific pathogen-free conditions. Colonies included C57BL/6, Clec4e-/- (B6.Cg-Clec4etm1.1Cfg) backcrossed more than 10 times to C57BL/6J-Crl (kindly provided by Scripps Research Institute, through R. Ashman and C. Wells, Griffiths University, Australia) (Wells et al., 2008), CD11cΔSyk (Iborra et al., 2012), Fcer1g-/- (B6;129P2-Fcer1gtm1Rav/J) from The Jackson Laboratory (Takai et al., 1994), CD11cΔSHP1 (Abram et al., 2013), and OT-II CD4+ TCR transgenic mice in C57BL/6 background (B6.Cg-Tg(TcraTcrb)425Cbn/J) and mated with B6/SJL expressing CD45.1 isoform to facilitate cell tracking. Animal studies were approved by the local ethics committee. All animal procedures conformed to EU Directive 2010/63EU and Recommendation 2007/526/EC regarding the protection of animals used for experimental and other scientific purposes, enforced in Spanish law under Real Decreto 1201/2005.
Leishmania parasite preparation, inoculation, and quantitation
For Leishmania challenge, parasites of different lines were cultured and kept in a virulent state as described (Martinez-Lopez et al., 2015). Mice were infected by i.d. inoculation of 1000 or 5×104 metacyclic L. major promastigotes into the dermis of both ears (Martinez-Lopez et al., 2015). Lesion size in the ear and number of viable parasites was determined as described (Martinez-Lopez et al., 2015). The parasite load is expressed as the number of parasites in the whole organ.
Parasite preparation of protein extracts and binding to Mincle-Fc chimera
For preparation of soluble Leishmania extract, also known as soluble Leishmania antigen (SLA), approximately 109 promastigotes were harvested and washed twice in PBS. After 3 cycles of freezing and thawing, the suspension was centrifuged at 13,000 × g for 20 min at 4°C, and supernatant containing SLA was collected and stored at −80°C. Protein concentration was estimated by the Bradford method.
Freeze-thawed L. major parasites were prepared by 3 cycles of freezing and thawing of 108 stationary parasites in complete RPMI medium or PBS. Fixed and permeabilized Leishmania parasites were prepared by fixing 108 parasites with 0.5 ml of 4% paraformaldehyde and immediate addition of 0.5 ml 1% NP-40. After incubation for 10 min at room temperature, parasites were extensively washed with PBS. To obtain culture supernatants, stationary promastigotes were washed 3 times in phosphate buffer saline (PBS), resuspended at 5x108 parasites/ ml in serum free DMEM, and incubated for 3 h at 37º C. Culture supernatants were collected by two steps of centrifugation, first at 1,500 ×g for 5 min at 4 °C, followed by a second step at 2,500 ×g for 10 min at 4 °C. Protein concentration was estimated by the Bradford method. For dot-blot determination of Mincle ligands in Leishmania extracts, protein samples were applied to 0.2 μm membranes (BioRad) using a vacuum dot blot apparatus (BioRad). To load different protein amounts in each dot, protein samples were serially diluted in PBS (1:3). Similarly, for ELISA, high-binding plates were loaded with protein samples serially diluted in PBS (1:3). Plates were incubated for 24 hours at 4ºC. Later, membranes and plates were washed with PBS and incubated with blocking solution (2% defatted milk in PBS) for 120 minutes at room temperature, followed by incubation with Mincle-Fc chimera or control Fc (2μg/ml) for 2 hours. Membranes and plates were then incubated with anti-human IgG (Fc gamma-specific) conjugated to biotin. Membranes were imaged with the LI-COR Odyssey Infrared Imaging System.
Generation and assay of B3Z cell lines expressing Mincle and FcRγ chain mutants
B3Z cells (kindly provided by N. Shastri, University of California) express a β-gal reporter for nuclear factor of activated T cells (NFAT) (Karttunen et al., 1992). B3Z cells were transduced with retroviruses expressing FcRγ chain, Syk and mouse Mincle. FcRγ chain ITAM tyrosine 65 and 76 phenylalanine mutants were generated using the QuickChange lightning site-directed mutagenesis kit (Agilent). Binding of ligands can be detected by NFAT reporter activation and induction of β-gal activity. B3Z cells were plated in 96 well plates and incubated with plated TDB or anti-Mincle (1B6) in the presence or absence of Leishmania extract. Lysed parasites used in B3Z assays were opsonized with fresh serum from infected Balb/c mice for 2 hours at RT and washed twice with cold PBS. Before B3Z cell plating, promastigotes were seeded on plates coated with 50 µg/ml poly-L-Lysine (Sigma), for 30 minutes at 37 ºC.
