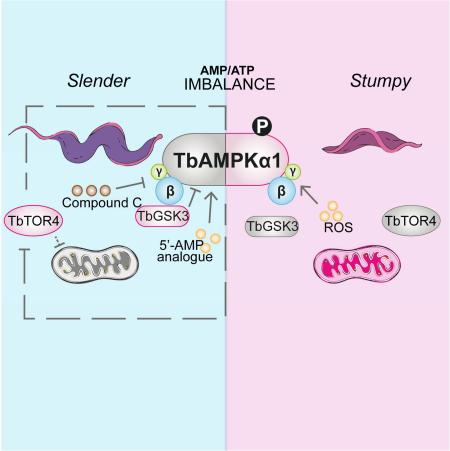
Abstract
During infection in mammals, the protozoan parasite Trypanosoma brucei transforms from a proliferative bloodstream form to a quiescent form that is pre-adapted to host transition. AMP analogs are known to induce quiescence and also inhibit TbTOR4. To examine the role of AMP-activated kinase (AMPK) in regulation of this developmental transition, we characterized trypanosome TbAMPK complexes. Expression of a constitutively active AMPKα1 induces quiescence of the infective form and TbAMPKα1 phosphorylation occurs during differentiation of wild-type pleomorphic trypanosomes to the quiescent stumpy form in vivo. Compound C, a well-known AMPK inhibitor inhibits parasite differentiation in mice. We also provide evidence linking oxidative stress to TbAMPKα1 activation and quiescent differentiation, suggesting that TbAMPKα1 activation balances quiescence, proliferation and differentiation.
Keywords: Trypanosoma brucei, developmental differentiation, TOR, AMPK, quiescence
Graphical Abstract
INTRODUCTION
All living organisms must adapt and respond to diverse environmental conditions, including nutritional and energetic stress (Fontana et al., 2010). Notably, protozoan parasites undergo developmental transitions in response to changing environmental conditions. Specifically, they ensure their own survival by sensing and responding to the availability of nutrients in the host environment (Goldenberg and Avila, 2011). Trypanosoma brucei is an extracellular parasite that causes sleeping sickness. Its life cycle alternates between a mammalian host and an arthropod vector, the tsetse fly. In the mammalian host, and in accordance with the emergence of high parasitemia, the bloodstream form can fluctuate between a proliferative slender form and a quiescent stumpy form, presumably to avoid damage to the host (Fenn and Matthews, 2007; Turner et al., 1995). The transition between forms is mediated by quorum-sensing in response to the stumpy inductor factor (SIF), a chemically uncharacterized signal secreted by trypanosomes (Vassella et al., 1997).
Despite the discovery of SIF-mediated differentiation in parasites, the signalling pathways underlying this process remain unclear. Studies have identified protein kinases that act as negative regulators in controlling parasite differentiation, such as the MAPK5, ZFK, and TbTOR4 kinases (Mony and Matthews, 2015). In addition, proteins associated with cAMP/AMP processing and purine balance may be involved, suggesting that the AMP/ATP ratio influences a finely tuned balance between energy consumption and differentiation processes (Barquilla et al., 2012; Laxman et al., 2006; Mony et al., 2014).
Most cells operate as self-sustaining systems, in which energy balance is maintained by a complex homeostatic system involving signalling pathways and nutrient sensors at multiple levels. In eukaryotes, the major “nutrient and energy” proteins are the target of rapamycin (TOR) and AMP-activated kinases (AMPK). Both kinases regulate the balance between catabolic and anabolic processes in accordance with cell requirements (Dunlop and Tee, 2013; Xu et al., 2012). T. brucei has an extensive family of TOR kinases, including TbTOR1 and TbTOR2, that are functional orthologs of yeast TOR proteins and control protein synthesis and actin polarization, respectively (Barquilla et al., 2008). In addition, a novel TOR kinase, TbTOR4, was identified that regulates the transition to quiescence in T. brucei. Ablation of TbTOR4 induces a molecular and biochemical phenotype that resembles the stumpy bloodstream form, which is pre-adapted to the insect host and to subsequent differentiation into the proliferative procyclic form (Barquilla et al., 2012). Conversely, TbTOR4 is significantly more phosphorylated in the procyclic form, suggesting that this kinase maintains the proliferation rate of the trypanosome and changes in response to nutritional requirements (Urbaniak et al., 2013). Furthermore, TbTOR4 activity is negatively regulated by AMP analogs, suggesting that reduced cellular energy (i.e. a high AMP/ATP ratio) promotes differentiation (Laxman et al., 2006; Barquilla et al., 2012).
In most eukaryotic cells, the main energy sensor seems to be AMPK, which senses changes in the AMP/ATP ratios during muscle contraction, oxidative stress and glucose starvation (Hardie, 2007). Thus AMPK operates as an on/off switch between energy-producing and energy-consuming metabolic processes (Daval et al., 2006). AMPK, which is highly conserved across eukaryotes, is a serine/threonine kinase that forms a heterotrimeric complex and includes a catalytic α subunit and regulatory β and γ subunits. In mammals, there are two isoforms of the α subunit, α1 and α2, two isoforms of the β subunit, β1 and β2, and three isoforms of the γ subunit, γ1–γ3, resulting in up to 12 possible heterotrimeric combinations (Hardie et al., 2012).
The α subunit has a kinase domain at the N-terminus that is activated in response to phosphorylation at Thr172 in the activation loop; thus, phosphorylation of this subunit is widely used as a marker of AMPK activation (Hawley et al., 1996). Kinase activity is triggered by several types of metabolic events that involve increases in AMP, ADP, or Ca2+ levels; more importantly, it is also triggered by the upstream kinase LKB1 and by CAMKKβ phosphorylation of Thr172 (Woods et al., 2005). Furthermore, AMP binding to AMPK leads to a conformational change, making AMPK a poorer substrate for phosphatases , and hence increasing its phosphorylation (Davies et al., 1995).
AMPK activation can initiate a series of signalling events that conserve energy globally by inhibiting mTOR activity (Kim et al., 2011). Thus, AMPK becomes active under nutrient-poor conditions and inactive under nutrient-rich conditions, the converse of what is observed for mTOR. The mechanisms underlying mTOR inhibition are either AMPK phosphorylation of the mTOR partner raptor (Gwinn et al., 2008) or AMPK phosphorylation of the tumour suppressor TSC2, a negative regulator of mTOR (Inoki et al., 2003). Accordingly, AMPK and mTOR represent diverse yet versatile energy sensors and metabolic regulators that regulate cell fate decisions in eukaryotes. Consequently, pathogens may have evolved to manipulate these pathways in order to achieve optimal levels of proliferation and cell fitness while avoiding exhaustion of host resources (Brunton et al., 2013).
In the present study, we report the biochemical characterization of AMPK complexes for the first time in T. brucei and describe the function of AMPK as a novel regulator of the development of quiescence bloodstream forms.
RESULTS
AMPK complexes in T. brucei contain AMPKα1 and AMPKα2
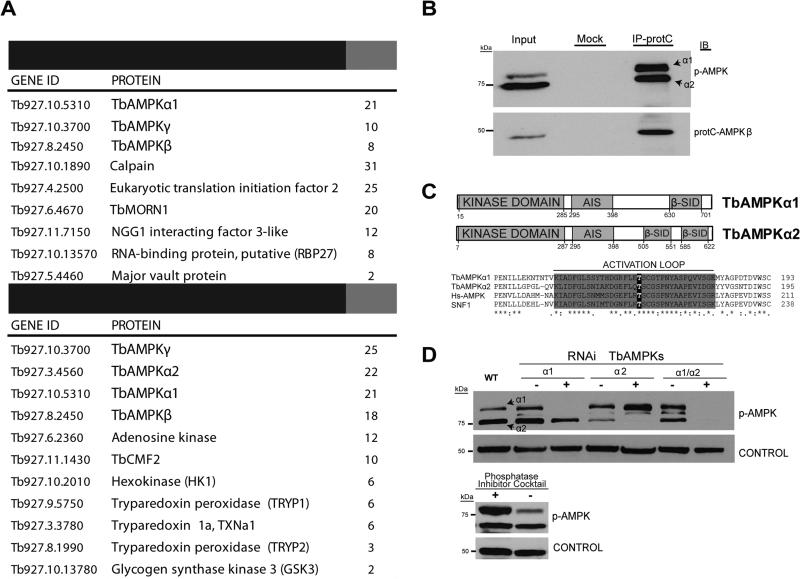
TbTOR4 activity is negatively regulated by AMP analogs (Barquilla et al., 2012); hence, AMPK may act as a sensor of AMP levels in trypanosomes. We searched the trypanosome genome database for orthologs of AMP-dependent kinases and found two proteins with significant homology to yeast SNF1, that we named TbAMPKα1 (Tb927.10.5310) and TbAMPKα2 (Tb927.3.4560). We also identified TbAMPKβ (Tb927.8.2450) and TbAMPKγ (Tb927.10.3700) regulatory subunit orthologs. To investigate whether these proteins formed a complex in T. brucei, as described for other eukaryotes, we used epitope-tagged versions of the TbAMPKα1 and TbAMPKα2 subunits (tagged with HA and protein C, respectively) and performed affinity purification followed by LC-MS/MS proteomic analyses. These analyses allowed us to identify independent complexes, since the TbAMPKα1 subunit is associated with the common subunits TbAMPKβ and TbAMPKγ, while the TbAMPKβ subunit is associated with both TbAMPKα1 and TbAMPα2 in addition to TbAMPKγ (Figure 1A and B). Interestingly, proteomics suggests additional proteins might interact with the TbAMPK core complexes that were previously identified as readouts of the AMPK pathway in other eukaryotes. Amongst these were some involved in crucial metabolic processes such as glycolysis (GSK3, hexokinase, and phosphofructokinase), and reactive oxygen species (ROS) metabolism (trypanothione peroxidase system TRYP1, TRYP2, TxN1a, and thioredoxin) (Brunton et al., 2013; Wu and Wei, 2012). Taken together, these results suggest that AMPK in T. brucei is represented by structurally and functionally conserved TbAMPKα1 and TbAMPKα2 complexes.
