Abstract
Familial dysautonomia (FD) is a rare genetic disease with extremely labile blood pressure due to baroreflex deafferentation. Patients have marked surges in sympathetic activity, frequently surrounding meals. We conducted an observational study to document the autonomic responses to eating in patients with FD, and to determine whether sympathetic activation was caused by chewing, swallowing or stomach distension.
Blood pressure and RR intervals were measured continuously while chewing gum (n= 15), swallowing food (n=20) and distending the stomach with a gastrostomy feed (n=9). Responses were compared to those of normal controls (n=10) and of patients with autonomic failure (n=10) who have chronically impaired sympathetic outflow.
In patients with FD, swallowing food was associated with a marked, but transient pressor response (p<0.0001) and additional signs of sympathetic activation including tachycardia, diaphoresis and flushing of the skin. Chewing gum evoked a similar increase in blood pressure that was higher in patients with FD than in controls (p=0.0001), but was absent in patients with autonomic failure. In patients with FD distending the stomach with a gastrostomy feed failed to elicit a pressor response.
The results provide indirect evidence that chewing triggers sympathetic activation. The increase in blood pressure that is exaggerated in patients with FD due to blunted afferent baroreceptor signalling. The chewing pressor response may be useful as a counter-manoeuvre to raise blood pressure and prevent symptomatic orthostatic hypotension in patients with FD.
Keywords: afferent baroreflex failure, familial dysautonomia, autonomic nervous system
Introduction
Familial dysautonomia (FD, OMIM #223900) is a rare autosomal recessive disease caused by a deficiency of the elongator 1 protein (ELP1/IKAP) (Slaugenhaupt et al., 2001). This results in a developmental defect affecting mostly primary sensory neurones (Macefield et al., 2011; Gutierrez et al., 2015), including those of the afferent baroreceptor pathways (Figure 1) (Norcliffe-Kaufmann et al., 2010). Efferent sympathetic neurones are reduced in number, but functionally intact and activated with particular emotional or physical stimuli (Norcliffe-Kaufmann et al., 2013a; Norcliffe-Kaufmann et al., 2013c). Without the normal baroreflex restraint of sympathetic outflow, (Macefield et al., 2012) blood pressure becomes extremely labile (Norcliffe-Kaufmann et al., 2011).
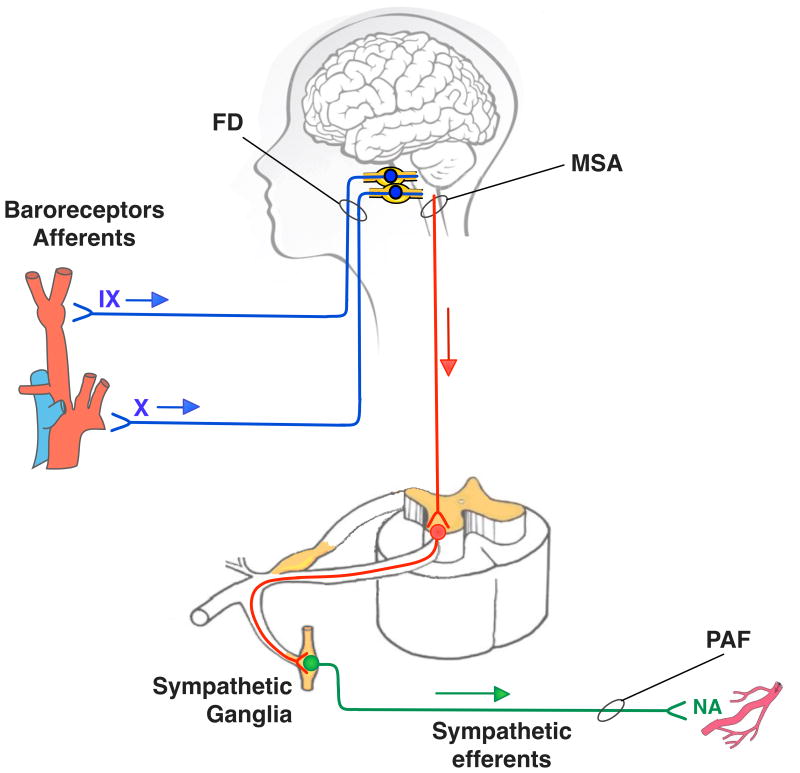
Figure 1.
Schematic showing lesions in neural autonomic pathways in familial dysautonomia (FD), pure autonomic failure (PAF) and multiple system atrophy (MSA). Afferent baroreceptor neurones are shown in blue, central neurones in red and efferent sympathetic neurones in green. Patients with FD have a selective deficit in afferent pathways relaying information from the arterial baroreceptors to the CNS (Norcliffe-Kaufmann et al., 2010). PAF affects peripheral efferent sympathetic fibres (Hague et al., 1997). MSA affects mostly neurones in the central autonomic network preventing descending spinal pathways from activating efferent sympathetic neurones (Tellez et al., 2009).
Patients with FD also have neurogenic dysphagia due to abnormal trigeminal brainstem reflexes. Newborns with FD are unable to coordinate swallowing (Geltzer et al., 1964) and when attempting to bottle-feed, infants show noticeable signs of sympathetic activation, becoming sweaty and mottled (Perlman et al., 1979). Feeding difficulties remain a life long problem (Axelrod et al., 1982; Axelrod et al., 1991) and aspiration pneumonia is still a leading cause of death in FD patients (Axelrod et al., 2002). Eighty-percent of patients eventually require a gastrostomy tube to ensure adequate caloric intake and gain weight (Dysautonomia Center Statistics).
