Abstract
Interindividual variability in drug response in nonalcoholic steatohepatitis (NASH) can be mediated by altered regulation of drug metabolizing enzymes and transporters. Among these is the mislocalization of multidrug resistance-associated protein (MRP2)/Mrp2 away from the canalicular membrane, which results in decreased transport of MRP2/Mrp2 substrates. The exact mechanism of this mislocalization is unknown, although increased activation of membrane retrieval processes may be one possibility. The current study measures the activation status of various mediators implicated in the active membrane retrieval or insertion of membrane proteins to identify which processes may be important in rodent methionine and choline deficient diet-induced NASH. The mediators currently known to be associated with transporter mislocalization are stimulated by oxidative stressors and choleretic stimuli, which play a role in the pathogenesis of NASH. The activation of protein kinases PKA, PKCα, PKCδ, and PKCε and substrates radixin, myristoylated alanine-rich C-kinase substrate, and Rab11 were measured by comparing the expression, phosphorylation, and membrane translocation between control and NASH. Many of the mediators exhibited altered activation in NASH rats. Consistent with membrane retrieval of Mrp2, NASH rats exhibited a decreased phosphorylation of radixin and increased membrane localization of PKCδ and PKCε, thought to be mediators of radixin dephosphorylation. Altered activation of PKCδ, PKA, and PKCα may impair the Rab11-mediated active insertion of Mrp2. Overall, these data suggest alterations in membrane retrieval and insertion processes that may contribute to altered localization of membrane proteins in NASH.
Introduction
Precision medicine is an initiative to ensure the right dose of therapeutic is delivered to a patient, based upon the patient’s individual needs, to guarantee pharmacologic efficacy with minimal adverse drug reactions. One emerging factor in precision medicine is nonalcoholic steatohepatitis (NASH), a severe stage of nonalcoholic fatty liver disease (NAFLD), characterized by fat deposition, hepatocellular injury, inflammation, and fibrosis (Marra et al., 2008). Global prevalence of NAFLD has risen significantly in recent years to 25.4% and generally presents alongside obesity, type II diabetes, and hypertension (Younossi et al., 2015). NASH livers undergo extensive remodeling of genes involved in drug disposition; for instance, many drug metabolizing enzyme families are misregulated in NASH, and transcriptomic analysis of human liver samples across the NAFLD spectrum revealed downregulation of uptake transporters and upregulation of efflux transporters (Fisher et al., 2009; Lake et al., 2011; Hardwick et al., 2011, 2013). Interestingly, the canalicular efflux transporter multidrug resistance-associated protein 2 (MRP2/Mrp2) is mislocalized, mediated by posttranslational events that impact the insertion of Mrp2 into the canalicular membrane (Hardwick et al., 2011, 2012). The mechanism by which this occurs has not been determined.
One mechanism that may be implicated is the active recycling of MRP2 to and from the canalicular membrane, termed membrane insertion and membrane retrieval. This process has been well-characterized in various in vitro and rodent models, shedding light on the acute stimuli and mediators that regulate this process for MRP2 and other membrane proteins. Retrieval of MRP2/Mrp2 has been shown to be stimulated by various oxidative stressors, including lipopolysaccharide, ethacrynic acid, and glutathione depletion (Ji et al., 2004; Sekine et al., 2008, 2010). Additionally, cholestatic stimuli also induce Mrp2 retrieval from the canalicular membrane, including estradiol-17β-d-glucuronide, taurolithocholate (TLC), and taurochenodeoxycholate (Mottino et al., 2002; Rost et al., 2008; Schonhoff et al., 2013). Conversely, choleretic stimuli such as taurodeoxycholic acid and tauroursodeoxycholic acid can activate Mrp2 insertion back into the canalicular membrane, in a cAMP-regulated process (Beuers et al., 2001; Schonhoff et al., 2008; Wimmer et al., 2008). Mrp2 mislocalization by oxidative stress is similarly reversible by replenishment of GSH and may even be prevented by antioxidants (Sekine et al., 2008, 2010). All of these oxidative stressors and cholestatic stimuli activate various pathways mediated by protein kinases (PK), including PKCα, PKCδ, PKCε, PKA, along with their substrates, radixin, MARCKS, Rab11 (Anwer, 2014).
Radixin is part of the ezrin-radixin-moesin protein family, which links membrane-localized proteins to the actin cytoskeleton, and plays a large role in membrane trafficking. Radixin, as the dominant hepatic ezrin-radixin-moesin protein, is implicated in the structure and function of the canalicular membrane, including localization of transporters (Wang et al., 2006). Radixin deficiency causes hyperbilirubinemia and other human cholestatic liver diseases, largely associated with loss of Mrp2 from the canalicular membrane (Kikuchi et al., 2002; Kojima et al., 2008). This loss is facilitated by the dephosphorylation of radixin at Threonine-564 and subsequent loss of association between MRP2/Mrp2 and radixin (Kikuchi et al., 2002; Kojima et al., 2008; Suda et al., 2011). Radixin dephosphorylation can be regulated by oxidative stress through the activation of protein phosphatase 1 (PP1), mediated by PKCs and PKA (Sekine et al., 2011). These studies point to the importance of radixin in facilitating the active retrieval of Mrp2 from the canalicular membrane.
MARCKS, another protein that crosslinks f-actin, has been implicated in the TLC-induced Mrp2 internalization pathway through its phosphorylation by PKCε or PKCδ, which stimulates MARCKS membrane retrieval and internalization of Mrp2 (Schonhoff et al., 2013). Rab11, involved in endo- and exocytic processes, is a substrate involved in tauroursodeoxycholic acid (TUDCA)-induced cAMP-mediated translocation of Mrp2 to the canalicular membrane, the disruption of which is associated with cholestasis (Zucchetti et al., 2013; Park et al., 2014). Two potential mediators of this process are PKCδ, which regulates Mrp2 membrane insertion via kinase activity, and the calcium-dependent PKA/PKCα pathway, which increases total biliary secretion (Wimmer et al., 2008; Park et al., 2012).
The purpose of this study was to identify whether activation of membrane retrieval and/or inactivation of membrane insertion occurs in NASH, because these processes may contribute to Mrp2 mislocalization. To determine whether these pathways exhibit altered activation, the individual activation states of the various mediators were measured in vivo by comparing membrane localization and phosphorylation state between healthy rats and rats fed a methionine and choline-deficient (MCD) diet to induce NASH. This is the first study to correlate Mrp2 mislocalization to active membrane recycling pathways in a rodent model of a chronic disease state.
Materials and Methods
Animals.
