Abstract
A single administration of the κ opioid receptor (KOR) antagonist, norbinaltorphimine (norBNI), produces long-term reduction in KOR function in heterologous expression systems and brain that is mediated by activation of c-Jun N-terminal kinase (JNK). In this study, we examined the long-term effects of norBNI on adult rat peripheral sensory neurons in vivo and ex vivo. Following a single intraplantar (i.pl.) injection of norBNI into the hind paw, peripheral KOR-mediated antinociception in the ipsilateral, but not the contralateral, hindpaw was abolished for at least 9 days. By contrast, the antinociceptive response to mu and delta opioid receptor agonists was unaltered. The long-term inhibitory effect on antinociception produced by pretreatment with norBNI required occupancy of peripheral KOR and was completely blocked by i.pl. injection of the JNK inhibitor, SP600125. In cultures of peripheral sensory neurons, norBNI activated JNK for at least 30 minutes. Furthermore, norBNI blocked KOR-mediated inhibition of adenylyl cyclase activity measured 24 hours later in a JNK-dependent manner, but did not block activation of extracellular signal-regulated kinase (ERK). The long-term inhibitory effect of norBNI on KOR function in vivo and ex vivo was blocked by inhibitors of mRNA translation, cycloheximide and rapamycin. These data suggest that in peripheral sensory neurons norBNI is a KOR-biased ligand for activation of JNK signaling, resulting in long-term blockade of some (antinociception, inhibition of adenylyl cyclase activity), but not all (ERK), KOR signaling. Importantly, norBNI elicits de novo protein synthesis in sensory neuron terminals that produces selective long-term regulation of KOR.
Introduction
Kappa opioid receptors (KORs) are expressed widely throughout the central nervous system (CNS) and regulate several physiologic functions and behaviors, including pain, depression, anxiety, and drug-seeking (Pfeiffer et al., 1986; Shippenberg and Herz, 1986; Todtenkopf et al., 2004; Bruchas et al., 2007a; Redila and Chavkin, 2008; Butelman et al., 2012; Zhou et al., 2013). KORs are also expressed by pain-sensing neurons (nociceptors) of the peripheral nervous system, where they function to inhibit transmission of pain stimuli to the CNS (Fields et al., 1980; Chen et al., 1997; Stein and Lang, 2009; Stein and Zollner, 2009; Berg et al., 2011; Jamshidi et al., 2015). It has been suggested that peripherally-restricted KOR agonists may provide for improved treatment of some forms of pain that would be devoid of CNS-mediated adverse effects (Kivell and Prisinzano, 2010; Vanderah, 2010; Berg et al., 2011; Vadivelu et al., 2011; Jamshidi et al., 2015), and some clinical studies have demonstrated efficacy of peripherally-restricted KOR agonists in a variety of pain conditions (Riviere, 2004; Arendt-Nielsen et al., 2009).
Opioid receptor systems expressed in nociceptors are regulated differently from their CNS counterparts. Although activation of opioid receptors by the CNS readily elicits antinociception (see Pasternak and Pan, 2013), local administration of opioids to nociceptors at peripherally-restricted doses does not produce antinociception in normal tissue (Joris et al., 1987; Przewlocki and Przewlocka, 2001; Obara et al., 2009; Rowan et al., 2009; Stein and Zollner, 2009; Berg et al., 2011, 2012). However, following inflammation or tissue injury, robust antinociceptive responses to peripheral administration of opioids occur (Fields et al., 1980; Chen et al., 1997; Obara et al., 2009; Rowan et al., 2009; Stein and Lang, 2009; Stein and Zollner, 2009; Berg et al., 2011; Sullivan et al., 2015). Similarly, opioid agonists do not produce inhibitory signaling in cultures of nociceptors unless the cells are first pretreated with an inflammatory mediator, such as bradykinin or arachidonic acid (Patwardhan et al., 2005; Berg et al., 2007, 2011; Sullivan et al., 2015).
Because KOR may be a viable peripheral target for pain pharmacotherapy, it is important to understand the regulation of KOR function in peripheral nociceptors. Several studies have shown that a single systemic injection of the prototypical KOR antagonist, norbinaltorphimine (norBNI), inhibits KOR-mediated antinociception for up to 3 weeks (Endoh et al., 1992; Horan et al., 1992; Jones and Holtzman, 1992; Butelman et al., 1993; Broadbear et al., 1994; Bruchas et al., 2007b; Melief et al., 2010, 2011). Similar long-term inhibition of KOR function occurs in pigeons (Jewett, 1995), heterologous expression systems (Bruchas et al., 2007b; Melief et al., 2010, 2011), and with other, but not all, KOR antagonists (Melief et al., 2011). Such long-term drug effects are often attributed to irreversible occupancy of a receptor. However, norBNI has been shown to be a selective, competitive KOR antagonist (Portoghese et al., 1987a,b), and the long-term effect of norBNI can be blocked by competitive antagonists (Bruchas et al., 2007b), suggesting that the long-term action of norBNI is not due to irreversible receptor occupancy. In an elegant series of experiments, Chavkin and colleagues (Bruchas et al., 2007b; Melief et al., 2010, 2011) demonstrated that norBNI acts as a KOR agonist to activate c-Jun N-terminal kinase (JNK) in a pertussis toxin-insensitive manner and that pharmacological inhibition of JNK, or JNK 1 knockout, prevents the long-term effects of norBNI in HEK cells and in mice. On the basis of this work, they proposed that a hypothetical JNK-sensitive substrate associates with KOR to block KOR signaling (Bruchas et al., 2007b; Bruchas and Chavkin, 2010; Melief et al., 2010, 2011).
Given that regulation of KOR function in nociceptors often differs from that in other tissues/cells, in this study we sought to determine whether long-term inhibitory control of KOR also occurred in nociceptors ex vivo and in vivo. We found that stimulation of KOR with norBNI produced a long-lasting reduction in some, but not all, KOR functional responses in nociceptors. Moreover, the long-term effects of norBNI were mediated by JNK-sensitive activation of protein translation in peripheral sensory neuron terminals. These results support the conclusion that norBNI is a biased KOR ligand, leading to activation of JNK (but not Gαi-mediated signaling), which initiates local, de novo protein synthesis in sensory neuron terminals and long-term inhibition of some aspects of KOR signaling.
Materials and Methods
Drugs and Chemicals.
The following compounds were purchased from Sigma-Aldrich (St. Louis, MO): bradykinin (BK) acetate salt, rolipram, norBNI dihydrochlorite, (-)-trans-(1S, 2S)-U50488 hydrochloride hydrate (U50488), JNK inhibitor (SP600125), and cycloheximide solution (CHX). Rapamycin (RAPA) was purchased from LC Laboratories (Woburn, MA). Prostaglandin E2 (PGE2) was purchased from Cayman Chemical (Ann Arbor, MI). [D-Pen2,5]-enkephalin (DPDPE) was purchased from Bachem (Torrance, CA). Salvinorin-A was a gift of T. Prisinzano (Kansas City, MO). 125I-cAMP was purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA). Nerve growth factor was purchased from Harlan Laboratories (Indianapolis, IN), collagenase from Worthington Biochemicals (Freehold, NJ), and all other tissue culture reagents were purchased from Life Technologies (Grand Island, NY).
Animals.
Adult male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA), weighing 250–300 g, were used in this study. The animal study protocol was approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio and conformed to the International Association for the Study of Pain and federal guidelines. Animals were housed for 1 week, with food and water available ad libitum, before experimentation.
Behavioral Assay.
Opioid agonist-mediated changes in paw withdrawal latency (PWL) to a radiant heat stimulus were measured with a plantar test apparatus (Hargreaves et al., 1988), as described previously (Berg et al., 2011; Jamshidi et al., 2015). The radiant heat stimulus was set to produce baseline PWL of 10 ± 2 seconds with a cutoff time of 25 seconds to prevent tissue damage. After baseline PWL was determined, animals were pretreated with BK (25 μg) via intraplantar (i.pl.) injection to induce KOR functional competence (Rowan et al., 2009; Berg et al., 2011, 2012; Jamshidi et al., 2015; Sullivan et al., 2015). To assess KOR-mediated antinociception, rats received coinjections (i.pl.) of PGE2 (0.3 µg) with vehicle or U50488 (0.1 µg) 15 minutes after the BK injection. PWL measurements were obtained in duplicate (at least 30 seconds apart) before and at 5-minute intervals following opioid/vehicle injection for 20 minutes. To assess long-term effects, norBNI (0.3–30 ng) or vehicle was injected (i.pl.) 2, 7, or 9 days before testing KOR function. In some experiments, SP600125 (1 µg) or vehicle was administered (i.pl.) 24 hours and 30 minutes prior to injection (i.pl.) of norBNI. Where indicated, CHX (25 µg) or RAPA (12 µg) was administered (i.pl.) 30 minutes prior to and 24 hours following injection (i.pl.) of norBNI. Time course data are expressed as the change (seconds) from individual PWL baseline values and represent mean ± S.E.M. of six rats per group. Area under the curve (AUC) data were quantified from each individual time course curve. All drugs were administered (i.pl.) at a volume of 50 μl. Investigators were blind to the treatment allocation.
Primary Sensory Neuronal Cultures.
