Abstract
Estimating the age distribution of mosquito populations is crucial for assessing their capacity to transmit disease and for evaluating the efficacy of available vector control programs. This study reports on the capacity of the near-infrared spectroscopy (NIRS) technique to rapidly predict the ages of the principal dengue and Zika vector, Aedes aegypti. The age of wild-type males and females, and males and females infected with wMel and wMelPop strains of Wolbachia pipientis were characterized using this method. Calibrations were developed using spectra collected from their heads and thoraces using partial least squares (PLS) regression. A highly significant correlation was found between the true and predicted ages of mosquitoes. The coefficients of determination for wild-type females and males across all age groups were R2 = 0.84 and 0.78, respectively. The coefficients of determination for the age of wMel and wMelPop infected females were 0.71 and 0.80, respectively (P< 0.001 in both instances). The age of wild-type female Ae. aegypti could be identified as < or ≥ 8 days old with an accuracy of 91% (N = 501), whereas female Ae. aegypti infected with wMel and wMelPop were differentiated into the two age groups with an accuracy of 83% (N = 284) and 78% (N = 229), respectively. Our results also indicate NIRS can distinguish between young and old male wild-type, wMel and wMelPop infected Ae. aegypti with accuracies of 87% (N = 253), 83% (N = 277) and 78% (N = 234), respectively. We have demonstrated the potential of NIRS as a predictor of the age of female and male wild-type and Wolbachia infected Ae. aegypti mosquitoes under laboratory conditions. After field validation, the tool has the potential to offer a cheap and rapid alternative for surveillance of dengue and Zika vector control programs.
Author Summary
Aedes aegypti is the principal vector for dengue, chikungunya and Zika viruses. These viruses require a period of development inside the mosquito before they can be transmitted to humans. Depending on environmental factors, dengue and Zika viruses take an average of 8–10 days to replicate inside the mosquito [1,2] whereas chikungunya virus takes 2–7 days to replicate [3]. The age of mosquitoes is therefore a critical determinant of disease transmission. A mosquito control strategy utilizing an endosymbiotic bacterium Wolbachia pipientis, has been proven effective at blocking dengue transmission in Ae. aegypti mosquitoes [4]. For effective virus blocking, mosquitoes infected with the wMel strain of Wolbachia must survive long enough to cover the extrinsic incubation period (EIP) of infected mosquitoes while those infected with wMelPop are expected to have a reduced lifespan to limit the period available for virus replication. These strategies therefore require routine monitoring using age grading techniques. In this study, we investigated the applicability of a rapid and cost effective near-infrared spectroscopy (NIRS) technique as an alternative age grading tool for wild-type and Wolbachia infected Ae. aegypti mosquitoes. We show that NIRS can rapidly predict on average, the age of male and female Ae. aegypti to ±3 days of their true age and can determine which mosquitoes are old enough to be potentially infectious with an accuracy of up to 91%.
Introduction
The mosquito Aedes aegypti is the primary vector for dengue, Zika and chikungunya viruses. Up to 100 million dengue cases occur annually [5,6] and an estimated 440,000–1,300,000 Zika cases were reported in early 2016. Notably, 3893 babies born to Zika infected mothers have been diagnosed with microcephaly [7,8]. Both Zika and dengue viruses are transmitted by female Ae. aegypti mosquitoes carrying viruses in their salivary glands. Due to the period required by the virus to replicate inside the mosquito, Ae. aegypti mosquitoes are in most cases only capable of transmitting the Zika or dengue viruses when they are at least 8 days old [1,2,9].
The success of existing arbovirus vector control programs for mosquito-borne viruses recommended by the World Health Organization such as targeted residual spraying and space spraying is dependent on their ability to shorten the lifespan of the mosquito and to limit the period available for virus development. Alternative biological control strategies under trial involve the release of Ae. aegypti mosquitoes transinfected with the wMel strain of Wolbachia pipientis for the suppression of arbovirus transmission [4] or the pathogenic wMelPop strain for arbovirus and/or vector population suppression [10–12]. Although the efficacy of Wolbachia induced arbovirus interference was first demonstrated against dengue and Chikungunya [13], it has now also been demonstrated against Zika virus [14,15]. Unlike the life-shortening strain wMelPop, the success of wMel as a biological control agent is dependent upon the survival of infected mosquitoes [4].
Following the outbreak of Zika virus in South America in 2015, there is renewed interest in defining mosquito survival characteristics as a means of evaluating vector control strategies that affect adult mosquito survival. The efficacy of the WHO recommended control interventions [16], would therefore be defined by their ability to effectively eliminate the old and potentially infectious population. To accurately define these characteristics, evaluations of current interventions would require assessments of mosquito populations on large-scale using rapid and accurate age grading techniques.