Male Sprague-Dawley rats weighing 200 to 250 g were obtained from Harlan (Indianapolis, IN). All animals were acclimated in 12-hour light/dark cycles in a University of Arizona Association for Assessment and Accreditation of Laboratory Animal Care-certified animal facility for at least 1 week before diet commencement and were allowed water and standard chow ad libitum. Housing and experimental procedures were in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996), and protocols were approved by the Arizona Institutional Animal Care and Use Committee. Rats were fed an isocaloric control diet with methionine and choline resupplemented, or a methionine and choline-deficient (MCD) diet (Dyets Inc., Bethlehem, PA) for 8 weeks (n = 13 each). Upon conclusion of the diet, animals were euthanized while under anesthesia, and liver slices for histologic analysis were collected and placed in 10% neutral-buffered formalin for 24 hours followed by 70% ethanol for paraffin embedding performed by the University of Arizona Histology Service Laboratory. H&E-stained samples were imaged with a Leica DM4000B microscope and a DFC450 camera (LeicaMicrosystems, Buffalo Grove, IL). The remaining liver tissue was snap-frozen in liquid nitrogen and stored at −80°C for protein preparations.
Protein Preparations.
Whole cell lysate preparations of rodent liver (n = 5 each) were prepared from 300 mg of tissue, homogenized with a Polytron PowerGen 1000 homogenizer (Fisher Scientific, Waltham, MA) in NP-40 buffer [20 mM Tris-HCl, 137 mM NaCl, 10% glycerol, 1% Nonidet P-40, and 2 mM EDTA with 1 Protease Inhibitor Cocktail Tablet (Roche, Indianapolis, IN) per 25 ml] at 4°C. Homogenized tissue was then agitated at 4°C for 2 hours and centrifuged at 10,000 g for 30 minutes, and the supernatant was transferred to a clean collection tube. For membrane samples (n = 8 each), liver was homogenized in 1 ml/100 mg tissue of buffer (50 mM Tris-HCl pH 7.4, 1 mM EDTA, 154 mM KCl with 1 protease inhibitor cocktail tablet/25 ml], centrifuged at 10,000 g for 30 minutes, and the supernatant was ultracentrifuged at 100,000 g for 70 minutes. The supernatant was collected and stored as a cytosolic fraction, and the pellet was resuspended in 100 mM sodium pyrophosphate pH 7.4, 0.1 mM EDTA by sonication. Solution underwent a second ultracentrifugation at 100,000 g for 70 minutes, and the pellet was resuspended in 10 mM KH2PO4 pH 7.4, 1.0 mM EDTA, 20% glycerol as a membrane fraction. Protein concentrations for whole cell lysate, membrane, and cytosol were determined using the Pierce BCA Protein Quantitation Assay (Thermo Fisher Scientific) per the manufacturer’s recommendations.
Immunofluorescence.
Immunofluorescent staining was performed on formalin-fixed, paraffin-embedded samples. Slides were deparaffinized with heat treatment (65°C) and xylene and rehydrated with isopropanol. The samples were then heated in an antigen retrieval buffer, Tris-EDTA (pH 9.0), and background was blocked in immunofluorescence buffer [1.0% bovine serum albumin (Sigma), 2.0% fetal calf serum in Tris-buffered saline/Tween 20] with 1.5% donkey and 1.5% goat sera. Samples were incubated in primary antibodies pan-Cadherin (ab16505, Abcam, Cambridge, UK) and Mrp2 (M8316, Sigma), followed by secondary antibodies AlexaFluor568 anti-mouse and AlexaFluor647 anti-rabbit (Life Technologies, Carlsbad, CA), respectively, and Hoechst 33 342 dye. Images were captured on a Leica DMI6000B fluorescence microscope equipped with a 16-bit Hamamatsu Flash 4.0 sCMOS camera using the 100×/1.40 oil immersion objective with the following filters: DAPI, FITC, rhodamine, and Cy5. The FITC channel had no label and was used as the control autofluorescence image, and subtraction methodology was developed using FIJI (Schneider et al., 2012).
Images from the FITC, rhodamine, and CY5 channels were opened in FIJI, and the mean gray value (MGV) measurement was acquired for each entire image. Because the amount of background autofluorescence in the rhodamine and CY5 channels was less than in the FITC channel, an adjustment factor was calculated to ensure that the background subtraction was appropriate: Adjustment factor = MGV-rhodamine/MGV-background-autofluorescence. The FITC image was then multiplied by the adjustment factor using “Process | Math | Multiply”. The adjusted background image was then subtracted from the rhodamine image using “Process | Image Calculator” and the subtract function. The resulting product shows the areas of staining in the rhodamine channel that can be observed by eye in the original image but are obscured by the high levels of background autofluorescence. The same procedure was followed for the Cy5 channel, and processed grayscale images were combined into a color image. This procedure yields a good approximation for removing the background autofluorescence characteristic of hepatocytes and improves the signal-to-noise ratio for the staining in these images.
Western Blot.
Fifty micrograms of whole cell lysate, membrane, or cytosolic preparation was reduced and denatured in Laemmli sample buffer (Bio-Rad Laboratories, Hercules, CA) and 5% 2-mercaptoethanol at 80°C for 5 minutes. Samples were separated on 10% SDS-PAGE and transferred to polyvinylidene fluoride membranes. The following antibodies were used to detect each protein: MARCKS (sc-6455), PKAα/β/γ cat, and ERK2 from Santa Cruz Biotechnology, Inc. (Dallas, TX); phospho-MARCKS (Ser152/156) (2741), phospho-PKA C (Thr197) (4781), PKCα (2056), PKCδ (2058), phospho-PKCδ (Thr505), PKCε (2683), and Rab11 (3539) from Cell Signaling Technology (Danvers, MA); phospho-PKCα (Ser657), phospho-ezrin(Thr567)/radixin(Thr564)/moesin(Thr558) from Millipore (Billerica, MA); phospho-PKCε (Ser729) and pan-cadherin from Abcam (Cambridge, UK); and radixin from Abnova (Taipei, Taiwan). Pan-cadherin and ERK2 were used as loading control for blots from membrane or whole/cytosolic preparations. Images were captured using the Image Laboratory Imaging System (Bio-Rad Laboratories) and relative protein quantities were determined using ImageJ software (National Institutes of Health, Bethesda, MD). Data reported are mean ± S.D., graphed in GraphPad Prism 5.0 (La Jolla, CA), with significance (*) defined to be P ≤ 0.05 after a Student’s t test.
Results
Mrp2 Mislocalization in NASH.