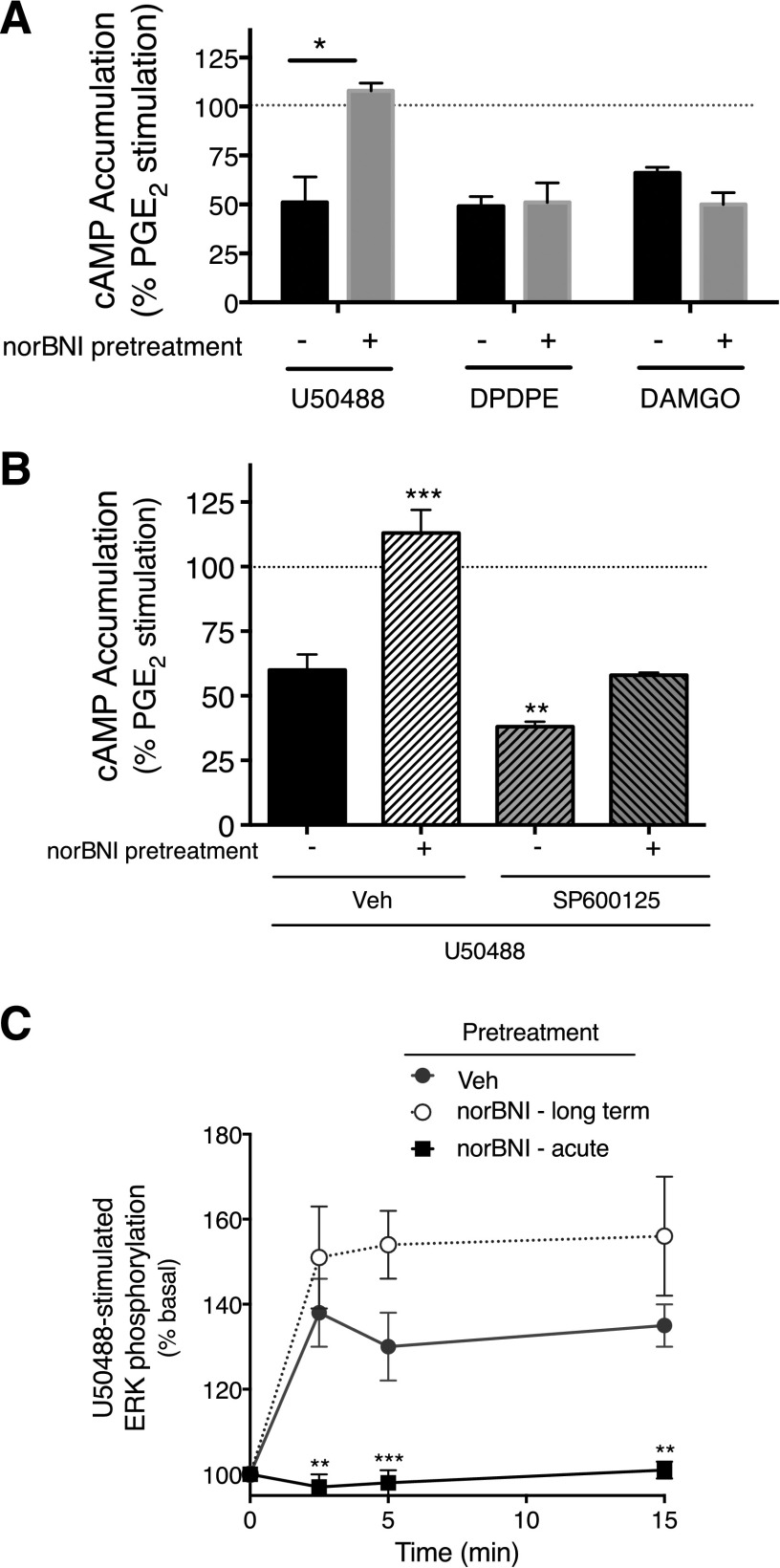
Primary cultures derived from rat trigeminal ganglion were prepared, as described previously (Patwardhan et al., 2005, 2006; Berg et al., 2007, 2011; Jamshidi et al., 2015; Sullivan et al., 2015), and maintained in culture for 5 days. For all experiments, cells were refed with serum-free Dulbecco’s modified Eagle's medium without nerve growth factor on day 5 and used for experiments on the sixth day of culture (i.e., after a 24-hour serum- and nerve growth factor-free period). Opioid receptors colocalize with bradykinin B2 receptors that are expressed on TRPV1-expressing peripheral sensory neurons (Patwardhan et al., 2005; Jeske et al., 2006; Patwardhan et al., 2006; Berg et al., 2007, 2011; Rowan et al., 2009; Gomez et al., 2011) along with receptors for prostaglandin E2 (Patwardhan et al., 2008). In addition, activation of bradykinin B2 receptors induces functional competence of opioid receptors expressed on peripheral pain-sensing neurons for reduction of PGE2-stimulated cAMP accumulation ex vivo and for reduction of PGE2-evoked thermal allodynia in vivo (Patwardhan et al., 2005; Berg et al., 2007, 2011; Rowan et al., 2009). In these experiments, we measured KOR-mediated inhibition of PGE2-mediated responses in vivo and ex vivo following induction of functional competence with bradykinin. Thus, we interpret the long-term effects of norBNI, both ex vivo (e.g., Fig. 5) and in vivo (e.g., Fig. 1), as due to the interaction of norBNI with KOR coexpressed with bradykinin B2 and PGE2 receptors on peripheral sensory neurons.
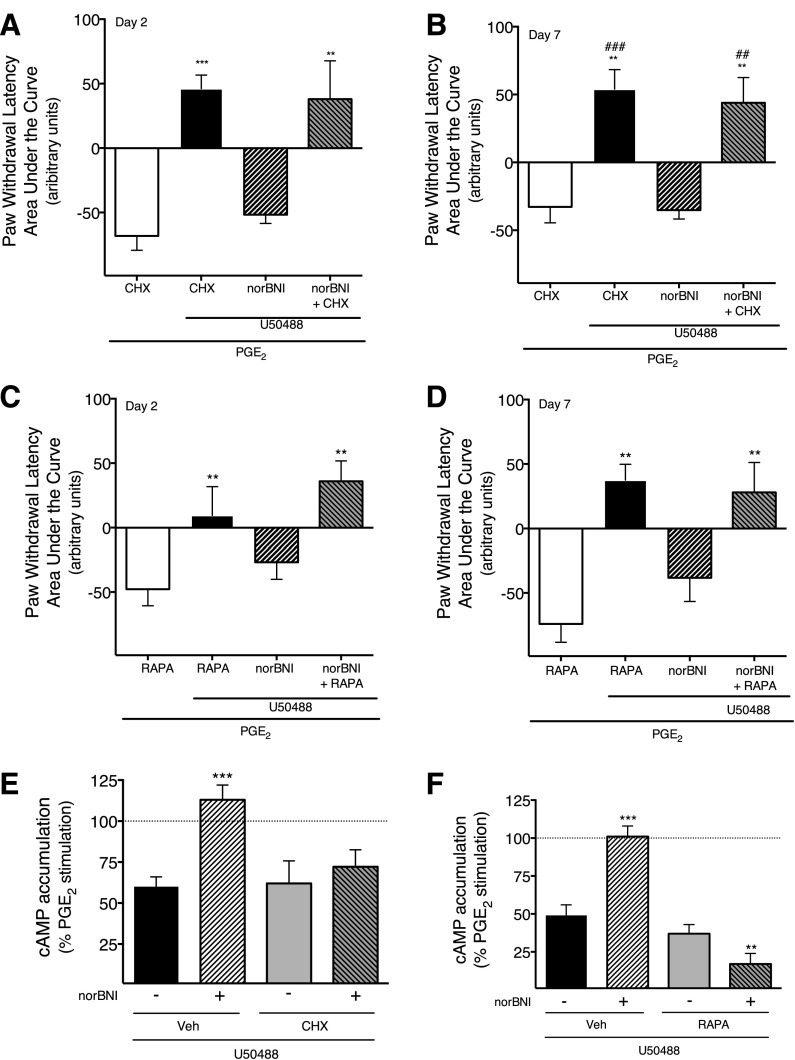
Fig. 5.
norBNI treatment produces long-term inhibition of U50488-mediated inhibition of PGE2-stimulated cAMP accumulation, but not U50488 stimulation of ERK. (A) Peripheral sensory neuron cultures were pretreated with norBNI (3 nM) or vehicle. After a 1-hour incubation, cells were washed thoroughly. Twenty-four hours later, cells were treated with PGE2 (1 µM) with U50488 (100 nM), DPDPE (100 nM), DAMGO (1 µM), or vehicle, and cellular cAMP levels were measured after 15 minutes. Data represent the mean ± S.E.M. of cAMP levels expressed as the percentage of PGE2-stimulated cAMP levels of three or four independent experiments. *P < 0.05 compared with vehicle-treated cells. (B) Cells were pretreated with SP600125 (1 µM) or vehicle (Veh) 30 minutes before addition of norBNI (3 nM) or vehicle. After a 1-hour incubation, cells were washed thoroughly and cellular cAMP was determined [as in (A)]. Data were analyzed with one-way ANOVA, followed by Dunnett’s post hoc test. ***P < 0.001, **P < 0.01 compared with vehicle-treated cells. (C) Cells were pretreated with norBNI (3 nM) or vehicle for 1 hour, and washed thoroughly [as in (A)]. Twenty-four hours later, levels of pERK were measured at the indicated time points following stimulation with U50488 (100 nM) in the absence or presence of norBNI (3 nM). pERK levels were measured using the pERK Surefire assay kit from PerkinElmer Life and Analytical Sciences, according to the manufacturer’s protocol. Data are expressed as the percentage increase in pERK over basal (no ligand) activity and represent the mean ± S.E.M. of four to six independent experiments. Data were analyzed with two-way ANOVA, followed by Bonferroni’s post hoc test. ***P < 0.001, **P < 0.01 compared with Veh-pretreated cells.