Our goal was to document the autonomic responses to eating in patients with FD, and to determine whether chewing, swallowing or stomach distension triggered sympathetic activation. Thus, we examined blood pressure and heart rate changes while chewing gum, swallowing food and distending the stomach with a gastrostomy-tube feed in patients with FD. Responses were compared to those of normal controls and of patients with autonomic failure who have chronically impaired sympathetic outflow.
Materials and Methods
Ethical approval
All study procedures were approved by the local Institutional Review Board of New York University School of Medicine. Participants gave informed written consent prior to participate or parental/guardian assent in the case of minors, with the understanding that they were free to withdraw consent at anytime. Procedures were performed in accordance with the standards of the Declaration of Helsinki. All measurements were non-invasive and carried out by trained clinical investigators carried out all procedures with medical staff supervision. All data was de-identified. No participants received financial compensation and their involvement in the study.
Subjects
Between February 2014 and March 2015, we studied 34 patients with FD (Table 1), 10 healthy controls (age 32±4 years, 5 males: 5 females) and 10 patients with chronic autonomic failure (7 with pure autonomic failure, 3 with multiple system atrophy, age 67±3, 7 males: 3 females). Patients were recruited from those registered at the Dysautonomia Center of New York University. Diagnosis of FD was based on molecular confirmation of the ikbkap gene mutation(Slaugenhaupt et al., 2001) and all patients had typical clinical features of the disease. The diagnosis of autonomic failure was based on established consensus criteria (Gilman et al., 2008; Freeman et al., 2011). Controls were volunteers that were not scheduled to undergo autonomic testing for any other reason than this study.
Table 1.
The characteristics of the FD subjects participating in the study and changes in systolic blood pressure (SBP) and heart rate (HR) during the three interventions: eating, PEG-tube feeding and chewing gum.
| ID | Age | ΔSBP eating | ΔSBP tube | ΔSBP chewing | ΔHR eating | ΔHR tube | ΔHR chewing | Gender | Eating | PEG-tube | Chewing |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 42 | 50 | - | - | 19 | - | - | F | YES | NO | NO |
| 2 | 25 | 8 | - | - | 16 | - | - | F | YES | NO | NO |
| 3 | 29 | 79 | - | - | 28 | - | - | F | YES | NO | NO |
| 4 | 32 | 22 | - | - | 3 | - | - | M | YES | NO | NO |
| 5 | 25 | 67 | - | - | 14 | - | - | F | YES | NO | NO |
| 6 | 33 | 87 | - | - | 17 | - | - | F | YES | NO | NO |
| 7 | 36 | 57 | - | 60 | 28 | - | 14 | M | YES | NO | YES |
| 8 | 16 | 25 | - | - | 10 | - | - | M | YES | NO | NO |
| 9 | 23 | -6 | - | 26 | -1 | - | -3 | F | YES | NO | YES |
| 10 | 18 | 36 | - | 50 | 28 | - | 20 | F | YES | NO | YES |
| 11 | 17 | 32 | - | 14 | 5 | - | 8 | F | YES | NO | YES |
| 12 | 5 | 60 | - | - | 60 | - | - | M | YES | NO | NO |
| 13 | 19 | 11 | - | - | 6 | - | - | F | YES | NO | YES |
| 14 | 38 | 41 | - | 8 | 17 | - | 5 | F | YES | NO | NO |
| 15 | 14 | 53 | - | - | 36 | - | - | F | YES | NO | NO |
| 16 | 14 | 6 | - | - | 2 | - | - | F | YES | NO | NO |
| 17 | 5 | 36 | 2 | - | 23 | -9 | - | F | YES | YES | NO |
| 18 | 18 | 26 | -19 | - | 20 | 6 | - | M | YES | YES | NO |
| 19 | 20 | 53 | 23 | 35 | 37 | 7 | 3 | F | YES | YES | YES |
| 20 | 23 | 31 | 7 | - | 17 | -1 | - | F | YES | YES | NO |
| 21 | 23 | - | 4 | - | - | -15 | - | M | NO | YES | NO |
| 22 | 26 | - | 15 | - | - | 5 | - | M | NO | YES | NO |
| 23 | 19 | - | 23 | - | - | -4 | - | M | NO | YES | NO |
| 24 | 23 | - | 6 | - | - | -3 | - | M | NO | YES | NO |
| 25 | 30 | - | 8 | - | - | 6 | - | M | NO | YES | NO |
| 26 | 35 | - | - | 47 | - | - | 11 | F | NO | NO | YES |
| 27 | 21 | - | - | 51 | - | - | 14 | F | NO | NO | YES |
| 28 | 42 | - | - | 17 | - | - | 0 | M | NO | NO | YES |
| 29 | 57 | - | - | 11 | - | - | 0 | F | NO | NO | YES |
| 30 | 37 | - | - | 19 | - | - | 17 | F | NO | NO | YES |
| 31 | 22 | - | - | 14 | - | - | 11 | F | NO | NO | YES |
| 32 | 51 | - | - | 28 | - | - | 8 | M | NO | NO | YES |
| 33 | 17 | - | - | 38 | - | - | -4 | F | NO | NO | YES |
| 34 | 39 | - | - | 25 | - | - | 9 | F | NO | NO | YES |
| Mean | 26 | 39 | 8 | 30 | 19 | -1 | 8 | Females:22 Males:12 | N=20 | N=9 | N=15 |
| SD | 12 | 25 | 13 | 16 | 14 | 8 | 7 |
Experimental protocol
Clonidine and benzodiazepines were tapered and discontinued prior to testing in the patients with FD, and midodrine was withheld in all patients. Subjects were instructed not to take caffeine, nicotine and alcohol from the night before and not to eat for 3 hours before the visit. Evaluations were carried out in the morning. All measurements were acquired in a quiet, temperature-controlled environment by trained staff. Patients were seated throughout.
