Abstract
P2X4 receptors (P2X4R) are a family of ATP-gated non-selective cation channels. We previously demonstrated that activation of P2X4R in spinal microglia is crucial for neuropathic pain, a highly debilitating chronic pain condition, suggesting that P2X4R is a potential therapeutic target for treating neuropathic pain. Thus, the identification of a compound that has a potent inhibitory effect on P2X4R is an important clinical challenge. In the present study, we screened a chemical library of clinically approved drugs and show for the first time that duloxetine, a serotonin and noradrenaline reuptake inhibitor, has an inhibitory effect on rodent and human P2X4R. In primary cultured microglial cells, duloxetine also inhibited P2X4R-, but not P2X7R-, mediated responses. Moreover, intrathecal administration of duloxetine in a model of neuropathic pain produced a reversal of nerve injury-induced mechanical allodynia, a cardinal symptom of neuropathic pain. In rats that were pretreated with a serotonin-depleting agent and a noradrenaline neurotoxin, the antiallodynic effect of duloxetine was reduced, but still remained. Based on these results, we suggest that, in addition to duloxetine’s primary inhibitory action on serotonin and noradrenaline transporters, an inhibitory effect on P2X4R may be involved at least in part in an antiallodynic effect of intrathecal duloxetine in a model of neuropathic pain.
Introduction
Neuropathic pain is a source of intractable chronic pain in conditions such as cancer, fibromyalgia, diabetic neuropathy and postherpetic neuralgia. A prominent symptom is abnormal pain hypersensitivity evoked by normally innocuous stimuli, known as mechanical allodynia. Analgesics such as pregabalin and opioids are used for treating neuropathic pain, however it has been estimated that only one in four patients experiences over 50% pain relief [1]. Furthermore, the clinical use of these drugs is often limited by side effects such as dizziness, obstipation and nausea [2]. Thus, there is a need to explore and develop analgesics with therapeutic potential for neuropathic pain.
Microglia are the resident immune cells in the central nervous system [3]. Following peripheral nerve injury (PNI), microglia critically contribute to the pathogenesis of neuropathic pain [4,5,6,7,8]. We have previously shown that the purinergic receptor P2X4 (P2X4R), a subtype of ATP-gated non-selective cation channels, is highly upregulated in spinal microglia after PNI, and blocking the function of the P2X4R produces a reversal of mechanical allodynia [4]. Furthermore, disruption of the P2X4R gene abolishes mechanical allodynia induced by PNI [9,10]. Interfering with microglial P2X4R upregulation by inhibiting interferon regulatory factor 8 and 5 also suppresses PNI-induced allodynia [6,7]. These findings indicate that activation of P2X4R in spinal microglia plays a pivotal role in the pathogenesis of neuropathic pain and suggest that P2X4R blockers are a promising therapeutic approach for neuropathic pain [11,12].
The aim of this study was to identify clinically approved drugs that inhibit P2X4R. In a recent small scale screening of clinically approved antidepressants and anticonvulsants, we showed that paroxetine, a selective serotonin (5-HT) reuptake inhibitor (SSRI), has an inhibitory effect on P2X4R and produces an antiallodynic effect in an animal model of neuropathic pain [13]. In this study, we performed a large scale screening of a chemical library (1979 clinically approved compounds) to identify a drug with a more potent inhibitory effect on P2X4R than paroxetine, and tested the effects in an animal model of neuropathic pain.
We found that duloxetine, a 5-HT and noradrenaline (NA) reuptake inhibitor (SNRI), has an inhibitory effect on the function of microglial P2X4R. Furthermore, intrathecal duloxetine attenuated PNI-induced mechanical allodynia, an effect that remained even in rats that had been pretreated with the 5-HT depleting agent parachlorophenylalanine (PCPA) and the NA neurotoxin N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4). Therefore, it is possible that, in addition to duloxetine’s inhibition of 5-HT and NA transporters, inhibition of P2X4R may be involved in its antiallodynic effect in a model of neuropathic pain.
Materials and Methods
Reagents
Reagents were obtained from the following sources: adenosine 5’-triphosphate disodium salt (ATP), 2’(3’)-O-(4-Benzoylbenzoyl)adenosine 5’-triphosphate triethylammonium salt (BzATP), adenosine 5'-diphosphate sodium salt (ADP), amitriptyline hydrochloride, bupropion hydrochloride, clomipramine hydrobromide, maprotiline hydrochloride, mirtazapine, ivermectin, 4-Chloro-DL-phenylalanine methyl ester hydrochloride (PCPA), and N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4) from Sigma-Aldrich (St. Louis, MO, USA); Fura-2-AM from Dojindo (Kumamoto, Japan); duloxetine hydrochloride and paroxetine hydrochloride from Toronto Research Chemicals (Toronto, Canada); pyridoxal phosphate-6-azophenyl-2’,4’-disulphonic acid (PPADS) tetrasodium salt from Tocris Bioscience (Bristol, UK). The approved drugs library of 1979 compounds were provided in the form of 96-well plate format by Drug Discovery Initiative, DDI, The University of Tokyo (Tokyo, Japan).
Cell cultures
1321N1 human astrocytoma cells stably expressing rat (r) P2X4R, rP2X7R and human (h) P2X4R were used. Cells were maintained in low-glucose DMEM supplemented with 10% heat-inactivated FBS, penicillin and streptomycin in a humidified atmosphere of 5% CO2 at 37°C. Primary cultured microglia were prepared according to a previously described method [14,15]. In brief, the mixed glial culture was prepared from brain of neonatal Wistar rats (Kyudo, Saga, Japan) and maintained for 9–24 days in DMEM with 10% heat-inactivated FBS. The mouse microglial cell line C8-B4 cells were maintained in high-glucose DMEM with 4 mM GlutaMax supplemented with 10% heat-inactivated FBS, penicillin and streptomycin in a humidified atmosphere of 5% CO2 at 37°C.
Animals
Male Wistar rats (250–270 g; Japan SLC, Shizuoka, Japan) were housed in individual cages. Neonatal Wistar rats (Kyudo, Saga, Japan) were used for experiments in primary cultured microglia. The animals were housed in groups of four per cage with wooden chip on the floor during habituation, and then in individual cages after intrathecal catheterization under controlled temperature (22 ± 1°C) and humidity (55 ± 10%). The room was lighted from 8:00 AM to 8:00 PM. Food and water were freely available. Standard food (Clea Japan, Tokyo, Japan) and tap water were freely available. The physical conditions of the animals were carefully monitored every other day during the animal experiments. In this study, no animals became ill or died prior to the experimental endpoint. All animals utilized in this study were euthanized by means of i.p. injection of pentobarbital after experiments. All animal experiments were conducted according to the national and international guidelines contained in the ‘Act on Welfare and Management of Animals’ (Ministry of Environment of Japan) and ‘Regulation of Laboratory Animals’ (Kyushu University) and under the protocols approved by the Institutional Animal Care and Use committee review panels at Kyushu University.