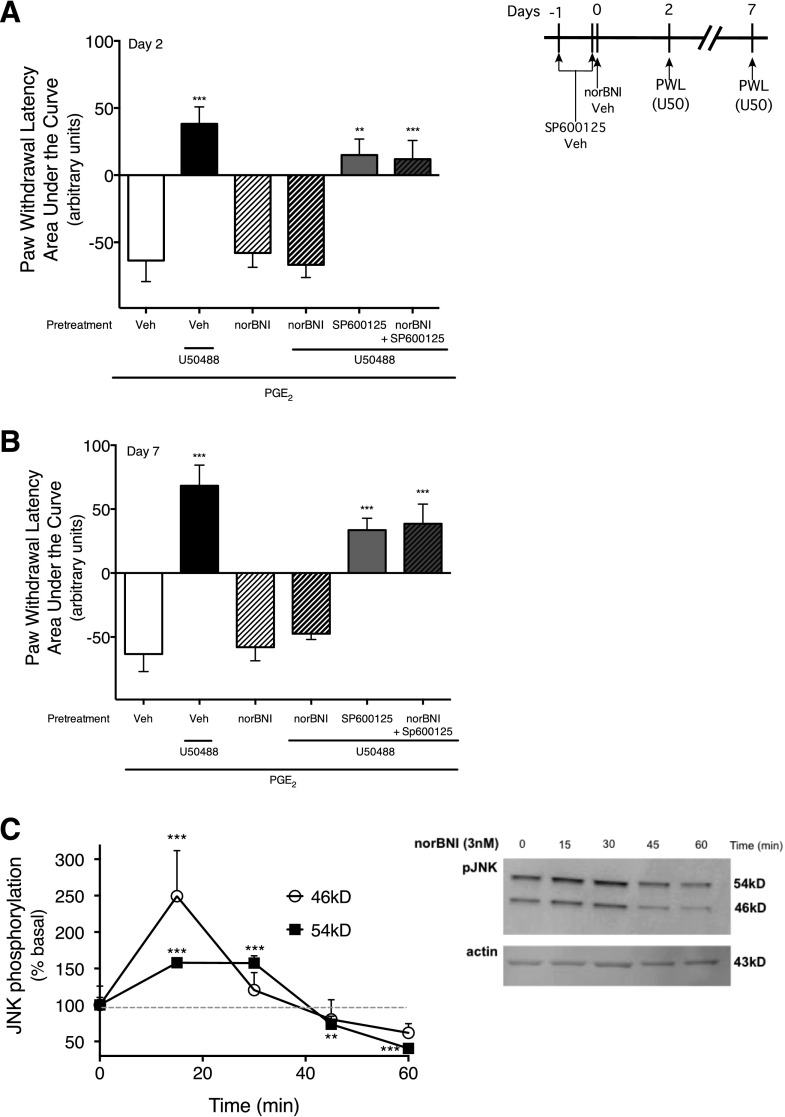
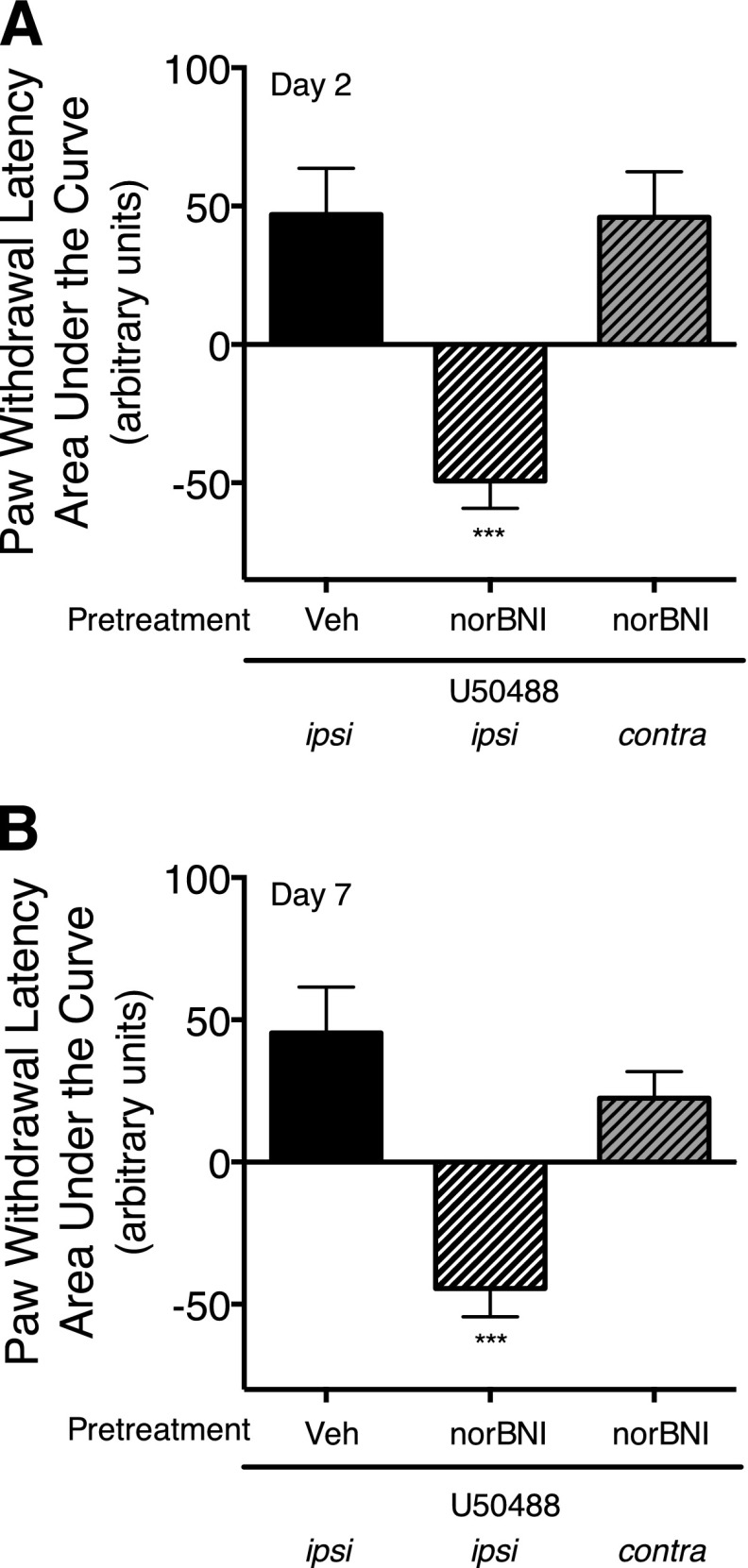
Fig. 1.
Long-term reduction in U50488-mediated inhibition of PGE2-stimulated thermal allodynia in the rat hind paw by norBNI is mediated by activation of JNK. (A and B) Rats received i.pl. injections of the JNK inhibitor, SP600125 (1 µg), or vehicle (Veh) 24 hours and 30 minutes before injection of norBNI (30 ng, i.pl.) or vehicle. Two days (A), and 7 days (B) after norBNI injection, rats received coinjections of PGE2 (0.3 µg, i.pl.) with either U50488 (0.1 µg, i.pl.) or vehicle. PWL in response to a radiant heat stimulus applied to the ventral surface of the hind paw were measured in duplicate before and at 5-minute intervals after the last injection for 20 minutes. Data are expressed as the mean ± S.E.M. of the AUC for each group of six animals. Negative values for AUC reflect allodynia (decreased PWL from baseline), and positive values reflect antinociception (increased PWL from baseline). Data were analyzed with one-way ANOVA and Dunnett’s post hoc test. ***P < 0.001, **P < 0.01 compared with PGE2 alone. Time course data are provided in Supplemental Fig. 1. (C) Treatment of peripheral sensory neurons in culture with norBNI activates JNK. Cells were treated with norBNI (3 nM) for 0–60 minutes, and the level of pJNK was measured using Western analysis. Immunoblots were analyzed using Li-Cor Odyssey Imaging software. Data points represent pJNK levels normalized to actin as a percentage of basal and represent the mean ± S.E.M. of four experiments. Inset: representative immunoblot of cells treated with norBNI and probed for pJNK and actin. *P < 0.05 versus basal activity (time 0).
Measurement of Cellular cAMP Accumulation.
Opioid receptor-mediated inhibition of adenylyl cyclase activity was determined by measuring the amount of cAMP accumulated (15 minutes) in the presence of the phosphodiesterase inhibitor, rolipram, and PGE2 (1 µM) with or without the indicated opioid receptor ligands, as described previously (Patwardhan et al., 2005, 2006; Berg et al., 2007, 2011; Jamshidi et al., 2015; Sullivan et al., 2015). For all experiments, cells were pretreated with BK (10 µM, 15 minutes, 37°C) to induce KOR functional competence (Berg et al., 2007, 2011, 2012; Jamshidi et al., 2015; Sullivan et al., 2015). To assess long-term effects of norBNI on KOR agonist-mediated inhibition of PGE2-stimulated cAMP accumulation, cells were treated with norBNI (3 nM) for 1 hour, washed three times with 500 µl/well serum-free media (a total wash period of 30 minutes), and then incubated further for 24 hours (37°C, 5% CO2) before testing KOR agonist efficacy. In some experiments, cells were pretreated with CHX (1 μM), RAPA (1 µM), or SP600125 (1 μM) 30 minutes prior to norBNI. Incubations were terminated by aspiration of the buffer and addition of 500 µl ice-cold absolute ethanol. The ethanol extracts from individual wells were dried under a gentle air stream and reconstituted in 100 µl 50 mM sodium acetate, pH 6.2. The cAMP content of each well was determined by radioimmunoassay.