When the patient appeared relaxed and calm, baseline blood pressure and RR intervals were recorded for 5 minutes in the seated position. To avoid the risk of aspiration, patients with FD were assigned to receive either a gastrostomy feed (n=9) or eat an oral snack (n=20) depending on how they would safely feed at home. Because of the different severities of neurogenic dysphagia, it was not possible to standardize the meals. Thus, patients taking food orally ate a snack of their choice with a consistency that was for them “safe to swallow” (supplementary table). Cardiovascular data were recorded during and after the meal for at least 30 minutes.
To investigate the haemodynamic responses to chewing, we measured blood pressure and RR intervals when chewing gum in patients with FD (n=15), autonomic failure (n=10) and controls (n=10). After instrumentation, participants rested in the seated position until a stable baseline was established. Autonomic measures were recorded for 5-minutes. Participants were given two pieces (1.5 g) of sugar-free peppermint flavoured chewing gum (sweetened with aspartame) and were verbally coached to chew forcefully for 5 minutes without swallowing while being monitored.
Measurements
Continuous blood pressure was measured using finger plethysmography (Ohmeda 2300 Finapres Medical Systems BV, Amsterdam, the Netherlands) with the hand supported at heart level in a sling. RR intervals were measured using surface electrodes (Covidien, Mansfield, MA). Intermittent blood pressure measurements were measured in the left arm using an automatic sphygmomanometer (Colin Press-Mate 7800, San Antonio, TX). Signals were acquired throughout digitally converted, sampled and analysed off-line (PowerLab, AD Instruments, USA).
Statistical analysis
Intermittent blood pressure values pre-intervention were averaged over the 5 minutes baseline. Hemodynamic responses were assessed as the peak change from baseline during feeding. To quantify the pressor response, area under the curve (AUC) was calculated as percentage change from baseline during the first five minutes of eating, gastrostomy feeding or chewing for each patient. Data were first tested for normality using the Shapiro-Wilk test (Prism, GraphPad, USA). Paired t-tests were used when appropriate to compare responses within groups. Differences between groups and interventions were compared using ANOVA. Tukey's HSD post hoc test was then applied to identify significant differences between groups. Pearson's correlation coefficient was used to establish if there was a linear correlation between jaw-jerk brainstem reflexes (as a measure of trigeminal afferent signalling from the jaw) or UPSIT scores (ie, olfactory function) and maximum systolic blood pressure reach while eating or chewing. Significance was set at p ≤ 0.05. All data are expressed as mean±SEM, unless otherwise stated.
Results
Patient characteristics
All patients with FD had typical neurological findings including indifference to pain and temperature and absent deep tendon reflexes. All had neurogenic dysphagia and documented histories of aspiration pneumonias in their medical records. All had characteristic features of afferent baroreflex failure, with documented hypotension on upright tilt as a result of the failure to release noradrenaline appropriately. All had paroxysmal hypertension in response to emotional and cognitive stresses. Ambulatory recordings were available in all patients and showed exaggerated blood pressure and heart rate lability.
All patients with autonomic failure complained of symptoms consistent with orthostatic hypotension-induced organ perfusion (Kaufmann et al., 2012). All met consensus criteria for orthostatic hypotension on upright tilt and had a blunted increase in heart rate (Freeman et al., 2011). Average resting systolic BP while supine was 151±5 mmHg and decreased by 55±5 mmHg after 3 minutes of tilt (SBP: 96±7 mmHg). Phase IV overshoot in blood pressure after release of the Valsalva strain was absent in all cases. Based on neurological examination and clinical consensus (JAP, HK), 7 were diagnosed with pure autonomic failure and 3 with multiple system atrophy.
Controls were all healthy, free of medication and were normotensive in the seated position (systolic BP: 120±4 mmHg).
Autonomic responses to food intake in FD
Swallowing food
Of the 34 patients with FD studied, 20 had less severe dysphagia and were able to eat by mouth (Table 1). Details of what the patients ate are listed in the supplementary table. As shown in Table 2, despite the patient appearing relaxed, one third of the patients (7/20) were hypertensive at rest with systolic blood pressures >140 mmHg (Chobanian et al., 2003). Seated blood pressure was on average 128/65 mmHg, this is typical for these patients. Sixty-percent reported having in the past experienced skin flushing and sweating whilst eating by mouth.
Table 2. Baseline and peak responses to chewing, swallowing and stomach distension in patients with FD.
| Condition | Parameter | Mean baseline | SD | Feeding maximum | SD | p-value |
|---|---|---|---|---|---|---|
| Eating (n=20) | SBP (mmHg) | 128 | 20 | 166 | 36 | <0.0001 |
| HR (bpm) | 81 | 10 | 101 | 13 | <0.0001 | |
| Tube (n=9) | SBP (mmHg) | 117 | 19 | 118 | 23 | 0.8651 |
| HR (bpm) | 75 | 13 | 75 | 9 | 0.7371 | |
| Chewing (n=15) | SBP (mmHg) | 108 | 22 | 139 | 21 | <0.0001 |
| HR (bpm) | 76 | 12 | 84 | 13 | 0.0005 |
SBP; systolic blood pressure, HR; heart rate, SD, standard deviation. Pre- and post comparison made with paired student-t-tests.
As showing in Figure 2, on eating systolic blood pressure increased abruptly by +39±6 mmHg and heart rate by +20±3 bpm (p<0.0001 and p<0.0001). Changes in diastolic pressure were similar (Figure 3). In addition to hypertension and tachycardia, 40% of patients showed other signs of sympathetic activation including skin flushing and diaphoresis. Patients were also short of breath. The increase in blood pressure and heart rate was transient and subsided immediately after the subject finished eating (Figure 3). The pressor response was not greater when eating food with a hard consistency (p=0.8711, Figure 3).