Figure 1. Characterisation of AMPK complexes in Trypanosoma brucei.
A. Proteomic analyses identify TbAMPK complexes and associated proteins. Proteins in tables were selected either by a high number of hits (peptides identified by MS/MS) or by a previously described functional or biochemical association with AMPK in other eukaryotes. Two independent affinity purifications using the catalytic subunit TbAMPKα1 or the regulatory subunit TbAMPKβ suggest that AMPKs form two independent and conserved complexes. Proteins present significantly in the negative control sample proteins were subtracted form the lists (See Supplementary Table 1 for the full list of co-purified proteins and control sample).
B. Co-IP of protein C-TbAMPKβ reveals that two AMPK catalytic alpha subunits (TbAMPKα1 and TbAMPKα2) bind to AMPKβ. The anti-phospho-Thr172 AMPK antibody recognizes both TbAMPKα subunits.
C. Schematic representation of the conserved regions of the TbAMPK orthologs. The TbAMPKα1 and TbAMPKα2 kinase domains have the conserved activation loop described in other organisms.
D. RNAi targeting TbAMPKα1 and TbAMPKα2 downregulates these proteins, and this downregulation can monitored by Western blot analysis using an anti-phospho-AMPKα (Thr172) antibody. To investigate the phosphospecificity of this antibody, protein extracts were incubated with or without a phosphatase inhibitor cocktail (PhosSTOP, Roche)
Functional analysis of T. brucei AMPKs
The proteomic analysis identified TbAMPKα1 and TbAMPKα2 as conserved kinases that co-purified with TbAMPKβ and TbAMPKγ (Figure 1B). While the metazoan TbAMPKα1 and TbAMPKα2 proteins have very similar molecular weights, the trypanosome AMPKs are predicted as 80.6 kDa (TbAMPKα1) and 70.6 kDa (TbAMPKα2) in size. Western blot analysis, using an anti-phospho-Thr172 antibody identified two bands corresponding to these sizes (Figure 1B). The anti-phospho-Thr172 antibody was developed against the AMPK amino-terminal region, which is conserved between human and trypanosome AMPKα (Figure 1C). To confirm that TbAMPKα1 and TbAMPKα2 were distinct proteins, we conducted RNAi experiments that depleted each kinase separately or both simultaneously. Western blot analysis demonstrated that the signal corresponding to the 80 kDa protein was reduced by RNAi targeting TbAMPKα1, while the lower 70 kDa band corresponding to TbAMPKα2 was reduced by RNAi targeting TbAMPKα2 (Figure 1D). Both signals were reduced by the RNAi targeting both kinases. These results indicate that the anti-phospho-Thr172 antibody recognized both trypanosome kinases, TbAMPKα1 and TbAMPKα2. They also confirmed the specificity of the antibody, since the signal obtained by Western blot analysis was reduced when we analyzed extracts in the absence of a phosphatase inhibitor cocktail (Figure 1D).
TbAMPKα1 activation by AMP analogs
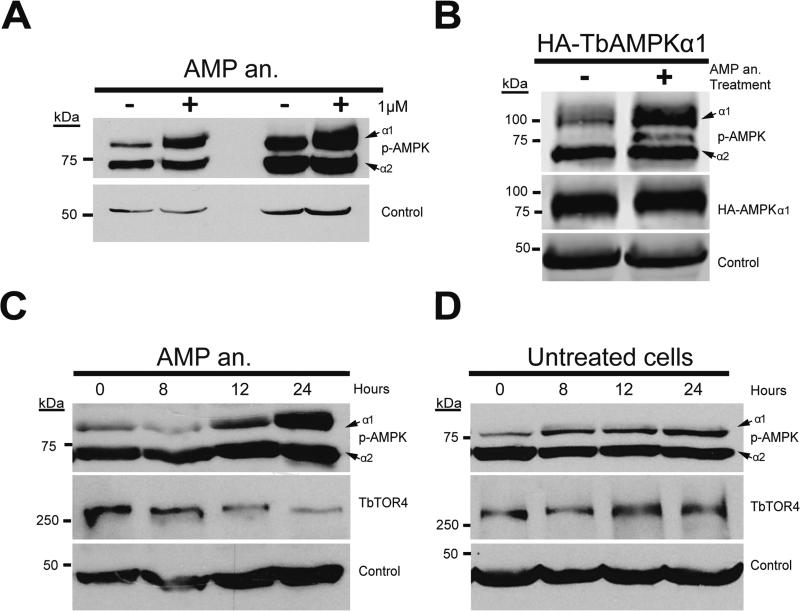
We next investigated whether an allosteric activator of AMPK, that mimics AMP, could activate TbAMPKs. Bloodstream cells were treated in culture for 18 h with 1 μM 8-pCPT-2’-O-Me-5’-AMP at different points during logarithmic growth. Western blot analysis using the anti-phospho-Thr172 AMPK antibody showed clear upregulation of TbAMPKα1 phosphorylation, but the TbAMPKα2 level was unaffected (Figure 2A). Increased TbAMPKα1 phosphorylation did not alter protein levels of HA-TbAMPKα1 (Figure 2B).
Figure 2. AMPKα1 kinase is activated in response to treatment with nucleotide analogs.
A. Bloodstream parasites were left untreated or were treated with 1 μM of AMP analog (AMP an.) (8-pCPT-2’-O-Me-5’-AMP) for 18 hours. The level of phosphorylated-TbAMPKα1 increased in treated cells compared to non-treated cells. The samples were collected in the early and mid-logarithmic phases of parasite growth to avoid a possible density-response effect due to AMPK activation.
B. As in (A), parasites were treated with 1 μM AMP analog (8-pCPT-2’-O-Me-5’-AMP) for 18 hours and collected for Western blot analysis. The level of HA-tagged TbAMPKα1 was not altered after treatment with AMP analog (8-pCPT-2’-O-Me-5’-AMP).
C. TbAMPKα1 activation in AMP analog-treated parasites increases in a time-dependent manner. TbTOR4 expression was reduced in parallel with the increase in TbAMPKα1 phosphorylation.
D. TbAMPKα1 phosphorylation depends on parasite density. Unlike the increase due to allosteric activation, this increase does not affect TbTOR4 expression. One representative Western blot is shown, results were repeated two times and some individual conditions were repeated numerous additional times.
We also investigated the kinetics of TbAMPKα1 activation by the AMP analog. TbAMPKα1 activation occurred in a time-dependent manner (Figure 2C). In addition, TbTOR4 protein levels were reduced after 24 h of treatment, as described previously (Barquilla et al., 2012). TbAMPKα1 showed a minor increase in phosphorylation due to parasite growth; however, this activation did not obviously affect TbTOR4 expression levels (Figure 2D).
