Abstract
Purpose of review
Spinal muscular atrophy (SMA) is an inherited childhood neurodegenerative disorder caused by ubiquitous deficiency of the survival motor neuron (SMN) protein—the hallmarks of which are the selective loss of motor neurons and skeletal muscle atrophy. Here we highlight recent progress in the understanding of SMA pathology and in the development of therapeutic approaches for its treatment.
Recent findings
Phenotypic characterization of mouse models of the disease, combined with analysis of SMN restoration or depletion in a spatially and temporally controlled manner, has yielded key insights into the normal requirement of SMN and SMA pathophysiology. Increasing evidence indicates a higher demand for SMN during neuromuscular development and extends the pathogenic effects of SMN deficiency beyond motor neurons to include additional cells both within and outside the nervous system. These findings have been paralleled by pre-clinical development of powerful approaches for increasing SMN expression through gene therapy or splicing modulation that are now in human trials.
Summary
Along with the availability of SMN-upregulating drugs, identification of the specific cell types in which SMN deficiency induces the disease and delineation of the window of opportunity for effective treatment are key advances in the ongoing path to SMA therapy.
Keywords: Motor neuron disease, SMA, SMN
Introduction
Spinal muscular atrophy (SMA), an autosomal recessive neurodegenerative disorder affecting 1 in about 10,000 newborns, is the leading hereditary cause of infant mortality [1,2]. SMA is characterized by the selective loss of motor neurons in the anterior horn of the spinal cord resulting in denervation, skeletal muscle atrophy and in severe cases, respiratory failure and death. In SMA patients, proximal muscles are preferentially affected and weakness is more pronounced in lower extremities. Based on the highest level of motor function achieved and age of disease onset, the wide spectrum of SMA phenotypes is classified into distinct clinical types spanning from the most frequent and severe type I SMA to the milder type IV form of the disease.
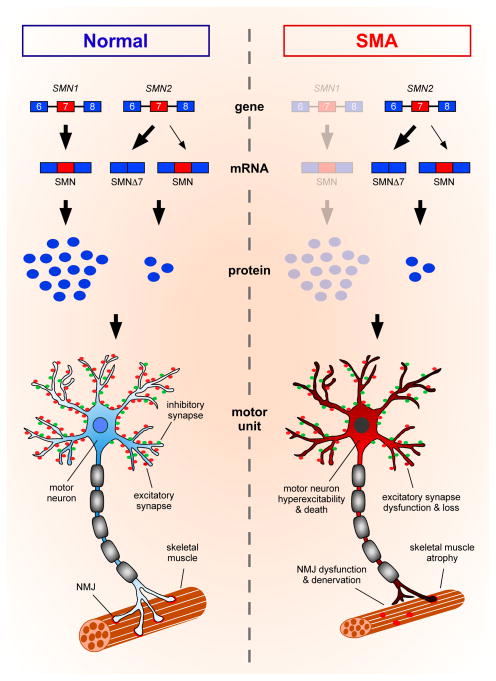
The homozygous deletion or mutation of the survival motor neuron 1 (SMN1) gene is the genetic cause of SMA [3]. Due to an evolutionarily recent duplication, two SMN genes (SMN1 and SMN2) exist on human chromosome 5q that only differ by five non-polymorphic nucleotide changes. One of these differences, a C to T transition located in exon 7, causes the majority of transcripts produced from SMN2 to lack exon 7 and produce a truncated, rapidly degraded form of the protein (Figure 1). While SMN2 produces sufficient protein to prevent embryonic lethality, it cannot fully compensate for the loss of SMN1, resulting in SMA [1,2]. Importantly, SMN2 copy number can vary among patients and serves as a critical modifier of SMA that inversely correlates with disease severity.
Figure 1. Genetic and cellular defects underlying motor system dysfunction in SMA.
SMA is the result of homozygous deletion or mutation of the SMN1 gene with retention of the nearly identical SMN2 gene. Most transcripts from SMN2 lack exon 7 due to alternative splicing and produce an unstable truncated form of the protein (SMNΔ7), leading to a setting of ubiquitous SMN deficiency in SMA. Motor neurons are particularly vulnerable to reduced levels of SMN expression and degenerate over the course of the disease. In addition to motor neuron death, several deficits that contribute to motor system dysfunction in SMA mouse models have been documented. These defects include: i) reduced density of synaptic vesicles, decreased neurotransmission, and increased denervation of the NMJs of vulnerable muscles; ii) reduced myofiber size and skeletal muscle atrophy; iii) defective excitatory neurotransmission and loss of glutamatergic synapses from proprioceptive neurons and local interneurons resulting in reduced excitatory drive; and iv) hyperexcitability of motor neurons.
In the two decades following the identification of SMN as the causative gene of SMA, significant advances have been made in defining key functions of SMN in the assembly of RNA-protein complexes and linking disruption of RNA processing induced by SMN deficiency with SMA pathogenesis [2,4]. This review will focus on advances in our understanding of the cellular basis of SMA from studies of mouse models and in the development of therapeutics now being tested in the clinic.
