Abstract
Bupropion sustained release is used to promote smoking cessation in males and nonpregnant females. However, its efficacy as a smoking cessation aid during pregnancy is not reported. The pregnancy-associated changes in maternal physiology may alter the pharmacokinetics and pharmacodynamics of bupropion and consequently its efficacy in pregnant smokers. Therefore, the aims of this study were to determine the steady-state pharmacokinetics of bupropion during pregnancy and the effect of functional genetic variants of CYP2B6 and CYP2C19 on bupropion pharmacokinetics in pregnant women. Plasma and urine concentrations of bupropion and its metabolites hydroxybupropion (OHBUP), threohydrobupropion, and erythrohydrobupropion were determined by liquid chromatography–mass spectrometry. Subjects were genotyped for five nonsynonymous single-nucleotide polymorphisms that result in seven CYP2B6 alleles, namely *2, *3, *4, *5, *6, *7, and *9, and for CYP2C19 variants *2, *3, and *17. The present study reports that the isoform-specific effect of pregnancy on bupropion-metabolizing enzymes along with the increase of renal elimination of the drug could collectively result in a slight decrease in exposure to bupropion in pregnancy. In contrast, pregnancy-induced increase in CYP2B6-catalyzed bupropion hydroxylation did not impact the plasma levels of OHBUP, probably due to a higher rate of OHBUP glucuronidation, and renal elimination associated with pregnancy. Therefore, exposure to OHBUP, a pharmacologically active metabolite of the bupropion, appears to be similar to that of the nonpregnant state. The predicted metabolic phenotypes of CYP2B6*6 and variant alleles of CYP2C19 in pregnancy are similar to those in the nonpregnant state.
Introduction
Bupropion (BUP) sustained release (SR), an antidepressant, is used clinically in a standardized dose of 150 mg twice per day to promote smoking cessation in males and nonpregnant females (Raupach and van Schayck, 2011). However, its efficacy as a smoking cessation aid for pregnant smokers is not reported.
The pharmacokinetic (PK) data for BUP in humans reported in the literature were obtained from males and nonpregnant females after single or multiple doses (Laizure et al., 1985; Hsyu et al., 1997; Benowitz et al., 2013). BUP is extensively metabolized via multiple pathways (Jefferson et al., 2005); three major metabolites of the drug in plasma, namely hydroxybupropion (OHBUP), threohydrobupropion (TB), and erythrohydrobupropion (EB), are pharmacologically active (Laizure et al., 1985). OHBUP is half as potent as the parent drug, whereas TB and EB have lower activity (Golden et al., 1988; Jefferson et al., 2005). At steady state, the plasma level of OHBUP greatly exceeds that of the parent drug; therefore, OHBUP is thought to be the major contributor to the pharmacologic activity of BUP (Golden et al., 1988).
CYP2B6 is the principal enzyme catalyzing the formation of OHBUP from BUP in liver (Hesse et al., 2000), and the formation of TB and EB is catalyzed by hepatic 11β-hydroxysteroid dehydrogenase 1 and carbonyl reductases (Molnari and Myers, 2012). In addition, CYP2C19 contributes to hydroxylation of BUP and its metabolites, TB and EB (Zhu et al., 2014). Both CYP2B6 and CYP2C19 genes are highly polymorphic, and some of the single-nucleotide polymorphisms (SNPs) have functional consequences (http://www.cypalleles.ki.se/). Specifically, the CYP2B6*6 allele of CYP2B6 represents the combination of 516Q>T and 785A>G SNPs and is associated with reduced protein expression and enzymatic activity (Zanger and Klein, 2013). CYP2B6 variants are associated with altered plasmaconcentrations of OHBUP (Benowitz et al., 2013; Høiseth et al., 2015) and BUP (Kirchheiner et al., 2003).
The positive correlation between levels of OHBUP and response to smoking cessation treatment with BUP was previously reported (Zhu et al., 2012). In contrast, carriers of the CYP2B6*6 variant, which is associated with slower metabolism of BUP, have higher abstinence rates than wild-type allele carriers (Lee et al., 2007). Therefore, it appears that the levels of BUP and its metabolite, OHBUP, could affect the quit rate in smokers treated with BUP for cessation.
During pregnancy, women experience numerous physiologic changes that could affect the PK profile of BUP (Loebstein et al., 1997). Pregnancy-induced increases in hepatic flow may accelerate BUP metabolic clearance (Loebstein et al., 1997). Furthermore, in vitro studies suggest the upregulation of hepatic CYP2B6 and downregulation of CYP2C19 by increased production of progestational hormones (Anderson, 2005; Mwinyi et al., 2010; Dickmann and Isoherranen, 2013). These in vitro findings were corroborated by observations in vivo: clearance of CYP2B6 substrates, namely methadone and efavirenz, was higher in pregnancy (Wolff et al., 2005; Olagunju et al., 2015), whereas clearance of the CYP2C19 substrate proguanil was decreased (McGready et al., 2003).
BUP and OHBUP are only moderately bound to plasma proteins (84% and 77%, respectively) (https://gsksource.com/zyban); therefore, pregnancy-associated declines in plasma albumin (<15%) and α-1-acid glycoprotein (50%) (Olagunju et al., 2012) should not alter the fraction of unbound BUP and OHBUP. In addition, the high lipophilicity of BUP suggests its preferential distribution into the tissue over the plasma compartment; therefore, pregnancy-induced increases in body water should not affect BUP biodisposition.
About 10% of the BUP dose is recovered in the urine as the unchanged drug or as its free or glucuronidated OHBUP, TB, and EB metabolites (Jefferson et al., 2005; Gufford et al., 2016). A recent study identified uridine glucuronosyl transferase (UGT) isoforms 2B7 and 1A9 as the primary enzymes catalyzing the glucuronidation of BUP metabolites (Gufford et al., 2016). Pregnancy-associated upregulation of UGT enzymes (Anderson, 2005) along with the increase in renal blood flow in pregnancy (Costantine, 2014) could accelerate renal elimination of BUP metabolites.
Taken together, it appears that, although the effect of pregnancy-induced changes in plasma volume and plasma protein concentrations on the PK of BUP is unlikely, changes in renal function, hepatic flow, and pregnancy-associated induction of CYP2B6 and reduced activity of CYP2C19 could affect the PK profile of BUP in pregnancy.
Therefore, the primary aim of this study was to determine the PK of BUP during pregnancy. The secondary aim was to explore the association between CYP2B6 and CYP2C19 genotypes and the metabolism of BUP during pregnancy. The data would provide evidence on the magnitudes of the effects of genetics and pregnancy on the biodisposition of BUP in pregnancy.
Materials and Methods
Subjects.
