Abstract
A mixing model derived from first principles describes the bulk density (BD) of intertidal wetland sediments as a function of loss on ignition (LOI). The model assumes that the bulk volume of sediment equates to the sum of self‐packing volumes of organic and mineral components or BD = 1/[LOI/k1 + (1‐LOI)/k2], where k1 and k2 are the self‐packing densities of the pure organic and inorganic components, respectively. The model explained 78% of the variability in total BD when fitted to 5075 measurements drawn from 33 wetlands distributed around the conterminous United States. The values of k1 and k2 were estimated to be 0.085 ± 0.0007 g cm−3 and 1.99 ± 0.028 g cm−3, respectively. Based on the fitted organic density (k1) and constrained by primary production, the model suggests that the maximum steady state accretion arising from the sequestration of refractory organic matter is ≤ 0.3 cm yr−1. Thus, tidal peatlands are unlikely to indefinitely survive a higher rate of sea‐level rise in the absence of a significant source of mineral sediment. Application of k2 to a mineral sediment load typical of East and eastern Gulf Coast estuaries gives a vertical accretion rate from inorganic sediment of 0.2 cm yr−1. Total steady state accretion is the sum of the parts and therefore should not be greater than 0.5 cm yr−1 under the assumptions of the model. Accretion rates could deviate from this value depending on variation in plant productivity, root:shoot ratio, suspended sediment concentration, sediment‐capture efficiency, and episodic events.
Keywords: accretion, marsh, sediment, bulk density, LOI
Key Points
Sediment bulk density can be largely explained by its organic matter concentration
Bulk volume approximates the summed volumes of the mineral and organic fractions
Upper bound of steady‐state accretion defines sustainability of coastal marshes under SLR
1. Introduction
Tidal wetlands have survived in place within the intertidal zone for millennia of rising sea level through biophysical processes that lead to the accumulation of mineral and organic matter, thereby increasing soil volume and surface elevation. Long‐term wetland stability requires that the soil surface elevation must increase at a rate similar to the local rate of relative sea‐level rise (SLR), i.e., they must be in equilibrium with the sea level. Many contemporary tidal wetlands formed during the late Holocene and accumulated up to several meters of deposits at a time when rates of SLR were relatively slow [Redfield and Rubin, 1962; Kelley et al., 1988; Engelhart and Horton, 2012; Engelhart et al., 2015]. With accelerating rates of relative SLR [Vermeer and Rahmstorf, 2009; Horton et al., 2014] and many examples of tidal wetlands converting to open water [Craft et al., 2009], there is an increasing need to understand the limits of the biophysical processes that expand soil volume and elevation.
Wetland elevation and its rate of change represent a balance between local rates of accretion and erosion as these factors ultimately determine the net change in sediment volume per unit area. Sediment volume decreases as a consequence of organic matter decomposition, compaction, and erosion. Sediment volume increases with additions of refractory root and rhizome tissue and deposition onto the sediment surface of mineral and refractory organic particles, which in turn is influenced by aboveground plant biomass. Empirical studies [e.g., Nyman et al., 1993; Turner et al., 2000; Neubauer, 2008] and simulation models [e.g., Kirwan et al., 2010] have illustrated the interactive roles of organic matter and mineral inputs on wetland accretion rates.
The annual surface deposition of mineral and organic material creates a lamination that can be conceptualized as a sediment cohort [Morris and Bowden, 1986], the volume of which will change over time as a consequence of compaction and the subsequent ingrowth and turnover of roots and rhizomes. The final volume of a cohort is achieved when its organic matter content has stabilized, a process that occurs over many years of burial by generations of younger cohorts until the cohort has reached a depth below the root zone [Morris and Bowden, 1986; Davis et al., 2015]. When the wetland is in equilibrium with a constant rate of SLR (i.e., the system is in steady state), the final mass of a cohort and its bulk density will determine the rate of vertical accretion. Many studies have described a variety of transient perturbations—including nutrient pollution, changing atmospheric chemistry, abrupt sea‐level change, and watershed development—that can alter or disrupt steady‐state accretion processes of tidal wetlands [Langley et al., 2009; Kirwan et al., 2011; Deegan et al., 2012; Kirwan and Megonigal, 2013; Mariotti and Fagherazzi, 2013; Weston, 2014; Ratliff et al., 2015]. Nevertheless, in light of the persistence of marsh habitat over centuries of rising sea level [Kemp et al., 2011, 2013], wetland survival must be viewed in the context of the steady state. Transient perturbations return to equilibrium with mean sea level, or they lead to wetland loss and a state change.
A central requirement in many empirical and mechanistic studies of wetland accretion is the need to convert mineral and organic mass to a volume (and therefore thickness) of sediment. The analysis presented here was motivated by the simple objective of describing the physical relationship between sediment dry bulk density (BD) and organic matter concentration expressed as loss on ignition (LOI) in coastal wetlands. A number of publications have explored this relationship for upland or wetland soils, using either empirical models or first principles [Jeffrey, 1970; Stewart et al., 1970; Adams, 1973; Harrison and Bocock, 1981; Gosselink et al., 1984; Grigal et al., 1989; Honeysett and Ratkowsky, 1989; Manrique and Jones, 1991; Federer et al., 1993; Turner et al., 2000; Neubauer, 2008; Périé and Ouime, 2008]. Common limitations of this previous work include datasets that are limited geographically, by a narrow range of possible soil conditions, and by sample size. Herein, we extend these previous analyses to a wide sample of herbaceous and forested tidal wetland ecosystems (>5000 samples across 33 riverine and estuarine systems) from the coastal region of the conterminous United States. The present study tests a quantitative mixing model wherein the proportions and compositions of the two end‐member components, organic and inorganic material, govern the density and volume of tidal wetland sediments and therefore influence steady‐state rates of vertical accretion in tidal wetlands. The results suggest a simple and robust rule for setting upper limits on steady‐state vertical accretion rates in coastal wetlands, a rule that is defensible from first principles.
2. Data and Methods
2.1. Sampling Protocol
We compiled a database of 5075 samples of sediment BD and LOI from 33 wetland sites in estuaries in Maine, Massachusetts, New York, Rhode Island, Delaware, Maryland, Virginia, North and South Carolina, Florida, Alabama, Mississippi, Louisiana, and California. The data were either unpublished and provided by the authors or published and publically available.
The methods for estimating bulk density and LOI in sediment vary in details, such as the operation, type, and dimension of the coring device, the volume of samples analyzed, etc. Despite this variation, they all share a common protocol of drying samples of known wet volume, weighing the dried samples, combusting the samples in a furnace, and reweighing to obtain the weight loss on ignition (LOI) [e.g., Dean, 1974; Percival and Lindsay, 1997]. There are numerous opportunities for error, including compression of the sediment when taking the core and/or when extracting the core from the core tube, imprecise sectioning of the core into known volumes, variation in drying temperature, furnace temperature, and presence of salts that precipitate when the pore water is evaporated from the sample [Heiri et al., 2001]. We think that compression during the coring operation is the most significant source of error, but to our knowledge, the relative sizes of these errors have not been evaluated.
2.2. Statistics
The Marquardt‐Levenberg method [Levenberg, 1944] of the SAS 9.4 PROC MODEL was used to estimate the unknown parameters in several alternative nonlinear models describing the relationship between sediment BD and LOI. PROC MODEL is an iterative procedure that finds the combination of parameters that minimizes the sum of squares of residuals. The procedure reports an adjusted R2 value for the model being fitted and significance tests with associated probabilities that the parameters differ significantly from zero. Nonlinear regression analysis has an advantage over linear regression, which requires that nonlinear data must first be linearized. Linear transformation introduces a bias by changing the relative weights of individual observations. Additional tests were performed (PROC REG, PROC GLM) with log‐transformed data to test for differences across sites, including homogeneity of slopes and LOI‐by‐site interactions. A massive dataset (N > 6000) from the Louisiana Coastwide Reference Monitoring System (CRMS) was randomly subsampled using PROC SURVEYSELECT to bring it in line with the number of samples from other sites and to prevent a deltaic bias.