The requirement of SMN within and beyond the motor system
SMN is a ubiquitously expressed protein and both invertebrate and vertebrate model organisms possess a single SMN gene, ablation of which is non viable [1,2]. The development and characterization of animal models has fundamentally advanced our knowledge of SMA pathogenesis [5]. One key feature of the human disease is the expression of low steady levels of SMN from the SMN2 gene. To date, this has only been accomplished in mice, which therefore represent the most accurate model currently available [1,2,5]. Without accounting for specific differences of individual models (for a detailed description see [5]), the general design principle used to generate severe mouse models of SMA was to combine homozygous ablation of murine Smn alleles, which alone results in early embryonic lethality, with the introduction of two copies of the human SMN2 gene. One of the most widely studied mouse models of SMA, the SMNΔ7 mouse model, additionally contains multiple transgenic copies of the SMNΔ7 cDNA.
Severe SMA mice are phenotypically indistinguishable from wild type littermates at birth, but soon display severe motor deficits and reduced body weight, and do not survive past the second postnatal week [1,2,5]. Importantly, the development of Smn alleles for either conditional depletion or restoration of SMN in SMA mice allowed delineation of the temporal requirement of SMN during development and the capacity of SMN restoration to rescue phenotypic deficits before and after disease onset [6••,7]. Through the use of an inducible Smn allele in the SMNΔ7 background, post-symptomatic restoration of SMN at postnatal day 4 corrected motor system pathology and survival, while SMN induction at later time points resulted in limited rescue [7], indicating the existence of an early window for maximum therapeutic efficacy of SMN induction. Similar conclusions were reached using either a drug-inducible SMN transgene or adeno-associated virus serotype 9 (AAV9)-mediated SMN gene delivery [8,9]. The converse experiment using a conditional Smn knockout allele to induce ubiquitous SMN depletion in mice demonstrated that early postnatal SMN reduction rapidly elicits an SMA-like phenotype, while reduction after the second postnatal week fails to generate an overt phenotype [6••]. Thus, following maturation of the neuromuscular system, the threshold requirement for SMN decreases to a lower level that is maintained throughout adulthood.
As in the human disease, the phenotypic characterization of mouse models of SMA has indicated that motor neurons are at the most vulnerable end of the spectrum of cell types affected and that the predominant effect of SMN deficiency is pathology of the motor system [1,2]. However, increasing evidence also points to functional deficits outside the central nervous system (CNS) [10]. Recent insights into the spatial requirement of SMN from studies of central and peripheral deficits associated with its deficiency in mouse models are discussed below.
Motor system dysfunction in SMA
Severe SMA mice demonstrate a pronounced loss of motor neurons (Figure 1), a hallmark of the human disease [1,2]. Alpha motor neurons are selectively affected while spindle-innervating gamma motor neurons and cholinergic spinal interneurons are largely spared in the disease [11]. Importantly, there are also differential susceptibilities of individual motor neuron pools along the rostro-caudal and medio-lateral axis of the spinal cord correlating with the vulnerability of distinct muscle groups [12]. For example, loss of motor neurons innervating vulnerable axial muscles can be detected by postnatal day 4 in the L1 segment of the lumbar spinal cord. However, in the L5 segment, loss of medial motor neurons innervating proximal muscles begins at postnatal day 9, while lateral motor neurons innervating resistant distal muscles are spared [12]. SMN deficiency induces additional pathological changes in the motor circuit. Morphological and functional changes at the neuromuscular junctions (NMJs) include reduced synaptic vesicle density and neurotransmission as well as denervation, which occur at early stages of disease and are more prominent in vulnerable than resistant muscles, correlating with impaired muscle growth [13–17]. In addition to distal deficits, several central defects have been documented in SMA mice, including selective and progressive loss of excitatory but not inhibitory synapses on both the somata and dendrites of vulnerable motor neurons (Figure 1), which represent one of the earliest pathological manifestations of the disease [12,18]. Loss of these synapses and the associated reduction in the excitatory drive from proprioceptive neurons and glutamatergic interneurons is thought to underlie a homeostatic response within motor neurons leading to their increased membrane excitability [12].
A key aspect for understanding SMA pathology and designing appropriate therapeutic approaches is to determine the cellular site of action in which SMN deficiency elicits deleterious effects on the motor system. Fundamental advances to this regard came from studies employing conditional Smn alleles that could be turned on or off in a spatially restricted manner. The combination of both approaches, i.e. the analysis of both depletion and restoration of SMN in specific cell types, is required to reach solid conclusions as to the cell type-specific requirement of SMN in vivo. To date, this has been effectively accomplished only for two candidate cellular targets of SMA pathology, the muscle and the motor neuron. Interestingly, while muscle atrophy is an ultimate outcome in SMA, depletion of SMN restricted to muscle had no effect on the size, strength and function of skeletal muscle [19•]. Moreover, selective restoration of SMN in muscle of SMA mice failed to induce major phenotypic improvement beyond a modest increase in weight [19•,20,21], although one study also reported enhanced myofiber growth [21]. Together these studies indicate that intrinsic, SMN-dependent deficits in skeletal muscle do not significantly contribute to SMA pathology. Conversely, two prominent hallmarks of the disease—death of motor neurons and dysfunction and loss of NMJs—originate cell autonomously within the motor neuron. The targeted depletion of SMN in motor neurons elicited specific pathological features of the disease such as impaired motor behavior, decreased functional output at the NMJ, loss of motor neurons and reduced myofiber size [22•,23]. Furthermore, selective restoration of SMN in motor neurons of SMA mice rescued motor neuron survival and corrected both innervation and neurotransmission deficits at the NMJ [21,22•,24]. However, motor neuron-specific depletion of SMN failed to elicit a severe SMA phenotype and selective restoration in motor neurons did not have a robust impact on the survival of SMA mice, despite a measurable improvement of motor unit function, [21,22•,23,24], clearly pointing to a role for cells beyond motor neurons in SMA pathogenesis.