TbAMPKα1 activation is associated with development of a stumpy-like form
During T. brucei infection, proliferation of the bloodstream form is reduced by differentiation to a quiescent stumpy form. This is presumed to prevent prevents exhaustion of host resources and pre-adapts the parasite for transition to the insect vector (Fenn and Matthews, 2007). Stumpy differentiation is thought to occur via a quorum-sensing mechanism in response to a chemically uncharacterized extracellular factor, or stumpy induction factor (SIF). Laboratory-adapted monomorphic strains are insensitive to SIF and unable to fully differentiate into the quiescent stumpy form (Turner et al., 1995). However, AMP and hydrolysable analogs of cAMP, but not hydrolysis-resistant analogs, do induce a stumpy-like quiescent form in monomorphic strains (Barquilla et al., 2012; Laxman et al., 2006; Vassella et al., 1997). As treatment with the AMP analog increases TbAMPKα1 phosphorylation (Figure 2), we investigated whether TbAMPKα1 activation paralleled the differentiation of the monomorphic strain to the stumpy-like form.
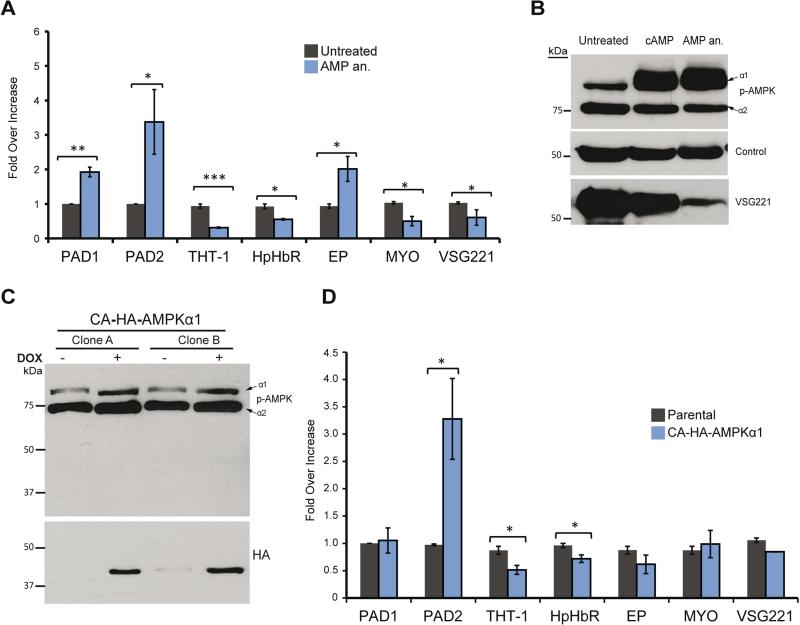
Differentiation to the stumpy form is characterized by specific changes in the T. brucei transcriptome. Thus, we examined the effect of TbAMPKα1 activation by the AMP analog on gene expression using quantitative RT-PCR (qRT-PCR) of transcripts that are selectively regulated in the stumpy form (Kabani et al., 2009). We compared transcript profiles in AMP analog-treated and untreated cells (Figure 3A). Levels of Hp-HbR and THT-1 mRNA decreased after AMP treatment, while levels of Protein-associated with differentiation 1 and 2 (PAD1 and PAD2) mRNAs significantly increased, as described previously (Kabani et al., 2009). Interestingly, AMP treatment also reduced the level of variant surface glycoprotein (VSG) mRNA derived from the active locus.
Figure 3. Activation of AMPKα1 kinase triggers remodeling of the quiescent stumpy transcriptome in bloodstream T. brucei.
A. Allosteric activation of TbAMPKα1 by 1 μM AMP analog (8-pCPT-2’-O-Me-5’-AMP) induces similar transcriptome changes that occur during slender to stumpy differentiation. mRNA analyzed by RT-qPCR in cells treated with 1 μM AMP analog for 18 hours and untreated control include genes previously associated with stumpy differentiation; PAD1, PAD2, THT-1, Hp-HbR, myosin (MYO), VSG221 (MITat 1.2/VSG2), and procyclin. The gene expression levels are represented as the fold-changes compared to untreated cells using comparative cycle threshold (Ct) calculations, with two internal standards; the PI3K-related protein (Tb927.2.2260) and the control (C1) protein (Tb10.389.0540).
B. Activation of AMPK using hydrolysable analogs of cyclic-AMP (cAMP) and 8-pCPT-2’-O-Me-5’-AMP (AMP an.) decreased the expression level of the VSG 221 protein as detected by Western blot analysis.
C. Constitutively active (CA) HA-TbAMPKα1 lacking a regulatory interaction domain was tagged with the HA epitope and expressed in T. brucei. Two independent clones (A & B) were analyzed by Western blot using anti-phospho-AMPKα (Thr172) antibody and anti-HA monoclonal antibody to detect the CA-HA-AMPK. CA-HA-AMPK expression seems to increase the basal phosphorylation level of TbAMPKα1.
D. Expression of CA-HA-AMPK induced gene expression changes characteristic of the stumpy form. Constitutively active AMPK expression results in a transcription remodelling similar to that induced by treatment with an AMP analog (A).
All data are reported as the mean ± SEM (n=3). *, p < 0.05; **, p < 0.01; ***, p < 0.0001 using the two-tailed t-test for paired observations.
Western blot analysis using the anti-phospho-Thr172 antibody showed that in T. brucei cells treated with either hydrolysable cAMP or with AMP analogs, TbAMPKα1 expression was upregulated, while TbAMPKα2 expression remained unaffected (Figure 3B). Furthermore, expression of VSG, the surface protein characteristic of the bloodstream form, was clearly reduced, while control protein levels were unaltered. These data suggest that AMP treatment triggers differentiation into the stumpy form, a form in which VSG expression is downregulated, as previously described (Amiguet-Vercher et al., 2004).
In order to further investigate the role of TbAMPKα1 in the regulation of the expression of stumpy-associated genes, we used a truncated version of the TbAMPKα1 kinase that is constitutively active (CA) (Um et al., 2013). Expressing CA-HA-TbAMPKα1 elicited changes in gene expression that were similar to those following activation with an AMP analog (Figures 3C and D), confirming that TbAMPKα1 has a positive role in activating the parasite differentiation process to the quiescent form.
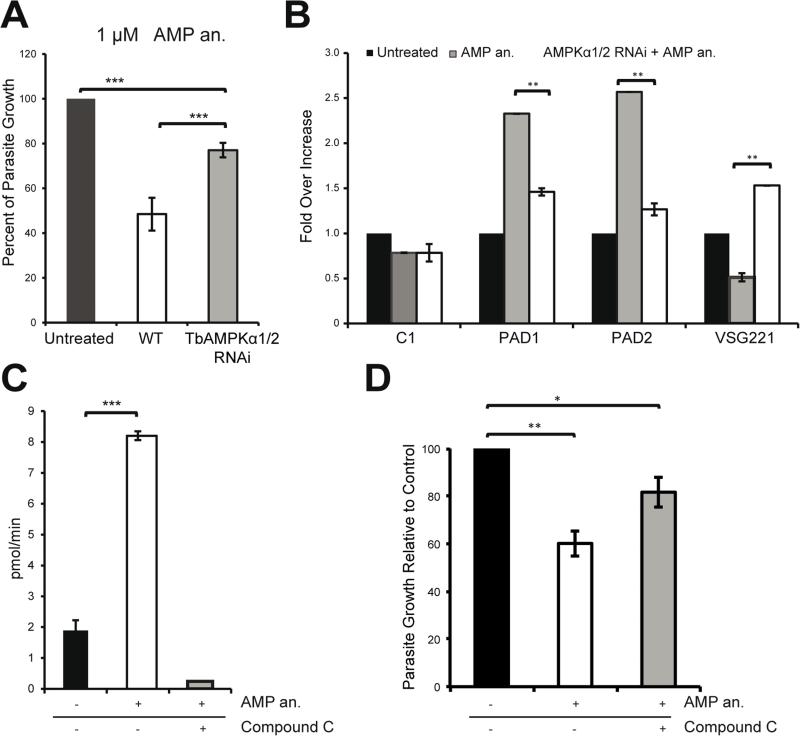
To investigate whether the observed AMP-mediated mRNA changes were dependent on TbAMPK, we used the AMP analog to treat cell lines in which the TbAMPKα1 and TbAMPKα2 kinases were depleted by RNAi. Cells depleted of each protein separately did not show significant changes in proliferation (Figure S1), nor did AMP treatment induce a stumpy-like phenotype, as shown previously (Barquilla et al., 2012; Laxman et al., 2006). Importantly, however, when both the TbAMPKα1 and TbAMPKα2 subunits were depleted using RNAi, the parasite showed resistance to AMP-induced growth inhibition, suggesting that the TbAMPK complex is the major target of this compound (Figure 4A). Double-RNAi treated cells did not show the same pattern of stumpy-specific mRNAs induced by AMP as in untreated cells (Figure 3A), suggesting that TbAMPKα1/2 are required for the transcription remodelling (Figure 4B). These findings indicate that TbAMPKα1/2 are involved in positive regulation of differentiation into the stumpy-like trypanosome form.