Measurement of Ca2+ responses in 96-well plates (FDSS 7000EX system)
Measurement of Ca2+ imaging in 96-well plates was performed using the Functional Drug Screening System 7000EX (FDSS7000EX, Hamamatsu, Japan). 1321N1 cells stably expressing rat P2X4R and rat P2X7R were cultured for 24 h at 37°C on 96-well plates (2.0 × 104 cells/well). Cells were loaded with 2.5 μM Fura-2-AM containing 0.02% pluronic (Molecular Probes) in a balanced salt solution (BSS; 150 mM NaCl, 5 mM KCl, 1.8 mM CaCl2, 1.2 mM MgCl2, 10 mM D-glucose and 25 mM HEPES, pH 7.4) at room temperature for 45 min and then washed with BSS. After pretreatment with the test drugs for 10 min, cells were stimulated with 10 μM ATP or 100 μM BzATP.
Measurement of Ca2+ responses in single cells (single cells analysis)
Measurement of Ca2+ responses in single cells were assessed by ratiometric images (F340/F380) of Fura-2 fluorescence, which were detected with Aquacosmos/HiSca (Hamamatsu Photonics, Hamamatsu, Japan). 1321N1 cells stably expressing human P2X4R were transferred to poly-L-lysine-coated glass coverslips, placed in silicon rubber walls (Flexiperm, Greiner Bio-One GmbH, Frickenhausen, Germany) (1.0 × 104 cells /well). 1321N1 cells were cultured for 24 h at 37°C and loaded with 2.5 μM Fura-2 -AM in BSS at room temperature for 45 min. Rat microglial cells were obtained as floating cells over the mixed glial culture. The floating cells were collected by gentle shaking and transferred to poly-L-lysine-coated glass coverslips, placed in Flexiperm (5.0 × 104 cells/well). Microglial cells were cultured for 3–6 h and were loaded with 5 μM Fura-2-AM in DMEM at 37°C for 30 min. Then, the cells were washed with BSS and mounted on an inverted fluorescence microscope (ECLIPSE TE2000-U; Nikon, Tokyo, Japan) equipped with a Xenonlamp (Xe75W; Nikon). Data were calculated using the relative increase ratio (F340/F380) from the basal level prior to ATP application with or without duloxetine. The effects of duloxetine were evaluated by the S2 (2nd [Ca2+]i response) / S1 (1st [Ca2+]i response), where [Ca2+]i represents intracellular Ca2+ concentration, or the ratio of the fluorescence intensity (F340/F380). Following addition of nucleotides (10, 50 or 100 μM ATP, 100 μM BzATP, or 10 μM ADP) for 30 s, the cells were pretreated with duloxetine for 10 min. The cells were pre-incubated with ivermectin (3 μM) for 3 min prior to ATP application.
Intrathecal administration
Under isoflurane (2% [v/v]) anesthesia, a 32-gauge intrathecal catheter (ReCathCo, Allison Park, PA) for intrathecal administration of duloxetine (20 and 50 μg/20 μL) was inserted through the atlanto-occipital membrane into the lumbar enlargement and externalized through the skin according to a method described previously [5,16].
Neuropathic pain model and behavioral test
We used the spinal nerve injury model [17] with some modifications. A unilateral L5 spinal nerve of rats was tightly ligated and cut just distal to the ligature under isoflurane (2.5%) anesthesia. To assess mechanical allodynia, calibrated von Frey filaments (0.4–15 g, Linton Instrumentation, Diss, Norfolk, UK) were applied to the plantar surface of the hindpaw from below the mesh floor. The 50% paw-withdrawal threshold (PWT) was determined by the up-down method [18]. Duloxetine (20 or 50 μg/20 μl) or vehicle (Phosphate buffered saline, 20 μl) was intrathecally administered to rats 7 days after PNI and mechanical allodynia was measured for 300 min. The paw withdrawal threshold at 180 min after intrathecal administration of duloxetine was converted into antiallodynic effect (%) which was calculated by the formula: antiallodynic effect (%) = 100 × (test value−control value)/(15 g−control value). Control and test values were paw withdrawal threshold at 0 and 180 min, respectively. PCPA (300 mg/kg, an inhibitor of 5-HT synthesis) was intraperitoneally administered once a day for 3 days from day 4 post-PNI, based on published studies [19]. DSP-4 (50 mg/kg, a NAergic neurotoxin) was intraperitoneally administered 3 days before PNI, based on published studies [20]. PCPA and DSP-4 were dissolved in saline.
Statistical analysis
Statistical analyses were performed using one-way ANOVA with Bonferroni’s multiple comparison test (Figs 1A–1B and 2), Student’s t test (Fig 3A–3B), one-way ANOVA with Bonferroni’s multiple comparison test (Figs 3C and 4A), Student’s t test (Fig 4B–4C), two-way ANOVA with post hoc Bonferroni test (Fig 5A) or one-way ANOVA with post hoc Tukey Multiple Comparison test (Fig 5B) using GraphPad Prism 4 software. Differences were considered significant at P < 0.05.
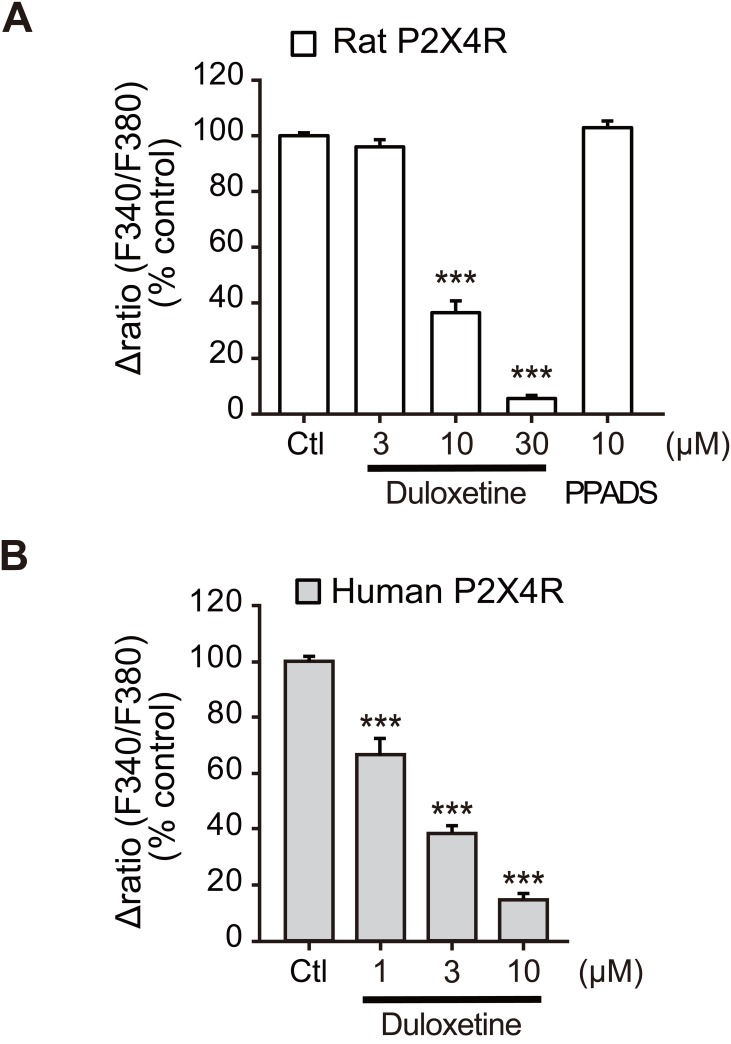
Fig 1. Duloxetine inhibits function of recombinant P2X4R.