Motor neurons function in the context of neural circuits that include local interneurons, proprioceptive afferents and descending pathways from the brain. Consistent with the contribution of motor circuit dysfunction to SMA, pan-neuronal depletion of SMN was more effective in inducing an SMA-like phenotype in mice, and pan-neuronal SMN restoration in SMA mice led to robust phenotypic correction including strong extension of survival [20,22•]. Together with studies in a Drosophila model of the disease [25,26], these findings indicate that neuronal restoration of SMN is critical for ameliorating SMA-related deficits in the motor system and point to SMA as a disease of motor circuits, not just motor neurons alone. However, elucidating the role of specific neural circuit components in the dysfunction of the SMA motor system requires direct testing through selective depletion and restoration approaches in mouse models.
Despite clear involvement of neurons in the disease process, genetic restoration of SMN in all neurons was unable to fully rescue the SMA phenotype in mice nor was neuronal depletion able to mimic the disease to the extent accomplished using ubiquitous drivers [6,7,19•,22•], arguing for additional cellular contributors. Indeed, recent studies have documented a variety of additional deficits in the CNS that may influence the severity of SMA. Astrocytes have critical roles in regulating synaptic function as well as providing trophic support to neurons. AAV9-mediated expression of SMN driven by the Glial Fibrillary Acidic Protein promoter in the astrocytes of SMA mice enhanced motor function and body weight as well as extended survival [27•]. Interestingly, the phenotypic benefit was associated with reduced loss of central synapses and partial restoration of NMJ innervation, but no improvement of motor neuron survival. An increased number of unmyelinated motor axons in vulnerable intercostal nerves, but not dorsal corticospinal tract neurons, has also been observed in severe SMA mice at symptomatic stages [28], which is proposed to reflect intrinsic SMN-dependent deficits in Schwann cells [29]. Lastly, SMN deficiency is also associated with reduced capillary density and abnormal development of the vascular architecture in the spinal cord and skeletal muscle as well as evidence of hypoxia at mid- to late-symptomatic stages in SMA mouse models [30•], indicating vascular deficits as potential exacerbating factors. However, conclusive evidence that intrinsic SMN-dependent deficits in these cell types contribute to SMA pathology awaits in vivo analysis after selective depletion and restoration of SMN with genetic approaches.
Peripheral tissue defects in SMA
SMN-deficiency has been shown to induce defects in tissues beyond the motor system. Altered cardiac function (bradycardia and dilated cardiomyopathy) was among the first deficits outside of the CNS to be documented in SMA mice [10]. More recently, additional deficits identified in peripheral organs include alterations in the normal development of the pancreas, testis and gastrointestinal system. Studies in an intermediate SMA mouse model uncovered progressive loss of insulin-producing β cells and an increase in glucagon-producing α cells in the pancreatic islets, as well as defects in glucose metabolism upon fasting [31], which appear to occur independent of neuromuscular pathology [32]. A reduction in testis size and spermatogenesis associated with decreased fertility has also been documented in a mild mouse model [33], indicating a requirement for SMN in postnatal development of the male reproductive organ. Lastly, the enteric nervous system controlling smooth muscle of the colon also exhibits vulnerability to SMN deficiency resulting in gastrointestinal symptoms [34•]. Collectively, these findings indicate that restoration of SMN in the periphery may be important for complete correction of the SMA phenotype. The potential contribution of peripheral deficits to SMA pathology is supported by the observation that peripheral SMN restoration in the absence of a measurable SMN increase in the CNS robustly rescues SMA mice [35•,36].
To date, however, two main issues remain to be addressed: i) the precise cellular origin of the peripheral organ deficits and ii) the clinical relevance of these defects for the human disease, as evidence of peripheral organ dysfunction has often been anecdotal and limited to small cohorts of SMA patients at the most extreme spectrum of disease severity. Future studies geared towards answering these questions are required to define tissues that need to be directly targeted for SMA therapy and to exclude the possibility that peripheral defects represent confounding elements inherent to modeling disease in mice rather than clinically relevant features in humans.
Progress in the development of SMA therapeutics
While there is currently no established treatment for SMA, basic research has led to critical findings enabling translation into clinical approaches. Studies in mice have indicated that early restoration of SMN levels is an effective therapeutic approach and that widespread expression of SMN is preferable for robust rescue. Among several different strategies tested to date, two main approaches have successfully emerged from pre-clinical studies and moved to clinical trials (visit https://clinicaltrials.gov/ for additional information): SMN upregulation through either gene therapy or modulation of SMN2 splicing.