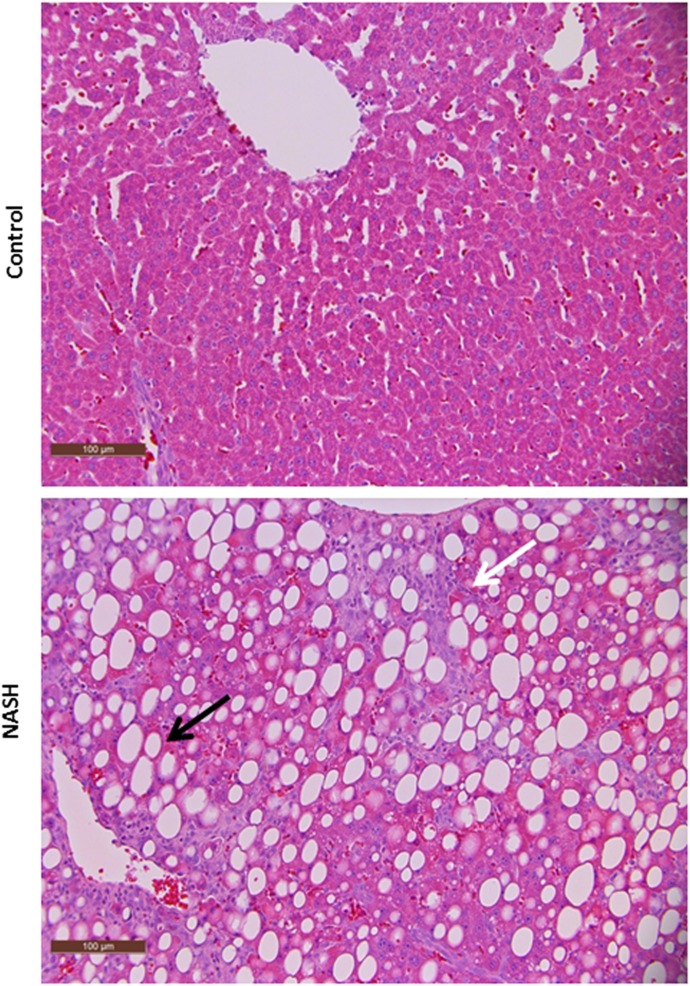
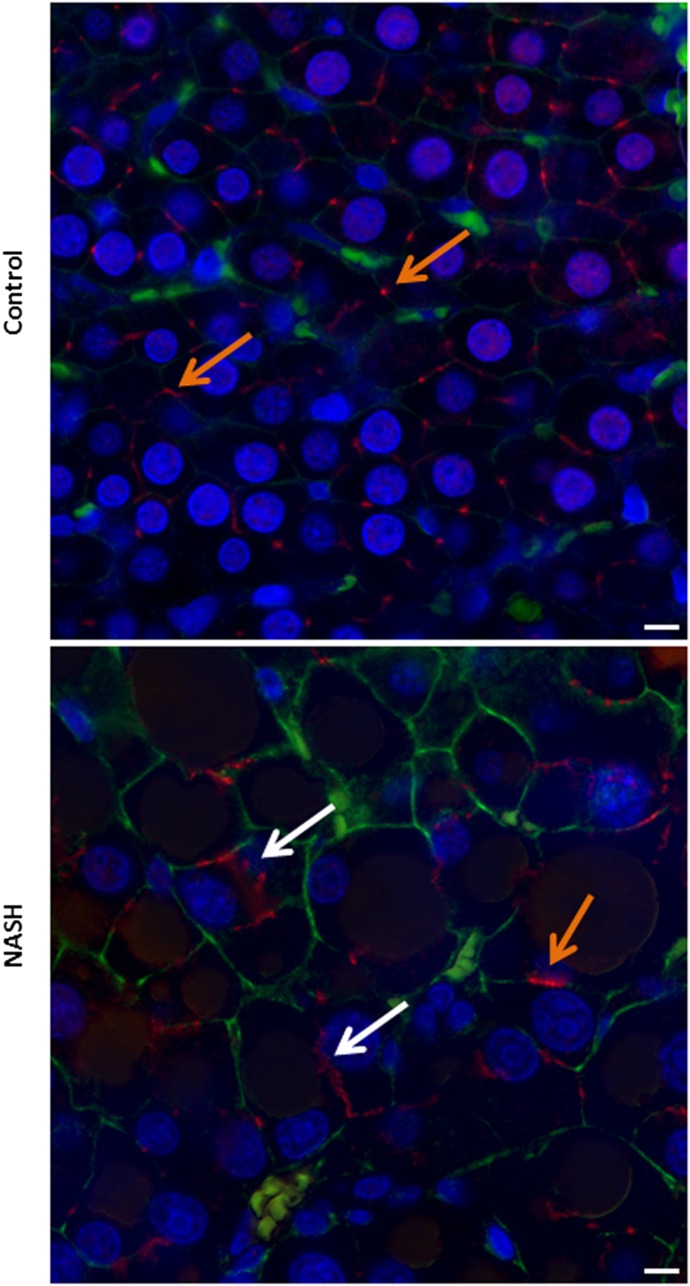
Hematoxylin and eosin-stained liver sections from representative control and MCD rats reveal that the 8-week MCD diet leads to the development of the NASH phenotype in Sprague-Dawley rats (Fig. 1). To visually ascertain the extent of Mrp2 mislocalization in NASH, dual-staining immunofluorescence was used to elicit fluorescence signals from Mrp2 and a plasma membrane marker, pan-cadherin, simultaneously (Fig. 2). Hepatocytes in control rats are clearly outlined with cadherin (green), which marks the plasma membrane, whereas Mrp2 is identified with punctate staining (red) localized to the canalicular membrane. NASH rats also have hepatocytes outlined with cadherin, indicating proper cellular structure in the disease state. Mrp2 in NASH appears to be a mix of properly localized staining on the canalicular membrane along with mislocalized staining as evidenced by the vesicularized pockets buckling into the hepatocyte.
Fig. 1.
Liver histopathology in control and NASH rats. Hematoxylin and eosin-stained liver sections from a representative MCD rat and its dietary control demonstrate that the characteristic features of NASH, including steatosis (black arrows) and inflammation (white arrows), appear in the MCD rat alone. Rats fed control diet had healthy livers with no evidence of steatosis. Original magnification, 20×. Scale bar, 100 µm.
Fig. 2.