Figure 4. Cells become resistant to AMP treatment upon downregulation of the AMPKα1/2 kinases.
A. Depletion of the AMPKα1/2 subunits using RNAi counteracts the growth defect caused by AMP treatment of bloodstream cells. After 48 hours of AMPKα1/2 depletion using RNAi, T. brucei were treated for 18 hours with an AMP analog.
B. Total RNA was purified from the cells shown in (A) and the expression of the PAD1, PAD2, and VSG 221 mRNA was quantified at the transcriptional level. The regulation of these canonical differentiation genes was compromised by kinase knockdown using RNAi.
C. AMPKα1 can phosphorylate the SAMS peptide in vitro in an AMP-dependent manner. Bloodstream parasites were incubated with or without 10 μM AMP analog (8-pCPT-2’-O-Me-5’-AMP) for 30 minutes. HA-TbAMPKα1 was immunoprecipitated with an anti-HA agarose matrix. AMPK activity was assayed by 32P-ATP incorporation into the SAMS peptide. The use of compound C (CC) abolished the activation of the kinase.
D. Parasites pretreated with compound C do not show the growth defect phenotype induced by the AMP analog (8-pCPT-2’-O-Me-5’-AMP). The parasites were pretreated with 0.25 μM compound C for 4 hours and then incubated with 1 μM AMP analog for 18 hours.
All data are reported as the mean ± SEM (n=3). *, p < 0.05; **, p < 0.01; ***, p < 0.0001 using the two-tailed t-test for paired observations.
Next we investigated whether TbAMPKα1 responds to the AMP analog treatment. Accordingly, we utilized a purified HA-TbAMPKα1 complex to perform a series of in vitro phosphorylation assays in the presence of γ-32P-ATP and the SAMS peptide, an AMPK substrate (Davies et al., 1989). In vitro phosphorylation assays using HA-TbAMPKα1 purified from cells showed moderate phosphorylation activity under control conditions. However, after cell treatment with 10 ⎧M of the AMP analog for 30 minutes, purified HA-TbAMPKα1 showed a significant increase in specific activity (pmol/min) over non-AMP-treated cells (Figure 4C). These data suggest that AMP activates TbAMPKα1. Nevertheless, additional analysis using an AMPK-specific inhibitor was required to rule out the possibility that the signal that was detected was due to a contaminating kinase. Cells treated with AMP were incubated with compound C, a well-known inhibitor of AMPK (Vucicevic et al., 2011). In vitro phosphorylation assays showed that compound C abolished the effects of the AMP analog (Figure 4C). To further analyze the effects of this inhibitor, we pre-treated parasites for 4 hours with compound C then treated them with AMP for 18 hours. Parasites pre-treated with compound C showed resistance to the AMP analog growth inhibition, confirming the specificity of AMP and compound C in targeting the AMPK kinase (Figure 4D). Taken together, our results suggest that AMP activates TbAMPKα1 and that compound C is an effective inhibitor of the kinase activity of TbAMPKα1.
AMPKα1 is regulated during the differentiation of pleomorphic cells
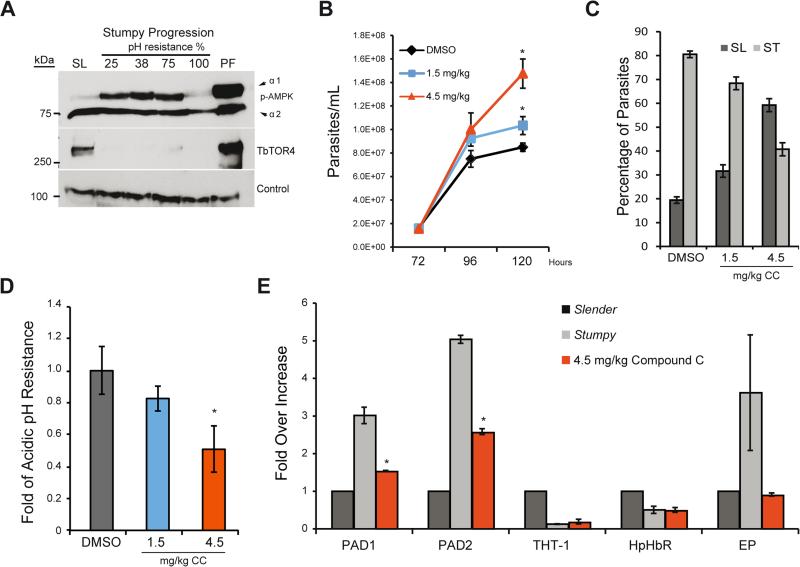
At high parasitemia in the mammalian host pleomorphic trypanosomes undergo the slender to stumpy differentiation process. Stumpy-like forms generated by AMP treatment of lab-adapted monomorphic strains show TbAMPKα1 activation in vitro (Figure 3). However, we considered it important to investigate this in wild-type pleomorphic trypanosomes during infection of mice to confirm biological relevance. To address this, we investigated whether TbAMPKα1 activation occurs during natural infection by isolating pleomorphic AnTat 1.1 bloodstream trypanosomes at different times during the course of mouse infection. During the course of an infection, the slender proliferative trypanosomes are sensitive to mild acidic pH 5.5 conditions, while the quiescent stumpy form is fully resistant, presumed as part of pre-adaptation to the insect vector mid-gut (Nolan et al., 2000). To monitor the degree of differentiation to the stumpy form, we isolated parasites from mice and analyzed cell resistance to mildly acidic pH. Western blot analysis during developmental differentiation clearly showed that TbAMPKα1 is not activated in the proliferative slender form, while robust TbAMPKα1 phosphorylation is detected at the initial stages of differentiation, suggesting that TbAMPKα1 activation occurs very early during stumpy differentiation and is maintained throughout the process (Figure 5A). Interestingly, when the parasites developed a full stumpy form differentiation (100% resistance to pH 5.5), TbAMPKα1 activation returned to the initial low levels. Finally, when the parasites develop into the proliferative procyclic insect form, which involves drastic metabolic changes, TbAMPKα1 phosphorylation increased dramatically. In addition, TbTOR4 decreased at the protein level during stumpy differentiation (Figure 5A), similar to the decrease observed after AMP treatment of the monomorphic strain (Figure 2C). Therefore, TbAMPKα1 is activated not only in in vitro generated stumpy-like cells but also in wild-type pleomorphic forms (Figure 5A). These results suggest that TbAMPKα1 activation is indeed associated with proliferative bloodstream differentiation to quiescent stumpy forms that are pre-adapted to the insect stage where TbAMPKα1 is active again.
Figure 5. AMPKα1 inactivation impairs the differentiation of T. brucei to the quiescent stumpy form during infection in mice.
A. Western blot analysis shows AMPKα1 regulation during differentiation from the proliferative slender (SL) form to the quiescent stumpy form in pleomorphic cells grown in vivo. One representative Western blot is shown. Experiments were repeated three times.
B. Inhibition of AMPK by compound C increases proliferation in parasites. The mice were treated daily intraperitoneal with 1.5 mg/kg or 4.5 mg/kg of compound C 72 hours after inoculation with the AnTat 1.1 pleomorphic strain.
C. Analysis of the distribution of the slender vs. stumpy form showed that 50% of the compound C-treated parasites remained slender and were less resistant to pH mild-to-low acidic fluctuations (D). In contrast, the non-treated parasites differentiated to the stumpy form.
E. Stumpy markers such as PAD1 and PAD2 were downregulated after inhibition of TbAMPK with compound C. The gene expression levels are shown as the fold-changes compared to untreated cells based on comparative cycle threshold (Ct) calculations using the PI3K-related gene as control. Genes analyzed: PAD1, PAD2, THT-1, Hp-HbR and control (C1) protein.
The error bars in B-C were calculated from independent mouse infections (3/mouse/group). All data are reported as the mean ± SEM (n=3). *, p < 0.05; **, p < 0.01; ***, p < 0.0001 using the two-tailed t-test for paired observations.