SMN gene therapy
One therapeutic approach for restoration of SMN levels is gene delivery with AAV9, which has been shown to efficiently transduce motor neurons and lead to phenotypic correction when delivered systemically or by intracerebroventricular (ICV) injection in neonatal SMA mice [9,37]. Further supporting the viability of gene therapy for treating SMA, viral-mediated delivery of human SMN was able to correct motor system pathology in a swine model of SMA induced by injection of an AAV vector driving RNAi-mediated knockdown of endogenous pig SMN [38•]. Additional pre-clinical studies demonstrated safety and tolerability of AAV9 gene delivery as well as proper targeting of motor neurons following intrathecal injection in non-human primates [39]. Encouraged by the results of the preclinical studies, AveXis is conducting an open-label, dose-escalating Phase 1 clinical trial (NCT02122952) to determine the safety, tolerability and efficacy of AAV9 mediated gene therapy in type I SMA patients following systemic administration.
SMN2 splicing modulation
Another targeted approach for SMA therapy is to increase SMN levels by modulating SMN2 splicing through antisense oligonucleotides (ASOs) designed to base pair to specific splicing regulatory sequences. While the efficacy of targeting other regulatory regions is also being explored [40], one optimal target for an ASO-based approach in SMA is the intronic splicing silencer element ISS-N1 located in intron 7 of SMN2, which can be blocked to promote exon 7 inclusion and restore SMN expression both in vitro and in vivo [36]. Delivery of 2′-O-methoxyethyl-modified or morpholino ASOs targeting ISS-N1 either systemically or by ICV injection led to remarkable improvement of both motor function and survival in severe SMA mice [36,41]. Intrathecal and ICV delivery in non-human primates was well-tolerated and provided further support to move this candidate approach into the clinic [42]. An open label Phase 1 trial using intrathecal delivery of Nusinersen—the ISS-N1 specific ASO developed by Ionis Pharmaceuticals in collaboration with Biogen—in type II and type III SMA patients demonstrated safety and increased motor function at the highest dosage [43]. An open label Phase 2 trial showed no safety concerns in type I SMA infants and that they lived longer and achieved more motor milestones than expected from the disease’s natural history. Two Phase 3 randomized double-blind studies are currently underway to assess efficacy and safety of intrathecal injection of Nusinersen in infants with early onset (NCT02193074) as well as in children with later-onset SMA (NCT02292537). Additional Phase 2 trials in pre-symptomatic SMA infants (NCT02386553) and an open access trial (NCT02462759) are ongoing.
In addition to ASO-mediated approaches, there has been tremendous recent progress in the development of small chemical compounds as a means to modulate SMN2 exon 7 splicing pharmacologically [2]. A series of orally bioavailable, blood-brain barrier-penetrant small molecules capable of promoting exon 7 splicing with high efficiency and selectivity have been developed through the concerted efforts of the SMA Foundation, PTC Therapeutics and Roche [44••]. Treatment of SMA mice with these compounds demonstrated remarkably robust phenotypic benefit, including enhanced motor function, weight gain and survival. This was associated with increased SMN expression, correction of SMN-dependent RNA processing deficits, and suppression of morphological defects in the motor system [44••,45]. Although the precise mechanism of action remains incompletely understood, these compounds corrected SMN2 splicing while globally altering the expression of only a handful of genes in human SMA fibroblasts [44••], reflecting an unprecedented selectivity for chemical modifiers of splicing. Along the same line, Novartis has developed another chemical modifier of SMN2 splicing with strong efficacy in SMA mice [46••]. Importantly, this compound appears to modulate splicing by stabilizing the binding of the U1 small nuclear ribonucleoprotein to the weak 5′ splice site of SMN2 exon 7 [46••]. Following the encouraging results of pre-clinical studies, both Roche and Novartis are now investigating the safety, tolerability, pharmacokinetics and pharmacodynamics of the SMN2 splicing modifiers in human trials. To date, Roche has conducted two randomized double-blind phase 1 trials in adult and pediatric SMA patients (NCT02240355) as well as in healthy volunteers (NCT02633709), while an open-label phase 1/2 study is being conducted by Novartis in type 1 SMA infants (NCT02268552).
Therapeutic complements to SMN upregulation
Despite the remarkable progress described above, SMA is still an incurable disease. SMN upregulating approaches are being tested in clinical trials, but they remain experimental strategies and it is unlikely that any of these alone will lead to a complete cure for SMA. In particular, SMN upregulation after disease onset might fall short of providing sufficient correction in SMA patients. Thus, it is conceivable that SMN-independent approaches designed to correct cellular deficits downstream of SMN deficiency within and outside the CNS might be particularly valuable for SMA treatment. To date, the only SMN-independent, candidate neuroprotective compound identified for SMA therapy is olesoxime—a cholesterol-like compound developed by Trophos that acts on mitochondrial membranes to promote neuronal survival and recovery from stress [47]. Olesoxime showed a beneficial effect on the maintenance of neuromuscular function in a pivotal phase 2 clinical trial in type II and non-ambulatory type III SMA patients (NCT01302600) and has been granted orphan drug designation by US and EU regulatory authorities. Roche is now conducting an open label, phase 2 study to evaluate long-term safety, tolerability, and effectiveness of olesoxime in SMA patients (NCT02628743).