This was a prospective, opportunistic study conducted at the University of Texas Medical Branch (UTMB). Eligible participants were pregnant women taking BUP to treat depression who agreed to participate in the PK studies in pregnancy and postpartum. Decisions about diagnosis and treatment were made by the subjects’ own healthcare provider(s) and were independent of participation in this study. The eligible participants were 18 years of age or older and in a pregnancy window of 10–14 weeks (early pregnancy), 22–26 weeks (mid-pregnancy), and 34–38 weeks (late pregnancy). Women were excluded from participation if there was anemia with hematocrit of less than 28% or a prior history of or current medical examination consistent with the presence of clinically significant alterations in hepatic, renal, or gastrointestinal functions. All procedures involving human subjects were conducted according to the International Conference on Harmonization–Good Clinical Practice guidelines in agreement with the Declaration of Helsinki. All women were enrolled with written informed consent under a protocol that was reviewed and approved by the Institutional Review Board of the UTMB. All subjects were compensated for participation.
Study Protocol.
Subjects in this opportunistic study received the following formulations and dosages of BUP: immediate-release, 100 mg three times daily (Mylan Pharmaceuticals, Cononsburg, PA); sustained-release (SR), 150 mg once per day (QD; Teva Pharmaceuticals USA Inc., North Wales, PA; Actavis Inc., Parsippany, NJ) and 150 mg twice per day (BID; Actavis Inc.; GlaxoSmithKline, Philadelphia, PA; Watson Laboratories Inc., Corona, CA); and extended release, 300 mg QD (Actavis Inc.; Zydus Pharmaceuticals Inc., Pennington, NJ). Prior to the PK study, all subjects completed at least 4 days of a dosing diary and were therefore presumed to be at steady state. Serial blood samples were collected prior to dosing (0 hours) and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, and up to 10, 12, and 24 hours, depending on the respective dosing intervals. All blood samples were collected in heparinized BD Vacutaner tubes, and plasma was separated immediately by centrifugation. Urine samples were collected within the same dose interval as the blood samples. All urine output was collected, and the volume was noted. Blood for genotyping was collected in BD Vacutaner ethylenediaminetetraacetic acid purple-top tubes. All samples were stored at −80°C until analysis.
Plasma and Urine Sample Analysis.
The concentrations of BUP, OHBUP, EB, and TB in plasma were determined simultaneously using a modified liquid chromatography–mass spectrometry (LC-MS/MS) method developed and validated in our laboratory, as previously reported (Wang et al., 2012). The concentrations of BUP and its metabolites, namely OHBUP, TB, and EB, in urine were determined separately using a modified LC-MS method (Wang et al., 2010). The urine samples for quantification of BUP metabolites were processed with and without glucuronide deconjugation using a modified method of Petsalo et al. (2007). The glucuronides of OHBUP, EB, and TB were quantified as the difference between the concentrations of the nonconjugated (free) drug and the total. The validation of the LC-MS methods was performed following the US Food and Drug administration guideline (http://www.fda.gov/downloads/Drugs/GuidanceCompilanceRegulatoryInformation/Guidances/ucm070107.pdf); detailed description of BUP and its metabolites assayed in plasma and urine is provided in Supplemental Materials and Methods 1. The concentrations of creatinine in serum were determined in the biochemical laboratories of UTMB.
CYP2B6 and CYP2C19 Genotyping.
Subjects were genotyped for five nonsynonymous SNPs that result in the seven common CYP2B6 variant alleles, namely CYP2B6*2 (64C>T), CYP2B6*3 (777C>A), CYP2B6*4 (785A>G), CYP2B6*5 (1459C>T), CYP2B6*6 (516G>T and 785A>G), and CYP2B6*7 (516G>T, 785A>G, and 1459C>T). SNPs were identified following the polymerase chain reaction–restriction fragment length polymorphism methods reported previously (Lang et al., 2001) and allele discrimination assays using TaqMan probes (Thermo Fisher Scientific, Waltham, MA); the specifics are provided in the Supplemental Materials and Methods Section 1. The most common alleles of CYP2C19 are the loss-of-function variants CYP2C19*2 (681G>A, rs4244285) and *3 (636G>A; rs4986893), and the gain-of-function variant CYP2C19*17 (-806C>T; rs12248560) (Fricke-Galindo et al., 2016). The identification of these alleles in our subjects was conducted as described by Zhu et al. (2014); details are provided in Supplemental Materials and Methods Section 2.
Data Analysis.
The PK parameters were computed using noncompartmental analysis (Kinetica software version 5.0; Thermo Scientific). Area under the plasma concentration-time curve for a dose interval at steady state (AUCss) served as the main measure of exposure to BUP and its metabolites. For the subjects whose plasma sampling times were terminated prior to the end of the respective dosing intervals, the remaining plasma concentration values were extrapolated from the best fit curve, and the AUCss values were computed as the sum of AUC0-n and AUCn-τ where n is the last measured time point. The apparent steady state oral clearance (CL/Fss) of BUP was estimated as dose/AUCss with and without normalization to the actual body weight (kg). The activity of CYP2B6 was estimated using the OHBUP/BUP metabolic ratio in plasma, calculated as a ratio of AUCss for OHBUP over that of the parent drug, corrected for mol. wt. difference. Clearance via reductive metabolic pathways was estimated in a similar fashion as TB/BUP and EB/BUP metabolic ratios. Renal clearance of BUP and its metabolites was calculated as:
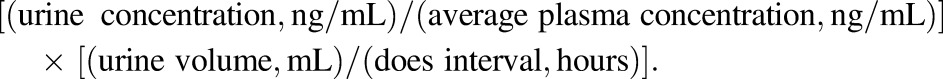 |
The molar percentage of BUP dose excreted as the parent drug and metabolites was calculated as (total excreted, mg)/(dose, mg) × 100%, corrected for the mol. wt. difference. Creatinine clearance was estimated using the Cockcroft-Gault formula:
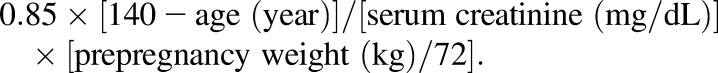 |
Statistical Analysis.
Results are presented as mean values ± S.D. Pairwise statistical comparisons were conducted using Wilcoxon signed rank test (SPSS Statistics, version 23; IBM, Armonk, NY). Mann–Whitney U test (SPSS Statistics, version 23; IBM) was used to compare PK data obtained from pregnant subjects homozygous for the CYP2B6 wild-type allele and carriers of the CYP2B6*6 allele, as well as to conduct comparisons between the metabolizer phenotypes of CYP2C19. P values < .05 were considered statistically significant. Post hoc analysis of statistical power was conducted using G*Power 3.1.9.2. (Faul et al., 2009).