After overnight culture, cells were washed in PBS, and LacZ activity was measured by lysis in CPRG (Roche)-containing buffer. Four hours later O.D. 595 nm was measured relative to O.D. 655 nm used as a reference.
Adoptive transfer and antigen presentation studies in vitro
For adoptive transfer experiments, CD4+ T cells were purified from pooled spleens and lymph nodes of OT-II CD4+ TCR transgenic mice by negative selection (Miltenyi Biotec). Purified CD4+ T cells were incubated at 5×106 cells/ml in PBS with 0.5 μM CellTrace™ Violet (Invitrogen) for 10 min at 37Cº. The reaction was stopped with 5% FCS PBS. CellTrace™ Violet -labeled purified CD4+ OT-II T cells (2–5×105) were transferred just after challenge in the ear dermis either with 5x104 metacyclic promastigotes of Leishmania-OVA, rVACV-OVA (kindly provided by J. Yewdell, NIAID, Bethesda) or dead Leishmania OVA (1x105). Four days after adoptive transfer, the dLNs were removed, LN cell suspensions were prepared and seeded in the presence of 10 μm I-Ab-restricted OVA peptide (323-339) and brefeldin A. LN cells were stained and analyzed by intracellular flow cytometry. In some experiments, T cells were purified from retromaxillary LNs of infected and healed mice and co-cultured with DCs enriched form dLNs of mice infected 48h before. IFN-γ release was determined in culture supernatants 72h later.
Statistical analysis
The statistical analysis was performed using Prism software (GraphPad Software, Inc). Statistical significance for comparison between two sample groups with a normal distribution (Shapiro-Wilk test for normality) was determined by unpaired two-tailed Student's t test. Comparisons of more than two groups were made by one way ANOVA and Bonferroni post-Hoc test. Differences were considered significant at p <0.05 was considered significant (* p < 0.05; ** p< 0.01; *** p < 0.001).
Supplementary Material
Acknowledgements
We are grateful to C. Reis e Sousa, C. Ardavín, A. Corbí, A. Hidalgo, and members of the D.S. laboratory for discussions and critical reading of the manuscript. We thank the CNIC facilities, personnel and to S. Bartlett for editorial assistance. We are indebted to G. Brown, J. Willment, A. Corbí, J.Yewdell, N. Shastri, C. Wells, R. Ashman, H. Miyoshi, RIKEN BRC and the Scripps Research Institute for providing reagents. SI is funded by grant SAF2015-74561-JIN. Work in the D.S. laboratory is funded by the CNIC and grants from the Spanish Ministry of Economy and Competitiveness (MINECO, SAF-2013-42920R), the European Commission (635122-PROCROP H2020) and the European Research Council (ERC-2010-StG 260414). The CNIC is supported by the MINECO and the Pro-CNIC Foundation, and is a Severo Ochoa Center of Excellence (MINECO award SEV-2015-0505).
Footnotes
Author contributions
S.I., M.M-L, F.J.C., C.d.F, H.M.I, R.C., Y.C., and D.S. did the experiments; C.L.A., C.A.L., D.M., B.K., S.Y., M.J.R., R.R., and M.S. provided essential reagents. S.I. and D.S. conceived and designed experiments, analyzed data and wrote the manuscript. All the authors discussed the results and the manuscript.
Conflict of interest: M.J.R. and B.K. are employees of MedImmune and shareholders in the parent company Astrazeneca. The other authors declare no competing financial interests.