Therapeutic application of any SMN-independent strategy focused on ameliorating a specific disease-relevant deficit would be most logically used in combination with SMN upregulating drugs as alone they may not have a strong clinical impact. To this end, two recent studies have set the stage for evaluation of combinatorial therapies by exploiting severe SMA mice treated with suboptimal doses of either ASO [48•] or SMN upregulating compounds [49•]. The resulting animals displayed incremental improvement with moderately extended survival and partially ameliorated motor system pathology, resembling pathological features of milder SMA forms. Interestingly, myostatin inhibition through AAV1-mediated follistatin gene delivery into skeletal muscle of SMA mice treated with suboptimal doses of an SMN-inducing compound improved muscle atrophy [49•]. These studies provided proof of concept for the utility of this approach to establish the therapeutic value of combinatorial treatments aimed to act synergistically with SMN enhancement. They also pointed to the potential benefit of muscle enhancing therapies in SMA. Accordingly, Cytokinetics is conducting a double-blind Phase 2 trial to investigate the efficacy of oral treatment with a troponin activator (CK-2127107) on skeletal muscle function or fatigability in types II-IV SMA patients (NCT02644668).
Conclusions
Much progress has been made in understanding SMA pathogenesis. It is becoming increasingly clear that deficits induced by SMN deficiency extend beyond the motor neuron to include neural networks of the motor circuit as well as additional cell types within and outside of the nervous system. However, the cell autonomy and clinical relevance of these deficits, especially those involving peripheral tissue, have yet to be conclusively established, and further insight is required to understand how they may inform therapeutic development. Moreover, many key steps between SMN dysfunction and the selective vulnerabilities associated with SMA pathology remain to be determined. In the future, closing this gap in knowledge will be fundamental not only to reach a comprehensive understanding of the disease but also to develop SMN-independent therapies that could complement current approaches for SMN upregulation. This will require determining the molecular and cellular cascade of events induced by SMN deficiency that contribute to the disease as well as targeted pharmacological approaches for their correction. As promising clinical trials are underway, continuing efforts in basic and translational SMA research are needed to ensure that the most effective and optimized treatments against this devastating disease can be established.
Key points.
Studies of conditional depletion or restoration of SMN in mouse models revealed a higher requirement for SMN during development of the neuromuscular system that coincides with the window of time in which SMN upregulation is most effective in correcting disease manifestations.
While classically defined as a pure motor neuron disease, increasing evidence implicates non-cell autonomous mechanisms in SMA pathology that extend beyond intrinsic defects induced by SMN deficiency in motor neurons to include dysfunction of neural networks of the motor circuit and additional deficits within the CNS as well as in peripheral tissue.
Extensive preclinical studies in animal models have identified gene therapy and modulation of SMN2 splicing as the most effective and promising therapeutic approaches for increasing SMN expression in SMA that are now being evaluated in clinical trials.
Future efforts aimed at advancing knowledge of the molecular and cellular basis of SMA will be important not only to provide key insight into disease mechanisms but also novel therapeutic avenues complementary to SMN upregulation.
Acknowledgments
We are grateful to Sarah Tisdale, Neil Shneider and Sergey Paushkin for critical reading and comments on this manuscript. We apologize to the authors in the field whose work could not be cited due to space limitations.
Financial support and sponsorship
Work in the L.P. laboratory was supported by grants from NIH-National Institute of Neurological Disorders and Stroke (NS069601, NS083831, and NS085612) and the Muscular Dystrophy Association (MDA382370).
Abbreviations
- SMA
spinal muscular atrophy
- SMN
survival motor neuron
- NMJ
neuromuscular junction
- AAV9
adeno-associated virus serotype 9
- ICV
intracerebroventricular
- ASO
antisense oligonucleotide
Footnotes
Conflicts of interest
None.
References and Recommended Reading
Papers of particular interest, published within the annual period of review, have been highlighted as: • of special interest, or •• of outstanding interest.