Measurement of Extracellular Signal-Regulated Kinase 1/2 Activation.
The κ agonist-mediated activation of extracellular signal-regulated kinase (ERK) was determined as described previously (Berg et al., 2011; Jamshidi et al., 2015). ERK activation was assessed by measuring the levels of phosphorylated ERK (pERK) produced in response to treatment of cells with U50488 (100 nM) for 0–15 minutes. pERK was measured with the AlphaScreen SureFire Phospho-ERK 1/2 Kit (PerkinElmer Life and Analytical Sciences), according to manufacturers’ instructions, and a Fluostar microplate reader equipped with AlphaScreen technology (BMG Labtech, Ortenberg, Germany). To assess the long-term effect of norBNI on KOR-mediated ERK activation, cells were pretreated with norBNI (3 nM) for 1 hour and then washed three times with 500 μl (per well) serum-free media (for a total of 30-minute wash period) and incubated for 24 hours (37°C, 5% CO2) before measurement of U50488 stimulation of ERK. To assess the effect of acute norBNI treatment, cells were pretreated with norBNI (3 nM) for 15 minutes before incubation with U50488 (100 nM).
Measurement of JNK Activation.
The levels of phosphorylated JNK (pJNK), as an index of JNK activity, in response to norBNI treatment were determined by Western analysis, as we have described previously (Jamshidi et al., 2015). Briefly, peripheral sensory neurons were plated in six-well plates and maintained in culture, as described above. Cells were washed with 4 ml/well Hanks' balanced salt solution containing 20 mM HEPES (pH 7.4) and incubated for 15 minutes at 37°C before addition of ligands. Cells were then treated with vehicle or norBNI (3 nM) for 5–60 minutes at 37°C in the presence of phosphatase inhibitor cocktail 3 (0.1%; Sigma-Aldrich) and okadaic acid (10 nM; Sigma-Aldrich). Incubation was terminated by aspiration of buffer and addition of 2 ml ice-cold phosphate-buffered saline to each well. Plates were placed on ice and phosphate-buffered saline aspirated, and cells were lysed by adding 50 μl lysis buffer (Pierce, Thermo Scientific, Rockford, IL), supplemented with 1% phosphatase inhibitor cocktail 3, 100 nM okadaic acid, and 1% protease inhibitor (Pierce, Thermo Scientific) per well. Cells were scraped and centrifuged 2000 rpm at 4°C for 3 minutes, and the supernatant was collected and prepared for SDS-PAGE following the NuPAGE protocol (Novex, Life Technologies, Grand Island, NY). Blots were probed with anti-rat phospho–stress-activated protein kinase/JNK (81E11) rabbit monoclonal antibody (catalog 4688; Cell Signaling, Danvers, MA), which is the same antibody that we have used to measure pJNK levels previously (Jamshidi et al., 2015) and has been used by the Chavkin group in their studies of KOR regulation of JNK (e.g., Melief et al., 2011). This antibody has been validated by the company for Western analysis using cell lysates of native HEK293, NIH3T3, and C6 cells, and results have been validated using JNK knockout mice (Melief et al., 2011). In all samples, actin was probed with an anti-rat actin goat polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) as a loading control. Goat anti-rabbit IR800 and donkey anti-goat IR680 secondary antibodies (Li-Cor Biosciences, Lincoln, NE) were used to detect pJNK and actin, respectively. Immunoblots were imaged using a Li-Cor infrared Odyssey Imager (Li-Cor Biosciences), and relative band intensities were quantified using Odyssey software (Li-Cor Biosciences).
Data Analysis.
For cell culture experiments, statistical significance was assessed using one-way analysis of variance (ANOVA), followed by Dunnett’s post hoc or Student’s t test (paired) using Prism software (GraphPad Software, San Diego, CA). P < 0.05 was considered statistically significant.
For behavioral experiments, time course data were analyzed with two-way ANOVA, followed by Bonferroni’s post hoc test to compare treatment effects over time using Prism software. Area under the time-response curves for individual rats was calculated, and mean values were analyzed with one-way ANOVA, followed by Dunnett’s post hoc test using Prism software. P < 0.05 was considered as statistically significant. Data are presented as mean ± S.E.M.
Results
Treatment with norBNI Produces Long-Term Reduction of KOR-Mediated Inhibition of PGE2-Evoked Thermal Allodynia by Activation of JNK.
Intraplantar injection of PGE2 produced a prolonged (>20 minutes) allodynia, as shown by a reduction in PWL (4–5 seconds) (Supplemental Fig. 1). Coinjection of U50488 completely inhibited PGE2-stimulated thermal allodynia and produced robust antinociception (PWL was above preinjection baseline values) (Fig. 1, A and B; Supplemental Fig. 1). A single injection (i.pl.) of norBNI administered 2 or 7 days earlier abolished the U50488-mediated inhibition of PGE2-stimulated thermal allodynia. norBNI did not alter the allodynia produced by PGE2.
Studies done in CNS neurons have shown that long-term reduction of KOR function by norBNI is due to activation of JNK (Bruchas et al., 2007b; Melief et al., 2010, 2011), so we next tested whether long-term effects of norBNI on U50488-mediated antithermal allodynia in the rat hind paw were JNK mediated. Pretreatment with the JNK inhibitor, SP600125 (i.pl.), completely blocked the long-term reduction in U50488-mediated antinociception by norBNI without affecting either PGE2-induced thermal allodynia (−63.6 ± 15.6 versus −46.51 ± 12.5; AUC values for vehicle versus SP600125 treatment, respectively) or U50488-mediated antinociception (38.2 ± 12.7 versus 15.0 ± 11.8 AUC values for vehicle versus SP600125, respectively). Consistent with blockade of the long-term effect of norBNI by pharmacological inhibition of JNK, treatment of primary sensory neuron cultures with norBNI increased pJNK levels (i.e., JNK activity) for about 30 minutes (Fig. 1C).
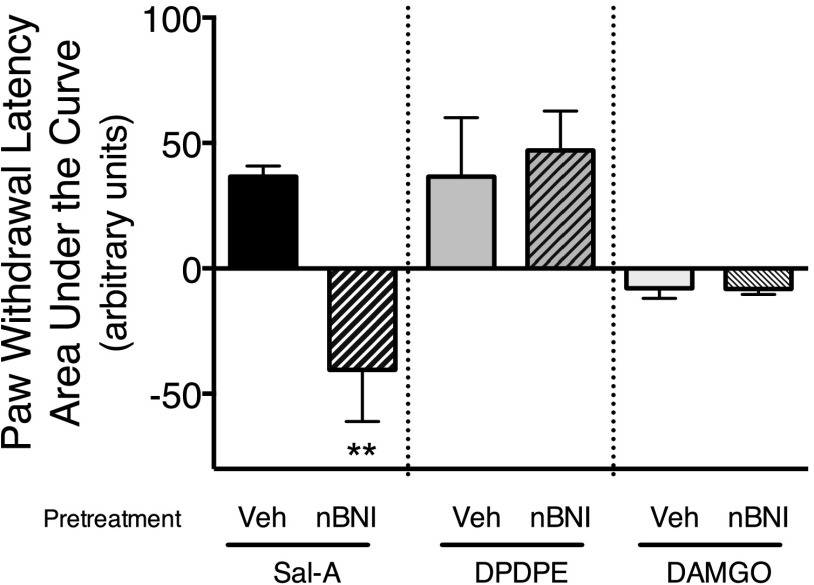
Figure 2 (and Supplemental Fig. 2) shows that injection of norBNI (i.pl.) also blocked the antinociceptive effect of the highly selective KOR agonist, Salvinorin-A, when tested 9 days after norBNI injection. By contrast, neither the antinociceptive response to the ∂ opioid receptor (DOR) agonist, DPDPE, nor to the µ opioid receptor (MOR) agonist, [D-Ala2,N-MePhe4,Gly-ol5]-enkephalin (DAMGO), was altered by norBNI.
Fig. 2.
Long-term reduction of Salvinorin-A–, but not DPDPE- or DAMGO-mediated inhibition of PGE2-stimulated thermal allodynia by norBNI. Rats were injected with norBNI (30 ng, i.pl.) or vehicle (Veh) 9 days before coinjection of PGE2 (0.3 µg, i.pl.) and the selective KOR agonist, Salvinorin-A (0.1 µg, i.pl.) or 2 days before coinjection of PGE2 (0.3 µg, i.pl.) and either the selective DOR agonist, DPDPE (20 µg, i.pl.), or the selective MOR agonist, DAMGO (8 µg, i.pl.). PWL in response to a radiant heat stimulus applied to the ventral surface of the hind paw was measured in duplicate before and at 5-minute intervals after the last injection. Data are expressed as the mean ± S.E.M. of the AUC for each group of six animals. Data were analyzed with one-way ANOVA and Dunnett’s post hoc test. **P < 0.01 compared with rats injected with vehicle (Veh). Time course data are provided in Supplemental Fig. 2.
Long-Term Effects of norBNI Are Mediated by KOR.