3. Results
3.1. Site Characteristics
The 33 sites analyzed (Table 1) represented varied climatic conditions, tidal amplitudes, soil compositions, and species. Most samples are from the near surface (50 cm), except for cores from Sand Hill, NC that were nearly 3 m in length and cores from Maine, Patuxent River, MD, and Otter Point, MD, where 1–2 m of sediment was sampled. Sediments were predominately siliciclastic, with varying amounts of organic matter, but also included calcareous sediments from the coastal Everglades (FCE). The sites spanned 14 degrees of latitude. Across sites, mean sediment LOI ranged from 0.12 g g−1 at Grand Bay, MS; China Camp, CA; and Otter Creek, MD to 0.79 g g−1 at GCREW near Edgewater, MD. Mean dry bulk density ranged from 0.12 g cm−3 at GCREW to 0.78 g cm−3 at North Inlet, SC.
Table 1.
Sites Included in the Analysis, Their Geographical and Physical Characteristics, Mean (±SD) Loss on Ignition (LOI) and Bulk Density (BD), Sample Size (N), and Data Sources
| Site | Community Type | Latitude (oN) | Tidal Amp (cm) | Loss on Ignition (g g−1) | Bulk Density (g cm−3) | N |
|---|---|---|---|---|---|---|
| Apcola, FLa | Tidal brackish marsh | 29.8 | 25 | 0.43 ± 0.12 | 0.14 ± 0.07 | 297 |
| FCEman, FLb | Everglades brackish mangrove | 25.2 | 10 | 0.26 ± 0.14 | 0.37 ± 0.14 | 72 |
| FCEmmm, FLb | Everglades mixed mangrove‐marsh | 25.2 | 5 | 0.17 ± 0.15 | 0.35 ± 0.13 | 41 |
| FCEbtf, FLb | Everglades brackish tidal forested | 25.4 | 20 | 0.81 ± 0.13 | 0.14 ± 0.03 | 39 |
| FCEbtma, FLb | Everglades brackish tidal mangrove | 25.4 | 30 | 0.37 ± 0.18 | .032 ± 0.14 | 80 |
| CRMS, LAh | Tidal salt to tidal fresh marsh | 29 | 0.38 ± 0.24 | 0.28 ± 0.22 | 500 | |
| GrandB, MSa | S. alterniflora/Juncus tidal marsh | 30 | 22 | 0.12 ± 0.04 | 0.39 ± 0.15 | 59 |
| WeeksB, ALa | Tidal brackish marsh | 30.4 | 20 | 0.17 ± 0.04 | 0.35 ± 0.08 | 60 |
| NorthI, SCa | Tidal salt marsh, S. alterniflora | 33.3 | 70 | 0.13 ± 0.08 | 0.78 ± 0.34 | 278 |
| Waccam, SCa | Tidal fresh marsh | 33.5 | 60 | 0.55 ± 0.16 | 0.13 ± 0.09 | 337 |
| Smyrna, NCa | Tidal brackish marsh Juncus roemerianus | 34.8 | 19 | 0.27 ± 0.15 | 0.43 ± 0.37 | 416 |
| SandH, NCa | Tidal brackish marsh Juncus roemerianus | 35 | 5 | 0.56 ± 0.20 | 0.23 ± 0.26 | 145 |
| James, VAa | Tidal fresh marsh, Polygonum, Peltandra, Zizania, others, & forest | 37.3 | 40 | 0.21 ± 0.06 | 0.44 ± 0.13 | 25 |
| Pamunk, VAa,e | Tidal fresh marsh | 37.6 | 44 | 0.21 ± 0.08 | 0.35 ± 0.15 | 401 |
| Browns Isl, CAl | Tidal brackish marsh, Schoenoplectus spp. | 38.0 | 46 | 0.35 ± 0.17 | 0.29 ± 0.15 | 113 |
| ChinaCp, CAl | Tidal salt marsh, Spartina foliosa, Sarcocornia pacifica. | 38.0 | 66 | 0.12 ± 0.04 | 0.50 ± 0.10 | 134 |
| CoonIs, CAl | Tidal salt marsh, S. foliosa, S. pacifica | 38.2 | 72 | 0.16 ± 0.09 | 0.44 ± 0.22 | 172 |
| MonieBay, MDc,d | Tidal brackish marsh, S. alterniflora | 38.2 | 15 | 0.27 ± 0.18 | 0.49 ± 0.49 | 189 |
| PetalumaR, CAl | Tidal salt marsh, S. foliosa, S. pacificia | 38.2 | 75 | 0.18 ± 0.08 | 0.4 ± 0.21 | 111 |
| RushRa, CAl | Tidal brackish marsh, Schoenoplectus spp. | 38.2 | 86 | 0.27 ± 0.15 | 0.38 ± 0.19 | 138 |
| Choptk, MDc | Tidal fresh marsh | 38.7 | 16 | 0.27 ± 0.05 | 0.30 ± 0.07 | 45 |
| PatuxR, MDc,g | Tidal fresh marsh, mixed | 38.7 | 28 | 0.34 ± 0.17 | 0.29 ± 0.14 | 230 |
| GCREW, MDa | Brackish tidal marsh, Schoenoplectus americanus, Spartina patens | 38.9 | 14 | 0.79 ± 0.05 | 0.12 ± 0.02 | 117 |
| OttCr, MDc | Tidal fresh marsh | 39.4 | 19 | 0.12 ± 0.02 | 0.68 ± 0.19 | 45 |
| DelRiv, DEf | Tidal fresh marsh | 39.8 | 91 | 0.14 ± 0.04 | 0.27 ± 0.17 | 11 |
| JamBay, NYj,k | S. alterniflora salt marsh | 40.6 | 80 | 0.41 ± 0.18 | 0.31 ± 0.32 | 133 |
| NarrBay, RIa,i | Tidal salt marsh, S. patens, D. spicata, S. alterniflora | 41.6 | 65 | 0.28 ± 0.19 | 0.41 ± 0.31 | 127 |
| Hudson, NYc | Tidal fresh marsh | 42 | 61 | 0.16 ± 0.1 | 0.58 ± 0.21 | 103 |
| PlumIs, MAa | S. patens, S. alterniflora salt marsh | 43 | 150 | 0.29 ± 0.14 | 0.26 ± 0.12 | 320 |
| Moody, MEa | Tidal salt marsh, S. patens, D. spicata, S. alterniflora | 43.2 | 134 | 0.31 ± 0.14 | 0.34 ± 0.22 | 52 |
| Webhannet, MEa | Tidal salt marsh, S. patens, D. spicata, S. alterniflora | 43.3 | 134 | 0.25 ± 0.13 | 0.44 ± 0.20 | 36 |
| Morse, MEa | Tidal salt marsh, S. patens, D. spicata, S. alterniflora | 43.7 | 139 | 0.22 ± 0.12 | 0.42 ± 0.21 | 37 |
| Sprague, MEa | Tidal salt marsh, S. patens, D. spicata, S. alterniflora | 43.7 | 139 | 0.20 ± 0.12 | 0.43 ± 0.24 | 212 |
| Grand Means | 0.30 ± 0.17 | 0.36 ± 0.14 | 5075 |
aoriginal data; b Chambers and Pederson, 2006 and http://fcelter.fiu.edu/data/; c Merrill, 1999; d Ward et al., 1988; e Neubauer, 2000; f Church et al., 2006; Orson et al., 1992; g Khan and Brush, 1994; h http://cims.coastal.la.gov/monitoring‐data/; i Wigand et al., 2003; j Wigand et al., 2014; k Swanson and Wilson, 2008; l Callaway et al., 2012
The dominant species vary among sites, with Spartina patens and Spartina alterniflora most common in northeastern and southeastern estuaries, respectively; Spartina foliosa and Schoenoplectus in the San Francisco estuary; a mix of Spartina species and brackish species (S. cynosuroides and/or Juncus roemerianus) in the Gulf Coast and North Carolina estuaries; and the red mangrove Rhizophora mangle in several Everglades sites. The tidal freshwater wetlands typically contained a diverse mixture of herbaceous species, including Peltandra virginica, Pontederia cordata, Zizania aquatica, and Zizaniopsis miliacea, with some locations also containing trees (e.g., Nyssa aquatica, Taxodium distichum). There was a weak, but insignificant (p = 0.25) decline of mean LOI by site with increasing latitude (−0.01 per degree) and a weak decline in LOI with tidal amplitude (−0.002 per cm, R 2 = 0.27, p = 0.05).