- 1.Burghes AHM, Beattie CE. Spinal muscular atrophy: why do low levels of survival motor neuron protein make motor neurons sick? Nat Rev Neurosci. 2009;10:597–609. doi: 10.1038/nrn2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tisdale S, Pellizzoni L. Disease Mechanisms and Therapeutic Approaches in Spinal Muscular Atrophy. J Neurosci. 2015;35:8691–8700. doi: 10.1523/JNEUROSCI.0417-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lefebvre S, Burglen L, Reboullet S, et al. Identification and characterization of a spinal muscular atrophy- determining gene. Cell. 1995;80:155–165. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- 4.Li DK, Tisdale S, Lotti F, Pellizzoni L. SMN control of RNP assembly: from post-transcriptional gene regulation to motor neuron disease. Semin Cell Dev Biol. 2014;32:22–29. doi: 10.1016/j.semcdb.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmid A, DiDonato CJ. Animal models of spinal muscular atrophy. J Child Neurol. 2007;22:1004–1012. doi: 10.1177/0883073807305667. [DOI] [PubMed] [Google Scholar]
- 6••.Kariya S, Obis T, Garone C, et al. Requirement of enhanced Survival Motoneuron protein imposed during neuromuscular junction maturation. J Clin Invest. 2014;124:785–800. doi: 10.1172/JCI72017. This study determined the temporal requirement for SMN by investigating the phenotypic effects of conditional depletion of murine Smn at distinct time points during postnatal mouse development. Remarkably, while reducing SMN to low levels in neonatal mice induces a severe SMA phenotype, a similar reduction in adults has little if any effect and the refractory phase coincides with completion of neuromuscular development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lutz CM, Kariya S, Patruni S, et al. Postsymptomatic restoration of SMN rescues the disease phenotype in a mouse model of severe spinal muscular atrophy. J Clin Invest. 2011;121:3029–3041. doi: 10.1172/JCI57291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le TT, McGovern VL, Alwine IE, et al. Temporal requirement for high SMN expression in SMA mice. Hum Mol Genet. 2011;20:3578–3591. doi: 10.1093/hmg/ddr275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robbins KL, Glascock JJ, Osman EY, et al. Defining the therapeutic window in a severe animal model of spinal muscular atrophy. Hum Mol Genet. 2014;23:4559–4568. doi: 10.1093/hmg/ddu169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamilton G, Gillingwater TH. Spinal muscular atrophy: going beyond the motor neuron. Trends Mol Med. 2013;19:40–50. doi: 10.1016/j.molmed.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Powis RA, Gillingwater TH. Selective loss of alpha motor neurons with sparing of gamma motor neurons and spinal cord cholinergic neurons in a mouse model of spinal muscular atrophy. J Anat. 2016;228:443–451. doi: 10.1111/joa.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mentis GZ, Blivis D, Liu W, et al. Early Functional Impairment of Sensory-Motor Connectivity in a Mouse Model of Spinal Muscular Atrophy. Neuron. 2011;69:453–467. doi: 10.1016/j.neuron.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnold W, McGovern VL, Sanchez B, et al. The neuromuscular impact of symptomatic SMN restoration in a mouse model of spinal muscular atrophy. Neurobiol Dis. 2016;87:116–123. doi: 10.1016/j.nbd.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kariya S, Park GH, Maeno-Hikichi Y, et al. Reduced SMN protein impairs maturation of the neuromuscular junctions in mouse models of spinal muscular atrophy. Hum Mol Genet. 2008;17:2552–2569. doi: 10.1093/hmg/ddn156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong L, Wang X, Choe DW, et al. Impaired synaptic vesicle release and immaturity of neuromuscular junctions in spinal muscular atrophy mice. J Neurosci. 2009;29:842–851. doi: 10.1523/JNEUROSCI.4434-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ling KKY, Gibbs RM, Feng Z, Ko C-P. Severe neuromuscular denervation of clinically relevant muscles in a mouse model of spinal muscular atrophy. Hum Mol Genet. 2012;21:185–195. doi: 10.1093/hmg/ddr453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray LM, Comley LH, Thomson D, et al. Selective vulnerability of motor neurons and dissociation of pre- and post-synaptic pathology at the neuromuscular junction in mouse models of spinal muscular atrophy. Hum Mol Genet. 2008;17:949–962. doi: 10.1093/hmg/ddm367. [DOI] [PubMed] [Google Scholar]
- 18.Ling KKY, Lin M-Y, Zingg B, et al. Synaptic defects in the spinal and neuromuscular circuitry in a mouse model of spinal muscular atrophy. PloS One. 2010;5:e15457–e15457. doi: 10.1371/journal.pone.0015457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19•.Iyer CC, McGovern VL, Murray JD, et al. Low levels of Survival Motor Neuron protein are sufficient for normal muscle function in the SMNΔ7 mouse model of SMA. Hum Mol Genet. 2015;24:6160–6173. doi: 10.1093/hmg/ddv332. This study analyzed transgenic mice in which depletion or restoration of SMN was selectively targeted to skeletal muscle and showed that intrinsic effects of SMN deficiency in muscle are not key contributors to the SMA phenotype. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gavrilina TO, McGovern VL, Workman E, et al. Neuronal SMN expression corrects spinal muscular atrophy in severe SMA mice while muscle-specific SMN expression has no phenotypic effect. Hum Mol Genet. 2008;17:1063–1075. doi: 10.1093/hmg/ddm379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez TL, Kong L, Wang X, et al. Survival motor neuron protein in motor neurons determines synaptic integrity in spinal muscular atrophy. J Neurosci. 