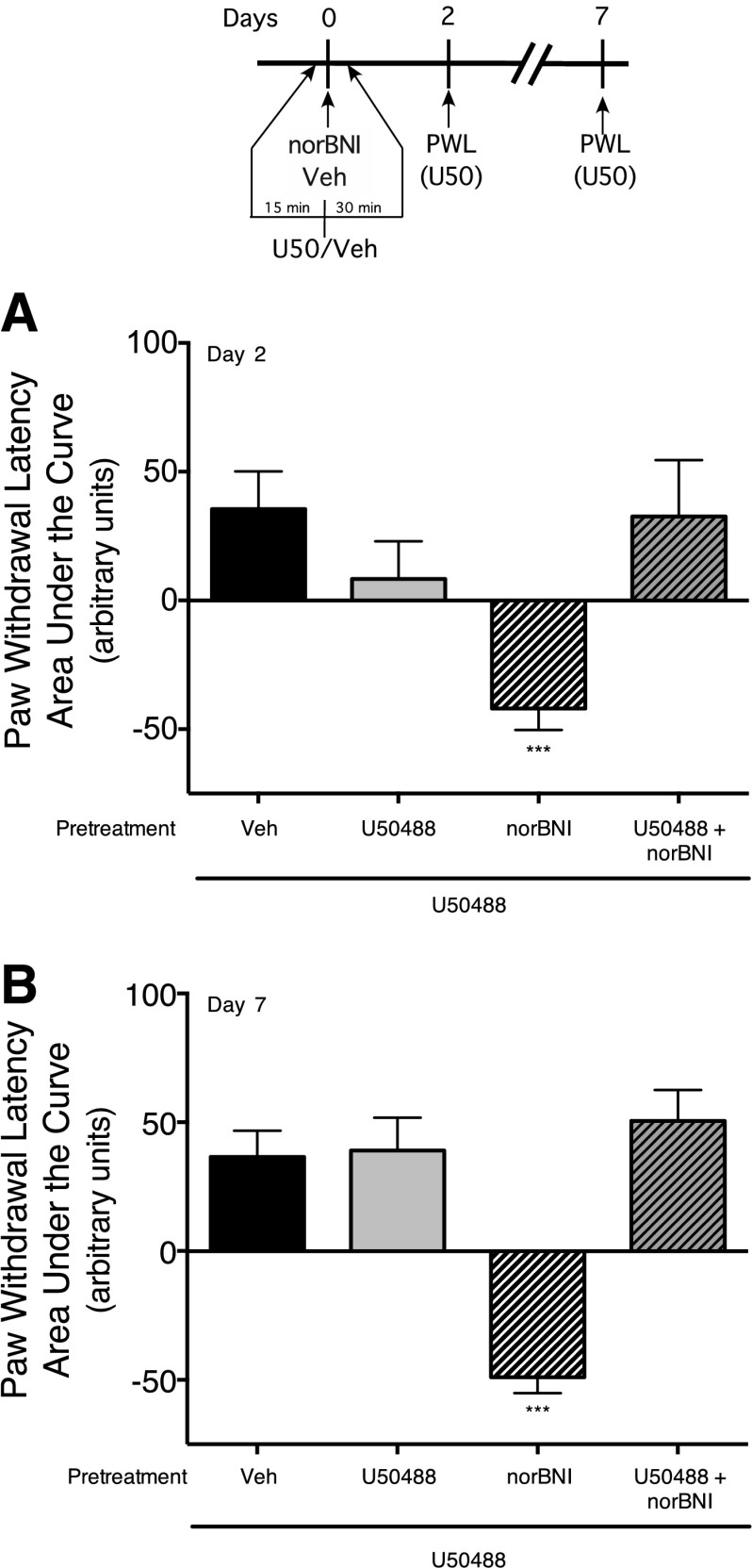
To determine whether the long-term effects of norBNI were due to interaction with KOR, we attempted to reduce occupancy of KOR by norBNI with a relatively higher dose of U50488. We first conducted a dose response for norBNI to produce long-term inhibition of KOR-mediated antinociception (Supplemental Fig. 3) and determined that a dose of 3 ng norBNI (i.pl.) was effective at blocking U50488-mediated antinociception 2 days after the norBNI injection. Based upon an assumption that the volume of distribution of norBNI in the hind paw following injection (i.pl.) was 1 ml, the concentration of norBNI, injected at a dose of 3 ng, would be 3 nM, which is 100 × Ki of norBNI for KOR. To reduce occupancy of KOR produced by this concentration of norBNI with U50488 (assuming the same volume of distribution and a Ki of U50488 of 11 nM; PDSP Ki Database; http://pdsp.med.unc.edu/pdsp.php) would require a concentration of U50488 at least 10,000 × Ki (110 µM). Consequently, for this experiment, we used a dose of U50488 of 100 µg (∼240 µM). As shown in Fig. 3 (and Supplemental Fig. 4), injection of U50488 (100 µg, i.pl.) completely abolished the long-term inhibitory effect of norBNI (3 ng, i.pl.) on U50488-mediated inhibition of PGE2-stimulated thermal allodynia measured 2 and 7 days after norBNI administration. Treatment with this high dose of U50488 alone did not produce a long-term effect on either basal or PGE2-mediated thermal allodynia, nor did U50488 alter the subsequent U50488-mediated antinociceptive response measured 2 and 7 days later.
Fig. 3.
Pretreatment with the KOR agonist, U50488, blocked the long-term inhibitory effect of norBNI. Rats received injections (i.pl.) of vehicle, U50488 (100 µg, 10,000 × Ki), norBNI (3 ng, 100 × Ki), or U50488 (100 µg) with norBNI (3 ng). Two (A) and seven (B) days later, U50488-mediated inhibition of PGE2-stimulated thermal allodynia was measured. Rats received coinjections (i.pl.) of PGE2 (0.3 µg) with U50488 (0.1 µg). PWL in response to application of a radiant heat stimulus to the ventral surface of the hind paw was measured in duplicate before and at 5-minute intervals following the coinjection of PGE2 plus U50488. Data are expressed as the mean ± S.E.M. of the AUC for each group of six animals. Data were analyzed with one-way ANOVA and Dunnett’s post hoc test. ***P < 0.001, compared with vehicle (Veh)-treated rats. Time course data are provided in Supplemental Fig. 3.
Long-Term Effects of norBNI Are Peripherally Restricted.
To verify the peripheral selectivity of the action of drugs injected into the hind paw, we routinely test for effects using the contralateral paw (e.g., Berg et al., 2011; Jamshidi et al., 2015). As shown in Fig. 4 and Supplemental Fig. 5, injection of norBNI to the paw contralateral to the injection of PGE2/U50488 did not alter U50488-mediated antinociception. Thus, the dose of norBNI administered to the hind paw was not sufficient to reach systemic concentrations that activate receptors in the CNS.
Fig. 4.
Long-term reduction of U50488-mediated inhibition of PGE2-stimulated thermal allodynia by norBNI is peripherally mediated. Rats received injections (i.pl.) of norBNI (30 ng) or vehicle (Veh). Two (A) and 7 (B) days later, Rats received coinjections (i.pl.) of PGE2 (0.3 µg) with U50488 (0.1 µg) in either the contralateral or the ipsilateral hind paw. PWL in response to application of a radiant heat stimulus to the ventral surface of the hind paw was measured in duplicate before and at 5-minute intervals following the coinjection of PGE2 plus U50488. Data are expressed as the mean ± S.E.M. of the AUC for each group of six animals. Data were analyzed with one-way ANOVA and Dunnett’s post hoc test. ***P < 0.001 compared with vehicle (Veh)-treated ipsilateral paw. Time course data are provided in Supplemental Fig. 4.
Long-Term Reduction in KOR Function by norBNI Ex Vivo Is Mediated by JNK.
In cultures of adult rat peripheral sensory neurons, U50488 inhibited PGE2-stimulated cAMP accumulation by 49 ± 13% (Fig. 5A), as we have reported before (Berg et al., 2011; Jamshidi et al., 2015). Similarly, the DOR agonist, DPDPE, and the MOR agonist, DAMGO, inhibited PGE2-stimulated cAMP accumulation by 51± 5% and 44 ± 3%, respectively. As shown in Fig. 5A, treatment of cells with norBNI (1 hour), followed by washing, abolished the response to U50488, but not to DPDPE or DAMGO, when tested 24 hours after norBNI.
Similar to the effect of norBNI in vivo (Fig. 1), the long-term effect of norBNI on U50488-mediated inhibition of PGE2-stimulated cAMP accumulation was blocked by pretreatment of cells with the JNK inhibitor, SP600125 (Fig. 5B). Supplemental Fig. 6 shows that acute treatment (15 minutes) of peripheral sensory neurons in culture with norBNI blocked U50488-mediated inhibition of cAMP accumulation and that norBNI can be washed away such that the U50488 response is restored.
Treatment with norBNI Does Not Produce Long-Term Inhibition of KOR-Mediated Activation of ERK.
We have shown before that activation of KOR with U50488 increases ERK activity in peripheral sensory neurons (Berg et al., 2011; Jamshidi et al., 2015). To determine whether long-term effects of norBNI extend to pathways other than cAMP signaling, we measured U50488-mediated activation of ERK after treatment with norBNI (3 nM) 24 hours earlier. As shown in Fig. 5C, treatment of peripheral sensory neurons with U50488 increased ERK phosphorylation (pERK). Interestingly and in contrast to the long-term inhibition of U50488-mediated inhibition of cAMP accumulation, norBNI treatment did not alter the U50488-mediated increases in ERK activity when tested 24 hours later.
Long-Term Reduction of KOR Function by norBNI Is Blocked by Inhibition of Protein Synthesis in Peripheral Sensory Neurons In Vivo and Ex Vivo.
Given the long-term nature of the effects of norBNI on KOR function and that JNK is a well-known activator of protein synthesis, we sought to determine whether protein synthesis was involved in the long-term effects of norBNI in peripheral sensory neurons. Figure 6 (and Supplemental Fig. 7) shows that injection (i.pl.) of the protein synthesis inhibitor, CHX, around the time of injection of norBNI, completely abolished the long-term inhibition of U50488-mediated inhibition of PGE2-stimulated thermal allodynia measured 2 and 7 days after norBNI injection. Similar results were also obtained with a second protein synthesis inhibitor, RAPA. Neither CHX nor RAPA alone altered PGE2-induced thermal allodynia or U50488-mediated antinociception.