Results
Subjects.
Twenty-nine subjects volunteered to participate in this opportunistic study. One subject was excluded from analysis due to deviations from the study protocol. Characteristics of the remaining 28 subjects are shown in Supplemental Table 1. At enrollment, the subjects had a mean age of 29.2 ± 6.9 (21–39) years, the mean gestational age was 27.5 ± 8.5 (13.1–38.0) weeks, and the average body weight was 86.8 ± 24.6 (50.4–168.8) kg. The majority of subjects were white/non-Hispanic (57%) and white/Hispanic (36%). Five subjects (18%) were enrolled during the early window, 11 (39%) during the middle window, and 12 (43%) during the late window. Depending on the time of enrollment and compliance, nine subjects (32%) completed one PK visit, 12 (43%) completed two PK visits, six (21%) completed three PK visits, and one subject completed four PK visits. Sixteen subjects were prescribed BUP SR 150 mg BID, five subjects took BUP SR 150 mg QD, three subjects took BUP immediate-release 100 mg three times daily, and two subjects took BUP extended release 300 mg QD. Having the same dose/formulation of BUP was an essential criterion for adequate paired comparison of PK parameters.
PK of BUP and Its Metabolites during Pregnancy and Postpartum.
Table 1 shows the paired estimated PK parameters of BUP and its metabolites for eight subjects in middle and late pregnancy, and for 12 subjects in late pregnancy and postpartum (lactating or non/postlactation period depending on availability). Only postlactating PK parameters were used for subjects 2 and 8, who participated during both postpartum studies (lactating and non/postlactating).
TABLE 1.
Paired estimated PK parameters for BUP during mid-pregnancy compared with late pregnancy; and late pregnancy compared with postpartum
Data presented as mean ± S.D. Mid-pregnancy, 22–26 weeks of gestation; late pregnancy, 34–38 weeks of gestation.
| Parameter | Mid-pregnancy (n = 8) | Late Pregnancy (n = 8) | Late Pregnancy (n = 12) | Postpartum (n = 12) | |
|---|---|---|---|---|---|
| BUP | AUCss BUP (ng × h/ml) | 640 ± 263 | 554 ± 214 | 654 ± 301 | 775 ± 291 |
| CL/Fss (L/h) | 359 ± 389 | 321 ± 152 | 259 ± 117 | 208 ± 93 | |
| CL/Fss (L/h/kg) | 4.37 ± 4.41 | 3.74 ± 2.29 | 3.10 ± 1.27 | 2.85 ± 1.79 | |
| OHBUP | AUCss OHBUP (ng × h/ml) | 9008 ± 3191 | 10,092 ± 3865 | 9499 ± 3893 | 9857 ± 6032 |
| OHBUP/BUP M.R. | 22.5 ± 28.1 | 21.3 ± 10.7 | 17.7 ± 10.7 | 14.1 ± 8.60 | |
| TB | AUCss TB (ng × h/ml) | 4843 ± 3196 | 3911 ± 2896 | 4105 ± 2564 | 4164 ± 3232 |
| TB/BUP M.R. | 7.91 ± 4.01 | 7.58 ± 4.63 | 6.79 ± 3.60 | 5.21 ± 3.10 | |
| EB | AUCss EB (ng × h/ml) | 759 ± 447 * | 541 ± 370 | 621 ± 387 | 871 ± 586 |
| EB/BUP M.R. | 1.33 ± 0.65 * | 1.06 ± 0.57 | 1.01 ± 0.51 | 1.10 ± 0.59 |
M.R., metabolic ratio, defined as the ratio of AUCs, corrected for mol. wt.
P < .05.
Paired analysis did not reveal any difference in the mean apparent oral clearance of BUP (CL/Fss) between the tested treatment windows, with or without adjustment to weight (Table 1). However, we observed that the mean value of AUCss of BUP in late pregnancy was slightly lower than that of postpartum (654 ± 301 ng × h/ml versus 775 ± 291 ng × h/ml, P = 0.099; Table 1). Furthermore, data analysis did not reveal any differences in the mean values of either OHBUP/BUP metabolic ratio or AUCss of OHBUP in mid- versus late pregnancy comparisons or late pregnancy versus postpartum (Table 1).
The mid-pregnancy mean value for TB AUCss was slightly higher than that in late pregnancy, although the results were not statistically significant (4843 ± 3196 ng × h/ml versus 3911 ± 2896 ng × h/ml, P = .068; Table 1). No difference in TB AUCss was observed in late pregnancy as compared with the nonpregnant state. However, the TB/BUP metabolic ratio in late pregnancy was slightly higher than that of postpartum (6.79 ± 3.60 versus 5.21 ± 3.10, P = .06; Table 1).
The mean values for EB AUCss and EB/BUP metabolic ratio in mid-pregnancy were higher than those of late pregnancy (EB AUCss: 759 ± 447 ng × h/ml versus 541 ± 370 ng × h/ml, P < .05; EB/BUP: 1.33 ± 0.65 versus 1.06 ± 0.57, P < .05; Table 2). Although we observed lower EB AUCss in late pregnancy than postpartum (621 ± 387 ng × h/ml versus 871 ± 586 ng × h/ml, P = .05; Table 1), no difference was revealed in the corresponding mean values of EB/BUP metabolic ratios (Table 1).
TABLE 2.
Urinary excretion of BUP and its metabolites over a dose interval. Paired analysis: mid-pregnancy versus late pregnancy, and late pregnancy versus postpartum
Data presented as mean ± S.D. Mid-pregnancy, 22–26 weeks of gestation; late pregnancy, 34–38 weeks of gestation.
| Parameter |
Mid-pregnancy (n = 4) |
Late Pregnancy (n = 4) |
Late Pregnancy (n = 11) |
Postpartum (n = 11) |
|
|---|---|---|---|---|---|
| Creatinine clearancea | (mL/min) | 185 ± 45* | 166 ± 33 | 175 ± 38* | 128 ± 23 |
| Renal clearance | CLR BUP (mL/min) | 23.1 ± 12.5 | 9.06 ± 5.80 | 17.2 ± 19.0 | 38.9 ± 77.7 |
| CLR OHBUP (mL/min) | 3.77 ± 3.19 | 1.34 ± 0.22 | 2.98 ± 3.58 | 3.08 ± 3.44 | |
| CLR TB (mL/min) | 72.1 ± 48.3 | 34.6 ± 12 6 | 56.8 ± 50.0 | 49.1 ± 32.1 | |
| CLR EB (mL/min) | 50.9 ± 40.3 | 20.4 ± 7.13 | 32.5 ± 32.4 | 27.8 ± 18.3 | |
| % of dose recovered as | BUP | 0.59 ± 0.24 | 0.25 ± 0.24 | 0.51 ± 0.59 | 0.87 ± 1.01 |
| OHBUP-free | 1.20 ± 1.03 | 0.53 ± 0.17 | 1.27 ± 1.63 | 1.47 ± 1.98 | |
| OHBUP-glucuronide | 7.97 ± 4.47 | 11.69 ± 8.10 | 13.8 ± 15.7* | 6.25 ± 5.47 | |
| TB-free | 15.9 ± 11.1 | 6.37 ± 6.71 | 10.0 ± 9.52 | 8.30 ± 6.30 | |
| TB-glucuronide | 1.07 ± 0.73 | 0.82 ± 0.83 | 3.10 ± 2.20* | 1.00 ± 1.15 | |
| EB-free | 1.71 ± 1.54 | 0.47 ± 0.48 | 0.76 ± 0.68 | 1.00 ± 0.76 | |
| EB-glucuronide | 2.55 ± 1.93 | 4.00 ± 3.88 | 0.56 ± 0.40 | 0.42 ± 0.37 |
The number of subjects in paired analysis of estimated renal creatinine clearance was the same as in Table 1.