(A) Effects of duloxetine (3–30 μM) and PPADS (10 μM) on ATP (10 μM)-induced [Ca2+]i responses in rP2X4R-1321N1 cells measured by the FDSS 7000EX system. Each column indicates the value as a percentage of control (Ctl: 10 μM ATP alone-induced [Ca2+]i responses) (n = 4, ***P < 0.001 vs. control). (B) Effect of duloxetine at various concentrations (1–10 μM) on ATP (10 μM)-induced [Ca2+]i respoznses in hP2X4R-1321N1 cells measured at a single cell level. Each column shows the effect of duloxetine evaluated by the S2 (2nd [Ca2+]i response) / S1 (1st [Ca2+]i response) (n = 4, ***P < 0.001 vs. control). Cells were pretreated with duloxetine and PPADS for 10 min prior to application of ATP. Data represent mean ± SEM for all groups.
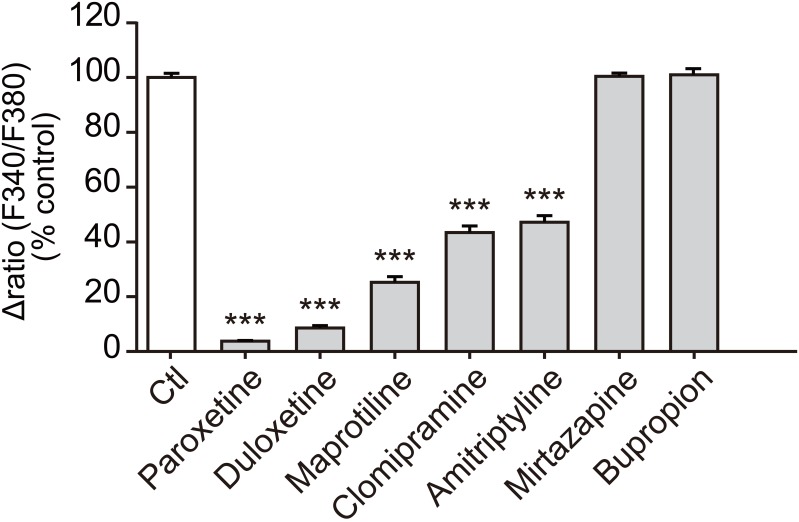
Fig 2. Comparison of the effect of duloxetine and other antidepressants on recombinant rP2X4R.
Effect of duloxetine and other antidepressants (30 μM) on ATP (10 μM)-induced [Ca2+]i in rP2X4R-1321N1 cells measured by the FDSS 7000EX system. Each column shows the value as a percentage of control (10 μM ATP alone-induced [Ca2+]i responses) (n = 6, ***P<0.001 vs. control). Cells were pretreated with duloxetine and other antidepressants 10 min prior to ATP application. Data represent mean ± SEM.
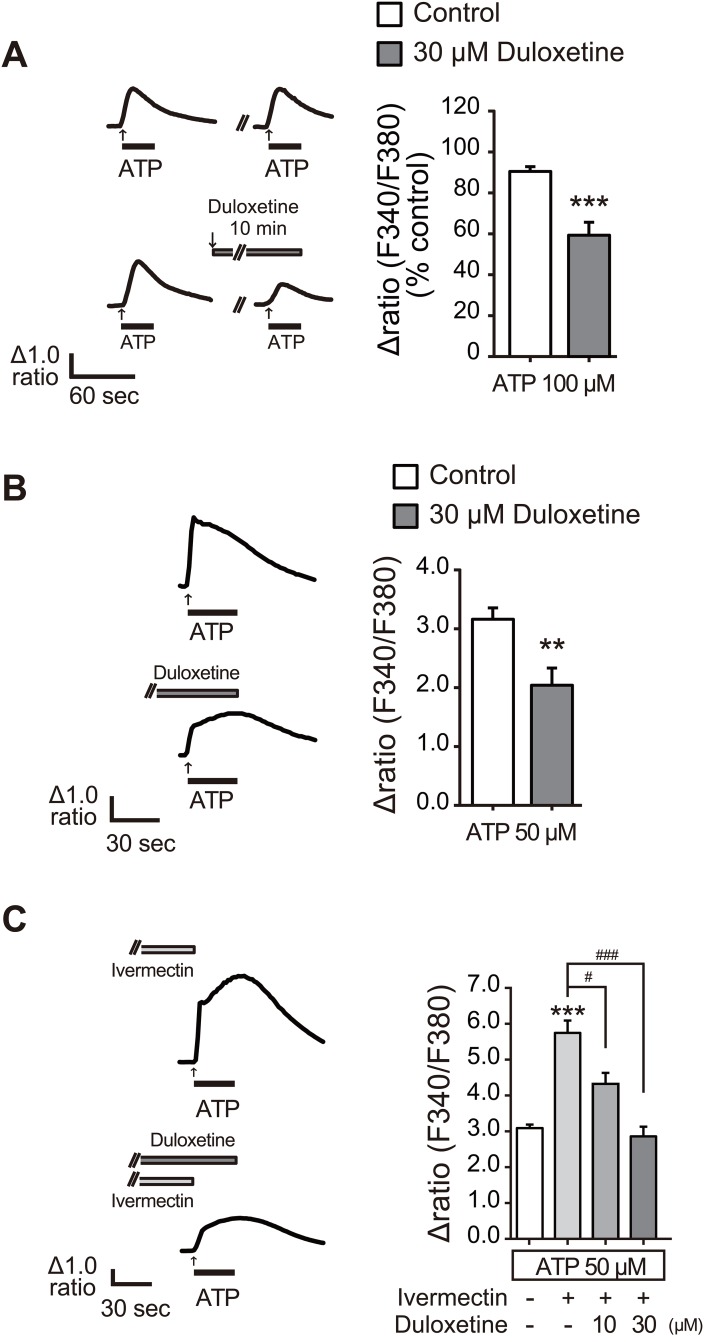
Fig 3. Duloxetine inhibited microglial P2X4R function.