Traditionally, entomologists have used techniques based on dissections of the female reproductive system to estimate mosquito age and survival. These include the Detinova technique for differentiating parous and nulliparous mosquitoes [17] and the Polovodova technique for determining the number of times mosquitoes have laid eggs [18]. However, these techniques are time consuming and labour-intensive. Moreover, interpretation of the diagnostic changes to ovarian morphology can be problematic [19]. As a result, estimates of population age structure based on these techniques often lack statistical power as only a small sample size can be dissected within a reasonable timeframe.
Analysis of age-related changes of cuticular hydrocarbons by gas chromatography [20–22] and transcriptional profiling [23] have been evaluated as alternative age grading techniques for Aedes mosquitoes. However, their high costs may restrict their utility and sustainability for large-scale field trials or control programs, particularly in areas where resources are limited. Although age related proteomic changes recently reported for Ae. aegypti [24] may offer cost effective and rapid alternative means of age assessments, these techniques are still early in development.
New approaches are required that can rapidly and cost-effectively assess large numbers of field specimens. In our previous studies, we reported the use of the near-infrared spectroscopy (NIRS) technique for age and species prediction. The tool was first applied to predict the age of female laboratory reared An. gambiae and An. arabiensis and to differentiate these sibling species [25]. It was then used to age grade and differentiate species of semi field reared An. gambiae and An. arabiensis [26], age grade laboratory reared An. arabiensis undergoing various physiological changes [27], preserved specimens [28–30] and to age grade Anopheles gambiae sensu lato mosquitoes reared from wild larvae that were either susceptible or resistant to pyrethroids [31]. More recently NIRS successfully predicted the ages of laboratory Ae. aegypti maintained on a varying larval and adult diets [32]. From laboratory and semi-field studies, we have shown that the accuracy of NIRS for An. gambiae s.s. and An. arabiensis ranges between 79–90% and 80–90% for age grading into <7 d and ≥ 7 d old age groups and for species differentiation, respectively.
Given its rapidity and relative ease of application, NIRS represents a unique opportunity to develop a viable alternative to current ovarian dissections and molecular techniques for age grading. The use of a NIR spectrometer for this purpose is non-destructive, rapid, and requires little training. NIRS facilitates the analysis of hundreds of mosquitoes just in one day because it takes 15 seconds to prepare and collect spectra from a mosquito, without reagents or sample preparation procedures. Moreover, samples can be scanned either fresh or preserved [28–30].
Here, we show that NIRS may be used to predict the age of both male and female wild-type Ae. aegypti mosquitoes up to 30 d old. We also examined the ability of NIRS to predict the age of Ae. aegypti females and males infected with wMel and wMelPop strains of Wolbachia pipientis.
Methods
Ethics approval
Ethics approvals were obtained for routine blood feeding of mosquito colonies from the QIMR Berghofer Medical Research Institute (QIMR HREC P1162). Written informed consent was provided by all adult volunteers involved in blood feeding, and volunteers were given the right to refuse to participate or withdraw from the experiment at any time.
Mosquito rearing
Colonies of wild-type Ae. aegypti and Ae. aegypti infected with wMel and wMelPop were acquired and maintained at the insectary of QIMR Berghofer Medical Research Institute as previously described [33]. Adults were given a 24 hr window to emerge. Wild-type females and males were collected at 1, 5, 9, 13, 17, 21, 25 and 30 d post emergence. Adult wMel strain mosquitoes were collected at 1, 5, 10, 15, 19 and 20 d post emergence. Adult wMelPop strain mosquitoes were collected at 1, 5, 10, 15 and 19 d post emergence. All mosquitoes were collected either unfed (1 and 5 d old) or blood fed but after oviposition (> 5 days old mosquitoes). Adults were knocked down with carbon dioxide and stored in RNAlater solution. To allow for RNAlater penetration, samples were stored overnight in a 4°C refrigerator and then stored at -20°C for 2 months before scanning [28].