Immunofluorescence of Mrp2 (red) and pan-cadherin (green) in control and NASH rats. Dual-staining immunofluorescence was used to identify Mrp2 and cadherin colocalization at the plasma membrane in control livers and Mrp2 mislocalization in NASH livers using Hoechst nuclear stain (blue) as a cellular reference. Orange arrows indicate properly localized Mrp2, white arrows indicate vesicularized staining and Mrp2 mislocalization. Images were taken using the 100×/1.40 oil immersion objective. Scale bar, 7.5 µm.
Active Mrp2 Membrane Retrieval and Inactive Membrane Insertion Processes in NASH.
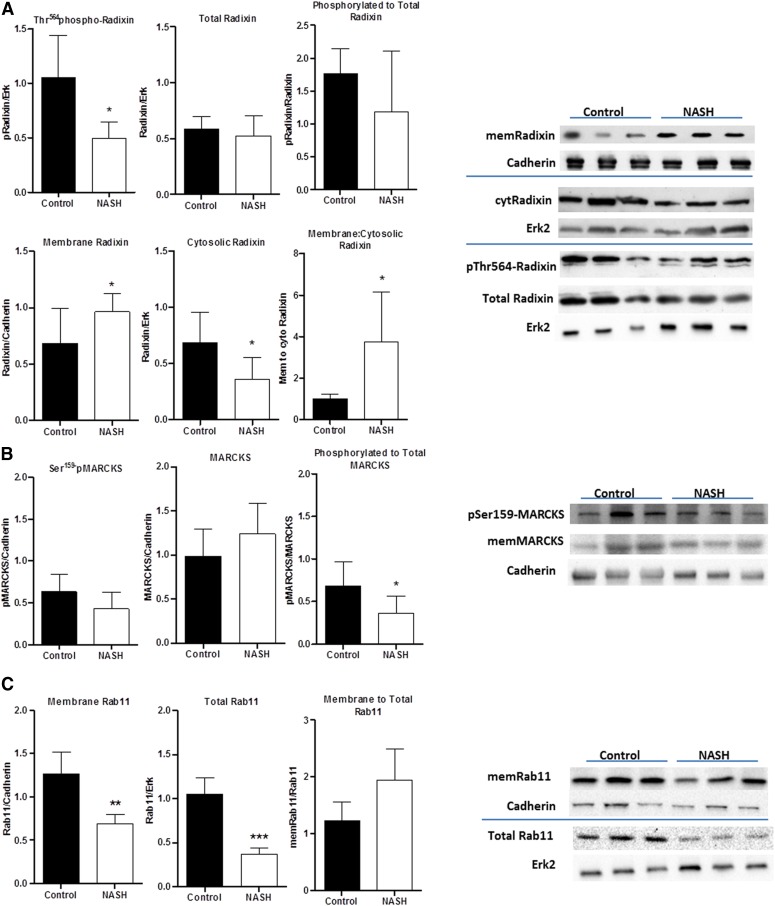
To identify pathways that are activated in NASH, a comprehensive Western blot approach was used to ascertain the activation status of various mediators involved in both the membrane insertion and membrane retrieval process for Mrp2. Activation status was assessed by comparing both the phosphorylation status and the membrane translocation of these mediators. Radixin, MARCKS, and Rab11 are substrates for protein kinases and involved in membrane recycling mechanisms for membrane-bound proteins, including Mrp2. Protein kinases analyzed that may regulate these substrates include PKA, PKCα, PKCδ, and PKCε.
Radixin was found to have a decreased phosphorylation status in NASH overall, with no change in total protein expression (Fig. 3A). It exhibited an increased membrane and decreased cytosolic protein expression, suggesting translocation to the plasma membrane. MARCKS does not exhibit any significant change in total phosphorylation or membrane localization in NASH, although there was a decreased phosphorylated-to-total MARCKS ratio. Rab11 has a decreased membrane and total protein expression (Figs. 3, B and C).
Fig. 3.
Western blot phosphorylation and translocation analyses for kinase substrates involved in Mrp2 membrane recycling. Radixin (A), MARCKS (B), and Rab11 (C) were analyzed in control and NASH rats for protein phosphorylation (n = 5) as well as membrane (n = 8) and total expression (n = 5). Data represent mean ± S.D. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 after a Student’s t test.
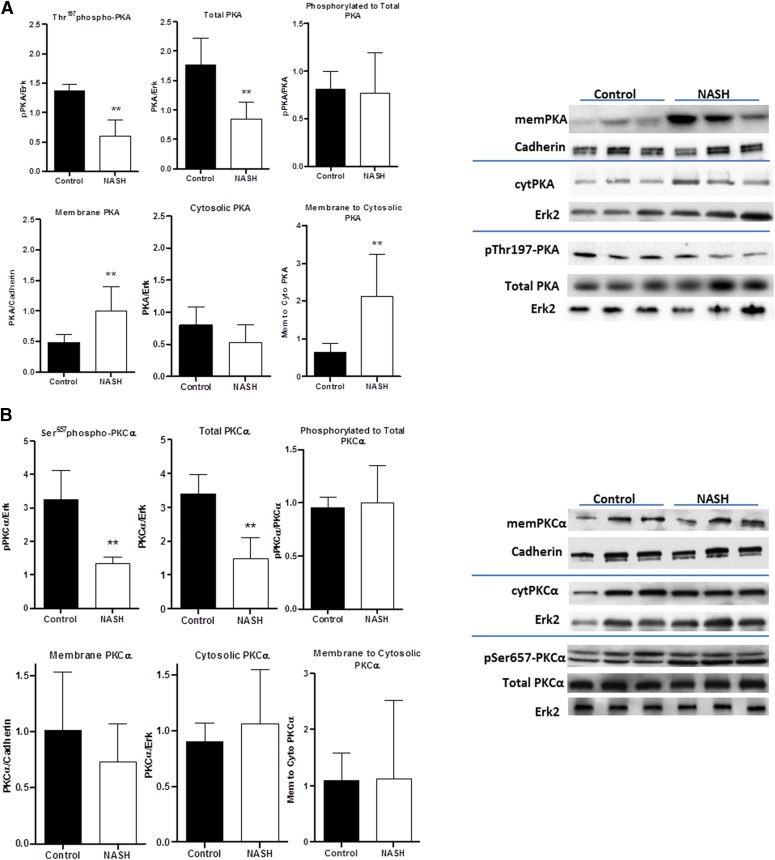
PKA and PKCα have been shown to work in tandem in a cAMP-dependent mechanism to prevent membrane retrieval of Mrp2, and the results of these analyses are shown in Fig. 4. PKA has a decreased overall phosphorylation status in the livers of NASH rats, which is directly correlated to a decreased expression. It does, however, exhibit an increase in membrane localization (Fig. 4A). PKCα, similarly, has a decreased phosphorylation and total expression in NASH, although it harbors no evidence of membrane translocation (Fig. 4B).