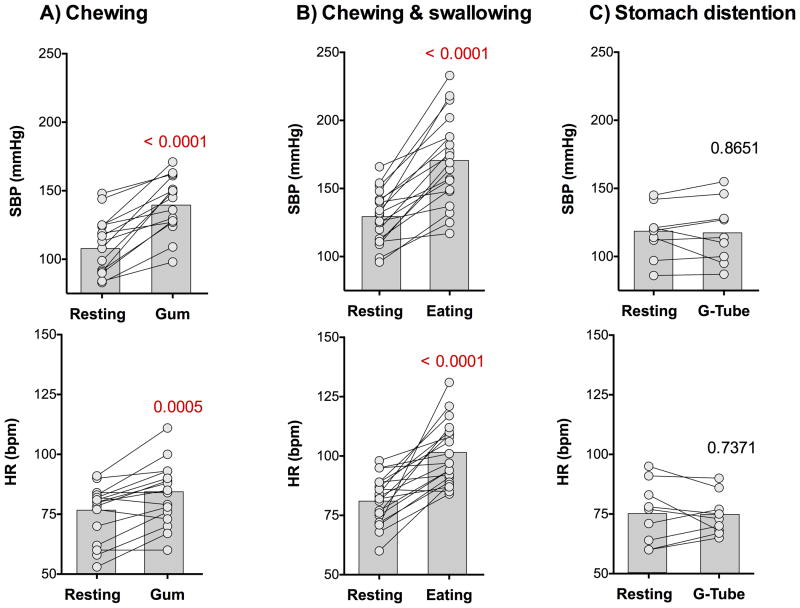
Figure 2. Effect of chewing, swallowing and stomach distension in familial dysautonomia.
Systolic blood pressure (SBP, top) and heart rate (HR, bottom) at rest and the maximum captured peak value when chewing (left), eating by mouth (middle) and distending the stomach with a gastrostomy feed (right). There was a significant increase in blood pressure and heart rate triggered by chewing and swallowing, but not when distending the stomach. All measurements were taken in the seated position. Individual data are linked by lines. Bars represent average values. Comparisons were made with paired student t-test.
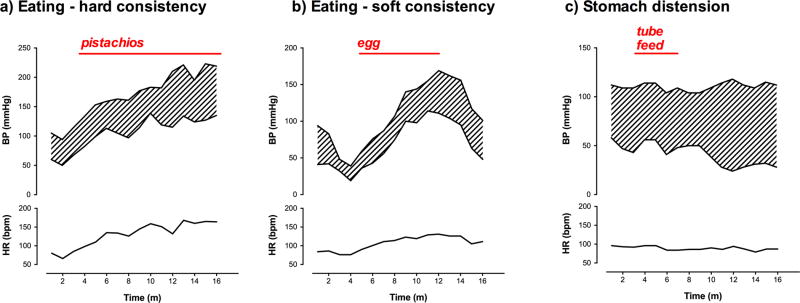
Figure 3. Examples of heart rate and blood pressure responses to eating and stomach distension in patients with familial dysautonomia.
A) Shows blood pressure (BP) and heart rate (HR) in a 29-year-old female patient while continuously eating pistachio nuts. Facial flushing and diaphoresis were noted while eating. B) Shows similar increase in blood pressure and heart rate when eating a soft-boiled egg in a 6 year old who followed a strictly low-tyramine diet. The patient also appeared flushed and sweaty. The pressor response did not appear to be affected by the consistency of the food. C) Shows the absence of a pressor response or tachycardia when food (300 ml of formula) was given directly into the stomach, bypassing the need to chew and swallow in a 20 year-old female patient. All measurements were taken while seated.
Stomach distension
To investigate whether the pressor response to eating involved distension of the stomach, we studied the cardiovascular responses to gastrostomy tube feedings in 9 patients with FD. There was no increase in blood pressure or in heart rate during the gastrostomy-tube feeding and none of the patients with FD showed signs of sympathetic activation during the feed (Table 2, Figures 2 & 3). In contrast, blood pressure was significantly lower than compared baseline at the end of the gastrostomy feed (-19±5 mmHg p= 0.0036). This suggested that sympathetic excitation during eating was unrelated to volume within the stomach itself and activation of mechanoreceptors sensing distension of the stomach wall.
Chewing
To differentiate whether chewing or swallowing was the trigger for the pressor response when eating by mouth, we studied cardiovascular autonomic responses to chewing gum (without swallowing) in 15 patients with FD. Immediately after the subjects were instructed to chew gum, their blood pressure and heart rate increased in parallel (p<0.0001 and p=0.0005 respectively, Table 2, Figures 2 & 3). Again, the pressor response and tachycardia were transient, subsiding the moment the masticatory movements ceased (Figure 3).
Pressor response to eating in FD: Chewing, swallowing or stomach distension?