(A) Representative average traces of [Ca2+]i increases in response to ATP (100 μM for 30 s) with or without duloxetine (30 μM for 10 min) measured by a single cell analysis in C8-B4 cells. Each column shows the effect of duloxetine evaluated by the S2 (2nd [Ca2+]i response) / S1 (1st [Ca2+]i response) (n = 4, ***P < 0.001 vs. control). (B) Representative average traces of [Ca2+]i increases in response to ATP (50 μM for 30 s) with or without duloxetine (30 μM for 10 min) measured by single cell analysis in rat microglial cells. Each column shows the relative increase ratio (F340/F380) from the basal level prior to ATP application (n = 4, **P < 0.01 vs. control). (C) Representative average traces of [Ca2+]i increases in response to ATP (50 μM for 30 s) in combination with ivermectin (3 μM, 3 min) with or without duloxetine (30 μM for 10 min) measured by single cell analysis in rat microglial cells. Each column shows the relative increase ratio (F340/F380) from the basal level prior to ATP application (n = 4, **P < 0.01, ***P < 0.001 vs. control, #P < 0.05, ###P < 0.001). Data represent mean ± SEM for all groups.
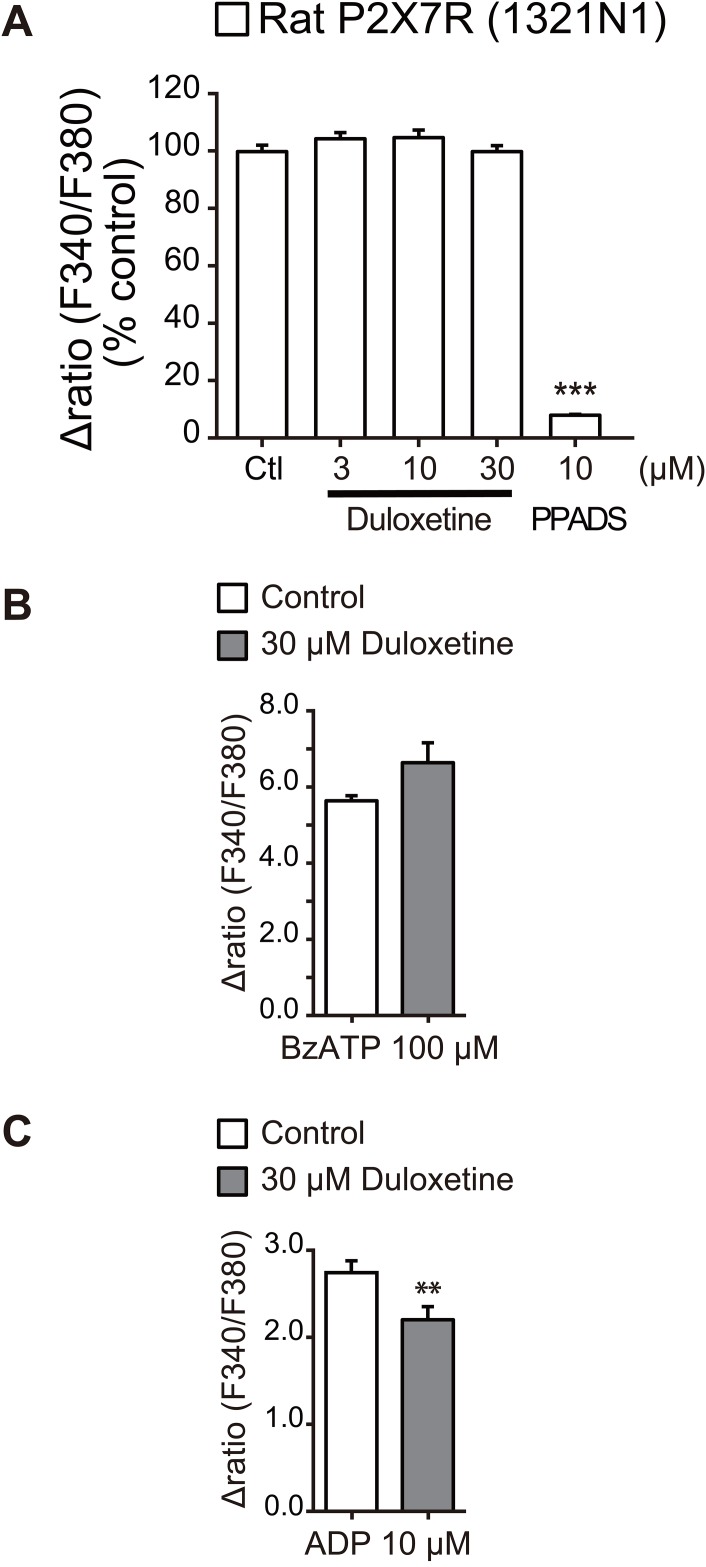
Fig 4. Effect of duloxetine on other P2 receptors.
(A) Effect of duloxetine at various concentrations (3–30 μM) and PPADS (10 μM) on BzATP (100 μM)-induced [Ca2+]i responses in rP2X7R-1321N1 cells measured by the FDSS 7000EX system. Each column shows the value as a percentage of control (100 μM BzATP alone-induced [Ca2+]i responses) (n = 4). (B, C) Effects of duloxetine (30 μM) on [Ca2+]i responses induced by BzATP (100 μM) (B) and ADP (10 μM) (C) measured by a single cell analysis in primary cultured rat microglial cells. Each column shows the relative increase ratio (F340/F380) from the basal level prior to BzATP or ADP application (n = 4, **P < 0.01). Cells were pretreated with duloxetine or PPADS for 10 min prior to application of BzATP or ADP. Data represent mean ± SEM for all groups.