Fig. 6.
The long-term inhibitory effect of norBNI in vivo (A–D) and in peripheral sensory neuron cultures (E and F) is blocked by inhibitors of protein translation. (A–D) Rats received injections (i.pl.) of CHX (25 µg; A and B), RAPA (12 µg; C and D), or vehicle (Veh) 30 minutes prior to and 24 hours following injection (i.pl.) of norBNI (30 ng) or vehicle. Two (A and C) and 7 days (B and D) following norBNI injection, rats received coinjections (i.pl.) of PGE2 (0.3 µg) with U50488 (0.1 µg) or vehicle. PWL in response to application of a radiant heat stimulus to the ventral surface of the hind paw was measured in duplicate before and at 5-minute intervals following the PGE2/U50488 coinjection. Data are expressed as the mean ± S.E.M. of the AUC for each group of six animals. Data were analyzed with one-way ANOVA and Dunnett’s post hoc test. ***P < 0.001, **P < 0.01, *p < 0.05 compared with vehicle-treated rats. Time course data are provided in Supplemental Fig. 6. (E and F) Peripheral sensory neurons in culture were treated with CHX (1 µM), RAPA (1 µM), or vehicle 30 minutes before norBNI (3 nM) or vehicle treatment of 1 hour, followed by washing. Twenty-four hours later, cells treated with PGE2 (1 µM) with or without U50488 (100 nM) and cellular levels of cAMP were measured. Data are expressed as percentage of PGE2-stimulated cAMP levels and represent the mean ± S.E.M. of four experiments. Data were analyzed with a one-way ANOVA with Dunnett’s post hoc test; ***P < 0.001, **P < 0.01 in comparison with vehicle-pretreated conditions, ##P < 0.01, ###P < 0.001 compared to norBNI treated rats.
To determine whether the long-term inhibitory effect of norBNI required ongoing protein synthesis, we administered CHX several days after norBNI injection. As shown in Supplemental Fig. 8, administration of CHX after norBNI injection (days 5–6) did not block the long-term effect of norBNI on U50488-mediated antinociception measured on day 7.
We next determined whether treatment with CHX or RAPA would block norBNI effects on KOR-mediated inhibition of cAMP accumulation ex vivo. As shown in Fig. 6, E and F, pretreatment of peripheral sensory neurons in culture with either CHX or RAPA completely abolished the long-term inhibitory effect of norBNI on U50488-mediated inhibition of adenylyl cyclase activity.
Discussion
Kappa opioid receptors expressed by peripheral pain-sensing neurons can produce robust antinociception; however, several cellular regulatory mechanisms, which may be unique to nociceptors, limit antinociceptive effectiveness of peripherally-restricted KOR agonists (Patwardhan et al., 2005; Berg et al., 2007, 2011; Rowan et al., 2009; Jamshidi et al., 2015; Sullivan et al., 2015). If peripheral KOR is to be a viable therapeutic target for peripherally-restricted analgesics, it is important to understand these regulatory mechanisms.
The prototypical KOR antagonist, norBNI, along with some other, but not all, KOR antagonists, can produce long-lasting inhibition of KOR function in the CNS and in heterologous expression systems (Endoh et al., 1992; Horan et al., 1992; Jones and Holtzman, 1992; Butelman et al., 1993; Broadbear et al., 1994; Bruchas et al., 2007b; Melief et al., 2010, 2011). In this study, a single injection of norBNI, injected locally into the plantar surface of the rat hind paw at peripherally-restricted doses, inhibited KOR agonist-mediated antinociception for up to 9 days. In cultures of adult rat peripheral sensory neurons, norBNI also produced long-lasting inhibition of KOR agonist-mediated inhibition of PGE2-stimulated cAMP accumulation, but did not inhibit U50488-stimulated ERK activation. The effect of norBNI was peripherally mediated, as U50488-mediated antinociception measured in the paw contralateral to norBNI injection was unaltered. Moreover, norBNI did not alter the antinociceptive effect of the DOR agonist, DPDPE, or the MOR agonist, DAMGO, suggesting that the long-lasting inhibitory effect of norBNI in the hind paw may be selective for KOR. The long-term effect of norBNI was mediated through interaction with KOR because occupancy of KOR with a relatively higher dose of U50488 blocked the long-term effect of norBNI in the hind paw. Chavkin’s group also found that protection of KOR by occupancy with naloxone or buprenorphine (in MOR-knockout mice) blocked the long-term inhibitory effect of systemic norBNI on KOR-mediated antinociception in the tail-flick assay (Bruchas et al., 2007b).
Long-lasting inhibitory effects of norBNI on central KOR function were found to be JNK dependent (Bruchas et al., 2007b; Melief et al., 2010, 2011). The long-term inhibitory effect of norBNI on peripheral KOR function was also dependent upon activation of JNK. norBNI also increased pJNK levels in cultured sensory neurons, as has been reported in mouse brain and HEK cells (Bruchas et al., 2007b; Melief et al., 2010, 2011). Although norBNI is generally considered to be a prototypical KOR antagonist (Portoghese et al., 1987a,b), these results indicate that norBNI is a biased KOR agonist, acting acutely as an antagonist for Gαi-mediated effects (antinociception and inhibition of adenylyl cyclase activity), but an agonist for the JNK signaling pathway.
It has been suggested that prolonged KOR antagonism may be due to pharmacokinetic and/or physiochemical properties of norBNI (Patkar et al., 2013). Decreases in maximal [3H]U69593 binding, with no change in KD, were found in mouse brain membranes up to 7 days following systemic injection of norBNI (10 mg/kg, i.p.) (Patkar et al., 2013). Although these data are consistent with prolonged occupancy of KOR, others have not observed changes in KOR agonist binding (Bruchas et al., 2007b). We found that although norBNI treatment blocked U50488-mediated inhibition of adenylyl cyclase activity measured 24 hours after administration in cultured sensory neurons, it did not block U50488-mediated increases in ERK activity, a response that is sensitive to acute norBNI antagonism. Together with the requirement for JNK activation, the results suggest that the long-lasting inhibitory effects of norBNI in peripheral sensory neurons are not due to persistent antagonism by occupancy of KOR, but via a cellular signaling mechanism that interferes with some (inhibition of adenylyl cyclase, antinociception), but not all (activation of ERK), KOR responses.
In addition to norBNI and some other antagonists (Bruchas et al., 2007b; Melief et al., 2011), activation of KOR with some prototypical agonist ligands (e.g., U50488, U69598) can also stimulate JNK activation (Kam et al., 2004; Bruchas et al., 2007b). However, U50488 does not elicit long-term inhibition of KOR function in rat peripheral sensory neurons or in mice (Bruchas et al., 2007b). It is notable that U50488-mediated JNK activation, but not that of norBNI, is sensitive to pertussis toxin (Bruchas et al., 2007b), suggesting that whereas U50488 activates JNK via KOR coupling to Gαi proteins, norBNI-mediated JNK activation is Gαi independent. It has been shown that the functional consequences of activation of the mitogen-activated protein kinase, ERK, can differ markedly depending upon the mechanism (Gαi protein versus non-Gαi protein mediation) by which ERK is activated (Azzi et al., 2003; Tohgo et al., 2003; Ahn et al., 2004; Kohout et al., 2004; Shenoy et al., 2006; Zheng et al., 2008), although, to our knowledge, similar studies have not been conducted for JNK.
The long-term inhibitory effect of norBNI on peripheral KOR function in vivo and ex vivo was blocked by two protein translation inhibitors with different mechanisms of action, CHX and RAPA. These results also suggest that the long-lasting effect of norBNI was not due to continued receptor occupancy by norBNI or a metabolite. Instead, a newly synthesized protein appears to mediate the long-term action of norBNI. Although effective when injected into the hind paw before norBNI, when protein translation inhibitors were administered 5–6 days after norBNI, they were ineffective. This suggests that a protein involved in norBNI-mediated long-term inhibition of KOR antinociceptive signaling is synthesized relatively early in response to activation of JNK, and it, or its effect, remains to inhibit KOR responsiveness for several days. Although norBNI produces long-term inhibitory effects in the CNS that are mediated by JNK activation, it is not known whether de novo protein synthesis is involved. Given that opioid receptor regulation in peripheral sensory neurons differs from that for receptors expressed on CNS neurons (e.g., see Sullivan et al., 2015), it is possible that mechanisms underlying long-term effects of norBNI may also differ.
In addition to its well-known role as a regulator of transcription, JNK also regulates protein translation (Swantek et al., 1997; Patel et al., 2012; Li et al., 2015). This suggests that norBNI activation of KOR increased JNK activity that in turn increased translation of mRNA in the nerve terminals of the hind paw to produce a protein that caused long-lasting inhibition of KOR agonist-mediated antinociception. Although once controversial, there is now substantial evidence that mRNA, ribosomes, and other elements required for protein translation are present and active in mammalian axons and nerve terminals (Bramham and Wells, 2007; Jimenez-Diaz et al., 2008; Melemedjian et al., 2010; Willis and Twiss, 2010; Obara et al., 2012; Ferrari et al., 2013). Considering the long distance between the nucleus and the nerve terminals in sensory neurons, it seems likely that the mRNA translated by KOR activation is resident in the nerve terminals awaiting a signal to initiate translation.