Fig. 4.
Western blot phosphorylation and translocation analyses for the PKA/PKCα pathway of Mrp2 membrane insertion. PKA (A) and PKCα (B) were analyzed in control and NASH rats for protein phosphorylation (n = 5) as well as membrane (n = 8), cytosolic (n = 8), and total expression (n = 5). Data represent mean ± S.D. **P # 0.01 after a Student’s t test.
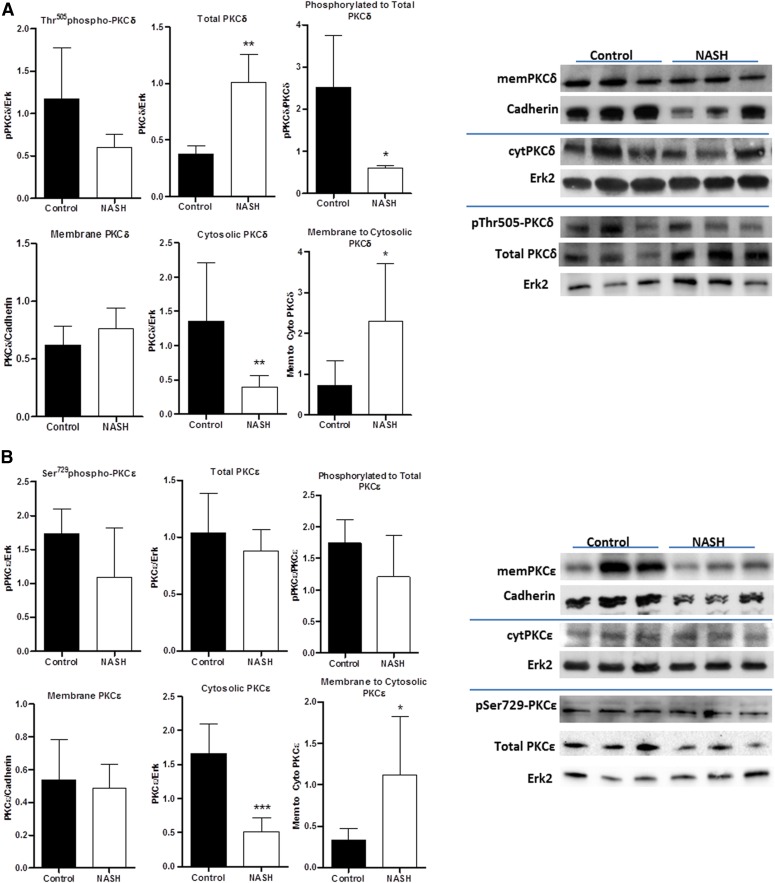
Finally, PKCδ and PKCε have been implicated in the regulation of membrane protein localization as well, with PKCδ playing an important role in the membrane insertion and PKCδ or PKCε required for the active retrieval. In NASH rats, PKCδ was shown to have increased overall protein expression, but a decreased phosphorylated protein-to-total protein ratio. However, it had an increased membrane-to-cytosolic expression ratio (Fig. 5A). PKCε also had an increased membrane-to-cytosolic expression ratio, with no change in phosphorylation status or total protein expression (Fig. 5B).
Fig. 5.
Western blot phosphorylation and translocation analyses for PKCδ and PKCε. PKCδ (A), and PKCε (B) were analyzed in control and NASH rats for protein phosphorylation (n = 5) as well as membrane (n = 8), cytosolic (n = 8), and total expression (n = 5). Data represent mean ± S.D. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 after a Student’s t test.
Discussion
Two important mechanisms regulating membrane localization are active recycling to (insertion) and from (retrieval) the membrane. Various stimuli activate multiple pathways that dictate membrane localization of transporters, including cholestatic and oxidative stress stimuli (Anwer, 2014). Many of these pathways overlap in the use of these mediators, creating a complex regulation of localization, even when looking at just one transporter. For example, activation of PKCα, a calcium-dependent protein kinase, has been shown to be involved in both TUDCA-stimulated Mrp2 insertion and estradiol-17β-d-glucuronide-induced Mrp2 retrieval (Crocenzi et al., 2008; Wimmer et al., 2008). Mrp2 membrane insertion by PKCα is dependent upon the cooperation of PKA; moreover, Mrp2 insertion is also mediated, in a cAMP-dependent manner, by PKCδ (Crocenzi et al., 2008; Park et al., 2012). Membrane retrieval of Mrp2 may also be regulated by novel protein kinases, specifically PKCε or PKCδ, when stimulated by cholestatic mediators such as TLC to phosphorylate its substrate, MARCKS (Schonhoff et al., 2013).
Mrp2 mislocalization has been reported in both human NASH and the MCD rat model of NASH and may have a profound impact on the proper elimination of xenobiotics (Hardwick et al., 2011, 2012; Dzierlenga et al., 2015). It is unknown what mechanisms dictate this mislocalization event, although impaired glycosylation has been suggested to play a role. In a previous study, human NASH liver samples exhibited increased overall protein expression of MRP2 and an increased expression of a lower molecular weight MRP2, suggestive of a deglycosylated species (Hardwick et al., 2011). This evidence may point to an impairment of MRP2 packaging and subsequent inability of a deglycosylated MRP2 species to be targeted to the canalicular membrane. However, there are other mechanisms of mislocalization that may play a complementary role to MRP2 protein packaging, namely, active membrane retrieval.
Most of the studies that have mapped out the signaling networks involved in the active recycling of Mrp2 membrane insertion were performed in primary hepatocytes and other in vitro models, with a few examples of rodent studies; moreover, they have used acute stressors to mediate insertion and retrieval (Anwer, 2014). It is unknown whether these same pathways may be altered in such a way as to maintain MRP2/Mrp2 mislocalization in a chronic disease scenario. Nevertheless, these processes can be mediated by oxidative stress, a hallmark of NASH progression, establishing the potential for mislocalization in the disease state (Marra et al., 2008). Glutathione levels in liver tissue (GSH:GSSG) decrease with progression of NAFLD (Hardwick et al., 2010), and glutathione depletion is also one of the stimuli shown to induce Mrp2 internalization (Sekine et al., 2008). In addition to oxidative stressors, cholestasis may accompany NASH progression; moreover, NAFLD progression contributes to a shift in bile acid composition and accompanied regulation (Lake et al., 2013; Jahnel et al., 2015). It is possible that these pathogenic hallmarks mediate MRP2 mislocalization in human NASH using the pathways described herein. In the rat MCD model of NASH, oxidative stress has also been suggested as a main driving force of the pathogenesis, including the upregulation of antioxidant response pathways (Lickteig et al., 2007). It is reasonable that similar stimuli may mediate the MRP2/Mrp2 mislocalization observed in both human NASH and rat MCD.