Tests of homogeneity of slopes of log‐normalized data were significant (p < 0.0001). Likewise, an analysis of covariance established that LOI varies among sites: the independent variables site, ln(LOI), and the ln(LOI) x site interaction term were all significant (p < 0.0001) for type I and type III errors, meaning that there is no reasonable chance that these samples behave in the same way with respect to the dependence of log‐transformed BD on log‐transformed LOI.
3.2. Model 1 Power Function
The first model tested (Figure 1) is one that is commonly used to estimate bulk density (BD) from LOI measurements. It is an empirical power function of the form
| (1) |
where a and b are empirical constants. The equation was fitted with an R 2 of 0.73. Parameter a = 0.141 ± 0.002 (p < 0.0001) and b = −0.59 ± 0.004 (p < 0.0001).
Figure 1.
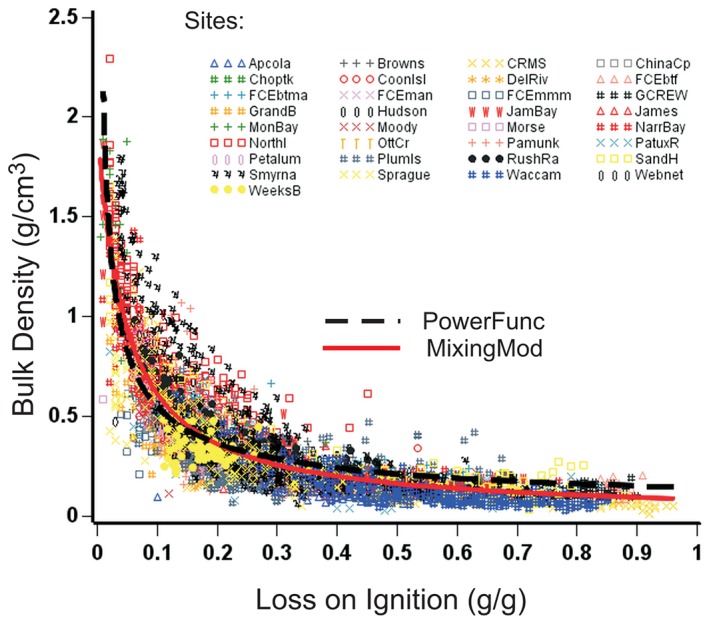
Best fits of the power function ( ) BD = a LOIb and ideal mixing model (
) BD = a LOIb and ideal mixing model ( ) BD = 1/[LOI/k1 + (1 − LOI)/k2)] to the complete collection of 5075 sediment samples from 33 tidal marshes and mangroves distributed around the United States. The data in Figure 1 are available in Appendix S1, Supporting Information
) BD = 1/[LOI/k1 + (1 − LOI)/k2)] to the complete collection of 5075 sediment samples from 33 tidal marshes and mangroves distributed around the United States. The data in Figure 1 are available in Appendix S1, Supporting Information
3.3. Model 2 Power Function With Intercept
This model differs from the first only in having a nonzero intercept c. It is an empirical power function of the form
| (2) |
where a, b, and c are empirical constants. The equation was fitted with an R 2 of 0.77. Parameter a = 0.569 ± 0.03 (p < 0.0001), b = −0.31 ± 0.01 (p < 0.0001), and c = −0.55 ± 0.04 (p < 0.0001).
3.4. Model 3 Ideal Mixing Model
Stewart et al. [1970] suggested that in natural soils containing organic matter, there is a relationship between soil apparent density and organic matter content, namely that the bulk volume of a soil approximates to the summed self‐packing volumes of the organic and mineral components. In other words, it is assumed that the bulk densities of pure organic matter and pure mineral matter are assumed constants, and in a mixture, the volumes occupied by the organic and mineral components are additive [Federer et al., 1993]. For example, starting with dry weights Wo and Wi of organic and inorganic matter having bulk densities of k1 and k2, and volumes Vo = Wo/k1 and Vi = Wi/k2, when the two are mixed, the resulting bulk density BD = (W o + Wi)/(W o/k1 + Wi/k2). The mixture will have a loss on ignition LOI = Wo/(W o + W i). Rearranging W o + W i = W o/LOI and substituting gives BD = (W o/LOI)/(W o/k1 + Wi/k2). Substituting for Wi gives BD = (W o/LOI)/[W o/k1 + (W o/LOI − W o)/k2] , which reduces to
| (3) |
This is known as the ideal mixing model [Adams, 1973]. Coefficients k1 and k2 are the bulk, self‐packing densities of pure organic and mineral matter, respectively.
The best fit over all sites (R 2 = 0.78) gave k1 and k2 coefficients of 0.085 ± 0.0007 g cm−3 (p < 0.0001) and 1.99 ± 0.028 g cm−3 (p < 0.0001), respectively (Figure 1). Note that when LOI = 0, Eq. 3 reduces to BD = k2, and Eq. 3 reduces to BD = k1 when LOI = 1. When fitted specifically to each site, the resulting k1 values varied from 0.05 to 0.168 g cm−3 and k2 from 0.59 to 2.87 g cm−3. Parameter estimates made from site‐specific data were unreliable because few sites possessed the full spectrum of BD and LOI values needed for a good fit.
3.5. Vertical Accretion
There have been several analyses of the relative contribution of organic and inorganic accretion to vertical accretion using a top–down approach [Nyman et al., 1993, 2006; Turner et al., 2000; Neubauer, 2008]. Starting with known accretion rates and composition, Turner et al. [2000] and Neubauer [2008] calculated the densities of organic and mineral matter that best fit the total accretion rates by linear regression. From their published inventories of mineral and organic matter above the 1963 137Cs maxima, we calculated vertical accretion by dividing the mass accretion rates (g cm−2 yr−1) by the k1 and k2 densities, 0.085 and 1.99 g cm−3, respectively, from the present study. Our calculated vertical accretion rates and their measured rates (Figure 2) were highly correlated (r 2 = 0.6, p < 0.0001).
Figure 2.