AMPKα1 inhibition reduces differentiation to the quiescent stumpy form during infection in mice
In vivo differentiation to the stumpy form correlates with TbAMPKα1 activation in pleomorphic trypanosomes (Figure 5A). Thus, we sought to investigate whether inhibition of TbAMPKα1 could halt stumpy form development during in vivo infection. In vitro phosphorylation assays showed that compound C inhibited TbAMPKα1 activity (Figure 4C). Thus, we decided to treat infected mice with two concentrations of compound C and then analyze parasitemia at different time points during the infection. Figure 5B shows that more parasites were isolated from mice treated with compound C, suggesting a delay in quiescence and continued proliferation. Furthermore, we isolated parasites from infected mice treated with different amounts of compound C and analyzed the cellular phenotype. First, living cells treated with compound C moved faster than controls (Supplemental videos 1 and 2), and the classic stumpy morphology decreased by 50% (Figure 5C). Second, when TbAMPKα1 was inhibited with compound C, the cells were less resistant to mildly acidic pH, contrary to what occurs in untreated cells with a stumpy phenotype (Figure 5D). Finally, we isolated mRNA from these cells and investigated changes in the expression levels of the PAD family mRNAs, considered stumpy marker genes. Figure 5E shows that PAD1 and PAD2 mRNA expression was reduced in parasites isolated from mice treated with compound C compared with expression in parasites isolated from untreated mice. Further, immunofluorescence analysis of PAD1 protein expression in compound C-treated cells showed clear downregulation (Figure S2). Collectively, these results suggesting that inhibition of TbAMPKα1 activity by compound C reduced differentiation to the stumpy form. Furthermore, TbAMPKα1 phosphorylation correlates with a decrease in TbTOR4 expression.
Upstream regulators of TbAMPKα1 activation
Next we sought to identify possible activators of TbAMPKα1. The proteomic analysis identified proteins that are well-known regulators of TbAMPKα1 activity in other eukaryotes, such as GSK3, as well as proteins involved in oxidative metabolism (Figure 1A). In addition to its role in regulating glycogen synthase, GSK3β is implicated in other aspects of glucose homeostasis in mammalian cells (Suzuki et al., 2013). Notably, glucose homeostasis is a vital feature of energy metabolism in the bloodstream. Co-immunoprecipitation (IP) experiments that used HA-epitope tagged TbGSK3 and protein C-tagged TbAMPKβ confirmed the interaction (Figure S3A). Knockdown of TbGSK3 using RNAi showed increased TbAMPKα1 phosphorylation as detected by Western blot analysis (Figure S3B). In addition, qRT-PCR analysis suggested that TbGSK3 depletion results in gene expression changes similar to those induced by TbAMPKα1 activation (Figure S3C). These results suggest that the functional interaction between GSK3 and AMPKβ is conserved in trypanosomes.
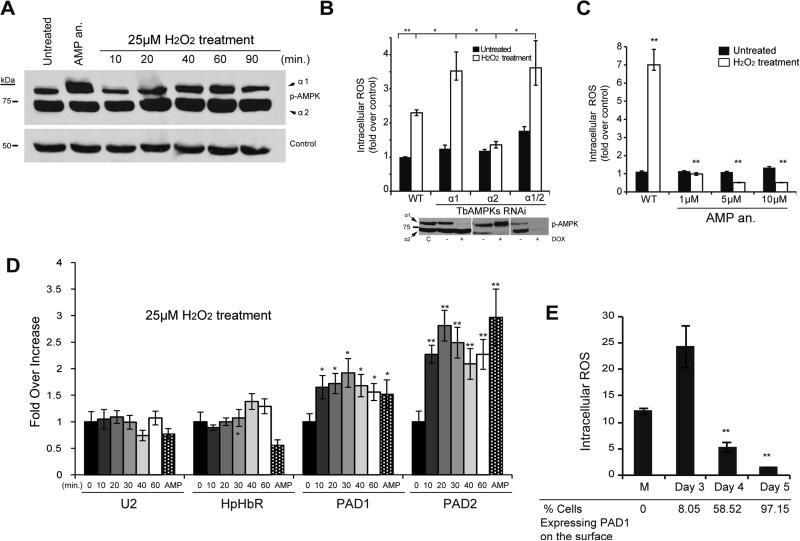
A bona fide developmental marker for the stumpy cell type is mitochondrial activation (Fenn and Matthews, 2007), suggesting that oxidative stress occurs during parasite differentiation. The AMPK pathway is an important signalling cascade involved in sensing the oxidative response (Duarte et al., 2015), whereby an increase in intracellular ROS induced AMPK activation (Zmijewski et al., 2010). To investigate this possibility, we treated parasites with H2O2 in a time course to mimic ROS increase. TbAMPKα1 phosphorylation gradually increased in response to the addition of exogenous H2O2, reaching a peak after one hour of treatment and decreasing later (Figure 6A). The dynamic regulation of AMPK phosphorylation suggests this kinase is functionally involved in T. brucei oxidative metabolism.
Figure 6. AMPKα1 is activated in response to oxidative stress.
A. AMPKα1 senses oxidative stress induced by exogenous H2O2. Cells were incubated with 25 μM of hydrogen peroxide and AMPKα1 phosphorylation analyzed by Western blot using phospho-specific antibody at the indicated time points in minutes. The level of phosphorylated AMPKα1 oscillated in AMP analog (AMP an.) (8-pCPT-2’-O-Me-5’-AMP) treated cells compared to untreated (UT) cells.
B. Depletion of AMPKα1 impairs ROS metabolism. AMPK subunits were depleted by RNAi for 24 hours and cells were subsequently incubated 20 minutes with 50 μM of H2O2. Intracellular ROS was assayed by flow cytometry of oxidized CM-H2DCFDA. The effectiveness of AMPK knockdown was examined by Western blot analysis.
C. AMPK〈1 activation by AMP analog (8-pCPT-2’-O-Me-5’-AMP) improves oxidative metabolism by reducing intracellular ROS levels. The parasites were incubated for 18 hours with 1–10 μM of AMP, washed, and then incubated with 100 μM of H2O2 during 30 min.
D. Hydrogen peroxide treatment of cells results in an increase of stumpy specific gene expression. Histogram showing the relative expression of mRNAs measured by qRT-PCR of U2, HpHbR, PAD1 and PAD2 mRNAs isolated from cells treated with H2O2. The gene expression levels are shown as the fold-changes compared to untreated cells (0 minute) based on comparative cycle threshold (Ct) calculations using the PI3K-related and Control 1 (C1) genes as control. The error bars were calculated from three independent experiments. All data are reported as the mean ± SEM (n=3). *, p < 0.05 and **, p < 0.01 using the two-tailed t-test for paired observations.
E. ROS fluctuation during in vivo development of parasites to the quiescence stumpy form. AnTat 1.1 pleomorphic strain was infected in mice and intracellular ROS levels were analyzed at day 3 to 5 post-infection. PAD1 surface expression IF analysis of blood-isolated parasites was used to detect stumpy form. Previous to the progression of the quiescence stumpy form intracellular ROS levels were higher than later when stumpy differentiation is detected. (M) Monomorphic proliferative strain growing in culture is used as control. Statically differences were found between mice at 4 and 5 days post-infection compared mice at 3 days. Data represent mean ± SEM (n=3) calculated from independent mouse infection (3/mouse/group). **, p < 0.01 using the two-tailed t-test for paired observations.
To further characterize the function of AMPKs in oxidative stress, we treated parasites with H2O2 after depleting TbAMPKα1, TbAMPKα2, or both using RNAi. In the absence of TbAMPKα1, the cells failed to efficiently metabolize H2O2 suggesting that TbAMPKα1 has an antioxidant function in trypanosomes, as in other eukaryotes (Figure 6B). Interestingly, depletion of TbAMPKα2, which increases the phosphorylation of TbAMPKα1 likely as a compensation mechanism, protected parasites from oxidative stress (Figure 6B). To confirm a putative antioxidant function, we activated TbAMPKα1 with the AMP analog and found a dramatic reduction of intracellular ROS in cells treated with H2O2 (Figure 6C). These results altogether suggest that oxidative stress induces TbAMPKα1 phosphorylation, which in turn functions as a feedback regulator for ROS internal levels.
To investigate whether a ROS increase is associated with differentiation to the stumpy form, we analyzed by RT-qPCR the mRNA levels for the differentiation markers PAD1 and PAD2 in cells treated with 25 μM of H2O2 (Figure 6D). A significant increase of the PAD expression after only 20-30 min treatment was detected (Figure 6D), concomitant with the AMPK phosphorylation (Figure 6A).