References
- Abram CL, Roberge GL, Pao LI, Neel BG, Lowell CA. Distinct Roles for Neutrophils and Dendritic Cells in Inflammation and Autoimmunity in motheaten Mice. Immunity. 2013;38:489–501. doi: 10.1016/j.immuni.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloulou M, Ben Mkaddem S, Biarnes-Pelicot M, Boussetta T, Souchet H, Rossato E, Benhamou M, Crestani B, Zhu Z, Blank U, et al. IgG1 and IVIg induce inhibitory ITAM signaling through FcγRIII controlling inflammatory responses. Blood. 2012;119:3084–3096. doi: 10.1182/blood-2011-08-376046. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Mendez S, Lira R, Kadambi N, Milon G, Sacks D. A natural model of Leishmania major infection reveals a prolonged “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity. J Immunol. 2000;165:969–977. doi: 10.4049/jimmunol.165.2.969. [DOI] [PubMed] [Google Scholar]
- Ben Mkaddem S, Hayem G, Jonsson F, Rossato E, Boedec E, Boussetta T, El Benna J, Launay P, Goujon JM, Benhamou M, et al. Shifting FcgammaRIIA-ITAM from activation to inhibitory configuration ameliorates arthritis. J Clin Invest. 2014;124:3945–3959. doi: 10.1172/JCI74572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank U, Launay P, Benhamou M, Monteiro RC. Inhibitory ITAMs as novel regulators of immunity. Immunol Rev. 2009;232:59–71. doi: 10.1111/j.1600-065X.2009.00832.x. [DOI] [PubMed] [Google Scholar]
- Dambuza IM, Brown GD. C-type lectins in immunity: recent developments. Curr Opin Immunol. 2015;32:21–27. doi: 10.1016/j.coi.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z, Ma S, Zhou H, Zang A, Fang Y, Li T, Shi H, Liu M, Du M, Taylor PR, et al. Tyrosine phosphatase SHP-2 mediates C-type lectin receptor-induced activation of the kinase Syk and anti-fungal TH17 responses. Nat Immunol. 2015;16:642–652. doi: 10.1038/ni.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duthie MS, Raman VS, Piazza FM, Reed SG. The development and clinical evaluation of second-generation leishmaniasis vaccines. Vaccine. 2012;30:134–141. doi: 10.1016/j.vaccine.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa A, Kamishikiryo J, Mori D, Toyonaga K, Okabe Y, Toji A, Kanda R, Miyake Y, Ose T, Yamasaki S, Maenaka K. Structural analysis for glycolipid recognition by the C-type lectins Mincle and MCL. Proc Natl Acad Sci USA. 2013;110:17438–17443. doi: 10.1073/pnas.1312649110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamerman JA, Ni M, Killebrew JR, Chu CL, Lowell CA. The expanding roles of ITAM adapters FcRgamma and DAP12 in myeloid cells. Immunol Rev. 2009;232:42–58. doi: 10.1111/j.1600-065X.2009.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iborra S, Izquierdo HM, Martinez-Lopez M, Blanco-Menendez N, Reis e Sousa C, Sancho D. The DC receptor DNGR-1 mediates cross-priming of CTLs during vaccinia virus infection in mice. J Clin Invest. 2012;122:1628–1643. doi: 10.1172/JCI60660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iborra S, Sancho D. Signalling versatility following self and non-self sensing by myeloid C-type lectin receptors. Immunobiology. 2015;220:175–184. doi: 10.1016/j.imbio.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa E, Ishikawa T, Morita YS, Toyonaga K, Yamada H, Takeuchi O, Kinoshita T, Akira S, Yoshikai Y, Yamasaki S. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J Exp Med. 2009;206:2879–2888. doi: 10.1084/jem.20091750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Itoh F, Yoshida S, Saijo S, Matsuzawa T, Gonoi T, Saito T, Okawa Y, Shibata N, Miyamoto T, Yamasaki S. Identification of distinct ligands for the C-type lectin receptors Mincle and Dectin-2 in the pathogenic fungus Malassezia. Cell Host Microbe. 2013;13:477–488. doi: 10.1016/j.chom.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Karttunen J, Sanderson S, Shastri N. Detection of rare antigen-presenting cells by the lacZ T-cell activation assay suggests an expression cloning strategy for T-cell antigens. Proc Natl Acad Sci U S A. 1992;89:6020–6024. doi: 10.1073/pnas.89.13.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerscher B, Willment JA, Brown GD. The Dectin-2 family of C-type lectin-like receptors: an update. Int Immunol. 