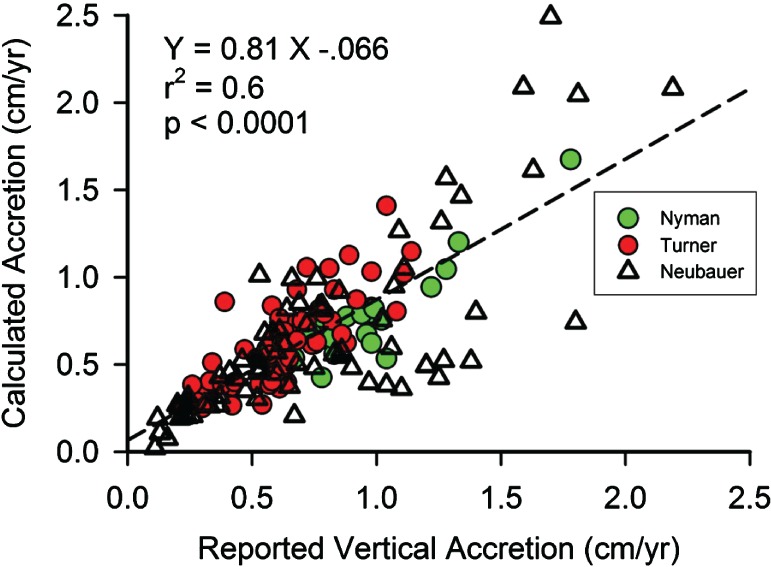
Mixing model‐calculated accretion rates and reported 137Cs‐based vertical accretion rates. Calculations used published mass accretion rates (g m−2 yr−1) of inorganic and organic matter as reported in Nyman et al. [1993, 2006], Turner et al. [2000], and Neubauer [2008].
With estimates of k1 and k2, using a bottom–up approach, it is possible to parse vertical accretion between its organic and mineral fractions and to reasonably estimate rates of vertical accretion across a range of suspended sediment concentrations and LOI (Figure 3). We used suspended sediment (TSS) concentrations ranging from 10 to 200 mg L−1 and assumed depths of high water over the marsh surface of 1, 5, and 20 cm—flood depths representative of high, mid, and low marsh. Following the logic of Krone [1987], the depth of high water determines the volume of water over the marsh surface and, together with the tide range, approximately defines the hydroperiod or duration of flooding, but as we are assuming a 100% capture efficiency, the explicit calculation of the hydroperiod is avoided. A marsh surface that captures all of the suspended sediment when flooded semi‐diurnally (704 floods per year) by 20 cm of water at high tide would receive an annual deposit of 0.42 g cm−2 yr−1 (=3 × 10−5 g cm−3 × 20 cm × 704 yr−1), using a flow‐weighted, average suspended sediment concentration of 30 mg/liter for East and eastern Gulf Coast estuaries [Weston, 2014]. Dividing by k2 (1.99 g cm−3) gives 0.2 cm/yr.
Figure 3.
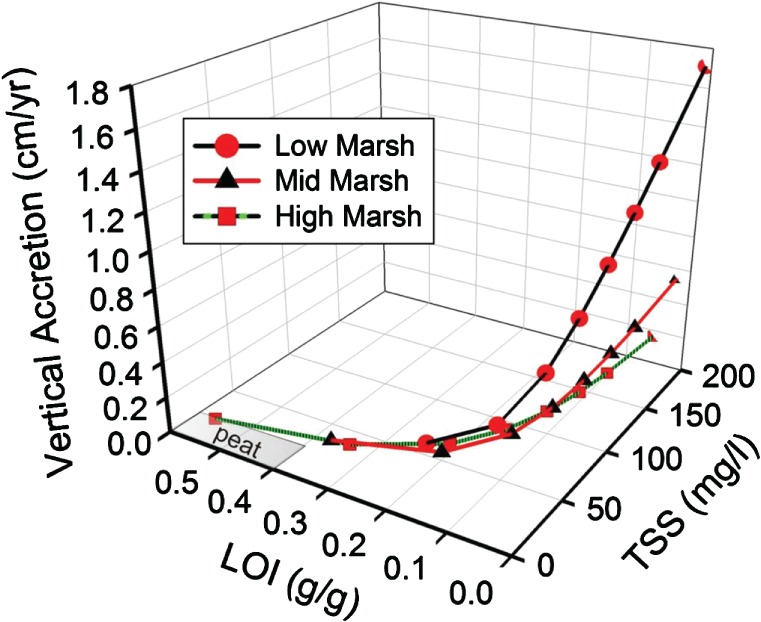
A curve family describing relationships among vertical accretion, soil organic content (LOI), and total suspended inorganic sediment (TSS) in hypothetical high‐, mid‐, and low‐marsh habitats. Calculations of vertical accretion due to organic production and mineral sedimentation are based on the pure packing densities k1 and k2 (see text) derived from a fit of the mixing model to the entire dataset. High, mid, and low marshes were defined as having high tides that flood the surface semi‐diurnally, with average water depths of 1, 5, and 20 cm, and with belowground refractory organic matter inputs of 80, 160, and 250 dry g m−2 yr−1, respectively. The grey area is the domain of peat formation, truncated at LOI = 0.6.
As the production of the vegetation is also sensitive to flood depth [Morris et al., 2002, 2013], we assumed belowground productivities of 800, 1600, and 2500 g dry weight m−2 yr−1 in high, mid, and low marsh, respectively, with 10% preservation. The organic contribution to vertical accretion is determined by the input of the refractory organic production, which should be nearly equal to the production of lignin. Using a lignin concentration of 10% [Hodson et al., 1984; Wilson et al., 1986; Buth and Voesenek, 1987], the vertical accretion due to a theoretical maximum belowground dry weight production of 2500 g m−2 yr−1 for Spartina alterniflora [Morris et al., 1984] should be about 0.3 cm yr−1 ≈ (0.25 g cm−2 yr−1 × 0.1 g lignin/g dry weight)/ 0.085 g cm−3. Shown here (Figure 3) are the vertical accretion rate and LOI resulting from various combinations of inputs. The results show that the highest vertical accretion rates are possible only at the highest TSS concentrations, that is, the highest inputs of mineral matter (Figure 3). However, for conditions typical of East Coast estuaries (high production, low TSS), organic matter probably contributes about 3/5 or 60% to vertical accretion, which is in line with Neubauer's [2008] estimate of 62%.
4. Discussion
The variability in BD not explained by the mixing model can be due to sampling and analytical errors, as discussed earlier, and to violations of the model assumptions, namely that k1 and k2 are constant and their volumes additive. Additivity is not strictly true because organic molecules encapsulated within the lattices of clay minerals or sorbed to the surfaces of clay minerals [Mortland, 1970; van Veen and Kuikman, 1990; Mayer, 1994; Hedges and Keil, 1995; Bergamaschi et al., 1997; Mayer et al., 2004] may raise the LOI without adding to the total volume. Furthermore, a portion of the variability also could be due to compaction or differences in sediment grain size. The mass per unit volume of the solid inorganic component, that is, excluding voids and water, is commonly taken to be 2.65 g cm−3 [Skopp, 2000; Blanco‐Canqui et al., 2004]. The difference between the solid inorganic density and k2 equates to an average porosity of 25%, but porosity and bulk density are functions of grain size [Manrique and Jones, 1991; Jackson et al., 2014], which is variable. However, the fidelity of the mixing model (Figure 1) suggests that these deviations from the model assumptions are minor, at least within the universe of data analyzed here.
There was a weak, but insignificant (p = 0.25) decline of mean LOI by site with increasing latitude (−0.01 per degree). If the concentration of organic matter in sediment was reduced by rising temperature, perhaps due to an increase in decay rate [Kirschbaum, 1995], then we would have expected an increase in LOI with latitude. Alternatively, a decline with latitude would be consistent with a trend of decreasing aboveground productivity in salt marshes with increasing latitude [Kirwan et al., 2009]. This decline with latitude is also consistent with the interpretation of Philben et al. [2014, 2015] who found that peat formed at more southerly latitudes and, during the Holocene Climatic Optimum, did not appear to be any more decomposed than peat formed in cooler latitudes or during subsequent cooler periods in younger sections of the cores. They suggested that decomposition in peatlands was regulated primarily by oxygen‐exposure time, not by temperature.