P < .05.
Urinary Elimination of BUP and Its Metabolites.
Data on the excretion of BUP and its metabolites in the urine are shown in Table 2. The mean value of creatinine clearance in mid-pregnancy was higher than that in late pregnancy (185 ± 45 mL/min versus 166 ± 33 mL/min, P < .05), whereas the mean value of creatinine clearance in late pregnancy was higher as compared with that of postpartum (175 ± 38 mL/min versus 128 ± 23 mL/min, P < .05).
Comparisons of renal clearance of the drug and its metabolites in late pregnancy versus postpartum did not reveal any differences (Table 2). Moreover, no difference was observed in renal clearance of OHBUP, EB, and TB in mid- versus late pregnancy comparisons, whereas renal clearance of BUP in mid-pregnancy was slightly elevated as compared with late pregnancy (23.1 ± 12.5 mL/min versus 9.06 ± 5.80 mL/min, P = .068; Table 2).
No statistically significant differences in the fractions of BUP dose eliminated in the urine as unchanged drug or as unconjugated metabolites OHBUP, TB, and EB were observed between the groups. The percentage of BUP dose recovered in the urine as unchanged drug in late pregnancy was slightly below that of postpartum (0.51 ± 0.59% versus 0.87 ± 1.01%, P = .059; Table 2). A similar trend was observed in late pregnancy versus postpartum comparison of the percentage of the drug dose excreted in urine in a form of unconjugated EB (0.76 ± 0.68% versus 1.00 ± 0.76%, P = .062; Table 2). Moreover, the percentage of BUP dose excreted as free TB and free EB metabolites in mid-pregnancy tended to exceed the percentages excreted in late gestation (for TB-free, 15.9 ± 11.1% versus 6.37 ± 6.71%, P = .068, and for EB-free, 1.71 ± 1.54% versus 0.47 ± 0.48%, P = .068; Table 2).
In pregnancy, 89 ± 9% of total OHBUP eliminated in the urine was excreted in a glucuronidated form, whereas TB and EB conjugates accounted for 28 ± 20% and 46 ± 23% of total excreted TB and EB, respectively. The fraction of BUP eliminated as OHBUP glucuronide was higher in late pregnancy than postpartum (13.8 ± 15.7% versus 6.25 ± 5.47%, P < .05; Table 2). Likewise, the fraction of BUP recovered in the urine as TB glucuronide in late pregnancy was higher than that of postpartum (3.10 ± 2.20% versus 1.00 ± 1.15%, P < .05; Table 2). In addition, OHBUP glucuronide as percentage of BUP dose recovered in urine in late pregnancy slightly exceeded that of mid-pregnancy (11.7 ± 8.10% versus 7.97 ± 4.47%, P = .068; Table 2). However, TB glucuronide recovered in urine as percentage of BUP dose in mid-pregnancy did not differ from that in late pregnancy (Table 2). The results showed no difference in the urinary excretion of EB glucuronide in pregnancy and postpartum.
CYP2B6 and CYP2C19 Genetic Variants and PK of BUP in Pregnancy.
BUP PK parameters were compared among the pregnant subjects with and without genetic variant alleles of CYP2B6 and CYP2C19. We aimed to conduct the comparisons in early, middle, and late pregnancy separately to minimize the effect of gestational age-associated changes. However, comparative analysis within the early pregnancy group was not possible due to an insufficient number of subjects (n = 5; Supplemental Table 1). In the remainder of the pregnancy groups, BUP clearance and metabolic ratios were examined irrespective of the drug dosing, whereas comparisons of the urinary excretion data and AUCss of BUP and its metabolites were restricted to those subjects treated with the same dose of the drug, 150 mg BID.
Thirteen pregnant women participated in the PK study during mid-pregnancy, and 21 participated during late pregnancy (Supplemental Table 1). The following CYP2B6 genotype combinations were observed in the study subjects: in mid-pregnancy (n = 10 total), five subjects were of *1/*1 wild-type for CYP2B6, three were *1/*6, one was *6/*6, and one was *1/*5. In late pregnancy (n = 19 total): 11 were *1/*1, four were*1/*6, two were*6/*6, one was *1/*9, and one was *4/*4 (Supplemental Table 1). Based on the CYP2B6 allele frequencies in both groups, we compared the PK parameters between carriers of *6 (which confers reduced activity) and wild-type carriers (Fig. 1; Supplemental Table 2).
Fig. 1.
The effect of CYP2B6*6 variant allele on the selected PK parameters of BUP in mid- and late pregnancy: OHBUP/BUP metabolic ratio (M.R.) (A), AUCss of OHBUP (B) and AUCss of BUP (C). M.R., defined as the ratio of AUCs, corrected for mol. wt.; mid-pregnancy, 22–26 weeks of gestation; late pregnancy, 34–38 weeks of gestation.
In mid-pregnancy, the OHBUP/BUP metabolic ratio tended to be lower in *6 carriers than in wild-type, (9.46 ± 4.4 versus 32.8 ± 34.0, P = .086; Fig. 1A), which is consistent with the reduced metabolic phenotype of CYP2B6*6 allele. Although no difference was observed in OHBUP AUCss (Fig. 1B, mid-pregnancy), the BUP AUCss trended higher in *6 carriers in mid-pregnancy (742 ± 114 ng × h/ml versus 414 ± 225 ng × h/ml, P = .077; Fig. 1C). The mid-pregnancy AUCss of TB and EB also appeared to be higher in *6 carriers than in subjects homozygous for the wild-type allele (7263 ± 3116 ng × h/ml versus 2553 ± 2084 ng × h/ml, for TB, P = .077, and 1119 ± 393 ng × h/ml versus 477 ± 340 ng × h/ml for EB, P < .05; Supplemental Table 2). Neither comparison in late pregnancy revealed any differences (Fig. 1; Supplemental Table 2). Moreover, no differences in urinary excretion data were observed between the *6 carriers and those with the wild-type CYP2B6 variant in mid- or late pregnancy comparisons (Supplemental Table 2).