Mosquito scanning using NIR spectrometer
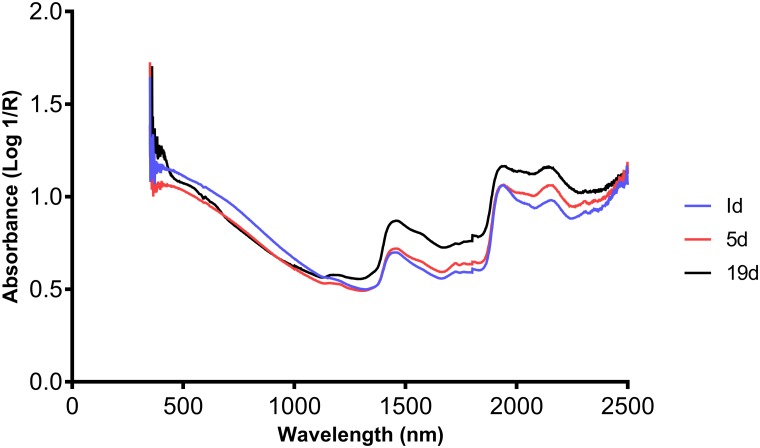
All mosquitoes collected were transferred to Ifakara Health Institute, Tanzania for scanning. Prior to scanning, residual RNAlater was removed from the mosquito specimens by blotting with paper towels. A maximum of 25 mosquitoes at a time were then positioned on a spectralon plate (ASD Inc, Boulder, CO), ventral side up. At least 40 mosquitoes at each age for all species were scanned using a LabSpec 5000 NIR spectrometer (ASD Inc, Boulder, CO), according to previously described protocols [14]. To collect the spectra, the heads and thoraces of mosquitoes were scanned under a 3 mm-bifurcated fibre optic probe containing six collection fibres and 33 illumination fibres. Typical raw spectra collected from the head and thorax of female wMel infected Ae. aegypti at 1, 5 and 19 d age points are shown in Fig 1.
Fig 1. Example of typical raw spectra collected from heads and thoraces of wMel infected female Ae. aegypti at 3 different age points.
Data analysis
Spectra were analyzed using Grams IQ software (Thermo Galactic, Salem, NH). Due to low light energy at shorter wavelengths and low sensor sensitivity at longer wavelengths, spectra appeared noisy outside the 500–2350 nm region. Therefore results were analyzed using spectra measured within this region. The relationship between spectra and age were analyzed by partial least squares (PLS) regression.
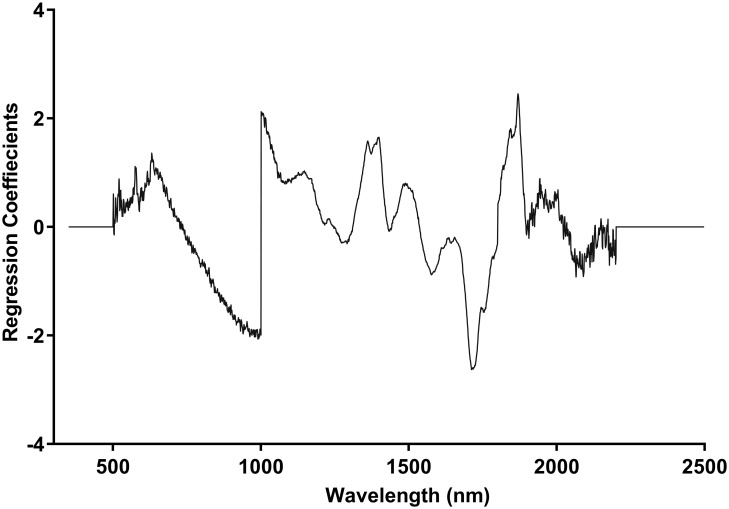
For wild-type Ae. aegypti, mosquitoes were divided into a training set and a validation set. The training set is used for developing a calibration model using cross validation analysis. Cross-validation is a “leave-one-out” self-prediction method where mosquitoes from a set are used to predict the age of mosquitoes from that same set. It is used to select the factors required for the calibration of the predictive model before running the validation set. All Wolbachia infected mosquitoes were analyzed using the cross validation method. The number of factors used in developing models was determined from the cross-validation prediction residual error sum of squares (PRESS) and regression coefficient plots. An example of a coefficient plot used for predicting the age of female wild-type Ae. aegypti is shown in Fig 2. Analysis of variance (ANOVA) was applied to test for statistical differences between mean predicted ages using the Statistical Package for Social Sciences 22 (IBM, Armonk, NY). Tukey post hoc analysis in ANOVA was applied to test for statistical differences between age groups. Actual age and predicted age were coded as independent and dependent variables, respectively. The relationship between true and predicted age was assessed by Spearman’s rank correlation coefficient analysis.
Fig 2. Regression coefficients for predicting the age of female wild-type Ae. aegypti using 9 partial least squares regression factors.
Results
Age prediction of male and female wild-type Ae. aegypti
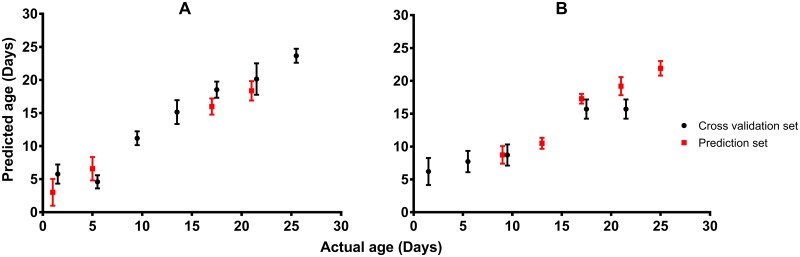
Except for 1 d old mosquitoes, the mean predicted age of female Ae. aegypti mosquitoes was within ± 2d of the actual age across all age groups (Table 1; Fig 3A). An accuracy of 91% (N = 501) was achieved if female mosquitoes excluded from the model were simply grouped into two age categories that separate mosquitoes that are unlikely to be infectious (< 8 d) from those that are old enough to have survived the dengue/Zika incubation period and therefore would be potentially infectious (≥ 8 d). Additionally, Spearman’s correlation analysis indicated a strong positive correlation between the predicted and the actual age for both the training set (R2 = 0.84; P <0.001) and the validation set (R2 = 0.83; P <0.001). Tukey Post hoc analysis indicated that female Ae. aegypti could be differentiated into 5 age groups (1–5, 6–9, 10–13, 14–17 and >17 d old).