An important question however, is whether oxidative stress occurs naturally during in vivo differentiation of a wild-type pleomorphic strain. Bloodstream pleomorphic trypanosomes undergo differentiation from the proliferative to the stumpy form very efficiently throughout mice infection. Thus, we analyzed intracellular ROS levels of parasites isolated from mice at days 3, 4 and 5 post infection. Significant levels of intracellular ROS were detected early during infection (day 3), when a low percentage of stumpy cells were detected (8.05% PAD1 positive, Figure 6E). However, parasites isolated on day 5 post infection, with 97% stumpy cells had dramatically reduced ROS levels. Unfortunately, we were unable to analyze ROS levels at day 3 due to low parasitemia. Notwithstanding, proliferative monomorphic strain displayed lower ROS levels that the pre-stumpy cells from day 3. These data show that slender cells committed to stumpy differentiation generate higher levels of intracellular ROS than stumpy cells (Figure 6E), suggesting that active TbAMPKα1 in pre-stumpy forms is involved in ROS detoxification (Figure 6B & 6E).
We next investigated possible cross talk between the TbTOR4 and TbAMPKα1 pathways. Allosteric activation of TbAMPKα1 by the AMP analog leads to TbTOR4 downregulation detected by Western bot analysis (Figure 2C). Thus, we investigated whether TbAMPKα1 activity is required in cells where TbTOR4 was depleted. Inhibition of TbAMPKα1 activity by Compound C treatment of cells depleted of TbTOR4 did not affect the developmental gene expression program (Figure S4). Next, we generated cell lines where both TbTOR4 and TbAMPKα1 were depleted by double inducible RNAi. Again, gene expression analysis of stumpy-specific marker genes showed no significant differences compare with the single TbTOR4 depletion (Figure S5). Thus, the developmental gene expression program triggered by TbTOR4 depletion occurs independently of TbAMPKα1 activity.
DISCUSSION
African trypanosomes need to precisely control their energy and proliferation in order to respond to changes in the host environment and to protect the viability of the host. Such control is fundamental to the host-parasite transition and to the efficiency of transmission. T. brucei maintains an integral link between cell cycle regulation and differentiation during its life cycle, which involves the response to parasite density and stumpy induction factor. Here we report a novel function for TbAMPK as a key factor in the regulation of differentiation from the proliferative to stumpy forms, and which suggests a link between metabolic activity, differentiation and control of parasitaemia.
The function of the TbAMPKα subunits in trypanosomes and the structure of possible TbAMPK complexes was previously totally unknown. However, depletion of the TbAMPK regulatory subunits TbAMPKβ and TbAMPKγ do induce changes in the expression of surface proteins in the insect (procyclic) stage of the parasite (Clemmens et al., 2009). Here, we first characterized the composition of the AMPK core complexes in T. brucei and found two alpha subunits that associate to form two distinct complexes together with the common TbAMPKβ and TbAMPKγ subunits. We used the name TbAMPKα1 (Tb927.10.5310) since this protein responds to an allosteric activator that mimics AMP and because it functions in metabolic regulation as described in other organisms (Aymerich et al., 2006; Gowans et al., 2013).
Proteomic analysis of the AMPK complex and co-IP experiments suggests that trypanosome AMPK also interacts with TbGSK3, a kinase involved in glucose metabolism and is associated with this pathway in other eukaryotes (Hardie et al., 2012). GSK3β inhibits AMPK activity by interacting with the AMPKβ regulatory subunit in a stable manner (Suzuki et al., 2013) and our evidence suggests a similar mechanism via association of TbGSK with the TbAMPK complex (Figure S3). In addition, trypanosome GSK3 depletion induced a similar stumpy phenotype as TbAMPKα1 activation, which suggests that TbGSK is a negative regulator of TbAMPK in trypanosomes. These data suggest that the AMPK/GSK interaction and control mechanism is conserved in T. brucei and that this acts to regulate energy balance metabolism.
In order to assess the relative contribution of AMPK activation to parasite metabolism, we used a highly permeable allosteric activator to mimic AMP. Treatment with the AMP analog resulted in a specific increase in TbAMPKα1 phosphorylation, but significantly phosphorylation of TbAMPKα2 remained unchanged (Figure 2). In other systems an increase in AMP alters the ATP/AMP ratio, and this is sensed mainly by AMPK where phosphorylation is promoted by upstream kinases (Carling et al., 2012). Our observations therefore suggest that in T. brucei AMPKα1 is a major player in sensing and coordinating the flux of energy in the parasite. Interestingly, the canonical AMPK upstream kinase, the LKB1-STRAD-MO25 complex, has yet to be described in trypanosomatids, but our proteomics identified a putative candidate that shares low but significant homology with the mammalian LKB1 catalytic domain (Supplemental Table 1), suggesting that part of this signalling pathway may also be conserved.
To compensate for fluctuations in ATP levels AMPK regulates a wide range of metabolic pathways to control energy fluctuations and maintain cell homeostasis. The parasite avoids overgrowth in the mammalian host by developing into a quiescent form (Vassella et al., 1997). In trypanosomes, TbAMPKα1 also senses changes in cell proliferation (Figure 2D), but this reversible TbAMPKα1 activation does not drive either differentiation or TbTOR4 downregulation. TbAMPKα1 allosteric activation (e.g. by AMP analogs) cannot be reversed and is effective in activating the kinase, triggering differentiation to quiescence and TbTOR4 downregulation (Figure 2C).
Activation of TbAMPKα1 promotes the initial steps of differentiation of T. brucei into stumpy-like forms in strains that do not normally fully differentiate in vitro. Activation by AMP analogs also induces the expression of developmentally regulated genes (Figure 3), suggesting that TbAMPK can modulate gene expression in order to modulate metabolism, similar to the case in other eukaryotes (Thomson et al., 2008). Importantly, expression of a constitutively active form of AMPK, CA-HA-AMPK, also induced stumpy-like differentiation (Figure 3C). In addition, inhibition of TbAMPK function, by either compound C or RNAi, significantly counteracted the effects of AMP on growth inhibition and the changes to expression of stumpy marker genes. Taken together, these results ruled out possible indirect effects of the AMP analog and suggest that TbAMPKα1 is a central regulator within the stumpy differentiation process.
Regardless of their differential responsiveness to differentiation signals, both pleomorphic and monomorphic strains respond to nucleoside analogs, suggesting that AMP regulation is downstream of the response to stumpy induction factor (SIF) (Mony and Matthews, 2015). We also analyzed TbAMPK regulation using an in vivo model of pleomorphic parasite differentiation in mice infections. Western blot analyses suggest that TbAMPKα1 is activated early during stumpy differentiation, while TbTOR4, as a negative regulator of differentiation, is undetectable at this time. Interestingly, TbAMPKα1 was not phosphorylated once stumpy population reached 100%, and TbTOR4 remained undetectable. These results, together with the downregulation of TbTOR4 upon TbAMPKα1 activation by the AMP analog (Figure 2C), suggest that these two events are connected and that TbAMPKα1 functions upstream of TbTOR4. Furthermore, the expression of stumpy genes was triggered regardless of TbAMPKα1 function in TbTOR4 depleted cells (Figures S4 & S5), is consistent with either TbAMPKα1 acting upstream of TbTOR4 to regulate the expression of stumpy-specific genes or that TbAMPKα1 and TbTOR4 are components of two parallel signalling pathways. Although we cannot formally rule out that these two signalling pathways operate independently, our results suggest crosstalk between these two kinases in trypanosomes.
In mammalian osteogenic differentiation, AMPK activation drives development by inhibiting the Akt/mTOR pathway (Pantovic et al., 2013). Similarly, activation of TbAMPKα1 induced parasite differentiation. The TbAMPKα1 activation that we detected during early differentiation correlates with the accumulation of intracellular AMP described in T. brucei differentiation (Graven et al., 2014). Previous results, as well as our own data, indicate that AMP is a key molecule in stumpy differentiation in trypanosomes. Mitochondrial activation is an important event in T. brucei differentiation as it enables the parasite to use proline as a new source of ATP. While proliferative forms obtain their metabolic energy almost entirely from glycolysis, stumpy forms reactivate mitochondria, restoring oxidative capacity, as a metabolic preadaptation to a different energy source (Priest and Hajduk, 1994). Accordingly, mitochondrion activity is considered a differentiation control point during the life cycle of T. brucei (Timms et al., 2002).