The study described herein determined the activation status of various mediators in the signaling pathways responsible for membrane protein insertion and retrieval, including protein kinases (PKA, PKCα, PKCδ, PKCε) and their putative substrates, radixin, MARCKS, and Rab11. Radixin is involved in membrane trafficking and cell polarity, and knockdown of radixin is associated with the development of hyperbilirubinemia through the impairment of Mrp2 localization (Kikuchi et al., 2002; Wang et al., 2006). The retrieval of Mrp2 from the canalicular membrane is dependent upon the desphosphorylation of radixin by PP1 and subsequent dissociation of Mrp2 and radixin (Saeki et al., 2011; Sekine et al., 2011; Suda et al., 2011). The current study found that MCD rats have an overall decrease in phosphorylated radixin, which is consistent with Mrp2 internalization. Studies using a lipopolysaccharide/oxidative stress-mediated Mrp2 retrieval have suggested a PKC-dependent mechanism for radixin dephosphorylation or a balance shift between PKA and PKC activation, due to a PKA-dependent phosphorylation of radixin (Sekine et al., 2011). The results herein do not indicate any increased activation of PKCs by looking at phosphorylation, although PKCδ exhibits an increased overall expression. PKCδ has been implicated in the parallel mechanism of Mrp2 internalization in HepG2 cells, mediated by ezrin phosphorylation (Chai et al., 2015). PKCδ and PKCε both exhibited an increased membrane localization compared with cytosolic, similarly implicating membrane retrieval of Mrp2 (Sekine et al., 2006). A decreased activation of PKA by expression level was observed, indicated by the decrease in phosphorylated and total PKA. This change in PKC-to-PKA activation ratio may contribute to the radixin-mediated retrieval process.
Another suggested substrate mediating the retrieval of Mrp2 from the membrane is MARCKS, which has been implicated in TLC-induced Mrp2 retrieval, via phosphorylation of MARCKS by PKCε (Schonhoff et al., 2013). The data herein indicate a decrease in membrane-associated phosphorylated MARCKS compared with total membrane MARCKS, with no detection of cytosolic MARCKS. This suggests a decreased activation of MARCKS and no dissociation from the membrane, which is inconsistent with the pathway described as leading to Mrp2 retrieval. Furthermore, an increase in membrane translocation of PKCε was observed, which suggests activation, although there was no change in phosphorylation. Although these data suggest that MARCKS may not be involved in NASH-related Mrp2 mislocalization, PKCε phosphorylates Mrp2 directly to alter the transport function, and the translocation of PKCε to the membrane may be related to this alternative function (Crocenzi et al., 2008). Furthermore, there may be other substrates for PKCε at the canalicular membrane that mediates Mrp2 retrieval in response to other stimuli.
Rab11 is a vesicular transport mediator, which is required for trans-golgi-to-plasma-membrane shuttling, and as another regulator of Mrp2 localization, it is more directly implicated in active endocytosis/exocytosis (Chen et al., 1998; Zucchetti et al., 2013; Park et al., 2014). TUDCA-induced Mrp2 retrieval was shown to be mediated by Rab11; furthermore, Mrp2 and other transporters are known to be shuttled between the membrane and Rab11-positive endosomes. Processes impacting Rab11 activation are likely to result in the misregulation of bile salt export pump as well (Zucchetti et al., 2013). Our data indicate a decreased expression of Rab11, which could indicate a global dysregulation of endocytic processes in NASH, impacting more than transporters alone. Interestingly, TUDCA-induced Mrp2 membrane insertion by Rab11 may be mediated by the kinase activity of PKCδ (Park et al., 2012). The studies herein show a significant decrease in the phosphorylated-to-total PKCδ ratio in NASH rats, despite an increased total expression and membrane localization. This suggests that translocation-related and kinase-related activation mechanisms may be at play. Overall, these data suggest that this pathway may also contribute to Mrp2 mislocalization in NASH.
The final putative mediator of Mrp2 membrane retrieval that was analyzed in this study was PKCα, which was found to have no change in membrane translocation but did exhibit a decrease in expression, resulting in a decreased total amount of phosphorylated PKCα. PKCα has been widely implicated in the targeting of Mrp2, and Bsep, to the canalicular membrane to promote the choloretic property of TUDCA, a process which also involves PKA (Wimmer et al., 2008). Decreased activation of PKCα in the current study is consistent with the mislocalization of Mrp2, suggesting that insertion mechanisms may be impaired. NASH rats also exhibited a decrease in phosphorylated and total PKA, which despite membrane translocation, may also indicate an overall decrease in the PKCα/PKA-dependent pathway of Mrp2 membrane insertion.
Taken together, the altered activation states of the mediators investigated in this study suggest an activation of membrane retrieval processes related to radixin dephosphorylation and an impairment of membrane insertion processes mediated by PKCα and Rab11 (Fig. 6). Mrp2 membrane retrieval has been shown to be mediated by these processes, specifically by dephosphorylation of radixin, a process that is redox dependent and mediated by PKCδ or PKCε, via PP1 (Sekine et al., 2006; Saeki et al., 2011). Decreased activation of PKCα and PKA in MCD rats also indicates a potential impairment of insertion mechanisms. This is the first study to characterize these mediators in an in vivo situation with a chronic stimulus. These mediators, largely activated by oxidative stressors, may also be stimulated in the chronic NASH state from oxidative stress, contributing to this phenomenon. Understanding the mechanism of MRP2/Mrp2 internalization in NASH and the causative stimuli may point to possible treatments, including the reversal of MRP2 mislocalization with restoration of redox balance. Mrp2 internalization induced by GSH depletion was shown to be reversed upon restoration of GSH (Sekine et al., 2008). Bile acids are similarly shown to have competing influence on Mrp2 membrane localization, as TUDCA stimulates Mrp2 insertion and TLC its retrieval from the membrane (Wimmer et al., 2008; Schonhoff et al., 2013). Overall, this study confirms the misregulation of mediators involved in membrane protein localization using the MCD model and suggests active membrane recycling as one possible mechanism for Mrp2 mislocalization in NASH.