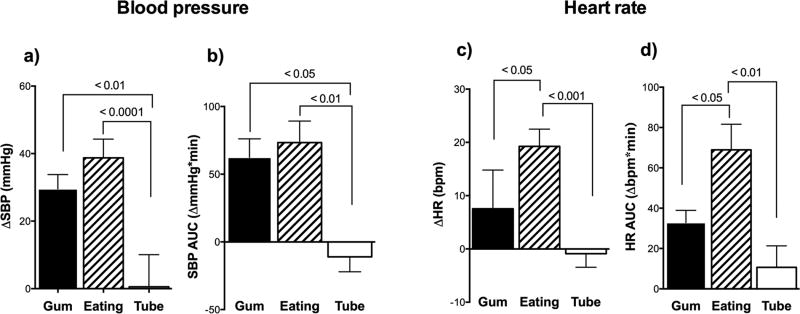
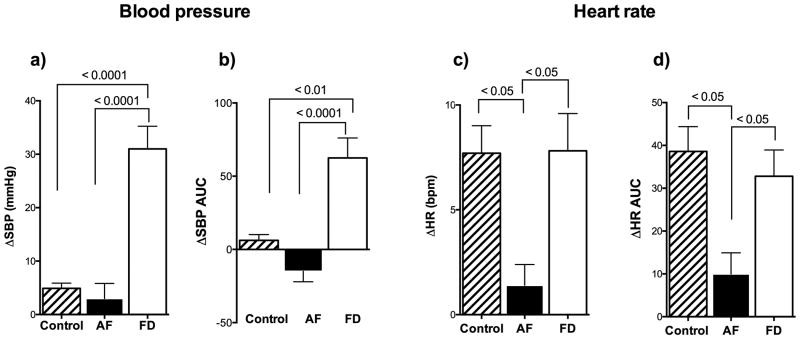
As shown in Figure 4, comparison of the three interventions revealed a significantly greater peak pressor response when eating by mouth and chewing, than when compared to feeding directly into the stomach through the gastrostomy-tube (p=0.0001). Area under the curve for change in systolic blood pressure showed similar findings (Figure 4). Heart rate was also significantly higher while eating than while chewing or gastrostomy-feedings (p=0.0001). Area under the curve for change in heart rate was highest when eating by mouth.
Figure 4. Comparison of responses to chewing, swallowing and stomach distension in patients with familial dysautonomia.
A) Shows significantly greater increase in systolic blood pressure (SBP) when chewing and swallowing compared to when distending the stomach alone during a tube feed. B) Shows area under the curve (AUC) for change in SBP in the first 5 minutes was greater when chewing gum and eating orally compared with feeding directly into the stomach (gastrostomy tube). C) Shows significantly greater increase in heart rate (HR) when eating by mouth compared with chewing gum and gastrostomy feedings. D) Shows area under the curve for change in HR in the first 5 minutes, which was greatest when eating orally. All measurements were taken in the seated position. p-values derived from ANOVA with post-hoc multiple comparisons.
Comparison with other autonomic disorders: A sympathetic response?
To investigate if the pressor response to chewing was sympathetically mediated, we also examined the blood pressure and heart rate changes to chewing gum in patients with chronically impaired sympathetic outflow (autonomic failure) and in healthy controls.
As previously reported, (Farella et al., 1999; Hasegawa et al., 2009) the normal response to chewing was an increase in heart rate (+7±1 bpm p= 0.0002). Careful examination of the chewing response in normal controls revealed a significant, albeit small, increase in blood pressure (+5±1 mmHg). In contrast, patients with autonomic failure had no increase in blood pressure or heart rate while chewing (p= 0.3152 and p= 0.1914, respectively, Figs 5 and 6). These findings indicate that the pressor response depends up on having functionally active sympathetic efferent neurons.
Figure 5. Responses to chewing in afferent baroreflex failure, efferent autonomic failure and controls.
A) Peak increase in systolic blood pressure (SBP) was exaggerated in patients with familial dysautonomia (FD) who fail to restrain sympathetic outflow due to baroreflex deafferentation. B) Area under the curve (AUC) for change in systolic blood pressure when chewing showed greater chewing pressor response in patients with afferent baroreflex failure (FD). C) Shows blunted HR increase in response to chewing in efferent autonomic failure. D) Area under the curve for change in heart rate when chewing was greater in afferent baroreflex failure and controls compared to patients with efferent autonomic failure. No increase in blood pressure or heart rate was observed in patients with efferent autonomic failure (AF) and chronically impaired sympathetic outflow – suggesting the chewing pressor response is sympathetically mediated.
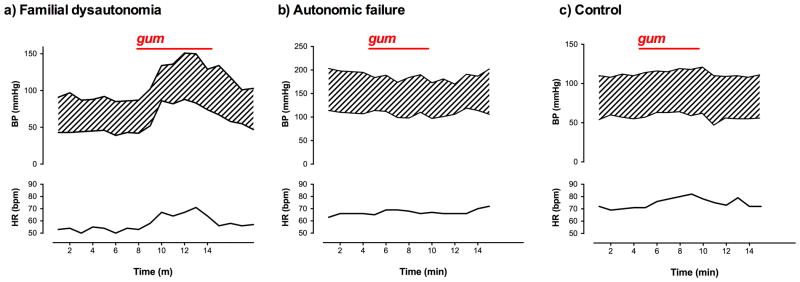
Figure 6. Examples of heart rate and blood pressure responses to eating and stomach distension in patients with familial dysautonomia.
A) Shows transient increase in blood pressure (BP) and heart rate (HR) in a 34-year-old male patient while chewing gum without swallowing. Facial flushing and diaphoresis were noted while eating. B) Shows absence of pressor effect and tachycardia in a 70-year old man with pure autonomic failure. C) Shows increase in heart rate without change in blood pressure in a 20-year old healthy female control. All measurements were taken while seated.
Discussion
The results of this study show that in patients with FD chewing markedly increases blood pressure and heart rate. The response appears to be sympathetically-mediated, as it is transient, accompanied by skin blotching and diaphoresis, and is not present in patients with chronic impairment in sympathetic neurones (autonomic failure).
This cardiovascular response occurs independently of swallowing or distension of the stomach, was not triggered by gastrostomy feeds. The lack of an increase in blood pressure with a gastrostomy feed in FD patients is in line with the observations in normal controls that noradrenaline release is blunted when the same food content is given by gavage (LeBlanc & Brondel, 1985). Only a very mild pressor response to chewing was present in healthy controls, suggesting that the pressor response to chewing is “unmasked” in FD due to the lack of afferent baroreceptor signalling and the failure to restrain sympathetic activity.