2012;32:8703–8715. doi: 10.1523/JNEUROSCI.0204-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.McGovern VL, Iyer CC, Arnold WD, et al. SMN expression is required in motor neurons to rescue electrophysiological deficits in the SMNΔ7 mouse model of SMA. Hum Mol Genet. 2015;24:5524–5541. doi: 10.1093/hmg/ddv283. Through restoration or depletion of SMN in select CNS cell types and consistent with other studies [20,21,23,24], this work showed that SMN is required cell autonomously in motor neurons for proper function of the motor unit. Importantly, it also showed that high levels of SMN in other neurons and glial cells, in addition to motor neurons, are essential to exert a sustained phenotypic impact and extend survival in SMA mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park G-H, Maeno-Hikichi Y, Awano T, et al. Reduced survival of motor neuron (SMN) protein in motor neuronal progenitors functions cell autonomously to cause spinal muscular atrophy in model mice expressing the human centromeric (SMN2) gene. J Neurosci. 2010;30:12005–12019. doi: 10.1523/JNEUROSCI.2208-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gogliotti RG, Quinlan KA, Barlow CB, et al. Motor neuron rescue in spinal muscular atrophy mice demonstrates that sensory-motor defects are a consequence, not a cause, of motor neuron dysfunction. J Neurosci. 2012;32:3818–3829. doi: 10.1523/JNEUROSCI.5775-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imlach WL, Beck ES, Choi BJ, et al. SMN is required for sensory-motor circuit function in Drosophila. Cell. 2012;151:427–439. doi: 10.1016/j.cell.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lotti F, Imlach WL, Saieva L, et al. An SMN-dependent U12 splicing event essential for motor circuit function. Cell. 2012;151:440–454. doi: 10.1016/j.cell.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Rindt H, Feng Z, Mazzasette C, et al. Astrocytes influence the severity of spinal muscular atrophy. Hum Mol Genet. 2015;24:4094–4102. doi: 10.1093/hmg/ddv148. This study identified a non-neuronal deficit in the CNS through the characterization of phenotypic improvement of a severe and mild SMA mouse model following the viral-mediated targeted restoration of SMN in astrocytes, suggesting glial cells contribute to SMA pathology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.Hunter G, Aghamaleky Sarvestany A, Roche SL, et al. SMN-dependent intrinsic defects in Schwann cells in mouse models of spinal muscular atrophy. Hum Mol Genet. 2014;23:2235–2250. doi: 10.1093/hmg/ddt612. This study described myelination abnormalities in select peripheral nerves of SMA mice and implicates dysfunction of Schwann cells induced by SMN deficiency in SMA pathology. [DOI] [PubMed] [Google Scholar]
- 29.Hunter G, Powis RA, Jones RA, et al. Restoration of SMN in Schwann cells reverses myelination defects and improves neuromuscular function in spinal muscular atrophy. Hum Mol Genet. 2016 doi: 10.1093/hmg/ddw141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30•.Somers E, Lees RD, Hoban K, et al. Vascular Defects and Spinal Cord Hypoxia in Spinal Muscular Atrophy. Ann Neurol. 2016;79:217–230. doi: 10.1002/ana.24549. This study described features of abnormal vascular development and hypoxia in the spinal cord of SMA mice as well as in muscle from both SMA patients and mouse models that may contribute to the disease process. [DOI] [PubMed] [Google Scholar]
- 31.Bowerman M, Swoboda KJ, Michalski JP, et al. Glucose metabolism and pancreatic defects in spinal muscular atrophy. Ann Neurol. 2012;72:256–268. doi: 10.1002/ana.23582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bowerman M, Michalski JP, Beauvais A, et al. Defects in pancreatic development and glucose metabolism in SMN-depleted mice independent of canonical spinal muscular atrophy neuromuscular pathology. Hum Mol Genet. 2014;23:3432–3444. doi: 10.1093/hmg/ddu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ottesen EW, Howell MD, Singh NN, et al. Severe impairment of male reproductive organ development in a low SMN expressing mouse model of spinal muscular atrophy. Sci Rep. 2016;6:20193–20193. doi: 10.1038/srep20193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Gombash SE, Cowley CJ, Fitzgerald JA, et al. SMN deficiency disrupts gastrointestinal and enteric nervous system function in mice. Hum Mol Genet. 2015;24:3847–3860. doi: 10.1093/hmg/ddv127. This study investigated the phenotypic effects of SMN reduction in the enteric nervous system of SMA mice revealing gastrointestinal deficits—a feature also observed in human patients— that are associated with intrinsic alterations in the neuronal control of gut functions but not neuronal loss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Hua Y, Liu YH, Sahashi K, et al. Motor neuron cell-nonautonomous rescue of spinal muscular atrophy phenotypes in mild and severe transgenic mouse models. Genes Dev. 2015;29:288–297. doi: 10.1101/gad.256644.114. Expanding on earlier findings from the same group [36], this study employed ASO-mediated approaches to address the impact of SMN restoration in the CNS relative to peripheral tissue on the SMA phenotype. Remarkably, upregulation of SMN in the periphery without a significant SMN increase in the CNS induces strong phenotypic benefit and robust lifespan extension in severe SMA mice, thereby linking peripheral pathology to reduced survival in a severe model of the disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hua Y, Sahashi K, Rigo F, et al. Peripheral SMN restoration is essential for long-term rescue of a severe spinal muscular atrophy mouse model. Nature. 