We found a weakly negative (−0.002 cm−1) but significant (p = 0.05) effect of tidal amplitude on LOI. Tidal amplitude could affect LOI in a number of ways, for example, by affecting dewatering and oxygen exposure, hydroperiod, and/or the effect of tidal energy on mineral sediment transport. For a fixed input of organic matter, the resulting LOI will be determined by the input of mineral matter. The latter would be controlled by the concentration of suspended sediment as well as the hydroperiod or settling time for suspended sediment.
Mixing model parameters k1 and k2 were used to parse total vertical accretion into its organic and mineral fractions and to place upper limits on vertical accretion. With respect to the contributions from organic production, it can be assumed that only belowground biomass contributes to soil organic matter in tidal salt marshes because we do not observe the accumulation of a litter layer on the marsh surface. Furthermore, excluding sites of marsh restoration or newly emerging marshes where the live biomass may build over a period of years, the input of labile organic matter does not add new volume because its production and decay rate are in equilibrium, that is, the volume of living and labile organic matter exists in steady state. The refractory fraction of belowground production, however, does contribute to the change in soil volume, and we can equate this to the production of lignin, which is considered to be the most refractory part of plant tissue and is a significant component of soil organic matter in anaerobic environments such as peatlands [Morita, 1962]. Its decomposition depends primarily on the supply of oxygen [Andriesse, 1988; Williams and Yavitt, 2003]. An upper limit for belowground dry weight production of 2500 g m−2 yr−1 containing 10% lignin gives a theoretical steady‐state vertical accretion of about 0.3 cm yr−1 (3 mm yr−1). Thus, peat marshes are not likely to indefinitely survive an accelerating SLR without a source of mineral sediment.
Empirical measures of belowground production sometimes exceed the generalized estimate above 2500 g m−2 yr−1, for example, 3500 g m−2 yr−1 in Great Sippewissett marsh [Valiela et al., 1976], 6500 g m−2 yr−1 in Delaware Bay [Roman and Daiber, 1984], and 7900 g m−2 yr−1 in Narragansett Bay [Wigand, 2008]. Measurements of belowground production are subject to significant sampling error [Singh et al., 1984], but theoretical estimates associated with model assumptions also have uncertainty. Moreover, maximum productivity varies greatly among plant communities and species. The productivity of tidal freshwater marshes, for example, exceeds that of salt marshes [Odum, 1988]. Primary production can also vary strongly with factors such as interannual variation in sea level [Morris et al., 2002], nitrogen loading, and atmospheric CO2 concentration [Langley et al., 2009]. Therefore, belowground production might provide greater accretion than the 0.3 cm yr−1 estimated here and also potentially greater rates of carbon sequestration.
The relative importance of inorganic and organic contributions to vertical accretion is dynamic over long timescales [Kearney et al., 1994; Drexler, 2011] and can vary spatially in contemporary settings [Craft et al., 1993]. Vertical accretion is ultimately limited by the mass inputs of mineral and refractory organic material, constrained by their bulk densities. Mass accretion rates of mineral and organic matter in tidal freshwater and salt water wetlands and 137Cs‐based vertical accretion have been reported by Nyman et al. [1993, 2006], Turner et al. [2000], and Neubauer [2008] among others, and these authors have advanced the argument that organic matter accretion is the principal means by which marshes accrete vertically. Our analysis supports this view, considering the low TSS concentrations typical of many estuaries, but we would argue that vertical accretion is limited by low availability of mineral sediment. Using a top–down approach, Turner et al. [2000] regressed the vertical accretion rate against organic matter accumulation and found a slope of 11 cm3 g−1. Its reciprocal is k1 and, ignoring a regression intercept, is equivalent to 0.09 g cm−3. His regression of vertical accretion rate against inorganic accumulation rate gave a slope of 1.36 cm3 g−1 or 0.73 g cm−3, which is significantly less than k2 derived in our study. Using multiple linear regression of vertical accretion rate against organic plus mineral accumulation, Neubauer [2008] derived densities equivalent to 0.182 g cm−3 and 0.847 g cm−3 for the organic and mineral fractions, respectively. The differences between coefficients derived by Turner et al. [2000] and Neubauer [2008] and our calculated values of k1 and k2 reflect the limited range of soil properties in those other studies, notably a near absence of high LOI samples.
Several generalities emerge from our bottom–up calculations of vertical accretion (Figure 3). Firstly, the theoretical maximum vertical accretion rate at steady state for a typical marsh on the East Coast and eastern Gulf is probably not greater than 0.5 cm yr−1. This is the expected vertical accretion from the combined inputs of organics and inorganics at the highest loading rates, that is, complete capture of 30 mg L−1 suspended sediment under 20 cm of water flooding the surface semi‐diurnally and with the addition of 250 g dry weight m−2 yr−1 of refractory organic matter. However, a significantly higher sediment load, like that available in a deltaic environment, for example, 200 mg L−1, could support a vertical accretion rate of 1.7 cm yr−1 (Figure 3). Secondly, the lowest concentration of sediment organic matter or LOI occurs at the highest vertical accretion rates for a marsh at steady‐state equilibrium with sea level, reflecting low marsh conditions with high mineral inputs. Conversely, the highest LOI occurs at the lowest accretion rates and in high marsh habitat with little flooding (Figure 3). High marsh habitat has the lowest opportunity for mineral loading from tidal flooding and, thus, little supplement to organic accretion by inorganic material.
Our analysis addresses marshes operating in steady state with typical rates of plant production and sediment availability. However, marshes often accrete sediment at different rates (Figure 2) because they are not always constrained by the limits we have assumed on mineral and organic matter accretion. Locally, there may be erosion of marsh edge and redeposition onto the remaining marsh platform [Wilson et al., 2014] or episodes of rapid sedimentation following hurricanes [Cahoon et al., 1995; Turner et al., 2006; McKee and Cherry, 2009; Baustian and Mendelssohn, 2015]. However, storm impacts also can be negative [Chabreck and Palmisano, 1973; Cahoon, 2006; Howes et al., 2010; Morton and Barras, 2011; Leonardi et al., 2016], and it seems unlikely that storms could contribute globally to a positive, steady‐state accretion or that they could accommodate an acceleration in sea‐level rise. There are also situations when a system may be far removed from the local equilibrium state. What follows is a rapid recovery and accretion toward the equilibrium [French and Burningham, 2003]. This could happen following a wetland restoration, an emergence of a bar at the mouth of a delta, or a sudden loss in relative elevation due to storm‐induced erosion or to mechanical means. These are transient conditions that could affect short‐term accretion rates but that should not affect the steady‐state accretion rate. Also possible are changes in state following a change in nutrient loading, which could affect organic matter production and preservation or a change in sediment loading. These could increase or decrease the steady‐state accretion rate, therefore affecting the ability of tidal wetlands to maintain elevation relative to a rising sea level.