Dependence on glycolysis to generate ATP is an unusual metabolic aspect of T. brucei, and glycolysis is far less efficient than oxidative metabolism, similar to the “Warburg effect” described in some T cells and cancer cells (Gogvadze et al., 2010). Taken together, our results suggest that stumpy differentiation is associated with oxidative stress, most likely induced by developmental mitochondrial activation, similar to the ROS-AMPK pathway described in cancer cells (Hart, et al 2015). Thus, activated TbAMPKα1 in stumpy forms helps to maintain oxidative flux during parasite development (Figure 6). In mammals AMPK has antioxidative properties (Colombo and Moncada, 2009), and our results suggest that trypanosome AMPKα1 activity prevents ROS increase, since TbAMPKα1 activation by AMP reduced intracellular ROS induced by H2O2 (Figure 6). Interestingly, the TbAMPK core complex co-purified with thioredoxin and tryparedoxin peroxidase, both components of the trypanothione peroxidase system (Diechtierow and Krauth-Siegel, 2011) (Supplementary Table 1). Thus, even though this interaction has yet to be confirmed by co-IP experiments, one TbAMPK readout could be on the trypanothione peroxidase system.
The data presented here suggest that T. brucei balances its differentiation and proliferation programs through the TbAMPKα1 pathway. Energy homeostasis and mitochondrial regulation are known to play key roles in the transition from proliferative blood stages to quiescent stumpy forms and the TbAMPKα1 pathway appears as one the mayor regulators of these process, coordinating multiple stimuli to maximize the potential for transmission. Furthermore, as AMPK is currently a major drug target for metabolic diseases and cancer, the trypanosome pathway may represent a novel anti-parasitic target.
EXPERIMENTAL PROCEDURES
Detailed materials and methods are provided in the Supplemental Information.
Trypanosome strains and AMP analogs treatment
Throughout this study monomorphic bloodstream Trypanosoma brucei brucei (Lister 427) and the pleomorphic AnTAT 1.1 were used. Parasites in culture at a low density (2×105 cells/ml) were incubated with 8-pCPT-2'-O-Me-5'-AMP (1 μM) and 8-pCPT-2'-O-Me-cAMP (10 μM) (c078 and c041 - Biolog Life Science Institute) during 18 hours. To avoid the AMPK activation caused by cell density, the control and treated cells were analyzed at the same cell density. Slender and stumpy forms of pleomorphic AnTAT 1.1 were purified from BALB/c mice blood (see Supplementary Information).
Phosphorylation assay
4×109 parasites were treated or not with 10 μM of 8-pCPT-2'-O-Me-5'-AMP during 30 minutes. After immunoprecipitation of HA-AMPKα1 (see Co-immunoprecipitation Assays in Supplementary Information), AMPK activity in the resulting immune complexes was determined by the incorporation of [γ-32P]ATP into the consensus substrate, the called SAMS peptide (HMRSAMSGLHLVKRR) (Abcam Biochemicals ab120182). In brief, purified HA-AMPKα1 was resuspended in 40 μL of final reaction buffer that contains 10X assay buffer (Hepes 250 mM, 200 μM ATP, 2 mM AMP, 10 mM EGTA, 10 mM DTT, 50 mM MgCl2), [γ-32P] ATP, 100 μM SAMS or 1 μM of Compound C (Abcam Biochemicals ab120843) and was incubated with shaking for 30 minutes at 37°C. The mixture was quickly pelleted and spotted into P81 Whatman paper. Unreacted ATP was removed washing the filter papers 4–5 times with 1% phosphoric acid. After the final wash, the filters were quickly dried with ethanol and counted in a scintillation counter. AMPK activity was calculated by phosphotransfer and specific activity of ATP by subtracting the average counts per minute (cpm) of the negative controls from the average cpm of AMPK samples and dividing this number by the specific activity of the ATP.
Detection of Intracellular Reactive Oxygen (ROS) Species
2×106 parasites were washed and resuspended in 1 mL of TBD glucose and incubated with 2 μM cell permeant 2',7'-dichlorodihydrofluorescein diacetate (H2DCFDA) (Life technologies C6827) for 20 minutes at 37°C. Finally, cells were centrifuged, resuspended in TDB glucose with 4μg/ml propidium iodide (to discard dead cells) and immediately analyzed by flow cytometry.
Peroxide hydrogen treatment
Logarithmic phase cells were incubated with 25μM H2O2 (diluted in miliQ water from the 10M stock solution, Sigma Aldrich H1009) from 10 to 90 minutes at 37°C. Cells were collected for WB and RT-qPCR analysis as described in the Supplemental Experimental Procedures.
AMPK inhibition by compound C
Monomorphic bloodstream parasites were incubated with 0.25 μM compound C for 4 or 24 hours at 37°C. For in vivo experiments, 1.5 or 4.5 mg.kg−1 compound C were daily intraperitoneally injected in mice 72 hours after inoculation with the AnTat 1.1 pleomorphic strain.
Supplementary Material
Highlights.
T. brucei AMPKs include α1 and α2 subunits that form two complexes with β and γ subunits.
AMPKα1 is a positive regulator of differentiation to insect pre-adapted quiescent form.
AMPKα1 inhibition reduces quiescence in WT trypanosomes infection in mice.
ROS increase in pleomorphic trypanosomes activates AMPKα1 cascade.
ACKNOWLEDGMENTS
We thank Prof. Mark Field (University of Dundee, UK) for critical reading and helpful comments of this manuscript. We thank to Keith Matthews (University of Edinburgh) for kindly supplying anti-PAD antibodies. We also thank Domingo Rojas, Andreu Saura and Isabel Vidal (IPBLN-CSIC, Granada) for experimental support. This work was supported by grants from the Spanish Ministerio de Ciencia e Innovación, (SAF2015-71444), Junta de Andalucia (CTS-5841) and Subdirección General de Redes y Centros de Investigación Cooperativa (RICET) RD12/0018/0015. This work was funded in part by the National Institutes of Health (R01AI114685).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
M. S. designed and performed experiments, prepared Figures and wrote the manuscript. G.C. and J.M.B. performed several experiments and Figures. M.N. designed and supervised the work and wrote the manuscript. All authors commented on the manuscript.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, five figures and one table and two videos that can be found with online at:
REFERENCES
- Amiguet-Vercher A, Perez-Morga D, Pays A, Poelvoorde P, Van Xong H, Tebabi P, Vanhamme L, Pays E. Loss of the mono-allelic control of the VSG expression sites during the development of Trypanosoma brucei in the bloodstream. Mol Microbiol. 2004;51:1577–1588. doi: 10.1111/j.1365-2958.2003.03937.x. [DOI] [PubMed] [Google Scholar]
- Aymerich I, Foufelle F, Ferre P, Casado FJ, Pastor-Anglada M. Extracellular adenosine activates AMP-dependent protein kinase (AMPK). J Cell Sci. 2006;119:1612–1621. doi: 10.1242/jcs.02865. [DOI] [PubMed] [Google Scholar]
- Barquilla A, Crespo JL, Navarro M. Rapamycin inhibits trypanosome cell growth by preventing TOR complex 2 formation. Proc Natl Acad Sci U S A. 2008;105:14579–14584. doi: 10.1073/pnas.0802668105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barquilla A, Saldivia M, Diaz R, Bart JM, Vidal I, Calvo E, Hall MN, Navarro M. Third target of rapamycin complex negatively regulates development of quiescence in Trypanosoma brucei. Proc Natl Acad Sci U S A. 2012;109:14399–14404. doi: 10.1073/pnas.1210465109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton J, Steele S, Ziehr B, Moorman N, Kawula T. Feeding uninvited guests: mTOR and AMPK set the table for intracellular pathogens. PLoS Pathog. 2013;9:e1003552. doi: 10.1371/journal.ppat.1003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carling D, Sanders MJ, Woods A. The regulation of AMP-activated protein kinase by upstream kinases. Int J Obes (Lond) 2008;32(Suppl 4):S55–59. doi: 10.1038/ijo.2008.124. [DOI] [PubMed] [Google Scholar]
- Carling D, Thornton C, Woods A, Sanders MJ. AMP-activated protein kinase: new regulation, new roles? Biochem J. 2012;445:11–27. doi: 10.1042/BJ20120546. [DOI] [PubMed] [Google Scholar]
- Clemmens CS, Morris MT, Lyda TA, Acosta-Serrano A, Morris JC. Trypanosoma brucei AMP-activated kinase subunit homologs influence surface molecule expression. Exp Parasitol. 2009;123:250–257. doi: 10.1016/j.exppara.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo SL, Moncada S. AMPKalpha1 regulates the antioxidant status of vascular endothelial cells. Biochem J. 2009;421:163–169. doi: 10.1042/BJ20090613. [DOI] [PubMed] [Google Scholar]