Exactly what is driving the apparent sympathetic response when chewing is an intriguing question. Masticatory muscle movements have been shown to trigger an increase in sympathetic outflow in humans (Bakke et al., 1996; Farella et al., 1999; Shiba et al., 2002; Abe et al., 2009; Hasegawa et al., 2009). Sensory information from the jaw is relayed to the brainstem by trigeminal afferent fibres, but these are severely depleted in patients with FD (Brown et al., 1964; Aguayo et al., 1971; Pearson & Pytel, 1978a). However, there is likely still some residual trigeminal sensory information arriving to the brainstem (Gutierrez et al., 2015). Against this trigeminal afferent signalling mechanism was the lack of correlation between electrophysiological measures of trigeminal function and the pressor response to chewing or eating (data not shown). Emotions induce exaggerated pressor responses in patients with FD (Riley et al., 1949; Norcliffe-Kaufmann et al., 2010; Norcliffe-Kaufmann et al., 2013c). Eating is challenging for these patients as neurogenic dysphagia and fear of aspiration pneumonia frequently create stress surrounding food intake and can lead to food phobias. Interestingly, many FD patients report much more pronounced blotching with meals that they enjoy, emphasizing the strong emotional charge surrounding food. Indeed, healthy controls have a increase in plasma noradrenaline spillover (a marker of sympathetic outflow), but only when eating an appetizing meal (LeBlanc & Brondel, 1985).
Another possibility to consider is that the cephalic phase of eating triggered by exposure to food cues (i.e., sight, thought and smell) may be producing the increase in blood pressure through gastric hormone secretion and changes in autonomic activity (Helman, 1988; Katschinski, 2000). Review of the recording reveal that the pressor response and tachycardia began as soon as the subject started chewing, not before – as would be expected if this was an anticipatory response. Whether the sight or thought of food was triggering sympathetic activation is unknown – although we were not able to provoke a pressor response by showing a patient food or having the patient talk about their favourite food, but further studies are needed to confirm this. Likewise, the majority of patients with FD have impaired sense of smell discrimination and we found no correlation between measures of olfactory function (University of Pennsylvania Smell test) and the magnitude of the pressor response (data not shown).
Nor do we think that the pressor response was triggered by taste. The deficiency of elongator-1 protein affects the development of sensory nerves of the tongue and taste buds (Smith et al., 1964; Pearson et al., 1970). As a consequence, patients have a very muted sense of taste with poor discrimination especially when it comes to sweet flavours (Henkin & Kopin, 1964).
The biological relevance of this rapid transient pressor response when eating is not immediately apparent. As a mechanism to counter postprandial hypotension, the timing is not correct as postprandial hypotension occurs after eating and lasts several hours, while the effect we observed was short lived (Berne & Fagius, 1993). Interestingly, animals also have a state of arousal while eating with a rapid increase in blood pressure, heart rate and respiratory rate mediated by the autonomic nervous system (Jones et al., 1993; Stinner & Ely, 1993). Fighting over food is a common behaviour in animals (Emeson & Morabito, 2005). A food arousal response may have served an evolutionary advantage especially at times when food was scarce, allowing animals and humans to be ready to defend a precious biological commodity. Such a response would be mediated by the cortex, and cortical responses are well known to be exaggerated in FD (Norcliffe-Kaufmann et al., 2013a).
Our study had some limitations. We were unable to provide direct measures of sympathetic nerve activity in this study. Microneurography recordings of muscle sympathetic nerve activity (MSNA) in patients with FD are not easy, because fibres are scarce (Pearson & Pytel, 1978b), cardiac rhythmicity is absent and MSNA fails to increase in response to known stimuli (Macefield et al., 2012). Lengthy invasive procedures often result in emotional upset, which results in tonic firing of sympathetic neurones, making it difficult to establish a stable resting state in these patients. The rapidity of the pressor response to eating suggests that this is a neurally-mediated response. Experimental evidence from animals also indicates that there is a sympathetic response to eating, as it can be blocked by ganglionic blockade with hexamethonium (Jones et al., 1993).
It was not possible to standardize the meal intake as not all patients can safely swallow the same consistencies due to varying degrees of neurogenic dysphagia. Although in controls, the pressor response to chewing harder consistencies is reportedly greater (Farella et al., 1999), we found no difference in the magnitude of the pressor response to chewing foods of hard and soft consistencies (p=0.8711). It was not possible to use a cross over study design, since not all patients that have gastrostomy tubes can eat by mouth without aspirating food into the lung. The dose of aspartame in the chewing gum was low and not previously associated with a pressor response in humans (Schiffman et al., 1987).
The study has clinical implications for the treatment of FD. Sympathetic activity cannot be appropriately restrained nor can it be activated by the baroreflex when necessary and hence patients have orthostatic hypotension (Norcliffe-Kaufmann et al., 2010). The rapid pressor response to chewing gum might be harnessed as a physical counter manoeuver to raise blood pressure in the upright position. It should be emphasized that the pressor response to eating is transient and subsides the moment chewing stops.
Supplementary Material
New Findings.
Our goal was to understand the autonomic responses to eating in patients with afferent baroreflex failure, by documenting changes in blood pressure and heart rate with chewing, swallowing and stomach distension.
Patients with lesions in the afferent baroreceptor pathways have an exaggerated pressor response to food intake. This appears to be a sympathetically-mediated response, triggered by chewing that occurs independently of swallowing or distension of the stomach. The chewing pressor response may be useful as a counter-manoeuvre to prevent orthostatic hypotension.