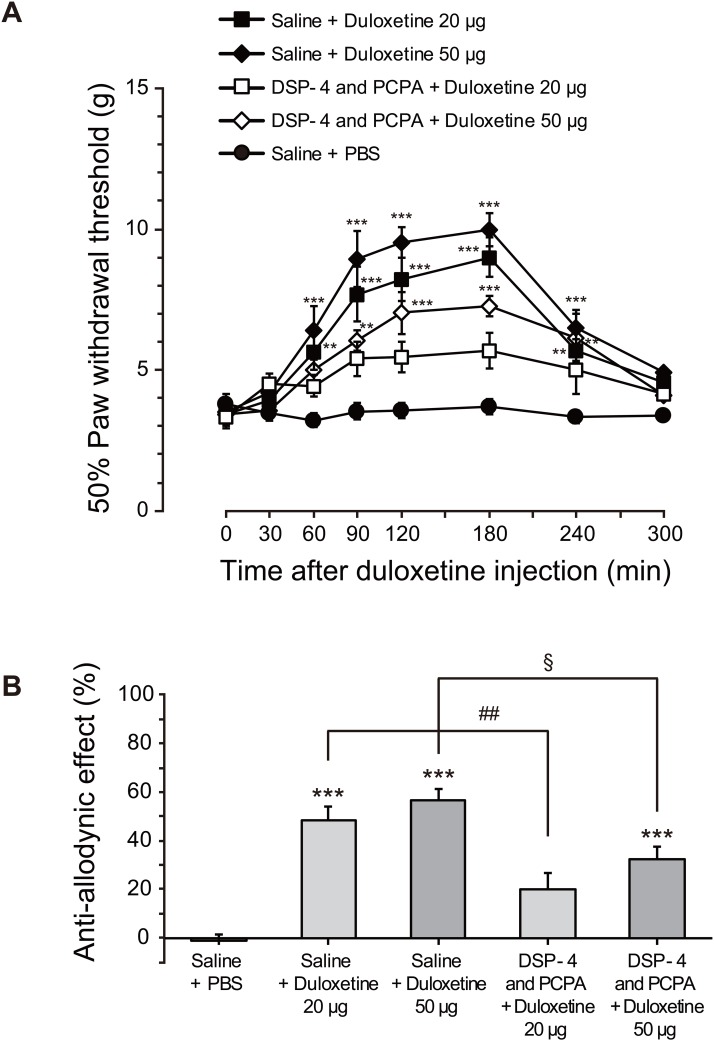
Fig 5. Intrathecal duloxetine attenuates allodynia after PNI: possible involvement of P2X4R.
(A) The paw withdrawal threshold (grams) of mechanical stimulation by von Frey filaments applied to the rat hindpaw after PNI. Duloxetine (20 or 50 μg/20 μl) or vehicle (Phosphate buffered saline, 20 μl) was intrathecally administered to rats on day 7 post-PNI. PCPA (300 mg/kg, i.p.) was administered once a day for 3 days from day 4 post-PNI, and DSP-4 (50 mg/kg, i.p.) was administered 3 days before PNI. The threshold was measured 300 min after duloxetine injection (n = 5–13, **P < 0.01, ***P < 0.001). Data represent mean ± SEM. (B) The antiallodynic effect (%) of duloxetine at 180 min after its intrathecal administration was calculated by the formula: antiallodynic effect (%) = 100 × (test value−control value)/(15 g−control value) [***P < 0.001 vs control group (Saline+PBS), §P < 0.05 and ##P < 0.01]. Data represent mean ± SEM.
Results
Duloxetine inhibits function of recombinant P2X4R expressed in 1321N1 cells
We screened 1979 compounds to identify clinically approved drugs that inhibit P2X4R. Using a high-throughput Ca2+ imaging apparatus, we measured [Ca2+]i levels in 1321N1 human astrocytoma cells (1321N1 cells) stably expressing rP2X4R (rP2X4R-1321N1) [13]. We identified the SNRI duloxetine as a compound that has an inhibitory effect on ATP-induced increases in [Ca2+]i, and that is known to possess both oral availability and the ability to cross the blood-brain barrier. The inhibitory effect of duloxetine was concentration dependent in rP2X4R-1321N1 cells (Fig 1A). In Ca2+ imaging analysis at the single cell level using hP2X4R-1321N1 cells, duloxetine produced similar inhibitory effects, with an IC50 value of 1.59 μM (Fig 1B). These results indicate that duloxetine has an inhibitory effect on recombinant rat and human P2X4R.
We also tested two other antidepressants categorized into different classes that had not been previously tested, mirtazapine (NAergic and specific 5-HTergic antidepressant) and bupropion (NA-dopamine reuptake inhibitor). However, these two antidepressants at 30 μM had no effect on the ATP-evoked [Ca2+]i responses in rP2X4R-1321N1 cells (Fig 2). In agreement with previous reports [13,21], paroxetine, maprotiline, clomipramine and amitriptyline significantly inhibited rat P2X4R-mediated [Ca2+]i responses, and the inhibition by duloxetine was similar to that of paroxetine (Fig 2).
Duloxetine inhibited microglial P2X4R function
In the central nervous system, microglia predominantly express P2X4R. Therefore, we examined the effect of duloxetine using C8-B4 cells, a cell line of immortalized mouse cerebellar microglial cells. We found that duloxetine reduced the ATP-induced [Ca2+]i increase (Fig 3A). We then assessed its effect in rat primary cultured microglial cells that endogenously express P2X4R [4,15]. Duloxetine (30 μM) significantly reduced the ATP-evoked [Ca2+]i responses in microglial cells (Fig 3B). To selectively detect a P2X4R-mediated component, we co-applied ATP with macrocyclic lactone antibiotic ivermectin, which is a positive allosteric modulator specific for P2X4R [22]. Consistent with previous studies, ivermectin enhanced the ATP-induced [Ca2+]i responses (Fig 3C). This enhancement was almost completely suppressed by duloxetine (30 μM) (Fig 3C), suggesting an inhibitory effect of duloxetine on endogenous P2X4R.
In addition to P2X4R, microglia express P2X7 receptors [23]. However, duloxetine (30 μM) had no effect on rP2X7R expressed in 1321N1 cells (Fig 4A). In rat primary cultured microglia, duloxetine (30 μM) had no effect on [Ca2+]i responses evoked by BzATP, an agonist for P2X7R (Fig 4B). Microglia also respond to ADP via P2Y12 receptors (P2Y12R), a subtype that is crucial for the motility of microglial processes [24]. ADP evoked [Ca2+]i responses, which were slightly, but significantly, reduced by duloxetine (Fig 4C). Taken together, these results suggest that duloxetine likely has an effect that is more selective to P2X4R.