The presence of mRNA and local protein translation machinery is energetically favorable and facilitates dynamic regulation of signaling in nerve terminals. Although regulation of protein synthesis in dendrites or nerve terminals is influenced by neuronal activity (Bramham and Wells, 2007; Willis and Twiss, 2010), there are very few reports that have demonstrated regulation of local protein synthesis in synaptic regions by G protein–coupled receptors (GPCRs). Increased local protein synthesis in synaptic regions of hippocampal neurons has been attributed to activation of metabotropic glutamate receptors in cultured neurons, hippocampal slices, and synaptoneurosomes (Shin et al., 2004; Mockett et al., 2011). Activation of dopamine D1/D5 receptors stimulates local synthesis of the GluR1 subunit of the AMPA receptor in dendrites of cultured hippocampal neurons (Smith et al., 2005) and increases the synthesis of synapse-associated protein 90/postsynaptic density protein 95-associated protein 3 in cultured prefrontal cortical neurons and slices (Wang et al., 2010). In this work, we provide evidence for local protein synthesis regulated by KOR activation with norBNI in sensory nerve terminals in vivo. Although evidence supporting regulation of local protein synthesis by GPCRs is scarce, it is well known that key mRNA translational control proteins, such as certain cytoplasmic polyadenylation element-binding proteins, eukaryotic translation initiation factors, and eukaryotic elongation factors, are regulated by cellular kinases that are known effectors of GPCRs, such as ERK, calcium-calmodulin–dependent protein kinase II, and mammalian target of rapamycin (Bramham and Wells, 2007). Thus, GPCR-mediated regulation of local protein synthesis in dendrites and nerve terminals may be more common than previously thought.
In summary, in peripheral sensory neurons, norBNI interaction with KOR activates JNK, resulting in long-term inhibition of KOR-mediated antinociception in vivo and inhibition of adenylyl cyclase activity in cultured neurons. These findings support and extend the work of the Chavkin group, who originally identified the role of JNK in the long-term regulation of KOR by norBNI and other antagonists in the CNS and in HEK cells (Bruchas et al., 2007b; Melief et al., 2010, 2011). KOR-dependent activation of JNK indicates that norBNI, and perhaps other long-acting KOR ligands, are not pure antagonists, but instead function as biased ligands. Importantly, the long-term effect of norBNI to selectively regulate KOR function required new protein synthesis in sensory nerve terminals. This new protein(s) functions to inhibit KOR-mediated antinociception and inhibition of adenylyl cyclase activity, but not activation of ERK. Currently, the identification of the protein(s) that regulates KOR function, and its mechanism of action, awaits further investigation. It is intriguing to speculate about the nature of the physiologic conditions that might lead to activation of this long-term inhibitory pathway.
Acknowledgments
We thank Dr. Tom Prisinzano for the generous gift of Salvinorin A, Dr. Elaine Jennings for help with experiments and for constructive criticisms, and Peter LoCoco for helpful comments.
Abbreviations
- ANOVA
analysis of variance
- AUC
area under the curve
- BK
bradykinin
- CHX
cycloheximide
- CNS
central nervous system
- DAMGO
[D-Ala2,N-MePhe4,Gly-ol5]-enkephalin
- DOR
∂ opioid receptor
- DPDPE
[D-Pen2,5]-enkephalin
- ERK
extracellular signal-regulated kinase
- GPCR
G protein–coupled receptor
- i.pl.
intraplantar
- JNK
c-Jun N-terminal kinase
- KOR
κ opioid receptor
- MOR
µ opioid receptor
- norBNI
norbinaltorphimine
- pERK
phosphorylated ERK
- PGE2
prostaglandin E2
- pJNK
phosphorylated JNK
- PWL
paw withdrawal latency
- RAPA
rapamycin
Authorship Contributions
Participated in research design: Berg, Clarke, Jamshidi.
Conducted experiments: Chavera, Jamshidi, Jacobs, Sullivan.
Performed data analysis: Berg, Clarke, Jamshidi.
Wrote or contributed to the writing of the manuscript: Berg, Clarke, Jamshidi.
Footnotes
This work was supported by National Institutes of Health Public Health Service [Grant R01 GM 106035]; National Institutes of Health National Institute of Dental and Craniofacial Research (COSTAR) [Training Grant T32DE14318 to B.A.J.]; and National Institutes of Health National Institute on Drug Abuse [Grant T32 DA 031115 to L.C.S.]. R.J.J. was supported in part by the Translational Science Training program at University of Texas Health Science Center at San Antonio [TST128233].
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. (2004) Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem 279:35518–35525. [DOI] [PubMed] [Google Scholar]
- Arendt-Nielsen L, Olesen AE, Staahl C, Menzaghi F, Kell S, Wong GY, Drewes AM. (2009) Analgesic efficacy of peripheral kappa-opioid receptor agonist CR665 compared to oxycodone in a multi-modal, multi-tissue experimental human pain model: selective effect on visceral pain. Anesthesiology 111:616–624. [DOI] [PubMed] [Google Scholar]
- Azzi M, Charest PG, Angers S, Rousseau G, Kohout T, Bouvier M, Piñeyro G. (2003) Beta-arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc Natl Acad Sci USA 100:11406–11411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KA, Patwardhan AM, Sanchez TA, Silva YM, Hargreaves KM, Clarke WP. (2007) Rapid modulation of micro-opioid receptor signaling in primary sensory neurons. J Pharmacol Exp Ther 321:839–847. [DOI] [PubMed] [Google Scholar]
- Berg KA, Rowan MP, Gupta A, Sanchez TA, Silva M, Gomes I, McGuire BA, Portoghese PS, Hargreaves KM, Devi LA, et al. (2012) Allosteric interactions between δ and κ opioid receptors in peripheral sensory neurons. Mol Pharmacol 81:264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KA, Rowan MP, Sanchez TA, Silva M, Patwardhan AM, Milam SB, Hargreaves KM, Clarke WP. (2011) Regulation of κ-opioid receptor signaling in peripheral sensory neurons in vitro and in vivo. J Pharmacol Exp Ther 338:92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Wells DG. (2007) Dendritic mRNA: transport, translation and function. Nat Rev Neurosci 8:776–789. [DOI] [PubMed] [Google Scholar]
- Broadbear JH, Negus SS, Butelman ER, de Costa BR, Woods JH. (1994) Differential effects of systemically administered nor-binaltorphimine (nor-BNI) on kappa-opioid agonists in the mouse writhing assay. Psychopharmacology 115:311–319. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Chavkin C. (2010) Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology 210:137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S, Chavkin C. (2007a) Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. J Neurosci 27:11614–11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Yang T, Schreiber S, Defino M, Kwan SC, Li S, Chavkin C. (2007b) Long-acting kappa opioid antagonists disrupt receptor signaling and produce noncompetitive effects by activating c-Jun N-terminal kinase. J Biol Chem 282:29803–29811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butelman ER, Negus SS, Ai Y, de Costa BR, Woods JH. (1993) Kappa opioid antagonist effects of systemically administered nor-binaltorphimine in a thermal antinociception assay in rhesus monkeys. J Pharmacol Exp Ther 267:1269–1276. [PubMed] [Google Scholar]
- Butelman ER, Yuferov V, Kreek MJ. (2012) κ-opioid receptor/dynorphin system: genetic and pharmacotherapeutic implications for addiction. Trends Neurosci 35:587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Dymshitz J, Vasko MR. (1997) Regulation of opioid receptors in rat sensory neurons in culture. Mol Pharmacol 51:666–673. [DOI] [PubMed] [Google Scholar]
- Endoh T, Matsuura H, Tanaka C, Nagase H. (1992) Nor-binaltorphimine: a potent and selective kappa-opioid receptor antagonist with long-lasting activity in vivo. Arch Int Pharmacodyn Ther 316:30–42. [PubMed] [Google Scholar]
- Ferrari LF, Bogen O, Chu C, Levine JD. (2013) Peripheral administration of translation inhibitors reverses increased hyperalgesia in a model of chronic pain in the rat. J Pain 14:731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL, Emson PC, Leigh BK, Gilbert RF, Iversen LL. (1980) Multiple opiate receptor sites on primary afferent fibres. Nature 284:351–353. [DOI] [PubMed] [Google Scholar]
- Gomez R, Por ED, Berg KA, Clarke WP, Glucksman MJ, Jeske NA. (2011) Metallopeptidase inhibition potentiates bradykinin-induced hyperalgesia. Pain 152:1548–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. (1988) A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32:77–88. [DOI] [PubMed] [Google Scholar]
- Horan P, Taylor J, Yamamura HI, Porreca F. (1992) Extremely long-lasting antagonistic actions of nor-binaltorphimine (nor-BNI) in the mouse tail-flick test. J Pharmacol Exp Ther 260:1237–1243. [PubMed] [Google Scholar]
- Jamshidi RJ, Jacobs BA, Sullivan LC, Chavera TA, Saylor RM, Prisinzano TE, Clarke WP, Berg KA. (2015) Functional selectivity of kappa opioid receptor agonists in peripheral sensory neurons. J Pharmacol Exp Ther 355:174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeske NA, Berg KA, Cousins JC, Ferro ES, Clarke WP, Glucksman MJ, Roberts JL. (2006) Modulation of bradykinin signaling by EP24.15 and EP24.16 in cultured trigeminal ganglia. J Neurochem 97:13–21. [DOI] [PubMed] [Google Scholar]
- Jewett DC, Woods JH. (1995) Nor-binaltorphimine: an ultra-long acting kappa-opioid antagonist in pigeons. Behav Pharmacol 6:815–820. [PubMed] [Google Scholar]