The observed CYP2C19 genotype combinations are presented in Supplemental Table 1 and were as follows: in mid-pregnancy (n = 12 total), four subjects were *1/*1 wild-type for CYP2C19, two were*1/*17, one was *17/*17, four were *1/*2, and one was *2/*2. In late pregnancy (n = 19 total), 10 were *1/*1, two were *1/*17, five were *1/*2, one was *2/*17, and one was *2/*2 (Supplemental Table 1). The subjects were stratified in two groups based on their metabolic phenotypes (Scott et al., 2013). Thus, PK parameters obtained from extensive metabolizers (EM) and ultrarapid metabolizers (UM), namely *1/*1, *1/*17, and *17/*17 carriers, were compared with those of poor metabolizers (PM) and intermediate metabolizers (IM), namely *2/*2 and *1/*2, including *2/*17 (Supplemental Table 3).
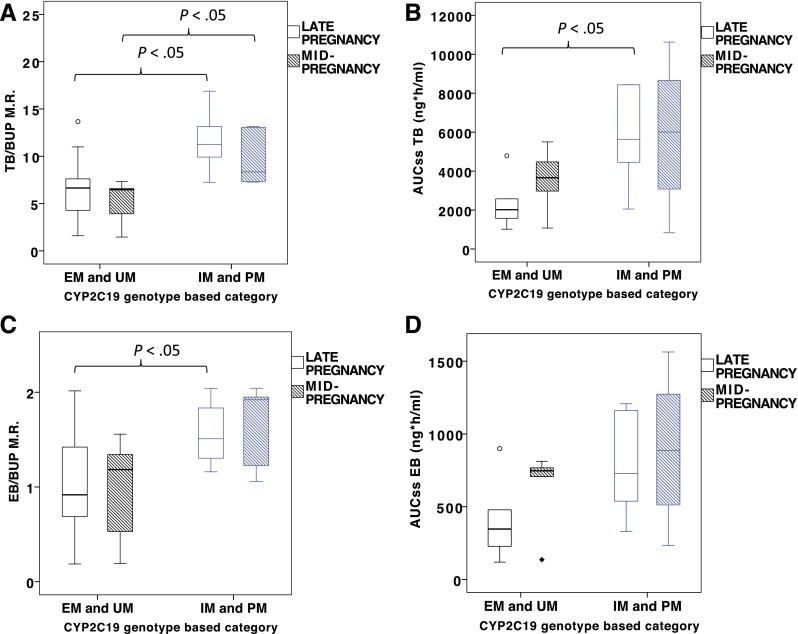
The TB/BUP metabolic ratio in the poor/intermediate metabolizers (PM + IM) group was higher than in the extensive/ultrarapid (EM + UM) group in both mid- and late pregnancy (mid-pregnancy, 11.6 ± 3.16 versus 6.58 ± 3.33, P < .05; late pregnancy, 11.6 ± 3.16 versus 6.58 ± 3.33, P < .05; Fig. 2A; Supplemental Table 3). We observed higher AUCss TB in PM + IM than in EM + UM in late pregnancy (5773 ± 2517 ng × h/ml versus 2333 ± 1313 ng × h/ml, P < .05; Fig. 2B; Supplemental Table 3); however, no difference in the AUCss of TB was observed between the groups in mid-pregnancy (Fig. 2B). Moreover, no difference in BUP AUCss was revealed between PM + IM and EM + UM groups in both mid- and late pregnancy comparisons.
Fig. 2.
The effect of CYP2C19 metabolic phenotype on the selected PK parameters of bupropion in mid- and late pregnancy: TB/BUP M.R. (A), AUCss of TB (B), EB/BUP metabolic ratio (M.R.) (C), AUCss of EB (D). M.R., defined as the ratio of AUCs, corrected for mol. wt.; mid-pregnancy, 22–26 weeks of gestation; late pregnancy, 34–38 weeks of gestation.
In a similar pattern, the EB/BUP metabolic ratio in PM + IM group was higher than in the EM + UM group in both mid- and late pregnancy, although statistical significance was not attained in mid-pregnancy comparisons (mid-pregnancy, 1.64 ± 0.46 versus 0.95 ± 0.56, P = .088; late pregnancy, 1.57 ± 0.34 versus 1.03 ± 0.52, P < .05; Fig. 2C; Supplemental Table 2). The EB AUCss in the PM + IM group was slightly higher than in the EM + UM group in late pregnancy (782 ± 350 ng × h/ml versus 403 ± 273 ng × h/ml, P = .055; Fig. 2D; Supplemental Table 3); however, no difference was observed in the mean value of EB AUCss in mid-pregnancy comparisons.
The percentage of BUP dose recovered in urine of PM + IM as conjugated TB in late pregnancy was 5.15 ± 2.17% and was higher than that of EM + UM (1.80 ± 1.18%, P < .05; Supplemental Table 3). In addition, the percentage of BUP dose recovered as unconjugated OHBUP and TB in the urine of PM + IM subjects in late pregnancy slightly exceeded that of the EM + UM group, although statistical significance was not reached (OHBUP-free: 0.71 ± 0.42% versus 0.35 ± 0.16%, P = .068; TB-free: 9.69 ± 6.01% versus 2.21 ± 1.41%, P = .068; Supplemental Table 3).
Discussion
The typical BUP SR dose for promoting cessation from smoking is 150 mg BID for 7–12 weeks in nonpregnant smokers. BUP is extensively metabolized, and its major product OHBUP contributes to the drug’s antismoking properties. Pregnancy-induced physiologic changes in the activity of hepatic enzymes metabolizing BUP—as well as increased hepatic blood flow and increased renal plasma flow—can alter the PK of BUP.
The OHBUP/BUP metabolic ratio has been historically used as a measure of CYP2B6 activity in BUP hydroxylation. Several studies suggest that the activity of CYP2B6 primarily affects the OHBUP levels, but not BUP (Zhu et al., 2012; Benowitz et al., 2013; Høiseth et al., 2015). The pregnancy-induced upregulation of CYP2B6 has been suggested based on in vitro and in vivo studies (Anderson, 2005; Olagunju et al., 2015). However, in our study, we did not observe any significant changes in the OHBUP/BUP metabolic ratio and OHBUP AUCss in pregnancy as compared with the nonpregnant state.