A tidal wetland with a high LOI and low accretion rate is not necessarily at its maximum rate of vertical accretion because the mineral loading rate and primary productivity will depend on its relative elevation, depth of flooding, and suspended sediment concentration. With an accelerated rate of SLR, the relative, equilibrium marsh elevation will fall, and the vertical accretion rate will rise [Morris et al., 2002]. It is only when the equilibrium elevation falls below the optimum for primary production that the marsh will fail to keep up with the acceleration in SLR. Our analysis, which assumes only chronic inputs, suggests that this will most likely occur when the local rate of SLR (including subsidence) exceeds about 0.5 cm yr−1 in most East Coast estuaries or 1.7 cm yr−1 in a sediment‐rich estuary. Thus, even current rates of SLR along the East Coast (mean = 0.34 cm yr−1, range = 0.18–0.60 cm yr−1) (http://tidesandcurrents.noaa.gov/sltrends/sltrends.html) are near the point that will lead to long‐term elevation loss and eventual drowning of coastal wetlands.
Supporting information
Supporting Information S1
Acknowledgments
This research was supported by NOAA grant NA10NOS4780146, NSF DEB grants 1052636, 0620409, 9910514, 1237517, 1127958, 0950090, 1457100 and 1355059, NSF EAR grants 1322859 and 1322658, NSF OCE‐1238212, NICCR grant DE‐FC02‐06ER64298, NASA grant NNH14AY67, the Bates College Student Research Fund, the Bryn Mawr College Summer Science Research Program, and the Blue Carbon Initiative coordinated by Conservation International, IUCN, and IOC‐UNESCO.
References
- Adams, W. A. (1973), The effect of organic matter on the bulk and true densities of some uncultivated podzolic soils, J. Soil Sci., 24, 10–17. [Google Scholar]
- Andriesse, J. P. (1988), Nature and management of tropical peat soils, FAO Soils Bull. 59, Food and Agriculture Organization United Nations, Rome.
- Baustian, J. J. , and Mendelssohn I. A. (2015), Hurricane‐induced sedimentation improves marsh resilience and vegetation vigor under high rates of relative sea level rise, Wetlands, 35, 795–802. [Google Scholar]
- Bergamaschi, B. A. , Tsamakis E., Keil R. G., Eglinton T. I., Montluçon D. B., and Hedges J. I. (1997), The effect of grain size and surface area on organic matter, lignin and carbohydrate concentration, and molecular compositions in Peru Margin sediments, Geochim. Cosmochim. Acta, 61, 1247–1260. [Google Scholar]
- Blanco‐Canqui, H. , Gantzer C. J., Anderson S. H., and Alberts E. E. (2004), Tillage and crop influences on physical properties for an Epiaqualf, Soil Sci. Soc. Am. J., 68, 567–576. [Google Scholar]
- Buth, G. J. C. , and Voesenek L. A. C. J. (1987), Decomposition of standing and fallen litter of halophytes in a Dutch salt marsh, in Geobotany 11: Vegetation Between Land and Sea, edited by Huiskes A. H. L., Blom C. W. P. M., and Rozema J., pp. 146–165, Dr. W. Junk Pub, Dordrecht, The Netherlands. [Google Scholar]
- Cahoon, D. R. , Reed D. J., Day J. W. Jr., Steyer G. D., Boumans R. M., Lynch J. C., McNally D., and Latif N. (1995), The influence of Hurricane Andrew on sediment distribution in Louisiana coastal marshes, J. Coastal Res., 21, 280–294. [Google Scholar]
- Cahoon, D. R. (2006), A review of major storm impacts on coastal wetland elevations, Estuaries Coasts, 29, 889–898. [Google Scholar]
- Callaway, J. C. , Borgnis E. L., Turner R. E., and Milan C. S. (2012), Carbon sequestration and sediment accretion in San Francisco Bay tidal wetlands, Estuar. Coast., 35, 1163–1181. [Google Scholar]
- Chabreck, R. H. , and Palmisano A. W. (1973), The effects of Hurricane Camille on the marshes of the Mississippi River Delta, Ecology, 54, 1118–1123. [Google Scholar]
- Chambers, R. M. , and Pederson K. A. (2006), Variation in soil phosphorus, sulfur, and iron pools among south Florida wetlands, Hydrobiologia, 569, 63–70. [Google Scholar]
- Church, T. M. , Sommerfield C. K., Velinsky D. J., Point D., Benoit C., Amouroux D., Plaa D., and Donard O. F. X. (2006), Marsh sediments as records of sedimentation, eutrophication and urban pollution in the urban Delaware Estuary, Mar. Chem., 102, 72–95. [Google Scholar]
- Craft, C. , Clough J., Ehman J., Joye S., Park R., Pennings S., Guo H., and Machmuller M. (2009), Forecasting the effects of accelerated sea‐level rise on tidal marsh ecosystem services, Frontiers Ecol., 7, 73–78. [Google Scholar]
- Craft, C. B. , Senica E. D., and Broome S. W. (1993), Vertical accretion in microtidal regularly and irregularly flooded estuarine marshes, Estuarine Coastal Shelf Sci., 37, 371–386. [Google Scholar]
- Davis, J. L. , Currin C. A., O'Brien C., Raffenburg C., and Davis A. (2015), Living shorelines: coastal resilience with a blue carbon benefit, PLoS One, 10(11), e0142595, doi:10.1371/journal.pone.0142595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean, W. E., Jr. (1974), Determination of carbonate and organic matter in calcareous sediments and sedimentary rocks by loss on ignition: Comparison with other methods, J. Sed. Res., 44, 242–248. [Google Scholar]
- Deegan, L. A. , Johnson D. S., Warren R. S., Peterson B. J., Fleeger J. W., Fagherazzi S., and Wollheim W. M. (2012), Coastal eutrophication as a driver of saltmarsh loss, Nature, 490, 388–392. [DOI] [PubMed] [Google Scholar]
- Drexler, J. Z. (2011), Peat formation processes through the millennia in tidal marshes of the Sacramento‐San Joaquin Delta, California, USA, Estuaries Coasts, 34, 900–911. [Google Scholar]
- Engelhart, S. E. , and Horton B. P. (2012), Holocene sea level database for the Atlantic coast of the United States, Quat. Sci. Rev., 54, 12–25. [Google Scholar]
- Engelhart, S. E. , Vacchi M., Horton B. P., Nelson A. R., and Kopp R. E. (2015), A sea‐level database for the Pacific coast of central North America, Quat. Sci. Rev., 113, 78–92, doi:10.1016/j.quascirev.2014.12.001. [Google Scholar]
- Federer, C. A. , Turcotte D. E., and Smith C. T. (1993), The organic fraction–bulk density relationship and the expression of nutrient content in forest soils, Can. J. Forest Res., 23, 1026–1032. [Google Scholar]
- French, J. R. , and Burningham H. (2003), Tidal marsh sedimentation versus sea‐level rise: a southeast England estuarine perspective, in Proceedings International Conference on Coastal Sediments 2003, edited by Davis R. A., pp. 1–14, World Scientific Publishing and East Meets West Productions, Corpus Christi, Tex. [Google Scholar]
- Gosselink, J. G. , Hatton R., and Hopkinson C. S. (1984), Relationship of organic carbon and mineral content to bulk density in Louisiana marsh soils, Soil Sci., 137, 177–180. [Google Scholar]
- Grigal, D. F. , Brovold S. L., Nord W. S., and Ohmann L. F. (1989), Bulk density of surface soils and peat in the North Central United States, Can. J. Soil Sci., 69, 895–900. [Google Scholar]
- Harrison, A. F. , and Bocock K. L. (1981), Estimation of soil bulk‐density from loss‐on‐ignition values, J. Appl. Ecol., 8, 919–927. [Google Scholar]
- Hedges, J. I. , and Keil R. G. (1995), Sedimentary organic matter preservation: an assessment and speculative synthesis, Mar. Chem., 49, 81–115. [Google Scholar]