Table 1. Mean age predictions of female and male wild-type Ae. aegypti mosquitoes using the cross validation method for samples used in the model and the prediction method for samples that were excluded from the model.
| Wild-type Ae. aegypti females | Wild-type Ae. aegypti males | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cross validation set1 [N = 101] | Prediction set2 [N = 501] | Cross validation set1 [N = 142] | Prediction set2 [N = 253] | ||||||||
| Actual age | Mean predicted age [95% CI] | SEM | Actual age | Mean predicted age [95% CI] | SEM | Actual age | Mean predicted age [95% CI] | SEM | Actual age | Mean predicted age [95% CI] | SEM |
| 1 | 3.0a[0.9–5.6] | 0.9 | 1 | 5.3a [3.8–6.7] | 0.7 | 1 | 5.7a[3.6–7.7] | 0.9 | 9 | 8.5a [7.1–10.0] | 0.7 |
| 5 | 6.5b[4.8–8.3] | 0.8 | 5 | 4.1a [3.1–5.1] | 0.5 | 5 | 7.2a,b[5.5–8.8] | 0.7 | 13 | 10.4a [9.5–11.4] | 0.5 |
| 17 | 15.9c[14.7–17.2] | 0.5 | 9 | 10.7b [9.6–11.7] | 0.5 | 9 | 8.2a,b[6.6–9.8] | 0.7 | 17 | 16.8b [15.9–17.8] | 0.5 |
| 21 | 18.3c[16.8–19.8] | 0.7 | 13 | 14.6c [12.8–16.4] | 0.9 | 17 | 15.2c[13.7–16.6] | 0.7 | 21 | 19.5c [18.3–20.8] | 0.6 |
| 17 | 18.0d,f [16.8–19.3] | 0.6 | 21 | 17.3c[15.9–18.7] | 0.6 | 25 | 21.5c [20.5–22.6] | 0.5 | |||
| 21 | 19.6c,d,e,f [17.3–22.0] | 0.9 | 30 | 28.1d[24.8–31.8] | 1.6 | 30 | |||||
| 25 | 23.2e [22.1–24.2] | 0.5 | |||||||||
| 30 | 19.9f[18.9–20.9] | 0.3 | |||||||||
Means followed by the same letter are not significantly different at P<0.05 when using Tukey post hoc test
Actual and mean predicted ages shown are in days
1 The accuracy of samples used to develop calibration models
2 The accuracy of samples used to validate models
Fig 3. Mean (95% CI) age prediction of female (A) and male (B) of wild-type Ae. aegypti mosquitoes using NIRS.
The age prediction of male mosquitoes that were excluded from the training set was generally within ± 3 d of the actual age. Moreover, an overall accuracy of 87% (N = 253) into < 8 d and ≥ 8 d old age groups was achieved. Spearman correlation analysis found a highly positive correlation between the predicted age and the actual age for both the training set (R2 = 0.77; P <0.001) and the validation set (R2 = 0.78; P <0.001). Tukey post hoc comparison test between age groups of male Ae. aegypti indicated that mosquitoes could be differentiated into four age groups (<9, 9–13, 14–17 and >17 d old) (Table 1; Fig 3B).
Age prediction of males and females infected with wMel and wMelPop
Using the cross validation analysis, females (N = 284) and males (N = 277) infected with the wMel strain were differentiated into < 8 d and ≥ 8 d old age groups with accuracies of 83%. Males (N = 234) and females (N = 229) infected with the wMelPop strain were both differentiated into the two groups with an accuracy of 78%. Spearman correlation analysis found a positive correlation between the predicted age and the actual age of females (R2 = 0.71; P<0.001) and males (R2 = 0.80; P<0.001) infected with wMel and females (R2 = 0.80; P<0.001) and males (R2 = 0.68; P<0.001) infected with wMelPop. All Wolbachia infected females and males were generally differentiated into 4 age groups (<5, 5–9, 10–15 and >15 d old) (Table 2).