Furthermore, the EB/BUP metabolic ratio in mid-pregnancy exceeded that of late pregnancy. Moreover, the TB/BUP metabolic ratio was slightly higher in late pregnancy than in postpartum, although not statistically significant. These data suggest an increase in the reductive metabolism of BUP in pregnancy. However, CYP2C19 also contributes to the hydroxylation of BUP and its metabolites, TB and EB (Chen et al., 2010; Zhu et al., 2014). We observed that, relative to late pregnancy, the AUCss of EB was higher in mid-pregnancy, suggesting a decreased rate of EB metabolism in mid-pregnancy, possibly due to pregnancy-induced downregulation of CYP2C19 (McGready et al., 2003). Hence, this could contribute to an increased EB/BUP metabolic ratio in mid-pregnancy. The slight increase in the AUCss of TB in mid-pregnancy relative to late pregnancy was not statistically significant and had no effect on the corresponding TB/BUP ratios. We cannot discount the potential decrease in CYP2C19-mediated metabolism of BUP during pregnancy; however, the accelerated CYP2B6-catalyzed hydroxylation of BUP could possibly counterbalance BUP metabolic clearance. As a net result, we detected no significant changes in the AUCss of BUP in pregnancy (slight decrease in late pregnancy versus postpartum, P = .099); and no effect of pregnancy on the BUP CL/Fss was observed.
Another factor that could affect the PK of BUP is urinary excretion of the drug and its metabolites due to pregnancy-induced increase in renal plasma flow. Our findings indicated a slight increase in the renal clearance of BUP in mid-pregnancy relative to late pregnancy, which is probably associated with the peaking increment of glomerular filtration rate around the second trimester of pregnancy (http://www.glowm.com/section_view/item/157). Moreover, the higher percentage of dose excreted as unconjugated TB and EB in mid-pregnancy as compared with late pregnancy could reflect the higher plasma levels (and consequently AUCss) of TB and EB in mid-pregnancy, in addition to the increased glomerular filtration rate. However, the results in the urine excretion of the drug and its metabolites in mid- versus late pregnancy comparisons were not statistically significant. The small sample size leads to low statistical power for some analysis. A potential carryover of the drug and its metabolites from the previous dose(s) was a limitation of the urine PK analysis in our study.
We measured the fraction of BUP recovered in the urine as the metabolites OHBUP, TB, and EB, in their free forms or as glucuronide conjugates. Of the OHBUP, TB, and EB metabolites quantified in the urine of pregnant subjects, OHBUP was the most appreciably conjugated, followed by TB and EB. Relative to postpartum, the percentage of BUP dose excreted as TB and OHBUP glucuronides was higher in late pregnancy, which was consistent with the hormone-mediated upregulation of several hepatic UGT enzymes during pregnancy, particularly in late trimester (Abernethy et al., 1982; Jeong et al., 2008; Ohman et al., 2008). The current sample size for late pregnancy versus postpartum comparisons (n = 12) was sufficient to achieve 93% statistical power for the TB glucuronide data analysis, although for the OHBUP glucuronide it was 43%. We did not measure the concentrations of conjugated metabolites in plasma, and that was one of the limitations in our study. However, the increased elimination rate of TB and OHBUP in their conjugated forms could contribute to the higher clearance of these metabolites. Therefore, it is possible that with pregnancy-induced upregulation of CYP2B6, the increase in the formation of OHBUP could not be observed due to a higher rate of OHBUP glucuronidation and its subsequent excretion. Likewise, the decrease in TB metabolism due to a pregnancy-associated downregulation of CYP2C19, along with an increase in TB clearance via glucuronidation, would result in no evident changes in TB levels during late pregnancy.
In the second part of our study, we investigated the influence of functional polymorphisms of CYP2B6 and CYP2C19 on BUP biodisposition in pregnancy, irrespective of pregnancy-induced changes. This was investigated to collectively understand the effects of both genetics and pregnancy on the PK of BUP. BUP and its metabolites exhibit linear PK at steady state (Findlay et al., 1981); therefore, the influence of genotype on BUP CL/Fss and OHBUP/BUP, TB/BUP, and EB/BUP metabolic ratios was examined irrespective of dosing.
Our results showed higher TB/BUP and EB/BUP metabolic ratios in pregnant CYP2C19 PM + IM subjects in both mid- and late pregnancy groups and higher TB and EB AUCss in these subjects in late pregnancy only. These results are consistent with the effect of CYP2C19 polymorphism on TB and EB in nonpregnant subjects (Zhu et al., 2014). However, it appears that CYP2C19 metabolizer phenotype did not influence the levels of BUP and, consequently, its AUCss and Cl/Fss in pregnant subjects in our study. Small sample size in both mid- and late pregnancy groups limited the power of statistical analysis. Moreover, the sample size in the mid-pregnancy group was insufficient to observe the effect of CYP2C19 metabolizer phenotypes on the AUCs of TB and EB as was detected in late pregnancy. In addition, our sample size in both mid- and late pregnancy was insufficient to differentiate individually between the different CYP2C19 metabolic phenotypes, namely, PM, IM, EM, and UM.
In our study, we did not observe any significant effect of the CYP2B6*6 variant on OHBUP/BUP metabolic ratio, and either BUP or OHBUP AUCs in pregnancy. However, the slight decrease in OHBUP/BUP metabolic ratio in carriers of CYP2B6*6 as compared with wild-type carriers in mid-pregnancy suggests that, in pregnant women, the CYP2B6*6 variant is associated with a reduced rate of BUP hydroxylation, as observed in men and nonpregnant women (Benowitz et al., 2013). Of note, in the mid-pregnancy group, the AUCss of EB was higher in *6 carriers than in subjects without that variant. The results could indicate an imbalance of CYP2B6 and CYP2C19 genotypes in these individuals. In addition to the insufficient sample size, a limitation we acknowledge is that we did not genotype for the CYP2B6*18-reduced activity variant allele that is present exclusively in individuals of African descent, with an allele frequency of 4–7% (Zanger and Klein, 2013). There were only two African-American pregnant subjects in our study, and neither of these two subjects was included in the CYP2B6 variant allele comparisons (Supplemental Table 1).
Due to the limited number of participants in our study, we could not investigate the impact of CYP2B6 and CYP2C19 polymorphism on the magnitude of pregnancy-induced changes in the PK of BUP and its metabolites. Nevertheless, it appears that decreased activity of CYP2C19 due to pregnancy, along with loss-of-function variants of CYP2C19, could contribute to higher steady-state exposure to TB and EB during pregnancy. The TB and EB metabolites of BUP have an inhibitory effect on the CYP2D6 enzyme (Parkinson et al., 2010), which is upregulated during pregnancy (Ke et al., 2013; Ryu et al., 2016). Therefore, possible drug–drug interactions of BUP and CYP2D6 substrates cannot be discounted in pregnancy, particularly in instances when dose adjustment of CYP2D6-metabolized medications is considered (Ryu et al., 2016).