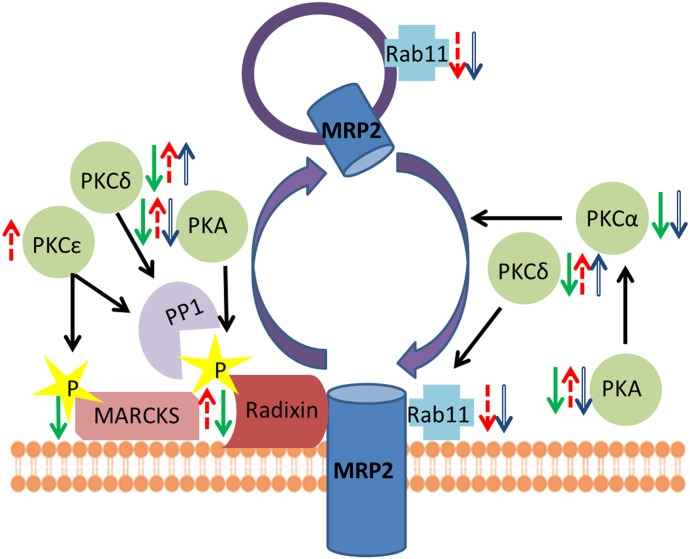
Fig. 6.
Active membrane retrieval and impaired membrane insertion of Mrp2 in NASH. This schematic outlines the summary of results for mediators that were shown to have altered activation in NASH. Arrows indicate increase (up) or decrease (down) in phosphorylation (green solid arrows), expression (blue hollow arrows) or membrane localization (red dashed arrows). These results support the model that decreased radixin phosphorylation is necessary for the internalization of Mrp2. This study suggests that decreased activation of PKA contributes to the loss of radixin phosphorylation or activation of PKCδ and/or PKCε contributes to active dephosphorylation of radixin by PP1. On the insertion side, the decreased kinase activity of PKCδ may contribute to a decrease in Rab11-mediated endocytosis. Additionally, an overall decrease in the activation of PKA and PKCα suggests and impairment of membrane insertion processes of Mrp2. MARCKS harbors a decreased phosphorylation ratio, but implications of this need to be more fully characterized.
Acknowledgments
The authors extend sincere gratitude to Dr. Rhiannon Hardwick for valued scientific advice during the outset of this study. The authors also thank Doug Cromey and the Southwest Environmental Health Sciences Microscopy Core Facility for advice and development of methodology for the dual-stained immunofluorescence experiments.
Abbreviations
- MARCKS
myristoylated alanine-rich C-kinase substrate
- MCD
methionine and choline deficient
- MGV
mean gray value
- MRP
multidrug resistance-associated protein
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- PK
protein kinase
- PP1
protein phosphatase 1
- TLC
taurolithocholate
- TUDCA
tauroursodeoxycholic acid
Author Contributions:
Participated in research design: Dzierlenga, Clarke, and Cherrington.
Conducted experiments: Dzierlenga.
Performed data analysis: Dzierlenga.
Wrote or contributed to the writing of the manuscript: Dzierlenga, Clarke, and Cherrington.
Footnotes
This work was supported by the National Institutes of Health National Institute of Environmental Health Sciences Toxicology Training Grant [ES007091], National Institute of Child Health and Human Development [Grant HD062489], and National Institute of Environmental Health Sciences [Grant ES019487].
References
- Anwer MS. (2014) Role of protein kinase C isoforms in bile formation and cholestasis. Hepatology 60:1090–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuers U, Bilzer M, Chittattu A, Kullak-Ublick GA, Keppler D, Paumgartner G, Dombrowski F. (2001) Tauroursodeoxycholic acid inserts the apical conjugate export pump, Mrp2, into canalicular membranes and stimulates organic anion secretion by protein kinase C-dependent mechanisms in cholestatic rat liver. Hepatology 33:1206–1216. [DOI] [PubMed] [Google Scholar]
- Chai J, Cai S-Y, Liu X, Lian W, Chen S, Zhang L, Feng X, Cheng Y, He X, He Y, et al. (2015) Canalicular membrane MRP2/ABCC2 internalization is determined by Ezrin Thr567 phosphorylation in human obstructive cholestasis. J Hepatol 63:1440–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Feng Y, Chen D, Wandinger-Ness A. (1998) Rab11 is required for trans-golgi network-to-plasma membrane transport and a preferential target for GDP dissociation inhibitor. Mol Biol Cell 9:3241–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocenzi FA, Sánchez Pozzi EJ, Ruiz ML, Zucchetti AE, Roma MG, Mottino AD, Vore M. (2008) Ca(2+)-dependent protein kinase C isoforms are critical to estradiol 17beta-D-glucuronide-induced cholestasis in the rat. Hepatology 48:1885–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzierlenga AL, Clarke JD, Hargraves TL, Ainslie GR, Vanderah TW, Paine MF, Cherrington NJ. (2015) Mechanistic basis of altered morphine disposition in nonalcoholic steatohepatitis. J Pharmacol Exp Ther 352:462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CDC, Lickteig AJA, Augustine LML, Ranger-Moore J, Jackson JPJ, Ferguson SS, Cherrington NJ. (2009) Hepatic cytochrome P450 enzyme alterations in humans with progressive stages of nonalcoholic fatty liver disease. Drug Metab Dispos 37:2087–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RN, Ferreira DW, More VR, Lake AD, Lu Z, Manautou JE, Slitt AL, Cherrington NJ. (2013) Altered UDP-glucuronosyltransferase and sulfotransferase expression and function during progressive stages of human nonalcoholic fatty liver disease. Drug Metab Dispos 41:554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RN, Fisher CD, Canet MJ, Lake AD, Cherrington NJ. (2010) Diversity in antioxidant response enzymes in progressive stages of human nonalcoholic fatty liver disease. Drug Metab Dispos 38:2293–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RN, Fisher CD, Canet MJ, Scheffer GL, Cherrington NJ. (2011) Variations in ATP-binding cassette transporter regulation during the progression of human nonalcoholic fatty liver disease. Drug Metab Dispos 39:2395–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RN, Fisher CD, Street SM, Canet MJ, Cherrington NJ. (2012) Molecular mechanism of altered ezetimibe disposition in nonalcoholic steatohepatitis. Drug Metab Dispos 40:450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnel J, Zöhrer E, Alisi A, Ferrari F, Ceccarelli S, De Vito R, Scharnagl H, Stojakovic T, Fauler G, Trauner M, et al. (2015) Serum bile acid levels in children with NAFLD: A biomarker for progression? J Pediatr Gastroenterol Nutr 61:85–90. [DOI] [PubMed] [Google Scholar]