Table 2. Mean age predictions of female and male wMel and wMelPop infected Ae. aegypti mosquitoes using the cross validation method.
| wMel infected Ae. aegypti: Cross Validation | wMelPop infected Ae. aegypti: Cross validation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| wMel infected females [N = 284] | wMel infected males [N = 277] | wMelPop infected females [N = 229] | wMelPop infected males [N = 234] | ||||||||
| Actual age | Mean predicted age [95% CI] | SEM | Actual age | Mean predicted age [95% CI] | SEM | Actual age | Mean predicted age [95% CI] | SEM | Actual age | Mean predicted age [95% CI] | SEM |
| 1 | 2.8a[1.6–3.9] | 0.5 | 1 | 4.8a[3.9–5.6] | 0.4 | 1 | 2.4a[1.4–3.9] | 0.5 | 1 | 3.9a[2.9–5.0] | 0.5 |
| 5 | 10.0b[9.2–10.7] | 0.3 | 5 | 6.4ab[5.6–7.2] | 0.3 | 5 | 9.0b[8.3–9.8] | 0.3 | 5 | 8.9b[7.9–9.8] | 0.4 |
| 10 | 13.2c[12.3–14.1] | 0.4 | 10 | 7.6b[6.6–8.6] | 0.4 | 10 | 9.7b[8.6–10.8] | 0.5 | 10 | 11.0c[10.1–11.9] | 0.4 |
| 15 | 13.6c[12.4–14.7] | 0.5 | 15 | 15.8c[15.1–16.5] | 0.3 | 15 | 12.6c[11.6–13.6] | 0.4 | 15 | 12.2c[11.0–13.4] | 0.5 |
| 19 | 14.6c,d[13.6–15.7] | 0.5 | 19 | 16.7c[15.4–18.1] | 0.6 | 19 | 16.4d[15.6–17.2] | 0.4 | 19 | 14.3d[13.3–15.2] | 0.4 |
| 20 | 15.8d[14.9–16.7] | 20 | 16.7c[15.9–17.6] | 0.4 | |||||||
Means followed by the same letter are not significantly different at P<0.05 when using Tukey post hoc test
Actual and mean predicted ages shown are in days
1 The accuracy of samples used to develop calibration models
Discussion
Our findings demonstrate the potential of the NIRS as a rapid technique for identifying the age of wild-type and Wolbachia infected Ae. aegypti mosquitoes. Mosquito survival is a fundamental parameter of vectorial capacity. As 1–2 days is required for blood feeding and at least 7 days is required for virus replication [2,9,34], the average infectious age of Ae. aegypti, the principal vector for Zika and dengue viruses, is considered to be at least 8 days. Wolbachia-based strategies utilizing the wMel strain require infected mosquitoes to survive and mate effectively with wild mosquitoes to drive the bacteria through populations. Alternatively, the life shortening properties of the pathogenic wMelPop strain may be harnessed to crash local vector populations [11]. The validation of either strategy will require accurate mosquito age grading techniques that can function against a background of Wolbachia infection.
Recently, Liebman and colleagues used NIRS to predict the age of laboratory reared female Ae. aegypti mosquitoes maintained on varying larval and adult diet up to 16 days post emergence [32]. We report on the ability of NIRS to age grade female and male wild-type Ae. aegypti up to 30 d old as well as females and males Ae. aegypti infected with Wolbachia up to 20 d old. Age predictions were determined on a continuous age scale and into < 8 d or ≥ 8 d old age groups. Although shorter EIPs have been reported for dengue viruses [35], eight days was the favored cut off point because it is widely quoted as the average age at which Ae. aegypti or Ae. albopictus are able to transmit dengue or Zika viruses [2,9,34] and the best accuracy was achieved at this cut off point.
Wavelengths ranging from 500–2350 nm were analyzed. This range comprises carbon-hydrogen (C-H) functional groups at 1220, 1450, 1700 and 1765 nm and protein (N-H) functional groups at 1510, 2055, 2060, 2180 and 2300 nm. Both cuticular hydrocarbons [20,21] and proteins [24] have previously yielded biomarkers suitable for age grading Ae. aegypti mosquito species. Overall, age prediction of wild-type females and Wolbachia infected females across all age categories assessed was within ±3–5 days of actual age. Five day old females infected with Wolbachia were the least accurately predicted. It may be that few developmental changes occur between 5 and10 days. It was recently reported by Hugo and colleagues, who examined changes in protein abundance with age of Ae. aegypti, that age-related changes for the majority of the proteins reported occurred between 1 and 5 d with little or no further protein changes occurring among older age groups [24]. These proteomic and transcriptome approaches may ultimately help identify biomarkers contributing to age related variation in NIRS spectra, provide further validation and improve the prediction accuracy of NIRS. We found strong positive correlation between the actual age and the predicted age for both wild-type and Wolbachia infected mosquitoes.