- Daval M, Foufelle F, Ferre P. Functions of AMP-activated protein kinase in adipose tissue. J Physiol. 2006;574:55–62. doi: 10.1113/jphysiol.2006.111484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SP, Carling D, Hardie DG. Tissue distribution of the AMP-activated protein kinase, and lack of activation by cyclic-AMP-dependent protein kinase, studied using a specific and sensitive peptide assay. Eur J Biochem. 1989;186:123–128. doi: 10.1111/j.1432-1033.1989.tb15185.x. [DOI] [PubMed] [Google Scholar]
- Davies SP, Helps NR, Cohen PT, Hardie DG. 5'-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C alpha and native bovine protein phosphatase-2AC. FEBS Lett. 1995;377:421–425. doi: 10.1016/0014-5793(95)01368-7. [DOI] [PubMed] [Google Scholar]
- Diechtierow M, Krauth-Siegel RL. A tryparedoxin-dependent peroxidase protects African trypanosomes from membrane damage. Free Radic Biol Med. 2011;51:856–868. doi: 10.1016/j.freeradbiomed.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Duarte FV, Amorim JA, Palmeira CM, Rolo AP. Regulation of Mitochondrial Function and its Impact in Metabolic Stress. Curr Med Chem. 2015 doi: 10.2174/0929867322666150514095910. [DOI] [PubMed] [Google Scholar]
- Dunlop EA, Tee AR. The kinase triad, AMPK, mTORC1 and ULK1, maintains energy and nutrient homoeostasis. Biochem Soc Trans. 2013;41:939–943. doi: 10.1042/BST20130030. [DOI] [PubMed] [Google Scholar]
- Fenn K, Matthews KR. The cell biology of Trypanosoma brucei differentiation. Curr Opin Microbiol. 2007;10:539–546. doi: 10.1016/j.mib.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogvadze V, Zhivotovsky B, Orrenius S. The Warburg effect and mitochondrial stability in cancer cells. Mol Aspects Med. 2010;31:60–74. doi: 10.1016/j.mam.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Goldenberg S, Avila AR. Aspects of Trypanosoma cruzi stage differentiation. Adv Parasitol. 2011;75:285–305. doi: 10.1016/B978-0-12-385863-4.00013-7. [DOI] [PubMed] [Google Scholar]
- Gowans GJ, Hawley SA, Ross FA, Hardie DG. AMP is a true physiological regulator of AMP-activated protein kinase by both allosteric activation and enhancing net phosphorylation. Cell Metab. 2013;18:556–566. doi: 10.1016/j.cmet.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graven P, Tambalo M, Scapozza L, Perozzo R. Purine metabolite and energy charge analysis of Trypanosoma brucei cells in different growth phases using an optimized ion-pair RP-HPLC/UV for the quantification of adenine and guanine pools. Exp Parasitol. 2014;141:28–38. doi: 10.1016/j.exppara.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart PC, Mao M, de Abreu AL, Ansenberger-Fricano K, Ekoue DN, Ganini D, Kajdacsy-Balla A, Diamond AM, Minshall RD, Consolaro ME, et al. MnSOD upregulation sustains the Warburg effect via mitochondrial ROS and AMPK-dependent signalling in cancer. Nature communications. 2015;6:6053. doi: 10.1038/ncomms7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Kabani S, Fenn K, Ross A, Ivens A, Smith TK, Ghazal P, Matthews K. Genome-wide expression profiling of in vivo-derived bloodstream parasite stages and dynamic analysis of mRNA alterations during synchronous differentiation in Trypanosoma brucei. BMC Genomics. 2009;10:427. doi: 10.1186/1471-2164-10-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxman S, Riechers A, Sadilek M, Schwede F, Beavo JA. Hydrolysis products of cAMP analogs cause transformation of Trypanosoma brucei from slender to stumpy-like forms. Proc Natl Acad Sci U S A. 2006;103:19194–19199. doi: 10.1073/pnas.0608971103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mony BM, MacGregor P, Ivens A, Rojas F, Cowton A, Young J, Horn D, Matthews K. Genome-wide dissection of the quorum sensing signalling pathway in Trypanosoma brucei. Nature. 2014;505:681–685. doi: 10.1038/nature12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mony BM, Matthews KR. Assembling the components of the quorum sensing pathway in African trypanosomes. Mol Microbiol. 2015;96:220–232. doi: 10.1111/mmi.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan DP, Rolin S, Rodriguez JR, Van Den Abbeele J, Pays E. Slender and stumpy bloodstream forms of Trypanosoma brucei display a differential response to extracellular acidic and proteolytic stress. Eur J Biochem. 2000;267:18–27. doi: 10.1046/j.1432-1327.2000.00935.x. [DOI] [PubMed] [Google Scholar]
- Pantovic A, Krstic A, Janjetovic K, Kocic J, Harhaji-Trajkovic L, Bugarski D, Trajkovic V. Coordinated time-dependent modulation of AMPK/Akt/mTOR signaling and autophagy controls osteogenic differentiation of human mesenchymal stem cells. Bone. 2013;52:524–531. doi: 10.1016/j.bone.2012.10.024. [DOI] [PubMed] [Google Scholar]
- Priest JW, Hajduk SL. Developmental regulation of mitochondrial biogenesis in Trypanosoma brucei. J Bioenerg Biomembr. 1994;26:179–191. doi: 10.1007/BF00763067. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Bridges D, Nakada D, Skiniotis G, Morrison SJ, Lin JD, Saltiel AR, Inoki K. Inhibition of AMPK catabolic action by GSK3. Mol Cell. 2013;50:407–419. doi: 10.1016/j.molcel.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson DM, Herway ST, Fillmore N, Kim H, Brown JD, Barrow JR, Winder WW. AMP-activated protein kinase phosphorylates transcription factors of the CREB family. J Appl Physiol (1985) 2008;104:429–438. doi: 10.1152/japplphysiol.00900.2007. [DOI] [PubMed] [Google Scholar]
- Timms MW, van Deursen FJ, Hendriks EF, Matthews KR. Mitochondrial development during life cycle differentiation of African trypanosomes: evidence for a kinetoplast-dependent differentiation control point. Mol Biol Cell. 2002;13:3747–3759. doi: 10.1091/mbc.E02-05-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CM, Aslam N, Dye C. Replication, differentiation, growth and the virulence of Trypanosoma brucei infections. Parasitology. 1995;111(Pt 3):289–300. doi: 10.1017/s0031182000081841. [DOI] [PubMed] [Google Scholar]
- Um JH, Brown AL, Singh SK, Chen Y, Gucek M, Lee BS, Luckey MA, Kim MK, Park JH, Sleckman BP, et al. Metabolic sensor AMPK directly phosphorylates RAG1 protein and regulates V(D)J recombination. Proc Natl Acad Sci U S A. 2013;110:9873–9878. doi: 10.1073/pnas.1307928110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbaniak MD, Martin DM, Ferguson MA. Global quantitative SILAC phosphoproteomics reveals differential phosphorylation is widespread between the procyclic and bloodstream form lifecycle stages of Trypanosoma brucei. J Proteome Res. 2013;12:2233–2244. doi: 10.1021/pr400086y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassella E, Reuner B, Yutzy B, Boshart M. Differentiation of African trypanosomes is controlled by a density sensing mechanism which signals cell cycle arrest via the cAMP pathway. J Cell Sci. 1997;110(Pt 21):2661–2671. doi: 10.1242/jcs.110.21.2661. [DOI] [PubMed] [Google Scholar]
- Vucicevic L, Misirkic M, Janjetovic K, Vilimanovich U, Sudar E, Isenovic E, Prica M, Harhaji-Trajkovic L, Kravic-Stevovic T, Bumbasirevic V, et al. Compound C induces protective autophagy in cancer cells through AMPK inhibition-independent blockade of Akt/mTOR pathway. Autophagy. 2011;7:40–50. doi: 10.4161/auto.7.1.13883. [DOI] [PubMed] [Google Scholar]
- Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Wu SB, Wei YH. AMPK-mediated increase of glycolysis as an adaptive response to oxidative stress in human cells: implication of the cell survival in mitochondrial diseases. Biochim Biophys Acta. 2012;1822:233–247. doi: 10.1016/j.bbadis.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Xu J, Ji J, Yan XH. Cross-talk between AMPK and mTOR in regulating energy balance. Crit Rev Food Sci Nutr. 2012;52:373–381. doi: 10.1080/10408398.2010.500245. [DOI] [PubMed] [Google Scholar]
- Zmijewski JW, Banerjee S, Bae H, Friggeri A, Lazarowski ER, Abraham E. Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. J Biol Chem. 2010;285:33154–33164. doi: 10.1074/jbc.M110.143685. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.