Intrathecal administration of duloxetine attenuates mechanical allodynia after PNI: a possible involvement of P2X4R
Our previous studies have implicated microglial P2X4R in mechanical allodynia after PNI [4,6,7,9,25,26]; therefore, we next investigated whether duloxetine has an antiallodynic effect. After PNI, the paw withdrawal threshold to mechanical stimulation markedly decreased, implying the development of mechanical allodynia. After intrathecally administered duloxetine (20 or 50 μg) to rats on day 7 post-PNI, we found that the decreased paw withdrawal threshold was significantly reversed (Fig 5A): at 180 min, 48% (20 μg) and 57% (50 μg) recovery of paw withdrawal threshold (Fig 5B). Furthermore, we examined the antiallodynic effect of duloxetine in rats that had been pretreated with PCPA to deplete 5-HT, and with DSP-4 to produce a toxic effect on NAergic neurons that fully depletes 5-HT and NA [20,27,28,29]. In these PCPA- and DSP-4-pretreated rats, we found that the reversal effect of intrathecal duloxetine on the PNI-induced allodynia (Fig 5A): at 180 min after the injection of duloxetine, the pretreatment with PCPA and DSP-4 suppressed the duloxetine’s effect (P < 0.05 and 0.01; Fig 5B). However, the antiallodynic effect of duloxetine still remained at a statistically significant level (Fig 5A and 5B). These results suggest it is possible that an antiallodynic effect of duloxetine in our model of neuropathic pain may be associated, at least in part, with its inhibitory effect on P2X4R.
Discussion
Our screening of clinically approved drugs revealed for the first time that the SNRI duloxetine has an inhibitory effect on P2X4R. We and others have previously reported that some antidepressants inhibit P2X4R-mediated Ca2+ responses [13,21,30]; in this study, the IC50 value of duloxetine for hP2X4R was 1.59 μM. This indicates duloxetine is as potent as paroxetine, which previously found to be the antidepressant with the most potent inhibition of P2X4R. Furthermore, at the concentration range eliciting P2X4R inhibition, duloxetine had no effect on P2X7R. This is in contrast to paroxetine which also inhibits P2X7R [13,31]. The tricyclic antidepressant amitriptyline has been reported to inhibit rat and mouse, but not human P2X4R [21]; in this study, the inhibitory effect of duloxetine was observed in rodent and human P2X4R, indicating the inhibition is not restricted to a specific species. Moreover, the P2X4R inhibition by duloxetine is not restricted to recombinant P2X4R. In our experiments, we showed the ability of duloxetine to inhibit P2X4R endogenously expressed in primary cultured microglial cells. It is well-known that microglia express several P2 receptors in addition to P2X4R [23], but by using ivermectin, a positive allosteric modulator specific for P2X4R [22], we showed a marked inhibition of P2X4R-mediated response by duloxetine. These results together indicate that duloxetine has an inhibitory effect on P2X4R expressed in microglial cells.
Studies have shown that duloxetine has attenuating effects on pain hypersensitivity in models of neuropathic pain associated with traumatic nerve injury [32,33,34,35], diabetic neuropathy [36,37] and fibromyalgia [38]. In addition to its clinical use for depression, duloxetine is frequently used for treating chronic pain such as diabetic neuropathic pain [39,40], chronic low back pain [41,42], osteoarthritis [41,43] and fibromyalgia [41,44,45]. It has been considered that the mechanism for pain relief by duloxetine might be distinct from its effect on depression [46]. The primary action of duloxetine is inhibition of 5-HT and NA reuptake into presynaptic terminals by their respective transporters. Thus, it is thought that the attenuating effects of duloxetine on neuropathic pain are related to an increase in the levels of 5-HT and NA in the descending pain inhibitory pathways from brainstem to the spinal dorsal horn [47,48]. Indeed, it has been reported that alleviation of mechanical allodynia in model of neuropathic pain by duloxetine was reduced by an intrathecal injection of a 5-HT2A receptor antagonist [36,49] or by an intraperitoneally injection of DSP-4 to produce a toxic effect on NAergic neurons [20]. Consistent with these findings, the present study showed that the suppressing effect of duloxetine on PNI-induced allodynia was reduced in rats that had been pretreated with PCPA and DSP-4, at the dose of these agents needed to fully deplete 5-HT and NA [20,27]. However, the antiallodynic effect of intrathecal duloxetine still remained in the rats treated with both PCPA and DSP-4. Thus, it is possible that, in addition to the primary pharmacological action of duloxetine to inhibit 5-HT and NA transporters, an inhibitory effect on microglial P2X4R in the spinal cord may be involved in the antiallodynic effect of duloxetine when administered intrathecally in a model of neuropathic pain. In support of this idea, we previously demonstrated that expression of the P2X4R is upregulated exclusively in microglia in the spinal cord after PNI and that pharmacological blockade and molecular knockdown of the P2X4R produces a reversal of mechanical allodynia [4]. P2X4R-deficient mice also display a marked suppression of the PNI-induced mechanical allodynia [9,10]. Furthermore, intrathecal administration of paroxetine and fluvoxamine, which inhibits P2X4R, also produces a similar reversal of the PNI-induced allodynia, but citalopram, a SSRI that has no effect on P2X4R, does not suppress the allodynia [13]. Thus, these data support our hypothesis that an antiallodynic effect of duloxetine may be associated, at least in part, with its inhibitory effect on P2X4R.
In models of neuropathic pain, expression of P2X7R and P2Y12R are also increased in spinal microglia, and pharmacological blockade and genetic knockout of these receptors reduces pain hypersensitivities after PNI [50,51,52,53,54,55,56]. In this study, we observed that duloxetine had no effect on P2X7R, and produced a weak inhibition of the ADP-induced [Ca2+]i responses in primary cultured microglial cells, which might be mediated by P2Y12R. Thus, it is possible that intrathecally administered duloxetine may slightly attenuate P2Y12R function in spinal microglia, which may also be involved, at least in part, in duloxetine’s effect on mechanical allodynia after PNI.
Conclusions
In this study, we provide evidence for the first time that the SNRI duloxetine has an inhibitory effect on recombinant P2X4R (rat and human) and microglial P2X4R (mouse and rat). Furthermore, intrathecally administered duloxetine produced a reversal of mechanical allodynia, and the effect was reduced but still remained in rats pretreated with both a 5-HT depleting agent and a NAergic neurotoxin. Thus, we hypothesized that, in addition to the primary action of duloxetine on monoamine transporters, inhibition of P2X4R may be involved in duloxetine’s antiallodynic effect in a model of neuropathic pain.