Age prediction accuracy of male wild-type and male mosquitoes infected with Wolbachia was impressive. NIRS predicted the ages of male wild-type, wMel and wMelPop mosquitoes into < 8 and ≥ 8 d age groups with 87%, 83% and 78% accuracy, respectively. This is the first investigation to report the ability of NIRS to predict the age of male Ae. aegypti mosquitoes. Despite the fact that they are not disease vectors, the survival of male mosquitoes would be a useful indicator of the success or failure of control strategies utilizing Wolbachia [10], genetic modification approaches requiring male competitiveness [36,37], a strategy to induce sex-ratio distortion of mosquito populations towards males [38] and strategies that seek to release sterile males [39]. The efficacy of any sterile male technique, including those that utilise Wolbachia infected males, relies on the ability of the male mosquito to compete and mate with the wild-type population. Nonetheless, to date only one age grading technique has been reported for male mosquitoes. A technique based on the frequency at which spermatocysts are found in male reproductive organs of An. gambiae s.s. and Anopheles culicifacies and the relative size of the sperm reservoir to differentiate mosquitoes into ≤ 4 d old and > 4 d old [40,41]. The difficult dissections and complex quantitative models involved in the application of this age grading technique, as pointed out by the authors, may limit its application on field related studies. Given its rapidity and simple prediction models, NIRS would be a suitable complementary age grading tool for rapidly differentiating young from old male mosquitoes.
NIRS offers a considerably higher throughput than alternative mosquito age grading approaches. We estimate that we can analyze approximately 1000 mosquitoes per day. Comparatively, the analysis of samples by cuticular hydrocarbon or transcriptional profiling age grading techniques currently allows an average of only 20–30 samples to be processed per day (averaged over preparation periods) [42]. With the capacity for larger sample sizes to be processed, NIRS has the potential to reduce sampling error associated with age prediction estimates in comparison to the alternative age grading techniques. The increased capacity and reduced sampling bias has the potential for substantially increasing the accuracy of population survivorship estimates, provided age prediction models are constructed without bias [22].
Accurate determination of mosquito population survivorship is key to understanding transmission risk and to the success of vector control strategies. Until recently, it has been impractical or impossible to accurately assess the age of Ae. aegypti in the field. Traditional dissection methods are technically challenging and only accurately differentiate parous from non-parous mosquitoes. Excitingly, our results that NIRS can accurately age Ae. aegypti mosquitoes over a 3 week lifespan gives us a tool for assessing transmission risk and understanding seasonality. NIRS has considerable advantages over conventional techniques given that results can be gained in real-time, without reagents or sample preparation. To improve the accuracy, alternative statistical methods are being developed. However, as certain age groups may be biochemically indistinguishable from each other and since NIRS relies on assessing alterations in biochemical signatures, it should be acknowledged that shortcomings in age prediction may stem from the underlying biochemistry as opposed to the statistical techniques applied. Although our study is still preliminary, our results demonstrate that upon further calibration, NIRS could be a potentially more accurate tool for predicting the age of Ae. aegypti compared to transcriptional profiling and cuticular hydrocarbon techniques. Follow up studies conducted under semi-field or field environments to improve the current calibration models have been envisaged. These and our recently published findings that NIRS can detect Wolbachia infections in Ae. aegypti [33], suggest a strong potential role for NIRS as a rapid surveillance tool for Ae. aegypti control programs.
Acknowledgments
wMelPop was provided to us courtesy of the Eliminate Dengue group, Monash University, Australia. We thank Nikita Lysenko for providing technical assistance in Tanzania and Jocalyn Clark of Grand Challenges Canada for editorial assistance. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by Grand Challenges Canada Stars for Global Health funded by the Government of Canada (grant 0439-01) awarded to MTSL. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Boorman J, Porterfield J. A simple technique for infection of mosquitoes with viruses transmission of Zika virus. Trans R Soc Trop Med Hyg. 1956;50(3):238–242. [DOI] [PubMed] [Google Scholar]
- 2.Watts DM, Burke DS, Harrison BA, Whitmire RE, Nisalak A. Effect of Temperature on the Vector Efficiency of Aedes aegypti for Dengue 2 Virus. Am J Trop Med Hyg. 1987;36(1):143–152. [DOI] [PubMed] [Google Scholar]
- 3.Dubrulle M, Mousson L, Moutailler S, Vazeille M, Failloux A-B. Chikungunya virus and Aedes mosquitoes: saliva is infectious as soon as two days after oral infection. PloS ONE. 2009;4(6):e5895 10.1371/journal.pone.0005895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476(7361):454–457. 10.1038/nature10356 [DOI] [PubMed] [Google Scholar]
- 5.Halstead SB. Dengue. Lancet. 2007;370(9599):1644–1652. 10.1016/S0140-6736(07)61687-0 [DOI] [PubMed] [Google Scholar]
- 6.CDC http://www.cdc.gov/dengue/epidemiology/.