- Ji B, Ito K, Sekine S, Tajima A, Horie T. (2004) Ethacrynic-acid-induced glutathione depletion and oxidative stress in normal and Mrp2-deficient rat liver. Free Radic Biol Med 37:1718–1729. [DOI] [PubMed] [Google Scholar]
- Kikuchi S, Hata M, Fukumoto K, Yamane Y, Matsui T, Tamura A, Yonemura S, Yamagishi H, Keppler D, Tsukita S, et al. (2002) Radixin deficiency causes conjugated hyperbilirubinemia with loss of Mrp2 from bile canalicular membranes. Nat Genet 31:320–325. [DOI] [PubMed] [Google Scholar]
- Kojima H, Sakurai S, Uemura M, Kitamura K, Kanno H, Nakai Y, Fukui H. (2008) Disturbed colocalization of multidrug resistance protein 2 and radixin in human cholestatic liver diseases. J Gastroenterol Hepatol 23:e120–e128. [DOI] [PubMed] [Google Scholar]
- Lake AD, Novak P, Fisher CD, Jackson JP, Hardwick RN, Billheimer DD, Klimecki WT, Cherrington NJ. (2011) Analysis of global and absorption, distribution, metabolism, and elimination gene expression in the progressive stages of human nonalcoholic fatty liver disease. Drug Metab Dispos 39:1954–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake AD, Novak P, Shipkova P, Aranibar N, Robertson D, Reily MD, Lu Z, Lehman-McKeeman LD, Cherrington NJ. (2013) Decreased hepatotoxic bile acid composition and altered synthesis in progressive human nonalcoholic fatty liver disease. Toxicol Appl Pharmacol 268:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lickteig AJ, Fisher CD, Augustine LM, Cherrington NJ. (2007) Genes of the antioxidant response undergo upregulation in a rodent model of nonalcoholic steatohepatitis. J Biochem Mol Toxicol 21:216–220. [DOI] [PubMed] [Google Scholar]
- Marra F, Gastaldelli A, Svegliati Baroni G, Tell G, Tiribelli C. (2008) Molecular basis and mechanisms of progression of non-alcoholic steatohepatitis. Trends Mol Med 14:72–81. [DOI] [PubMed] [Google Scholar]
- Mottino AD, Cao J, Veggi LM, Crocenzi F, Roma MG, Vore M. (2002) Altered localization and activity of canalicular Mrp2 in estradiol-17beta-D-glucuronide-induced cholestasis. Hepatology 35:1409–1419. [DOI] [PubMed] [Google Scholar]
- Park SW, Schonhoff CM, Webster CRL, Anwer MS. (2012) Protein kinase Cδ differentially regulates cAMP-dependent translocation of NTCP and MRP2 to the plasma membrane. Am J Physiol Gastrointest Liver Physiol 303:G657–G665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SW, Schonhoff CM, Webster CRL, Anwer MS. (2014) Rab11, but not Rab4, facilitates cyclic AMP- and tauroursodeoxycholate-induced MRP2 translocation to the plasma membrane. Am J Physiol Gastrointest Liver Physiol 307:G863–G870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rost D, Kloeters-Plachky P, Stiehl A. (2008) Retrieval of the rat canalicular conjugate export pump Mrp2 is associated with a rearrangement of actin filaments and radixin in bile salt-induced cholestasis. Eur J Med Res 13:314–318. [PubMed] [Google Scholar]
- Saeki J, Sekine S, Horie T. (2011) LPS-induced dissociation of multidrug resistance-associated protein 2 (Mrp2) and radixin is associated with Mrp2 selective internalization in rats. Biochem Pharmacol 81:178–184. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonhoff CM, Gillin H, Webster CRL, Anwer MS. (2008) Protein kinase Cdelta mediates cyclic adenosine monophosphate-stimulated translocation of sodium taurocholate cotransporting polypeptide and multidrug resistant associated protein 2 in rat hepatocytes. Hepatology 47:1309–1316. [DOI] [PubMed] [Google Scholar]
- Schonhoff CM, Webster CRL, Anwer MS. (2013) Taurolithocholate-induced MRP2 retrieval involves MARCKS phosphorylation by protein kinase Cϵ in HUH-NTCP Cells. Hepatology 58:284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine S, Ito K, Horie T. (2008) Canalicular Mrp2 localization is reversibly regulated by the intracellular redox status. Am J Physiol Gastrointest Liver Physiol 295:G1035–G1041. [DOI] [PubMed] [Google Scholar]
- Sekine S, Ito K, Horie T. (2006) Oxidative stress and Mrp2 internalization. Free Radic Biol Med 40:2166–2174. [DOI] [PubMed] [Google Scholar]
- Sekine S, Ito K, Saeki J, Horie T. (2011) Interaction of Mrp2 with radixin causes reversible canalicular Mrp2 localization induced by intracellular redox status. Biochim Biophys Acta 1812:1427–1434. [DOI] [PubMed] [Google Scholar]
- Sekine S, Yano K, Saeki J, Hashimoto N, Fuwa T, Horie T. (2010) Oxidative stress is a triggering factor for LPS-induced Mrp2 internalization in the cryopreserved rat and human liver slices. Biochem Biophys Res Commun 399:279–285. [DOI] [PubMed] [Google Scholar]
- Suda J, Zhu L, Karvar S. (2011) Phosphorylation of radixin regulates cell polarity and Mrp-2 distribution in hepatocytes. Am J Physiol Cell Physiol 300:C416–C424. [DOI] [PubMed] [Google Scholar]
- Wang W, Soroka CJ, Mennone A, Rahner C, Harry K, Pypaert M, Boyer JL. (2006) Radixin is required to maintain apical canalicular membrane structure and function in rat hepatocytes. Gastroenterology 131:878–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer R, Hohenester S, Pusl T, Denk GU, Rust C, Beuers U. (2008) Tauroursodeoxycholic acid exerts anticholestatic effects by a cooperative cPKC alpha-/PKA-dependent mechanism in rat liver. Gut 57:1448–1454. [DOI] [PubMed] [Google Scholar]
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. (2016) Global Epidemiology of Non-Alcoholic Fatty Liver Disease-Meta-Analytic Assessment of Prevalence, Incidence and Outcomes. Hepatology 64:73–84. [DOI] [PubMed] [Google Scholar]
- Zucchetti AE, Barosso IR, Boaglio AC, Luquita MG, Roma MG, Crocenzi FA, Sánchez Pozzi EJ. (2013) Hormonal modulation of hepatic cAMP prevents estradiol 17β-D-glucuronide-induced cholestasis in perfused rat liver. Dig Dis Sci 58:1602–1614. [DOI] [PubMed] [Google Scholar]