Acknowledgments
This work was supported by a research grant from Eli Lilly Japan (M.T.), Platform for Drug Discovery, Informatics, and Structural Life Science from Japan Agency for Medical Research and Development (AMED) (K.I.), the Research Project on Elucidation of Chronic Pain from AMED (M.T.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by a research grant from Eli Lilly Japan (M.T.), Platform for Drug Discovery, Informatics, and Structural Life Science from Japan Agency for Medical Research and Development (AMED) (K.I.), and the Research Project on Elucidation of Chronic Pain from AMED (M.T.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Nightingale S (2012) The neuropathic pain market. Nat Rev Drug Discov 11: 101–102. 10.1038/nrd3624 [DOI] [PubMed] [Google Scholar]
- 2.O'Connor AB, Dworkin RH (2009) Treatment of neuropathic pain: an overview of recent guidelines. Am J Med 122: S22–32. [DOI] [PubMed] [Google Scholar]
- 3.Kreutzberg GW (1996) Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19: 312–318. [DOI] [PubMed] [Google Scholar]
- 4.Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, et al. (2003) P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 424: 778–783. 10.1038/nature01786 [DOI] [PubMed] [Google Scholar]
- 5.Tsuda M, Masuda T, Kitano J, Shimoyama H, Tozaki-Saitoh H, et al. (2009) IFN-gamma receptor signaling mediates spinal microglia activation driving neuropathic pain. Proc Natl Acad Sci U S A 106: 8032–8037. 10.1073/pnas.0810420106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masuda T, Tsuda M, Yoshinaga R, Tozaki-Saitoh H, Ozato K, et al. (2012) IRF8 is a critical transcription factor for transforming microglia into a reactive phenotype. Cell Rep 1: 334–340. 10.1016/j.celrep.2012.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masuda T, Iwamoto S, Yoshinaga R, Tozaki-Saitoh H, Nishiyama A, et al. (2014) Transcription factor IRF5 drives P2X4R+-reactive microglia gating neuropathic pain. Nat Commun 5: 3771 10.1038/ncomms4771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan Z, Kuhn JA, Wang X, Colquitt B, Solorzano C, et al. (2016) Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat Neurosci 19: 94–101. 10.1038/nn.4189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuda M, Kuboyama K, Inoue T, Nagata K, Tozaki-Saitoh H, et al. (2009) Behavioral phenotypes of mice lacking purinergic P2X4 receptors in acute and chronic pain assays. Mol Pain 5: 28 10.1186/1744-8069-5-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, et al. (2008) Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci 28: 11263–11268. 10.1523/JNEUROSCI.2308-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beggs S, Trang T, Salter MW (2012) P2X4R+ microglia drive neuropathic pain. Nat Neurosci 15: 1068–1073. 10.1038/nn.3155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuda M, Masuda T, Tozaki-Saitoh H, Inoue K (2013) P2X4 receptors and neuropathic pain. Front Cell Neurosci 7: 191 10.3389/fncel.2013.00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagata K, Imai T, Yamashita T, Tsuda M, Tozaki-Saitoh H, et al. (2009) Antidepressants inhibit P2X4 receptor function: a possible involvement in neuropathic pain relief. Mol Pain 5: 20 10.1186/1744-8069-5-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakajima K, Shimojo M, Hamanoue M, Ishiura S, Sugita H, et al. (1992) Identification of elastase as a secretory protease from cultured rat microglia. J Neurochem 58: 1401–1408. [DOI] [PubMed] [Google Scholar]
- 15.Toyomitsu E, Tsuda M, Yamashita T, Tozaki-Saitoh H, Tanaka Y, et al. (2012) CCL2 promotes P2X4 receptor trafficking to the cell surface of microglia. Purinergic Signal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsushita K, Tozaki-Saitoh H, Kojima C, Masuda T, Tsuda M, et al. (2014) Chemokine (C-C motif) receptor 5 is an important pathological regulator in the development and maintenance of neuropathic pain. Anesthesiology 120: 1491–1503. 10.1097/ALN.0000000000000190 [DOI] [PubMed] [Google Scholar]
- 17.Kim SH, Chung JM (1992) An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 50: 355–363. [DOI] [PubMed] [Google Scholar]
- 18.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 19.Ugedo L, Grenhoff J, Svensson TH (1989) Ritanserin, a 5-HT2 receptor antagonist, activates midbrain dopamine neurons by blocking serotonergic inhibition. Psychopharmacology (Berl) 98: 45–50. [DOI] [PubMed] [Google Scholar]
- 20.Kinoshita J, Takahashi Y, Watabe AM, Utsunomiya K, Kato F (2013) Impaired noradrenaline homeostasis in rats with painful diabetic neuropathy as a target of duloxetine analgesia. Mol Pain 9: 59 10.1186/1744-8069-9-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sim JA, North RA (2010) Amitriptyline does not block the action of ATP at human P2X4 receptor. Br J Pharmacol 160: 88–92. 10.1111/j.1476-5381.2010.00683.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khakh B, Proctor W, Dunwiddie T, Labarca C, Lester H (1999) Allosteric control of gating and kinetics at P2X(4) receptor channels. The Journal of neuroscience: the official journal of the Society for Neuroscience 19: 7289–7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burnstock G (2008) Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov 7: 575–590. 10.1038/nrd2605 [DOI] [PubMed] [Google Scholar]
- 24.Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, et al. (2006) The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci 9: 1512–1519. 10.1038/nn1805 [DOI] [PubMed] [Google Scholar]
- 25.Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, et al. (2005) BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438: 1017–1021. 10.1038/nature04223 [DOI] [PubMed] [Google Scholar]
- 26.Biber K, Tsuda M, Tozaki-Saitoh H, Tsukamoto K, Toyomitsu E, et al. (2011) Neuronal CCL21 up-regulates microglia P2X4 expression and initiates neuropathic pain development. EMBO J 30: 1864–1873. 10.1038/emboj.2011.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kornum BR, Licht CL, Weikop P, Knudsen GM, Aznar S (2006) Central serotonin depletion affects rat brain areas differently: a qualitative and quantitative comparison between different treatment schemes. Neurosci Lett 392: 129–134. 10.1016/j.neulet.2005.09.013 [DOI] [PubMed] [Google Scholar]
- 28.Ross SB (1976) Long-term effects of N-2-chlorethyl-N-ethyl-2-bromobenzylamine hydrochloride on noradrenergic neurones in the rat brain and heart. Br J Pharmacol 58: 521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koe BK, Weissman A (1966) p-Chlorophenylalanine: a specific depletor of brain serotonin. J Pharmacol Exp Ther 154: 499–516. [PubMed] [Google Scholar]