- Heiri, O. , Lotter A. F., and Lemcke G. (2001), Loss on ignition as a method for estimating organic and carbonate content in sediments: Reproducibility and comparability of results, J. Paleolimnol., 25, 101–110. [Google Scholar]
- Hodson, R. E. , Christian R. R., and Maccubbin A. E. (1984), Lignocellulose and lignin in the salt marsh grass Spartina alterniflora: Initial concentrations and short‐term, post‐depositional changes in detrital matter, Marine Biol., 81, 1–7. [Google Scholar]
- Honeysett, J. L. , and Ratkowsky D. A. (1989), The use of ignition loss to estimate bulk density of forest soils, J. Soil Sci., 40, 1365–2389. [Google Scholar]
- Horton, B. P. , Rahmstorf S., Engelhart S. E., and Kemp A. C. (2014), Expert assessment of sea‐level rise by AD 2100 and AD 2300, Quat. Sci. Rev., 84, 1–6. [Google Scholar]
- Howes, N. C. , FitzGerald D. M., Hughes Z. J., Georgiou I. Y., Kulp M. A., Miner M. D., Smith J. M., and Barras J. A. (2010), Hurricane‐induced failure of low salinity wetlands, Proc. Natl. Acad. Sci. U. S. A., 210, 14014–14019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, C. R. , Thompson J. A., and Kolka R. K. (2014), Wetland soils, hydrology and geomorphology, Chapter 2, in Ecology of Freshwater and Estuarine Wetlands, edited by Batzer D. and Sharitz R. , pp. 23–60 , University of California Press, Berkeley. [Google Scholar]
- Jeffrey, D. W. (1970), A note on the use of ignition loss as a means for the approximate estimation of soil bulk density, J. Ecol., 58, 297–299. [Google Scholar]
- Kearney, M. S. , Stevenson J. C., and Ward L. G. (1994), Spatial and temporal changes in marsh vertical accretion rates at Monie Bay: Implications for sea‐level rise, J. Coastal Res., 10, 1010–1020. [Google Scholar]
- Kelley, J. T. , Belknap D. F., Jacobson G. L., and Jacobson H. A. (1988), The morphology and origin of salt marshes along the glaciated coastline of Maine, USA, J. Coastal Res., 4, 649–665. [Google Scholar]
- Kemp, A. C. , Horton B. P., Donnelly J. P., Mann M. E., Vermeer M., and Rahmstorf S. (2011), Climate related sea‐level variations over the past two millennia, Proc. Natl. Acad. Sci. U. S. A., 108, 11017–11022, doi:10.5670/oceanog.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp, A. C. , Horton B. P., Vane C. H., Bernhardt C. E., Corbett D. R., Engelhart S. E., Anisfeld S. C., Parnell A. C., and Cahill N. (2013), Sea‐level change during the last 2500 years in New Jersey, USA, Quat. Sci. Rev., 81, 90–104, doi:10.1016/j.quascirev.2013.09.024. [Google Scholar]
- Khan, H. , and Brush G. S. (1994), Nutrient and metal accumulation in a freshwater tidal marsh, Estuaries, 17, 345–360. [Google Scholar]
- Kirschbaum, M. U. F. (1995), The temperature dependence of soil organic matter decomposition, and the effect of global warming on soil organic storage, Soil Biol. Biochem., 27, 753–760. [Google Scholar]
- Kirwan, M. L. , Guntenspergen G. R., and Morris J. T. (2009), Latitudinal trends in Spartina alterniflora productivity and the response of coastal marshes to global change, Global Change Biol., 15, 1982–1989. [Google Scholar]
- Kirwan, M. L. , and Megonigal J. P. (2013), Tidal wetland stability in the face of human impacts and sea‐level rise, Nature, 504, 53–60, doi:10.1038/nature12856. [DOI] [PubMed] [Google Scholar]
- Kirwan, M. L. , Guntenspergen G. R., D'Alpaos A., Morris J. T., Mudd S. M., and Temmerman S. (2010), Limits on the adaptability of coastal marshes to rising sea level, J. Geophys. Res., 37, L23401, doi:10.1029/2010GL045489. [Google Scholar]
- Kirwan, M. L. , Murray A. B., Donnelly J. P., and Corbett D. R. (2011), Rapid wetland expansion during European settlement and its implication for marsh survival under modern sediment delivery rates, Geology, 39, 507–510, doi:10.1130/G31789.1. [Google Scholar]
- Krone, R. B. (1987), A method for simulating historic marsh elevations, in Coastal Sediments '87, edited by Krause N. C., pp. 316–323, New York, American Society of Civil Engineers. [Google Scholar]
- Langley, J. A. , McKee K. L., Cahoon D. R., Cherry J. A., and Megonigal J. P. (2009), Elevated CO2 stimulates marsh elevation gain, counterbalancing sea‐level rise, Proc. Natl. Acad. Sci. U. S. A., 106, 6182–6186, doi:10.1073/pnas.0807695106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi, N. , Ganju N. K., and Fagherazzi S. (2016), A linear relationship between wave power and erosion determines salt‐marsh resilience to violent storms and hurricanes, Proc. Natl. Acad. Sci. U. S. A., 113, 64–68, doi:10.1073/pnas.1510095112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenberg, K. (1944), A method for the solution of certain non‐linear problems in least squares, Q. Appl. Math., 2, 164–168. [Google Scholar]
- Manrique, L. A. , and Jones C. A. (1991), Bulk density of soils in relation to soil physical and chemical properties, Soil Sci. Soc. Am. J., 55, 476–481. [Google Scholar]
- Mariotti, G. , and Fagherazzi S. (2013), Critical width of tidal flats triggers marsh collapse in the absence of sea‐level rise, Proc. Natl. Acad. Sci. U. S. A., 110, 5353–5356, doi:10.1073/pnas.1219600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer, L. M. (1994), Relationships between mineral surfaces and organic carbon concentrations in soils and sediments, Chem. Geol., 114, 347–363. [Google Scholar]
- Mayer, L. M. , Schick L. L., Hardy K. R., Wagai R., and McCarthy J. (2004), Organic matter in small mesopores in sediments and soils, Geochim. Cosmochim. Acta, 68, 3863–3872. [Google Scholar]
- McKee, K. L. , and Cherry J. A. (2009), Hurricane Katrina sediment slowed elevation loss in subsiding brackish marshes of the Mississippi River Delta, Wetlands, 29, 2–15. [Google Scholar]
- Merrill, J. Z. (1999), Tidal freshwater marshes as nutrient sinks: Particulate nutrient burial and denitrification, PhD dissertation, Univ. of Maryland.
- Morita, H. (1962), Composition of peat humus and its derivatives. Oxidative conversion to lignin model compounds, J. Org. Chem., 27, 1079–1080. [Google Scholar]
- Morris, J. T. , and Bowden W. B. (1986), A mechanistic, numerical model of sedimentation, mineralization, and decomposition for marsh sediments, Soil. Sci. Soc. Am. J., 50, 96–105. [Google Scholar]
- Morris, J. T. , Houghton R. A., and Botkin D. B. (1984), Theoretical limits of belowground production by Spartina alterniflora: An analysis through modelling, Ecol. Modell., 26, 155–175. [Google Scholar]
- Morris, J. T. , Sundareshwar P. V., Nietch C. T., Kjerfve B., and Cahoon D. R. (2002), Responses of coastal wetlands to rising sea level, Ecology, 83, 2869–2877. [Google Scholar]
- Morris, J. T. , Sundberg K., and Hopkinson C. S. (2013), Salt marsh primary production and its responses to relative sea level and nutrients in estuaries at Plum Island, Massachusetts, and North Inlet, South Carolina, USA, Oceanography, 26, 78–84. [Google Scholar]
- Mortland, M. M. (1970), Clay‐organic complexes and interactions, Adv. Agron., 22, 75–117. [Google Scholar]
- Morton, R. A. , and Barras J. A. (2011), Hurricane impacts on coastal wetlands: A half‐century record of storm‐generated features from Southern Louisiana, J. Coastal Res., 27, 27–43. [Google Scholar]
- Neubauer, S. C. (2000), Carbon dynamics in a tidal freshwater marsh, PhD dissertation, College of William and Mary, Williamsburg, Va.