Acknowledgments
We would like to thank all the participants of this study and the Dysautonomia Foundation for their support. We also would like to thank all staff at the Dysautonomia Center.
Funding: This research was funded by grants from the Dysautonomia Foundation Inc. (CFM, LNK, JAP and HK) and NIH (U54NS065736 to LNK and HK).
Footnotes
Competing interests: The authors declare that there is no competing interest.
Author contributions: CFM contributed to the conception and design of experiments, collection, analysis and data interpretation and manuscript writing. LNK contributed to the conception and design of experiments, data interpretation, and manuscript writing. JAP contributed to data collection and manuscript revision. HK contributed to the conception and design of experiments and manuscript revision.
Disclosures: There are no conflicts of interest
References
- Abe N, Yashiro K, Hidaka O, Takada K. Influence of gum-chewing on the haemodynamics in female masseter muscle. Journal of Oral Rehabilitation. 2009;36:240–249. [Google Scholar]
- Aguayo AJ, Nair CP, Bray GM. Peripheral nerve abnormalities in the Riley-Day syndrome. Findings in a sural nerve biopsy. Arch Neurol. 1971;24:106–116. doi: 10.1001/archneur.1971.00480320034003. [DOI] [PubMed] [Google Scholar]
- Axelrod FB, Goldberg JD, Ye XY, Maayan C. Survival in familial dysautonomia: Impact of early intervention. J Pediatr. 2002;141:518–523. doi: 10.1067/mpd.2002.127088. [DOI] [PubMed] [Google Scholar]
- Axelrod FB, Gouge TH, Ginsburg HB, Bangaru BS, Hazzi C. Fundoplication and gastrostomy in familial dysautonomia. J Pediatr. 1991;118:388–394. doi: 10.1016/s0022-3476(05)82152-3. [DOI] [PubMed] [Google Scholar]
- Axelrod FB, Schneider KM, Ament ME, Kutin ND, Fonkalsrud EW. Gastroesophageal fundoplication and gastrostomy in familial dysautonomia. Ann Surg. 1982;195:253–258. doi: 10.1097/00000658-198203000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakke M, Thomsen CE, Vilmann A, Soneda K, Farella M, Møller E. Ultra sonographic assessment of the swelling of the human masseter muscle after static and dynamic activity. Archives of Oral Biology. 1996;41:133–140. doi: 10.1016/0003-9969(95)00135-2. [DOI] [PubMed] [Google Scholar]
- Berne C, Fagius J. Metabolic regulation of sympathetic nervous system activity: lessons from intraneural nerve recordings. Int J Obes Relat Metab Disord. 1993;17(Suppl 3):S2–6. discussion S22. [PubMed] [Google Scholar]
- Brown WJ, Beauchemin JA, Linde LM. A Neuropathological Study of Familial Dysautonomia (Riley-Day Syndrome) in Siblings. J Neurol Neurosurg Psychiatry. 1964;27:131–139. doi: 10.1136/jnnp.27.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ Joint National Committee on Prevention DE; Treatment of High Blood Pressure. National Heart L; Blood I; National High Blood Pressure Education Program Coordinating C. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- Emeson RB, Morabito MV. Food fight: the NPY-serotonin link between aggression and feeding behavior. Sci STKE. 2005;2005:pe12. doi: 10.1126/stke.2772005pe12. [DOI] [PubMed] [Google Scholar]
- Farella M, Bakke M, Michelotti A, Marotta G, Martina R. Cardiovascular responses in humans to experimental chewing of gums of different consistencies. Archives of Oral Biology. 1999;44:835–842. doi: 10.1016/s0003-9969(99)00074-6. [DOI] [PubMed] [Google Scholar]
- Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, Goldstein DS, Hainsworth R, Hilz MJ, Jacob G, Kaufmann H, Jordan J, Lipsitz LA, Levine BD, Low PA, Mathias C, Raj SR, Robertson D, Sandroni P, Schatz I, Schondorff R, Stewart JM, van Dijk JG. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69–72. doi: 10.1007/s10286-011-0119-5. [DOI] [PubMed] [Google Scholar]
- Geltzer AI, Gluck L, Talner NS, Polesky HF. Familial Dysautonomia; Studies in a Newborn Infant. N Engl J Med. 1964;271:436–440. doi: 10.1056/NEJM196408272710903. [DOI] [PubMed] [Google Scholar]
- Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Durr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez JV, Norcliffe-Kaufmann L, Kaufmann H. Brainstem reflexes in patients with familial dysautonomia. Clin Neurophysiol. 2015;126:626–633. doi: 10.1016/j.clinph.2014.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hague K, Lento P, Morgello S, Caro S, Kaufmann H. The distribution of Lewy bodies in pure autonomic failure: autopsy findings and review of the literature. Acta neuropathologica. 1997;94:192–196. doi: 10.1007/s004010050693. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Sakagami J, Ono T, Hori K, Zhang M, Maeda Y. Circulatory response and autonomic nervous activity during gum chewing. European Journal of Oral Sciences. 2009;117:470–473. doi: 10.1111/j.1600-0722.2009.00637.x. [DOI] [PubMed] [Google Scholar]
- Helman CA. Chewing gum is as effective as food in stimulating cephalic phase gastric secretion. American Journal of Gastroenterology. 1988;83:3. [PubMed] [Google Scholar]