- 30.Toulme E, Garcia A, Samways D, Egan T, Carson M, et al. (2010) P2X4 receptors in activated C8-B4 cells of cerebellar microglial origin. The Journal of general physiology 135: 333–353. 10.1085/jgp.200910336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dao-Ung P, Skarratt KK, Fuller SJ, Stokes L (2015) Paroxetine suppresses recombinant human P2X7 responses. Purinergic Signal 11: 481–490. 10.1007/s11302-015-9467-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iyengar S, Webster AA, Hemrick-Luecke SK, Xu JY, Simmons RM (2004) Efficacy of duloxetine, a potent and balanced serotonin-norepinephrine reuptake inhibitor in persistent pain models in rats. J Pharmacol Exp Ther 311: 576–584. 10.1124/jpet.104.070656 [DOI] [PubMed] [Google Scholar]
- 33.Kiso T, Watabiki T, Tsukamoto M, Okabe M, Kagami M, et al. (2008) Pharmacological characterization and gene expression profiling of an L5/L6 spinal nerve ligation model for neuropathic pain in mice. Neuroscience 153: 492–500. 10.1016/j.neuroscience.2008.02.031 [DOI] [PubMed] [Google Scholar]
- 34.Le Cudennec C, Castagne V (2014) Face-to-face comparison of the predictive validity of two models of neuropathic pain in the rat: analgesic activity of pregabalin, tramadol and duloxetine. Eur J Pharmacol 735: 17–25. 10.1016/j.ejphar.2014.04.003 [DOI] [PubMed] [Google Scholar]
- 35.Hoshino H, Obata H, Saito S (2015) Antihyperalgesic effect of duloxetine and amitriptyline in rats after peripheral nerve injury: Influence of descending noradrenergic plasticity. Neurosci Lett 602: 62–67. 10.1016/j.neulet.2015.06.041 [DOI] [PubMed] [Google Scholar]
- 36.Mixcoatl-Zecuatl T, Jolivalt CG (2011) A spinal mechanism of action for duloxetine in a rat model of painful diabetic neuropathy. Br J Pharmacol 164: 159–169. 10.1111/j.1476-5381.2011.01334.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuhad A, Bishnoi M, Chopra K (2009) Anti-nociceptive effect of duloxetine in mouse model of diabetic neuropathic pain. Indian J Exp Biol 47: 193–197. [PubMed] [Google Scholar]
- 38.Nagakura Y, Oe T, Aoki T, Matsuoka N (2009) Biogenic amine depletion causes chronic muscular pain and tactile allodynia accompanied by depression: A putative animal model of fibromyalgia. Pain 146: 26–33. 10.1016/j.pain.2009.05.024 [DOI] [PubMed] [Google Scholar]
- 39.Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S (2005) Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain 116: 109–118. 10.1016/j.pain.2005.03.029 [DOI] [PubMed] [Google Scholar]
- 40.Mico JA, Ardid D, Berrocoso E, Eschalier A (2006) Antidepressants and pain. Trends Pharmacol Sci 27: 348–354. 10.1016/j.tips.2006.05.004 [DOI] [PubMed] [Google Scholar]
- 41.Skljarevski V, Zhang S, Iyengar S, D'Souza D, Alaka K, et al. (2011) Efficacy of Duloxetine in Patients with Chronic Pain Conditions. Curr Drug ther 6: 296–303. 10.2174/157488511798109592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williamson OD, Sagman D, Bruins RH, Boulay LJ, Schacht A (2014) Antidepressants in the treatment for chronic low back pain: questioning the validity of meta-analyses. Pain Pract 14: E33–41. 10.1111/papr.12119 [DOI] [PubMed] [Google Scholar]
- 43.Wang ZY, Shi SY, Li SJ, Chen F, Chen H, et al. (2015) Efficacy and Safety of Duloxetine on Osteoarthritis Knee Pain: A Meta-Analysis of Randomized Controlled Trials. Pain Med 16: 1373–1385. 10.1111/pme.12800 [DOI] [PubMed] [Google Scholar]
- 44.Bennett RM (2009) Fibromyalgia: a new treatment option for fibromyalgia. Nat Rev Rheumatol 5: 188–190. 10.1038/nrrheum.2009.48 [DOI] [PubMed] [Google Scholar]
- 45.Arnold LM, Lu Y, Crofford LJ, Wohlreich M, Detke MJ, et al. (2004) A double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder. Arthritis Rheum 50: 2974–2984. 10.1002/art.20485 [DOI] [PubMed] [Google Scholar]
- 46.Perahia DG, Pritchett YL, Desaiah D, Raskin J (2006) Efficacy of duloxetine in painful symptoms: an analgesic or antidepressant effect? Int Clin Psychopharmacol 21: 311–317. 10.1097/01.yic.0000224782.83287.3c [DOI] [PubMed] [Google Scholar]
- 47.Stamford JA (1995) Descending control of pain. Br J Anaesth 75: 217–227. [DOI] [PubMed] [Google Scholar]
- 48.Smith T, Nicholson RA (2007) Review of duloxetine in the management of diabetic peripheral neuropathic pain. Vasc Health Risk Manag 3: 833–844. [PMC free article] [PubMed] [Google Scholar]
- 49.Sun YH, Li HS, Zhu C, Hu W, Yang J, et al. (2014) The analgesia effect of duloxetine on post-operative pain via intrathecal or intraperitoneal administration. Neurosci Lett 568: 6–11. 10.1016/j.neulet.2014.03.046 [DOI] [PubMed] [Google Scholar]
- 50.Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, et al. (2005) Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain 114: 386–396. 10.1016/j.pain.2005.01.002 [DOI] [PubMed] [Google Scholar]
- 51.Honore P, Donnelly-Roberts D, Namovic MT, Hsieh G, Zhu CZ, et al. (2006) A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat. J Pharmacol Exp Ther 319: 1376–1385. 10.1124/jpet.106.111559 [DOI] [PubMed] [Google Scholar]
- 52.Tozaki-Saitoh H, Tsuda M, Miyata H, Ueda K, Kohsaka S, et al. (2008) P2Y12 receptors in spinal microglia are required for neuropathic pain after peripheral nerve injury. J Neurosci 28: 4949–4956. 10.1523/JNEUROSCI.0323-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kobayashi K, Yamanaka H, Fukuoka T, Dai Y, Obata K, et al. (2008) P2Y12 receptor upregulation in activated microglia is a gateway of p38 signaling and neuropathic pain. J Neurosci 28: 2892–2902. 10.1523/JNEUROSCI.5589-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kobayashi K, Takahashi E, Miyagawa Y, Yamanaka H, Noguchi K (2011) Induction of the P2X7 receptor in spinal microglia in a neuropathic pain model. Neurosci Lett 504: 57–61. 10.1016/j.neulet.2011.08.058 [DOI] [PubMed] [Google Scholar]
- 55.He WJ, Cui J, Du L, Zhao YD, Burnstock G, et al. (2012) Spinal P2X(7) receptor mediates microglia activation-induced neuropathic pain in the sciatic nerve injury rat model. Behav Brain Res 226: 163–170. 10.1016/j.bbr.2011.09.015 [DOI] [PubMed] [Google Scholar]
- 56.Xu J, Chen XM, Zheng BJ, Wang XR (2016) Electroacupuncture Relieves Nerve Injury-Induced Pain Hypersensitivity via the Inhibition of Spinal P2X7 Receptor-Positive Microglia. Anesth Analg 122: 882–892. 10.1213/ANE.0000000000001097 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.