- 7.Control ECfDPa. Zika virus epidemic in the Americas: potential association with microcephaly and Guillain-Barré syndrome. Stockholm, Sweden: European Centre for Disease Prevention and Control: http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association-with-microcephaly-rapid-risk-assessment.pdf. 2015. [Google Scholar]
- 8.Mlakar J, Korva M, Tul N, Popović M, Poljšak-Prijatelj M, Mraz J, et al. Zika virus associated with microcephaly. New Eng J Med. 2016;374(10):951–958. 10.1056/NEJMoa1600651 . [DOI] [PubMed] [Google Scholar]
- 9.Wong P-SJ, Li M-zI, Chong C-S, Ng L-C, Tan C-H. Aedes Stegomyia albopictus(Skuse): A potential vector of Zika Virus in Singapore. PLoS Negl Trop Dis. 2013;7(8):e2348 10.1371/journal.pntd.0002348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMeniman CJ, Lane RV, Cass BN, Fong AWC, Sidhu M, Wang Y-F, et al. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science. 2009;323(5910):141–144. 10.1126/science.1165326 [DOI] [PubMed] [Google Scholar]
- 11.Ritchie SA, Townsend M, Paton CJ, Callahan AG, Hoffmann AA. Application of wMelPop Wolbachia strain to crash local populations of Aedes aegypti. PLoS Negl Trop Dis. 2015;9(7):e0003930 10.1371/journal.pntd.0003930. PMC4512704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen TH, Le Nguyen H, Nguyen TY, Vu SN, Tran ND, Le T, et al. Field evaluation of the establishment potential of wMelPop Wolbachia in Australia and Vietnam for dengue control. Parasit Vector. 2015;8(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, et al. A Wolbachia symbiont in Aedes aegypti limits infection with Dengue, Chikungunya, and Plasmodium. Cell. 2009;139(7):1268–1278. 10.1016/j.cell.2009.11.042 [DOI] [PubMed] [Google Scholar]
- 14.Dutra HLC, Rocha MN, Dias FBS, Mansur SB, Caragata EP, Moreira LA. Wolbachia Blocks Currently Circulating Zika Virus Isolates in Brazilian Aedes aegypti Mosquitoes. Cell host microbe. 2016;19(6):771–774. 10.1016/j.chom.2016.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aliota MT, Peinado SA, Velez ID, Osorio JE. The wMel strain of Wolbachia Reduces Transmission of Zika virus by Aedes aegypti. Scientific reports. 2016;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO. Mosquito Control: can it stop Zika at source? http://www.who.int/emergencies/zika-virus/articles/mosquito-control/en/ Accessed 13/04/2016. 2016.
- 17.Detinova T. Age-grouping methods in Diptera of medical importance, with special reference to some vectors of malaria. Monogr Ser World Health Organ. 1962;47:13–191. [PubMed] [Google Scholar]
- 18.Polovodova VP. The determination of the physiological age of female Anopheles by number of gonotrophic cycles completed. Med Parazitol Parazitar Bolezni. 1949;18:352–355. [Google Scholar]
- 19.Hugo LE, Quick-miles S, Kay BH, Ryan PA. Evaluations of mosquito age grading techniques based on morphological changes. J Med Entomol. 2008;45(3):353–369. 10.1603/0022-2585(2008)45[353:EOMAGT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 20.Desena ML, Clark JM, Edman JD, Symington SB, Scott TW, Clark GG, et al. Potential for aging female Aedes aegypti (Diptera: Culicidae) by gas chromatographic analysis of cuticular hydrocarbons, including a field evaluation. J Med Entomol. 1999;36(6): 811–823. [DOI] [PubMed] [Google Scholar]
- 21.Desena ML, Edman JD, Clark JM, Symington SB, Scott TW. Aedes aegypti (Diptera: Culicidae) age determination by cuticular hydrocarbon analysis of female legs. J Med Entomol. 1999;36:824–830. [DOI] [PubMed] [Google Scholar]
- 22.Hugo LE, Kay BH, Eaglesham GK, Holling N, Ryan PA. Investigation of cuticular hydrocarbons for determining the age and survivorship of Australian mosquitoes. Am J Trop Med Hyg. 2006;74(3):462–474. [PubMed] [Google Scholar]
- 23.Cook PE, Hugo LE, Iturbe-Ormaetxe Ia, Williams CR, Chenoweth SF, Ritchie SA, et al. The use of transcriptional profiles to predict adult mosquito age under field conditions. Proc Nat Acad Sci. 2006;103(48):18060–18065. 10.1073/pnas.0604875103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hugo LE, Monkman J, Dave KA, Wockner LF, Birrell GW, Norris EL, et al. Proteomic biomarkers for ageing the mosquito Aedes aegypti to determine risk of pathogen transmission. PloS one. 2013;8(3):e58656 10.1371/journal.pone.0058656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayagaya VS, Michel K, Benedict MQ, Killeen GF, Wirtz RA, Ferguson HM, et al. Non-destructive determination of age and species of Anopheles gambiae s.l. Using near-infrared spectroscopy. Am J Trop Med Hyg. 2009;81(4):622–630. 10.4269/ajtmh.2009.09-0192 [DOI] [PubMed] [Google Scholar]