2011;478:123–126. doi: 10.1038/nature10485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foust KD, Wang X, McGovern VL, et al. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat Biotechnol. 2010;28:271–274. doi: 10.1038/nbt.1610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38•.Duque SI, Arnold WD, Odermatt P, et al. A large animal model of spinal muscular atrophy and correction of phenotype. Ann Neurol. 2015;77:399–414. doi: 10.1002/ana.24332. This study used intrathecal injection of an AAV9 vector expressing an shRNA against SMN1 in piglets to establish the first large animal model of SMA and to demonstrate effective phenotypic correction by SMN gene delivery. Postnatal reduction of SMN in pigs induced SMA-like pathology including proximal weakness, muscle denervation and death of motor neurons, which could be robustly corrected by AAV9-SMN gene therapy not only pre-symptomatically but also after onset of symptoms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyer K, Ferraiuolo L, Schmelzer L, et al. Improving single injection CSF delivery of AAV9-mediated gene therapy for SMA: a dose-response study in mice and nonhuman primates. Mol Ther. 2015;23:477–487. doi: 10.1038/mt.2014.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osman EY, Miller MR, Robbins KL, et al. Morpholino antisense oligonucleotides targeting intronic repressor Element1 improve phenotype in SMA mouse models. Hum Mol Genet. 2014;23:4832–4845. doi: 10.1093/hmg/ddu198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Porensky PN, Mitrpant C, McGovern VL, et al. A single administration of morpholino antisense oligomer rescues spinal muscular atrophy in mouse. Hum Mol Genet. 2012;21:1625–1638. doi: 10.1093/hmg/ddr600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rigo F, Chun SJ, Norris DA, et al. Pharmacology of a central nervous system delivered 2′-O-methoxyethyl-modified survival of motor neuron splicing oligonucleotide in mice and nonhuman primates. J Pharmacol Exp Ther. 2014;350:46–55. doi: 10.1124/jpet.113.212407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chiriboga CA, Swoboda KJ, Darras BT, et al. Results from a phase 1 study of nusinersen (ISIS-SMNRx) in children with spinal muscular atrophy. Neurology. 2016;86:890–897. doi: 10.1212/WNL.0000000000002445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44••.Naryshkin NA, Weetall M, Dakka A, et al. Motor neuron disease. SMN2 splicing modifiers improve motor function and longevity in mice with spinal muscular atrophy. Science. 2014;345:688–693. doi: 10.1126/science.1250127. This study reported the discovery of the first orally bioavailable, CNS permeable, and highly selective small chemical modifiers of SMN2 splicing that strongly promote exon 7 inclusion both in vitro and in vivo. Importantly, these compounds induce SMN upregulation and the robust phenotypic correction of SMA pathology in mice, pointing to pharmacological correction of SMN2 splicing as a promising approach for the treatment of SMA. [DOI] [PubMed] [Google Scholar]
- 45.Zhao X, Feng Z, Ling KKY, et al. Pharmacokinetics, pharmacodynamics, and efficacy of a small-molecule SMN2 splicing modifier in mouse models of spinal muscular atrophy. Hum Mol Genet. 2016 doi: 10.1093/hmg/ddw062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46••.Palacino J, Swalley SE, Song C, et al. SMN2 splice modulators enhance U1-pre-mRNA association and rescue SMA mice. Nat Chem Biol. 2015;11:511–517. doi: 10.1038/nchembio.1837. This study identified a novel small molecule that potently enhances SMN2 splicing in SMA mice and described its mechanism of action through sequence-specific stabilization of the RNA duplex structure formed by U1 snRNP with the 5′ splice site of SMN2 exon 7, highlighting the therapeutic potential of selective splicing modulation with small molecules. [DOI] [PubMed] [Google Scholar]
- 47.Sunyach C, Michaud M, Arnoux T, et al. Olesoxime delays muscle denervation, astrogliosis, microglial activation and motoneuron death in an ALS mouse model. Neuropharmacology. 2012;62:2346–2352. doi: 10.1016/j.neuropharm.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 48•.Zhou H, Meng J, Marrosu E, et al. Repeated low doses of morpholino antisense oligomer: an intermediate mouse model of spinal muscular atrophy to explore the window of therapeutic response. Hum Mol Genet. 2015;24:6265–6277. doi: 10.1093/hmg/ddv329. This study established a mouse model with an intermediate SMA phenotype that mimics milder forms of the human disease by treating severe SMA mice with low doses of morpholino oligonucleotides that enhance SMN2 splicing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49•.Feng Z, Ling KKY, Zhao X, et al. Pharmacologically induced mouse model of adult spinal muscular atrophy to evaluate effectiveness of therapeutics after disease onset. Hum Mol Genet. 2016;25:964–975. doi: 10.1093/hmg/ddv629. This study employed pharmacological treatment of severe SMA mice with suboptimal doses of an SMN2 splicing modifier to generate a mouse model of later-onset SMA. This model was then used to demonstrate the therapeutic efficacy of post-symptomatic upregulation of SMN or muscle-enhancing inhibition of myostatin in a context more akin to that of patients with type II and III SMA forms. [DOI] [PubMed] [Google Scholar]