2013;25:271–277. doi: 10.1093/intimm/dxt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerscher B, Wilson GJ, Reid DM, Mori D, Taylor JA, Besra GS, Yamasaki S, Willment JA, Brown GD. Mycobacterial receptor, Clec4d (CLECSF8, MCL), is coregulated with Mincle and upregulated on mouse myeloid cells following microbial challenge. Eur J Immunol. 2016;46:381–389. doi: 10.1002/eji.201545858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefèvre L, Lugo-Villarino G, Meunier E, Valentin A, Olagnier D, Authier H, Duval C, Dardenne C, Bernad J, Lemesre JL, et al. The C-type lectin receptors dectin-1, MR, and SIGNR3 contribute both positively and negatively to the macrophage response to Leishmania infantum. Immunity. 2013;38:1038–1049. doi: 10.1016/j.immuni.2013.04.010. [DOI] [PubMed] [Google Scholar]
- Leon B, Lopez-Bravo M, Ardavin C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity. 2007;26:519–531. doi: 10.1016/j.immuni.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Lobato-Pascual A, Saether PC, Fossum S, Dissen E, Daws MR. Mincle, the receptor for mycobacterial cord factor, forms a functional receptor complex with MCL and FcepsilonRI-gamma. Eur J Immunol. 2013;43:3167–3174. doi: 10.1002/eji.201343752. [DOI] [PubMed] [Google Scholar]
- Martinez-Lopez M, Iborra S, Conde-Garrosa R, Sancho D. Batf3-dependent CD103+ dendritic cells are major producers of IL-12 that drive local Th1 immunity against Leishmania major infection in mice. Eur J Immunol. 2015;45:119–129. doi: 10.1002/eji.201444651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Tanaka T, Kaisho T, Sanjo H, Copeland NG, Gilbert DJ, Jenkins NA, Akira A novel LPS-inducible C-type lectin is a transcriptional target of NF-IL6 in macrophages. J Immunol. 1999;163:5039–5048. [PubMed] [Google Scholar]
- Miyake Y, Masatsugu OH, Yamasaki S. C-Type Lectin Receptor MCL Facilitates Mincle Expression and Signaling through Complex Formation. J Immunol. 2015;194:5366–5374. doi: 10.4049/jimmunol.1402429. [DOI] [PubMed] [Google Scholar]
- Miyake Y, Toyonaga K, Mori D, Kakuta S, Hoshino Y, Oyamada A, Yamada H, Ono K, Suyama M, Iwakura Y, et al. C-type lectin MCL is an FcRgamma-coupled receptor that mediates the adjuvanticity of mycobacterial cord factor. Immunity. 2013;38:1050–1062. doi: 10.1016/j.immuni.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Ng LG, Hsu A, Mandell MA, Roediger B, Hoeller C, Mrass P, Iparraguirre A, Cavanagh LL, Triccas JA, Beverley SM, et al. Migratory dermal dendritic cells act as rapid sensors of protozoan parasites. PLoS Pathog. 2008;4:e1000222. doi: 10.1371/journal.ppat.1000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagán AJ, Peters NC, Debrabant A, Ribeiro-Gomes F, Pepper M, Karp CL, Jenkins MK, Sacks DL. Tracking antigen -specific CD4 +T cells throughout the course of chronic Leishmania majorinfection in resistant mice. Eur J Immunol. 2013;43:427–438. doi: 10.1002/eji.201242715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquier B, Launay P, Kanamaru Y, Moura IC, Pfirsch S, Ruffié C, Hénin D, Benhamou M, Pretolani M, Blank U, Monteiro RC. Identification of FcalphaRI as an inhibitory receptor that controls inflammation: dual role of FcRgamma ITAM. Immunity. 2005;22:31–42. doi: 10.1016/j.immuni.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Peters N, Sacks D. Immune privilege in sites of chronic infection: Leishmania and regulatory T cells. Immunol Rev. 2006;213:159–179. doi: 10.1111/j.1600-065X.2006.00432.x. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Gomes FL, Peters NC, Debrabant A, Sacks DL. Efficient capture of infected neutrophils by dendritic cells in the skin inhibits the early anti-leishmania response. PLoS Pathog. 2012;8:e1002536. doi: 10.1371/journal.ppat.1002536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho D, Joffre OP, Keller AM, Rogers NC, Martínez D, Hernanz-Falcón P, Rosewell I, Reis e Sousa C. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458:899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho D, Reis e Sousa C. Signaling by Myeloid C-Type Lectin Receptors in Immunity and Homeostasis. Annu Rev Immunol. 