- 26.Sikulu M, Killeen G, Hugo L, Ryan P, Dowell K, Wirtz R, et al. Near-infrared spectroscopy as a complementary age grading and species identification tool for African malaria vectors. Parasit Vector. 2010;3(1):49 10.1186/1756-3305-3-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ntamatungiro A, Mayagaya V, Rieben S, Moore S, Dowell F, Maia M. The influence of physiological status on age prediction of Anopheles arabiensis using near infra-red spectroscopy. Parasite Vector. 2013;6(1):298 10.1186/1756-3305-6-298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dowell FE, Noutcha AEM, Michel K. The effect of preservation methods on predicting mosquito age by near Infrared spectroscopy. Am J Trop Med Hyg. 2011;85(6):1093–1096. 10.4269/ajtmh.2011.11-0438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sikulu M, Dowell K, Hugo L, Wirtz R, Michel K, Peiris K, et al. Evaluating RNAlater as a preservative for using near-infrared spectroscopy to predict Anopheles gambiae age and species. Malar J. 2011;10(1):186 10.1186/1475-2875-10-186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayagaya VS, Ntamatungiro AJ, Moore SJ, Wirtz RA, Dowell FE, Maia MF. Evaluating preservation methods for identifying Anopheles gambiae ss and Anopheles arabiensis complex mosquitoes species using near infra-red spectroscopy. Parasit Vector. 2015;8(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sikulu MT, Majambere S, Khatib BO, Ali AS, Hugo LE, Dowell FE. Using a Near-infrared spectrometer to estimate the age of Anopheles mosquitoes exposed to pyrethroids. PLoS One. 2014;9(3):e90657 10.1371/journal.pone.0090657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liebman K, Swamidoss I, Vizcaino L, Lenhart A, Dowell F, Wirtz R. The Influence of diet on the use of Near-infrared spectroscopy to determine the age of female Aedes aegypti mosquitoes. Am J Trop Med Hyg. 2015;92(5):1070–1075. 10.4269/ajtmh.14-0790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sikulu-Lord MT, Maia MF, Milali MP, Henry M, Mkandawile G, Kho EA, et al. Rapid and non-destructive detection and identification of two strains of Wolbachia in Aedes aegypti by Near-infrared spectroscopy. PLoS Negl Trop Dis. 2016;10(6):e0004759 10.1371/journal.pntd.0004759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schule PA. Dengue fever: Transmission by Aedes aegypti. Am J Trop Med Hyg. 1928;1(3):203–213. [Google Scholar]
- 35.Chan M, Johansson MA. The incubation periods of dengue viruses. PLoS One. 2012;7(11):e50972 10.1371/journal.pone.0050972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferguson HM, John B, Ng'habi K, Knols BG. Redressing the sex imbalance in knowledge of vector biology. Trends ecol evolution. 2005;20(4):202–209. [DOI] [PubMed] [Google Scholar]
- 37.Scott TW, Takken W, Knols BG, Boëte C. The ecology of genetically modified mosquitoes. Science. 2002;298(5591):117–119. 10.1126/science.298.5591.117 [DOI] [PubMed] [Google Scholar]
- 38.Galizi R, Doyle LA, Menichelli M, Bernardini F, Deredec A, Burt A, et al. A synthetic sex ratio distortion system for the control of the human malaria mosquito. Nat Commun. 2014;5. 10.1038/ncomms4977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tabachnick WJ. Reflections on the Anopheles gambiae genome sequence, transgenic mosquitoes and the prospect for controlling malaria and other vector borne diseases. J Med Entomol. 2003;40(5):597–606. [DOI] [PubMed] [Google Scholar]
- 40.Huho B, Ng'habi K, Killeen G, Nkwengulila G, Knols B, Ferguson H. A reliable morphological method to assess the age of male Anopheles gambiae. Malar J. 2006;5:62 10.1186/1475-2875-5-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahmood F, Reisen WK. Anopheles culicifacies: effects of age on the male reproductive system and mating ability of virgin adult mosquitoes. Med Vet Entomol. 1994;8(1):31–37. [DOI] [PubMed] [Google Scholar]
- 42.Cook P, Hugo L, Iturbe-Ormaetxe I, Williams C, Chenoweth S, Ritchie S, et al. Predicting the age of mosquitoes using transcriptional profiles. Nat Protoc. 2007;2:2796–2806. 10.1038/nprot.2007.396 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.