- Henkin RI, Kopin IJ. Abnormalities of Taste and Smell Thresholds in Familial Dysautonomia: Improvement with Methacholine. Life Sci. 1964;3:1319–1325. doi: 10.1016/0024-3205(64)90051-7. [DOI] [PubMed] [Google Scholar]
- Jones SA, Langille BL, Frise S, Adamson SL. Nonadrenergic noncholinergic autonomic mediation of pressor response to feeding in lambs. Am J Physiol. 1993;265:R530–536. doi: 10.1152/ajpregu.1993.265.3.R530. [DOI] [PubMed] [Google Scholar]
- Katschinski M. Nutritional implications of cephalic phase gastrointestinal responses. Appetite. 2000;34:189–196. doi: 10.1006/appe.1999.0280. [DOI] [PubMed] [Google Scholar]
- Kaufmann H, Malamut R, Norcliffe-Kaufmann L, Rosa K, Freeman R. The Orthostatic Hypotension Questionnaire (OHQ): validation of a novel symptom assessment scale. Clin Auton Res. 2012;22:79–90. doi: 10.1007/s10286-011-0146-2. [DOI] [PubMed] [Google Scholar]
- LeBlanc J, Brondel L. Role of palatability on meal-induced thermogenesis in human subjects. American Journal of Physiology. 1985;248:4. doi: 10.1152/ajpendo.1985.248.3.E333. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Norcliffe-Kaufmann L, Gutierrez J, Axelrod FB, Kaufmann H. Can loss of muscle spindle afferents explain the ataxic gait in Riley-Day syndrome? Brain. 2011;134:3198–3208. doi: 10.1093/brain/awr168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield VG, Norcliffe-Kaufmann LJ, Axelrod F, Kaufmann H. Cardiac-Locked Bursts of Muscle Sympathetic Nerve Activity Are Absent in Familial Dysautonomia. J Physiol. 2012 doi: 10.1113/jphysiol.2012.246264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norcliffe-Kaufmann L, Axelrod F, Kaufmann H. Afferent baroreflex failure in familial dysautonomia. Neurology. 2010;75:1904–1911. doi: 10.1212/WNL.0b013e3181feb283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norcliffe-Kaufmann L, Axelrod FB, Kaufmann H. Developmental abnormalities, blood pressure variability and renal disease in Riley Day syndrome. J Hum Hypertens. 2011;27:51–55. doi: 10.1038/jhh.2011.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norcliffe-Kaufmann L, Martinez J, Axelrod F, Kaufmann H. Hyperdopaminergic crises in familial dysautonomia: a randomized trial of carbidopa. Neurology. 2013a;80:1611–1617. doi: 10.1212/WNL.0b013e31828f18f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norcliffe-Kaufmann LJ, Axelrod FB, Kaufmann H. Cyclic vomiting associated with excessive dopamine in Riley-day syndrome. J Clin Gastroenterol. 2013c;47:136–138. doi: 10.1097/MCG.0b013e3182582cbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J, Finegold MJ, Budzilovich G. The tongue and taste in familial dysautonomia. Pediatrics. 1970;45:739–745. [PubMed] [Google Scholar]
- Pearson J, Pytel B. Quantitative studies of ciliary and sphenopalatine ganglia in familial dysautonomia. J Neurol Sci. 1978a;39:123–130. doi: 10.1016/0022-510x(78)90193-4. [DOI] [PubMed] [Google Scholar]
- Pearson J, Pytel BA. Quantitative studies of sympathetic ganglia and spinal cord intermedio-lateral gray columns in familial dysautonomia. J Neurol Sci. 1978b;39:47–59. doi: 10.1016/0022-510x(78)90187-9. [DOI] [PubMed] [Google Scholar]
- Perlman M, Benady S, Saggi E. Neonatal diagnosis of familial dysautonomia. Pediatrics. 1979;63:238–241. [PubMed] [Google Scholar]
- Riley CM, Day RA, Greeley DM, Landford WS. Central autonomic dysfunction with defective lacrimation: I. Report of five cases. Pediatrics. 1949;3:468–478. [PubMed] [Google Scholar]
- Schiffman SS, Buckley CE, 3rd, Sampson HA, Massey EW, Baraniuk JN, Follett JV, Warwick ZS. Aspartame and susceptibility to headache. N Engl J Med. 1987;317:1181–1185. doi: 10.1056/NEJM198711053171903. [DOI] [PubMed] [Google Scholar]
- Shiba Y, Nitta E, Hirono C, Sugita M, Iwasa Y. Evaluation of mastication-induced change in sympatho-vagal balance through spectral analysis of heart rate variability. Journal of Oral Rehabilitation. 2002;29:956–960. doi: 10.1046/j.1365-2842.2002.00964.x. [DOI] [PubMed] [Google Scholar]
- Slaugenhaupt SA, Blumenfeld A, Gill SP, Leyne M, Mull J, Cuajungco MP, Liebert CB, Chadwick B, Idelson M, Reznik L, Robbins C, Makalowska I, Brownstein M, Krappmann D, Scheidereit C, Maayan C, Axelrod FB, Gusella JF. Tissue-specific expression of a splicing mutation in the IKBKAP gene causes familial dysautonomia. Am J Hum Genet. 2001;68:598–605. doi: 10.1086/318810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Farbman A, Dancis J. Absence of Taste-Bud Papillae in Familial Dysautonomia. Science. 1964;147:1040–1041. doi: 10.1126/science.147.3661.1040. [DOI] [PubMed] [Google Scholar]
- Stinner JN, Ely DL. Blood pressure during routine activity, stress, and feeding in black racer snakes (Coluber constrictor) Am J Physiol. 1993;264:R79–84. doi: 10.1152/ajpregu.1993.264.1.R79. [DOI] [PubMed] [Google Scholar]
- Tellez MJ, Norcliffe-Kaufmann LJ, Lenina S, Voustianiouk A, Kaufmann H. Usefulness of tilt-induced heart rate changes in the differential diagnosis of vasovagal syncope and chronic autonomic failure. Clin Auton Res. 2009;19:375–380. doi: 10.1007/s10286-009-0039-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.