- Neubauer, S. C. (2008), Contributions of mineral and organic components to tidal freshwater marsh accretion, Estuarine Coastal Shelf Sci., 78, 78–88. [Google Scholar]
- Nyman, J. A. , Delaune R. D., Walters R. J., and Patrick W. H. Jr. (1993), Relationship between vegetation and soil formation in a rapidly submerging coastal marsh, Mar. Ecol. Prog. Ser., 96, 269–279. [Google Scholar]
- Nyman, J. A. , Walters R. J., Delaune R. D., and Patrick W. H. Jr. (2006), Marsh vertical accretion via vegetative growth, Estuarine Coastal Shelf Sci., 69, 370–380. [Google Scholar]
- Odum, W. E. (1988), Comparative ecology of tidal freshwater and salt marshes, Annu. Rev. Ecol. Syst., 19, 147–176. [Google Scholar]
- Orson, R. A. , Simpson R. L., and Good R. E. (1992), A mechanism for the accumulation and retention of heavy metals in tidal freshwater marshes of the upper Delaware River estuary, Estuarine Coastal Shelf Sci., 34, 171–186. [Google Scholar]
- Percival, J. B. , and Lindsay P. J. (1997), Measurement of physical properties of sediments (Chapter 2), in Manual of Physico‐Chemical Analysis of Aquatic Sediments, edited by Mudroch A., Azcue J. M., and Mudroch P. , pp. 7–45 , CRC Lewis Publishers, Boca Raton, Fla. [Google Scholar]
- Périé, C. , and Ouime R. (2008), Organic carbon, organic matter and bulk density relationships in boreal forest soils, Can. J. Soil Sci., 88, 315–325. [Google Scholar]
- Philben, M. , Kaiser K., and Benner R. (2014), Does oxygen exposure time control the extent of organic matter decomposition in peatlands? J. Geophys. Res. Biogeosci., 119, 897–909, doi:10.1002/2013JG002573. [Google Scholar]
- Philben, M. , Holmquist J., MacDonald G., Duan D., Kaiser K., and Benner R. (2015), Temperature, oxygen, and vegetation controls on decomposition in a James Bay peatland, Global Biogeochem. Cycles, 29, 729–743, doi:10.1002/2014GB004989. [Google Scholar]
- Ratliff, K. M. , Braswell A. E., and Marani M. (2015), Spatial response of coastal marshes to increased atmospheric CO2 , Proc. Natl. Acad. Sci. U. S. A., 112, 15580–15584, doi:10.1073/pnas.1516286112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfield, A. C. , and Rubin M. (1962), The age of salt marsh peat and its relation to recent changes in sea level at Barnstable Massachusetts, Proc. Natl. Acad. Sci. U. S. A., 48, 1728–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman, C. T. , and Daiber F. C. (1984), Aboveground and belowground primary production dynamics of two Delaware Bay tidal marshes, Bull. Torrey Bot. Club, 3, 34–41. [Google Scholar]
- Singh, J. S. , Lauenroth W. K., Hunt H. W., and Swift D. M. (1984), Bias and random errors in estimators of net root production: A simulation approach, Ecology, 65, 1760–1764. [Google Scholar]
- Skopp, J. M. (2000), Physical properties of primary particles, in Handbook of Soil Science, edited by Sumner M. E., pp. A1–A17, CRC Press, Boca Raton, Fla. [Google Scholar]
- Stewart, V. I. , Adams W. A., and Abdulla H. H. (1970), Quantitative pedological studies on soils derived from Silurian mudstones, J. Soil Sci., 21, 248–255. [Google Scholar]
- Swanson, R. L. , and Wilson R. E. (2008), Increased tidal ranges coinciding with Jamaica Bay development contribute to marsh flooding, J. Coastal Res., 24, 1565–1569. [Google Scholar]
- Turner, R. E. , Swenson E. M., and Milan C. S. (2000), Organic and inorganic contributions to vertical accretion in salt marsh sediments, in Concepts and Controversies in Tidal Marsh Ecology, edited by Weinstein M. P. and Kreeger D. A., pp. 583–595, Kluwer Academic Publishers, Berlin, Germany. [Google Scholar]
- Turner, R. E. , Baustian J. J., Swenson E. M., and Spicer J. S. (2006), Wetland sedimentation from Hurricanes Katrina and Rita, Science, 314, 449–452. [DOI] [PubMed] [Google Scholar]
- Valiela, I. , Teal J. M., and Persson N. Y. (1976), Production and dynamics of experimentally enriched salt marsh vegetation: belowground biomass, Limnol. Oceanogr., 21, 245–252. [Google Scholar]
- Van Veen, J. A. , and Kuikman P. J. (1990), Soil structural aspects of decomposition of organic matter by micro‐organisms, Biogeochemistry, 11, 213–233. [Google Scholar]
- Vermeer, M. , and Rahmstorf S. (2009), Global sea level linked to global temperature, Proc. Natl. Acad. Sci. U. S. A., 106, 21527–21532, doi:10.1073/pnas.0907765106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, L. G. , Kearney M. S., and Stevenson J. C. (1988), Assessment of Marsh Stability at the Estuarine Sanctuary Site at Monie Bay: Implications for Management. Final Rep., National Oceanic and Atmospheric Administration, National Ocean Service, Office of Ocean Resource Management, Sanctuary Program Division, Washington, D. C.
- Weston, N. B. (2014), Declining sediments and rising seas: An unfortunate convergence for tidal wetlands, Estuaries Coasts, 37, 1–23. [Google Scholar]
- Wigand, C. (2008), Coastal salt marsh community change in Narragansett Bay in response to cultural eutrophication (Chapter 17), in Science for Ecosystem‐based Management: Narragansett Bay in the 21st Century, edited by Costa Pierce B. A. and Desbonnet A., pp. 499–522 , Springer Publishing, New York. [Google Scholar]
- Wigand, C. , McKinney R., Chintala M., Charpentier M., and Thursby G. (2003), Relationships of nitrogen loadings, residential development, and physical characteristics with plant structure in New England salt marshes, Estuaries, 26, 1494–1504. [Google Scholar]
- Wigand, C. , et al. (2014), Below the disappearing marshes of an urban estuary: Historic nitrogen trends and soil structure, Ecol. Appl., 24, 633–649. [DOI] [PubMed] [Google Scholar]
- Williams, C. J. , and Yavitt J. B. (2003), Botanical composition of peat and degree of peat decomposition in three temperate peatlands, Ecoscience, 10, 85–95. [Google Scholar]
- Wilson, C. A. , Hughes Z. J., FitzGerald D. M., Hopkinson C. S., Valentine V., and Kolker A. S. (2014), Saltmarsh pool and tidal creek morphodynamics: Dynamic equilibrium of northern latitude saltmarshes? Geomorphology, 213, 99–115. [Google Scholar]
- Wilson, J. O. , Buchsbaum R., Valiela I., and Swain T. (1986), Decomposition in salt marsh ecosystems: phenolic dynamics during decay of litter of Spartina a1terniflora , Mar. Ecol. Prog. Ser., 29, 177–187. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1