In summary, we reported the effect of pregnancy on the pharmacologic profile of BUP, as well as the impact of CYP2B6 and CYP2C19 functional polymorphisms on BUP biodisposition during mid- and late pregnancy. It appears that the pregnancy-induced increase in CYP2B6-catalyzed BUP hydroxylation did not impact the plasma levels of OHBUP in pregnancy, probably due to a higher rate of OHBUP glucuronidation and renal elimination associated with pregnancy. Therefore, although maternal exposure to BUP could be slightly decreased in pregnancy, the exposure to its pharmacologically active metabolite OHBUP appears similar to that of the nonpregnant state. The predicted metabolic phenotypes of CYP2B6*6 and variant alleles of CYP2C19 in pregnancy are similar to those in the nonpregnant state. The association of the CYP2B6*6 variant with quit rates among pregnant smokers treated with BUP for smoking cessation remains to be investigated.
Acknowledgments
The authors appreciate the support of the physicians and nurses of the Labor and Delivery Ward and Perinatal Research Division of UTMB (Galveston, TX). The authors also appreciate the help of Susan Abdel-Rahman, who has contributed to revising the manuscript.
Abbreviations
- AUCss
area under the plasma concentration-time curve for a dose interval at steady state
- BID
twice per day
- BUP
bupropion
- CL/Fss
apparent steady state oral clearance
- CLR
renal clearance
- EB
erythrohydrobupropion
- EM
extensive metabolizer
- IM
intermediate metabolizer
- LC-MS
liquid chromatography–mass spectrometry
- OHBUP
hydroxybupropion
- PK
pharmacokinetic
- PM
poor metabolizer
- QD
once per day
- SNP
single-nucleotide polymorphism
- SR
sustained release
- TB
threohydrobupropion
- UGT
uridine glucuronosyl transferase
- UM
ultrarapid metabolizer
- UTMB
University of Texas Medical Branch
Authorship Contributions
Participated in research design: Rytting, Abdel-Rahman, Oncken, Clark, Ahmed, Hankins, Nanovskaya.
Conducted experiments: Fokina, Xu, West.
Contributed new reagents or analytic tools: Fokina.
Performed data analysis: Fokina, Xu, Rytting, Abdel-Rahman, Ahmed, Hankins, Nanovskaya.
Wrote or contributed to the writing of the manuscript: Fokina, Xu, Rytting, Abdel-Rahman, Oncken, West, Clark, Ahmed, Hankins, Nanovskaya.
Footnotes
This work was supported by National Institutes of Health National Institute on Drug Abuse [Grant RO1 DA030998] and Obstetric-Fetal Pharmacology Research Unit Network of National Institutes of Health National Institutes of Child Health and Human Development [Grant U10-HD47891].
The authors report no conflict of interest.
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Abernethy DR, Divoll M, Ochs HR, Ameer B, Greenblatt DJ. (1982) Increased metabolic clearance of acetaminophen with oral contraceptive use. Obstet Gynecol 60:338–341. [PubMed] [Google Scholar]
- Anderson GD. (2005) Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet 44:989–1008. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Zhu AZ, Tyndale RF, Dempsey D, Jacob P., 3rd (2013) Influence of CYP2B6 genetic variants on plasma and urine concentrations of bupropion and metabolites at steady state. Pharmacogenet Genomics 23:135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Liu HF, Liu L, Nguyen K, Jones EB, Fretland AJ. (2010) The in vitro metabolism of bupropion revisited: concentration dependent involvement of cytochrome P450 2C19. Xenobiotica 40:536–546. [DOI] [PubMed] [Google Scholar]
- Costantine MM. (2014) Physiologic and pharmacokinetic changes in pregnancy. Front Pharmacol 5:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickmann LJ, Isoherranen N. (2013) Quantitative prediction of CYP2B6 induction by estradiol during pregnancy: potential explanation for increased methadone clearance during pregnancy. Drug Metab Dispos 41:270–274. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, Lang A-G. (2009) Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 41:1149–1160. [DOI] [PubMed] [Google Scholar]
- Findlay JW, Van Wyck Fleet J, Smith PG, Butz RF, Hinton ML, Blum MR, Schroeder DH. (1981) Pharmacokinetics of bupropion, a novel antidepressant agent, following oral administration to healthy subjects. Eur J Clin Pharmacol 21:127–135. [DOI] [PubMed] [Google Scholar]
- Fricke-Galindo I, Céspedes-Garro C, Rodrigues-Soares F, Naranjo ME, Delgado Á, de Andrés F, López-López M, Peñas-Lledó E, LLerena A. (2016) Interethnic variation of CYP2C19 alleles, ‘predicted’ phenotypes and ‘measured’ metabolic phenotypes across world populations. Pharmacogenomics J 16:113–123. [DOI] [PubMed] [Google Scholar]
- Golden RN, De Vane CL, Laizure SC, Rudorfer MV, Sherer MA, Potter WZ. (1988) Bupropion in depression. II. The role of metabolites in clinical outcome. Arch Gen Psychiatry 45:145–149. [DOI] [PubMed] [Google Scholar]
- Gufford BT, Lu JB, Metzger IF, Jones DR, Desta Z. (2016) Stereoselective glucuronidation of bupropion metabolites in vitro and in vivo. Drug Metab Dispos 44:544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse LM, Venkatakrishnan K, Court MH, von Moltke LL, Duan SX, Shader RI, Greenblatt DJ. (2000) CYP2B6 mediates the in vitro hydroxylation of bupropion: potential drug interactions with other antidepressants. Drug Metab Dispos 28:1176–1183. [PubMed] [Google Scholar]
- Høiseth G, Haslemo T, Uthus LH, Molden E. (2015) Effect of CYP2B6*6 on steady-state serum concentrations of bupropion and hydroxybupropion in psychiatric patients: a study based on therapeutic drug monitoring data. Ther Drug Monit 37:589–593. [DOI] [PubMed] [Google Scholar]
- Hsyu PH, Singh A, Giargiari TD, Dunn JA, Ascher JA, Johnston JA. (1997) Pharmacokinetics of bupropion and its metabolites in cigarette smokers versus nonsmokers. J Clin Pharmacol 37:737–743. [DOI] [PubMed] [Google Scholar]
- Jefferson JW, Pradko JF, Muir KT. (2005) Bupropion for major depressive disorder: pharmacokinetic and formulation considerations. Clin Ther 27:1685–1695. [DOI] [PubMed] [Google Scholar]