2012;30:491–529. doi: 10.1146/annurev-immunol-031210-101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho D, Reis e Sousa C. Sensing of cell death by myeloid C-type lectin receptors. Curr Opin Immunol. 2013;25:46–52. doi: 10.1016/j.coi.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenen H, Bodendorfer B, Hitchens K, Manzanero S, Werninghaus K, Nimmerjahn F, Agger EM, Stenger S, Andersen P, Ruland J, et al. Cutting Edge: Mincle Is Essential for Recognition and Adjuvanticity of the Mycobacterial Cord Factor and its Synthetic Analog Trehalose-Dibehenate. J Immunol. 2010;184:2756–2760. doi: 10.4049/jimmunol.0904013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert L, Werba G, Tiwari S, Giao Ly NN, Alothman S, Alqunaibit D, Avanzi A, Barilla R, Daley D, Greco SH, et al. The necrosome promotes pancreatic oncogenesis via CXCL1 and Mincle-induced immune suppression. Nature. 2016;532:245–249. doi: 10.1038/nature17403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenderov K, Barber DL, Mayer-Barber KD, Gurcha SS, Jankovic D, Feng CG, Oland S, Hieny S, Caspar P, Yamasaki S, et al. Cord Factor and Peptidoglycan Recapitulate the Th17-Promoting Adjuvant Activity of Mycobacteria through Mincle/CARD9 Signaling and the Inflammasome. J Immunol. 2013;190:5722–5730. doi: 10.4049/jimmunol.1203343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa MdG, Reid DM, Schweighoffer E, Tybulewicz V, Ruland J, Langhorne J, Yamasaki S, Taylor PR, Almeida SR, Brown GD. Restoration of pattern recognition receptor costimulation to treat chromoblastomycosis, a chronic fungal infection of the skin. Cell Host Microbe. 2011;9:436–443. doi: 10.1016/j.chom.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastav S, Kar S, Chande AG, Mukhopadhyaya R, Das PK. Leishmania donovani exploits host deubiquitinating enzyme A20, a negative regulator of TLR signaling, to subvert host immune response. J Immunol. 2012;189:924–934. doi: 10.4049/jimmunol.1102845. [DOI] [PubMed] [Google Scholar]
- Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Walker PS, Scharton-Kersten T, Krieg AM, Love-Homan L, Rowton ED, Udey MC, Vogel JC. Immunostimulatory oligodeoxynucleotides promote protective immunity and provide systemic therapy for leishmaniasis via IL-12- and IFN-gamma-dependent mechanisms. Proc Natl Acad Sci USA. 1999;96:6970–6975. doi: 10.1073/pnas.96.12.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells CA, Salvage-Jones JA, Li X, Hitchens K, Butcher S, Murray RZ, Beckhouse AG, Lo Y-L-S, Manzanero S, Cobbold C, et al. The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J Immunol. 2008;180:7404–7413. doi: 10.4049/jimmunol.180.11.7404. [DOI] [PubMed] [Google Scholar]
- Wevers BA, Kaptein TM, Zijlstra-Willems EM, Theelen B, Boekhout T, Geijtenbeek TB, Gringhuis SI. Fungal engagement of the C-type lectin mincle suppresses dectin-1-induced antifungal immunity. Cell Host Microbe. 2014;15:494–505. doi: 10.1016/j.chom.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Woelbing F, Kostka SL, Moelle K, Belkaid Y, Sunderkoetter C, Verbeek S, Waisman A, Nigg AP, Knop J, Udey MC, von Stebut E. Uptake of Leishmania major by dendritic cells is mediated by Fcgamma receptors and facilitates acquisition of protective immunity. J Exp Med. 2006;203:177–188. doi: 10.1084/jem.20052288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuthrich M, Wang H, Li M, Lerksuthirat T, Hardison SE, Brown GD, Klein B. F. pedrosoi-induced Th17-cell differentiation in mice is fostered by Dectin-2 and suppressed by Mincle recognition. Eur J Immunol. 2015 doi: 10.1002/eji.201545591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol. 2008;9:1179–1188. doi: 10.1038/ni.1651. [DOI] [PubMed] [Google Scholar]
- Yamasaki S, Matsumoto M, Takeuchi O, Matsuzawa T, Ishikawa E, Sakuma M, Tateno H, Uno J, Hirabayashi J, Mikami Y, et al. C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. Proc Natl Acad Sci USA. 2009;106:1897–1902. doi: 10.1073/pnas.0805177106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdanbakhsh M, Sacks DL. Why does immunity to parasites take so long to develop? Nat Rev Immunol. 2010;10:80–81. doi: 10.1038/nri2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.