- Jiménez-Díaz L, Géranton SM, Passmore GM, Leith JL, Fisher AS, Berliocchi L, Sivasubramaniam AK, Sheasby A, Lumb BM, Hunt SP. (2008) Local translation in primary afferent fibers regulates nociception. PLoS One 3:e1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DN, Holtzman SG. (1992) Long term kappa-opioid receptor blockade following nor-binaltorphimine. Eur J Pharmacol 215:345–348. [DOI] [PubMed] [Google Scholar]
- Joris JL, Dubner R, Hargreaves KM. (1987) Opioid analgesia at peripheral sites: a target for opioids released during stress and inflammation? Anesth Analg 66:1277–1281. [PubMed] [Google Scholar]
- Kam AY, Chan AS, Wong YH. (2004) Phosphatidylinositol-3 kinase is distinctively required for mu-, but not kappa-opioid receptor-induced activation of c-Jun N-terminal kinase. J Neurochem 89:391–402. [DOI] [PubMed] [Google Scholar]
- Kivell B, Prisinzano TE. (2010) Kappa opioids and the modulation of pain. Psychopharmacology 210:109–119. [DOI] [PubMed] [Google Scholar]
- Kohout TA, Nicholas SL, Perry SJ, Reinhart G, Junger S, Struthers RS. (2004) Differential desensitization, receptor phosphorylation, beta-arrestin recruitment, and ERK1/2 activation by the two endogenous ligands for the CC chemokine receptor 7. J Biol Chem 279:23214–23222. [DOI] [PubMed] [Google Scholar]
- Li D, Chen D, Zhang X, Wang H, Song Z, Xu W, He Y, Yin Y, Cao J. (2015) c-Jun N-terminal kinase and Akt signalling pathways regulating tumour necrosis factor-α-induced interleukin-32 expression in human lung fibroblasts: implications in airway inflammation. Immunology 144:282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melemedjian OK, Asiedu MN, Tillu DV, Peebles KA, Yan J, Ertz N, Dussor GO, Price TJ. (2010) IL-6- and NGF-induced rapid control of protein synthesis and nociceptive plasticity via convergent signaling to the eIF4F complex. J Neurosci 30:15113–15123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melief EJ, Miyatake M, Bruchas MR, Chavkin C. (2010) Ligand-directed c-Jun N-terminal kinase activation disrupts opioid receptor signaling. Proc Natl Acad Sci USA 107:11608–11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melief EJ, Miyatake M, Carroll FI, Béguin C, Carlezon WA, Jr, Cohen BM, Grimwood S, Mitch CH, Rorick-Kehn L, Chavkin C. (2011) Duration of action of a broad range of selective κ-opioid receptor antagonists is positively correlated with c-Jun N-terminal kinase-1 activation. Mol Pharmacol 80:920–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockett BG, Guévremont D, Wutte M, Hulme SR, Williams JM, Abraham WC. (2011) Calcium/calmodulin-dependent protein kinase II mediates group I metabotropic glutamate receptor-dependent protein synthesis and long-term depression in rat hippocampus. J Neurosci 31:7380–7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara I, Géranton SM, Hunt SP. (2012) Axonal protein synthesis: a potential target for pain relief? Curr Opin Pharmacol 12:42–48. [DOI] [PubMed] [Google Scholar]
- Obara I, Parkitna JR, Korostynski M, Makuch W, Kaminska D, Przewlocka B, Przewlocki R. (2009) Local peripheral opioid effects and expression of opioid genes in the spinal cord and dorsal root ganglia in neuropathic and inflammatory pain. Pain 141:283–291. [DOI] [PubMed] [Google Scholar]
- Pasternak GW, Pan YX. (2013) Mu opioids and their receptors: evolution of a concept. Pharmacol Rev 65:1257–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MR, Sadiq AA, Jay-Dixon J, Jirakulaporn T, Jacobson BA, Farassati F, Bitterman PB, Kratzke RA. (2012) Novel role of c-jun N-terminal kinase in regulating the initiation of cap-dependent translation. Int J Oncol 40:577–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patkar KA, Wu J, Ganno ML, Singh HD, Ross NC, Rasakham K, Toll L, McLaughlin JP. (2013) Physical presence of nor-binaltorphimine in mouse brain over 21 days after a single administration corresponds to its long-lasting antagonistic effect on κ-opioid receptors. J Pharmacol Exp Ther 346:545–554. [DOI] [PubMed] [Google Scholar]
- Patwardhan AM, Berg KA, Akopain AN, Jeske NA, Gamper N, Clarke WP, Hargreaves KM. (2005) Bradykinin-induced functional competence and trafficking of the delta-opioid receptor in trigeminal nociceptors. J Neurosci 25:8825–8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan AM, Diogenes A, Berg KA, Fehrenbacher JC, Clarke WP, Akopian AN, Hargreaves KM. (2006) PAR-2 agonists activate trigeminal nociceptors and induce functional competence in the delta opioid receptor. Pain 125:114–124. [DOI] [PubMed] [Google Scholar]
- Patwardhan AM, Vela J, Farugia J, Vela K, Hargreaves KM. (2008) Trigeminal nociceptors express prostaglandin receptors. J Dent Res 87:262–266. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. (1986) Psychotomimesis mediated by kappa opiate receptors. Science 233:774–776. [DOI] [PubMed] [Google Scholar]
- Portoghese AS, Lipkowski AW, Takemori AE. (1987a) Bimorphinans as highly selective, potent kappa opioid receptor antagonists. J Med Chem 30:238–239. [DOI] [PubMed] [Google Scholar]
- Portoghese PS, Lipkowski AW, Takemori AE. (1987b) Binaltorphimine and nor-binaltorphimine, potent and selective kappa-opioid receptor antagonists. Life Sci 40:1287–1292. [DOI] [PubMed] [Google Scholar]
- Przewłocki R, Przewłocka B. (2001) Opioids in chronic pain. Eur J Pharmacol 429:79–91. [DOI] [PubMed] [Google Scholar]
- Redila VA, Chavkin C. (2008) Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system. Psychopharmacology 200:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivière PJ. (2004) Peripheral kappa-opioid agonists for visceral pain. Br J Pharmacol 141:1331–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan MP, Ruparel NB, Patwardhan AM, Berg KA, Clarke WP, Hargreaves KM. (2009) Peripheral delta opioid receptors require priming for functional competence in vivo. Eur J Pharmacol 602:283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ. (2006) Beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta2 adrenergic receptor. J Biol Chem 281:1261–1273. [DOI] [PubMed] [Google Scholar]
- Shin CY, Kundel M, Wells DG. (2004) Rapid, activity-induced increase in tissue plasminogen activator is mediated by metabotropic glutamate receptor-dependent mRNA translation. J Neurosci 24:9425–9433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Herz A. (1986) Differential effects of mu and kappa opioid systems on motivational processes. NIDA Res Monogr 75:563–566. [PubMed] [Google Scholar]
- Smith WB, Starck SR, Roberts RW, Schuman EM. (2005) Dopaminergic stimulation of local protein synthesis enhances surface expression of GluR1 and synaptic transmission in hippocampal neurons. Neuron 45:765–779. [DOI] [PubMed] [Google Scholar]
- Stein C, Lang LJ. (2009) Peripheral mechanisms of opioid analgesia. Curr Opin Pharmacol 9:3–8. [DOI] [PubMed] [Google Scholar]
- Stein C, Zollner C. (2009) Opioids and sensory nerves. Handb Exp Pharmacol 194:495-518. [DOI] [PubMed] [Google Scholar]
- Sullivan LC, Berg KA, Clarke WP. (2015) Dual regulation of δ-opioid receptor function by arachidonic acid metabolites in rat peripheral sensory neurons. J Pharmacol Exp Ther 353:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swantek JL, Cobb MH, Geppert TD. (1997) Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for lipopolysaccharide stimulation of tumor necrosis factor alpha (TNF-alpha) translation: glucocorticoids inhibit TNF-alpha translation by blocking JNK/SAPK. Mol Cell Biol 17:6274–6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr (2004) Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology 172:463–470. [DOI] [PubMed] [Google Scholar]
- Tohgo A, Choy EW, Gesty-Palmer D, Pierce KL, Laporte S, Oakley RH, Caron MG, Lefkowitz RJ, Luttrell LM. (2003) The stability of the G protein-coupled receptor-beta-arrestin interaction determines the mechanism and functional consequence of ERK activation. J Biol Chem 278:6258–6267. [DOI] [PubMed] [Google Scholar]
- Vadivelu N, Mitra S, Hines RL. (2011) Peripheral opioid receptor agonists for analgesia: a comprehensive review. J Opioid Manag 7:55–68. [DOI] [PubMed] [Google Scholar]
- Vanderah TW. (2010) Delta and kappa opioid receptors as suitable drug targets for pain. Clin J Pain 26 (Suppl 10):S10–S15. [DOI] [PubMed] [Google Scholar]
- Wang H, Kim SS, Zhuo M. (2010) Roles of fragile X mental retardation protein in dopaminergic stimulation-induced synapse-associated protein synthesis and subsequent alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-4-propionate (AMPA) receptor internalization. J Biol Chem 285:21888–21901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis DE, Twiss JL. (2010) Regulation of protein levels in subcellular domains through mRNA transport and localized translation. Mol Cell Proteomics 9:952–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Loh HH, Law PY. (2008) Beta-arrestin-dependent mu-opioid receptor-activated extracellular signal-regulated kinases (ERKs) translocate to nucleus in contrast to G protein-dependent ERK activation. Mol Pharmacol 73:178–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Leri F, Grella SL, Aldrich JV, Kreek MJ. (2013) Involvement of dynorphin and kappa opioid receptor in yohimbine-induced reinstatement of heroin seeking in rats. Synapse 67:358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]