- Jeong H, Choi S, Song JW, Chen H, Fischer JH. (2008) Regulation of UDP-glucuronosyltransferase (UGT) 1A1 by progesterone and its impact on labetalol elimination. Xenobiotica 38:62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke AB, Nallani SC, Zhao P, Rostami-Hodjegan A, Isoherranen N, Unadkat JD. (2013) A physiologically based pharmacokinetic model to predict disposition of CYP2D6 and CYP1A2 metabolized drugs in pregnant women. Drug Metab Dispos 41:801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchheiner J, Klein C, Meineke I, Sasse J, Zanger UM, Mürdter TE, Roots I, Brockmöller J. (2003) Bupropion and 4-OH-bupropion pharmacokinetics in relation to genetic polymorphisms in CYP2B6. Pharmacogenetics 13:619–626. [DOI] [PubMed] [Google Scholar]
- Laizure SC, DeVane CL, Stewart JT, Dommisse CS, Lai AA. (1985) Pharmacokinetics of bupropion and its major basic metabolites in normal subjects after a single dose. Clin Pharmacol Ther 38:586–589. [DOI] [PubMed] [Google Scholar]
- Lang T, Klein K, Fischer J, Nüssler AK, Neuhaus P, Hofmann U, Eichelbaum M, Schwab M, Zanger UM. (2001) Extensive genetic polymorphism in the human CYP2B6 gene with impact on expression and function in human liver. Pharmacogenetics 11:399–415. [DOI] [PubMed] [Google Scholar]
- Lee AM, Jepson C, Hoffmann E, Epstein L, Hawk LW, Lerman C, Tyndale RF. (2007) CYP2B6 genotype alters abstinence rates in a bupropion smoking cessation trial. Biol Psychiatry 62:635–641. [DOI] [PubMed] [Google Scholar]
- Loebstein R, Lalkin A, Koren G. (1997) Pharmacokinetic changes during pregnancy and their clinical relevance. Clin Pharmacokinet 33:328–343. [DOI] [PubMed] [Google Scholar]
- McGready R, Stepniewska K, Seaton E, Cho T, Cho D, Ginsberg A, Edstein MD, Ashley E, Looareesuwan S, White NJ, et al. (2003) Pregnancy and use of oral contraceptives reduces the biotransformation of proguanil to cycloguanil. Eur J Clin Pharmacol 59:553–557. [DOI] [PubMed] [Google Scholar]
- Molnari JC, Myers AL. (2012) Carbonyl reduction of bupropion in human liver. Xenobiotica 42:550–561. [DOI] [PubMed] [Google Scholar]
- Mwinyi J, Cavaco I, Pedersen RS, Persson A, Burkhardt S, Mkrtchian S, Ingelman-Sundberg M. (2010) Regulation of CYP2C19 expression by estrogen receptor α: implications for estrogen-dependent inhibition of drug metabolism. Mol Pharmacol 78:886–894. [DOI] [PubMed] [Google Scholar]
- Ohman I, Luef G, Tomson T. (2008) Effects of pregnancy and contraception on lamotrigine disposition: new insights through analysis of lamotrigine metabolites. Seizure 17:199–202. [DOI] [PubMed] [Google Scholar]
- Olagunju A, Bolaji O, Amara A, Else L, Okafor O, Adejuyigbe E, Oyigboja J, Back D, Khoo S, Owen A. (2015) Pharmacogenetics of pregnancy-induced changes in efavirenz pharmacokinetics. Clin Pharmacol Ther 97:298–306. [DOI] [PubMed] [Google Scholar]
- Olagunju A, Owen A, Cressey TR. (2012) Potential effect of pharmacogenetics on maternal, fetal and infant antiretroviral drug exposure during pregnancy and breastfeeding. Pharmacogenomics 13:1501–1522. [DOI] [PubMed] [Google Scholar]
- Parkinson A, Kazmi F, Buckley DB, Yerino P, Ogilvie BW, Paris BL. (2010) System-dependent outcomes during the evaluation of drug candidates as inhibitors of cytochrome P450 (CYP) and uridine diphosphate glucuronosyltransferase (UGT) enzymes: human hepatocytes versus liver microsomes versus recombinant enzymes. Drug Metab Pharmacokinet 25:16–27. [DOI] [PubMed] [Google Scholar]
- Petsalo A, Turpeinen M, Tolonen A. (2007) Identification of bupropion urinary metabolites by liquid chromatography/mass spectrometry. Rapid Commun Mass Spectrom 21:2547–2554. [DOI] [PubMed] [Google Scholar]
- Raupach T, van Schayck CP. (2011) Pharmacotherapy for smoking cessation: current advances and research topics. CNS Drugs 25:371–382. [DOI] [PubMed] [Google Scholar]
- Ryu RJ, Eyal S, Easterling TR, Caritis SN, Venkataraman R, Hankins G, Rytting E, Thummel K, Kelly EJ, Risler L, et al. (2016) Pharmacokinetics of metoprolol during pregnancy and lactation. J Clin Pharmacol 56:581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SA, Sangkuhl K, Stein CM, Hulot JS, Mega JL, Roden DM, Klein TE, Sabatine MS, Johnson JA, Shuldiner AR, Clinical Pharmacogenetics Implementation Consortium (2013) Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther 94:317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Abdelrahman DR, Zharikova OL, Patrikeeva SL, Hankins GD, Ahmed MS, Nanovskaya TN. (2010) Bupropion metabolism by human placenta. Biochem Pharmacol 79:1684–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Vernikovskaya DI, Abdelrahman DR, Hankins GD, Ahmed MS, Nanovskaya TN. (2012) Simultaneous quantitative determination of bupropion and its three major metabolites in human umbilical cord plasma and placental tissue using high-performance liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal 70:320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff K, Boys A, Rostami-Hodjegan A, Hay A, Raistrick D. (2005) Changes to methadone clearance during pregnancy. Eur J Clin Pharmacol 61:763–768. [DOI] [PubMed] [Google Scholar]
- Zanger UM, Klein K. (2013) Pharmacogenetics of cytochrome P450 2B6 (CYP2B6): advances on polymorphisms, mechanisms, and clinical relevance. Front Genet 4:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu AZ, Cox LS, Nollen N, Faseru B, Okuyemi KS, Ahluwalia JS, Benowitz NL, Tyndale RF. (2012) CYP2B6 and bupropion’s smoking-cessation pharmacology: the role of hydroxybupropion. Clin Pharmacol Ther 92:771–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu AZ, Zhou Q, Cox LS, Ahluwalia JS, Benowitz NL, Tyndale RF. (2014) Gene variants in CYP2C19 are associated with altered in vivo bupropion pharmacokinetics but not bupropion-assisted smoking cessation outcomes. Drug Metab Dispos 42:1971–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]