Abstract
Orexin/hypocretin (Orx/Hcrt) projections from the lateral hypothalamus to the paraventricular nucleus of the thalamus (PVT) are implicated in drug addiction. Specifically, the posterior section of the PVT (pPVT) innervates brain structures that modulate motivated behavior. This study investigated the role of pPVT-Orx/Hcrt transmission in cocaine-seeking behavior. Because the effects of Orx/Hcrt are mediated by two Orx/Hcrt receptors (Hcrt-r1 and Hcrt-r2), we examined the extent to which Hcrt-r1 and Hcrt-r2 are involved in Orx/Hcrt-induced cocaine seeking. Male Wistar rats were made cocaine dependent by self-administering cocaine 6 hours/day (long access) for 21 days. After self-administration training, the rats underwent daily extinction training, during which cocaine was withheld. After extinction, the rats were injected into the pPVT with Orx-A/Hcrt-1 (0–2 µg) alone or, using a single dose of 0.5 µg, in combination with an Hcrt-r1 antagonist (SB334867; 0–15 µg) or an Hcrt-r2 antagonist (TCSOX229; 0–15 µg). Orx-A/Hcrt-1 alone reinstated (primed) cocaine seeking. Unexpectedly, coadministration of Orx-A/Hcrt-1 with SB334867 did not have any effects on Orx-A/Hcrt-1-induced reinstatement, whereas when coadministered with Orx-A/Hcrt-1, TCSOX229 prevented cocaine-seeking behavior. These results indicate that Hcrt-r2 in the pPVT mediates the reinstating effect of Orx-A/Hcrt-1 in animals with a history of cocaine dependence and further identify Hcrt-r2 as a possible molecular target that can guide future therapeutic approaches for the prevention of drug-seeking behavior.
Introduction
A growing amount of evidence supports the significant implication of the paraventricular nucleus of the thalamus (PVT) in drug addiction because of its projections to limbic and cortical structures and involvement in the neurocircuitry that mediates craving and drug seeking (Everitt et al., 2001; McFarland and Kalivas, 2001; Ito et al., 2002; Kalivas and Volkow, 2005; Belin and Everitt, 2008; Steketee and Kalivas, 2011). Indeed, excitotoxic lesions of the PVT blocked beer-seeking behavior (Hamlin et al., 2009), attenuated reinstatement of cocaine seeking induced by a prime injection (James et al., 2010), and prevented the expression of cocaine conditioned place preference (Browning et al., 2014). Moreover, PVT neurons are activated by an acute dose of cocaine (Brown et al., 1992) and contextual stimuli that were conditioned to alcohol (Wedzony et al., 2003; Dayas et al., 2008; Perry and McNally, 2013), cocaine (Brown et al., 1992; Franklin and Druhan, 2000), and methamphetamine availability (Rhodes et al., 2005). Additionally, a correlation was observed between cocaine-seeking behavior and PVT activation and not during a highly palatable food-seeking behavior (Matzeu et al., 2015a), whereas presentation of stimuli previously paired with sucrose availability did not produce PVT activation (Wedzony et al., 2003).
Although frequently acknowledged as a homogeneous nucleus, the PVT consists of two structural portions—anterior (aPVT) and posterior (pPVT) (Vertes and Hoover, 2008)—that mediate distinct functions associated with reward-seeking behavior. For example, injection of TCSOX229, a hypocretin receptor 2 (Hcrt-r2) antagonist, into the aPVT decreased ethanol drinking but had no effect when injected into the pPVT (Barson et al., 2015). Additionally, voluntary ethanol drinking has been shown to activate the aPVT, but not the pPVT, to elevate Hcrt-r2 gene expression, whereas intragastrically administered ethanol increased Fos and Hcrt-r2 expression in the aPVT but not in the pPVT (Barson et al., 2015), suggestive of a role of Hcrt-r2 in the aPVT in modulating ethanol intake. On the contrary, temporary inactivation of the pPVT totally and selectively blocked the reinstatement of cocaine seeking (Matzeu et al., 2015b), suggesting that the pPVT is a key structure that regulates cocaine-seeking behavior.
It is known that the PVT receives abundant projections from the hypothalamus that contains numerous peptides, including orexin/hypocretin (Orx/Hcrt; Freedman and Cassell, 1994; Otake, 2005; Parsons et al., 2006; Baimel et al., 2015). The Orx/Hcrt peptides orexin A and B (Orx-A and Orx-B, or Hcrt-1 and Hcrt-2) are synthetized only in neurons from the dorsal tuberal hypothalamic nuclei, including the lateral hypothalamus (LH), perifornical nucleus, and dorsomedial hypothalamus (de Lecea et al., 1998; Sakurai et al., 1998). Although Orx/Hcrt neurons localization is restricted to hypothalamic nuclei, they send projections across the entire brain (de Lecea et al., 1998; Baldo et al., 2003) and are involved in many biologic functions, such as energy homeostasis and feeding, neuroendocrine and autonomic functions, arousal and wakefulness (Sutcliffe and de Lecea, 2000; Mieda and Yanagisawa, 2002; de Lecea, 2012). More recently, Orx/Hcrt has been described to be involved in drug addiction and drug seeking (Harris et al., 2005; Dayas et al., 2008; Martin-Fardon et al., 2010; Jupp et al., 2011), but what remains unclear is the participation of the pPVT Orx/Hcrt transmission in drug-seeking behavior after dependence.
Extended access to cocaine self-administration results in an increased (escalation) cocaine intake (Johanson et al., 1976; Bozarth and Wise, 1985). Specifically, rats with extended access to the drug exhibit a gradual daily increase in cocaine intake and an upward shift in the cocaine dose-effect function indicative of the need to increase the amount of cocaine intake to reach the desire effects (Ahmed and Koob, 1998; Ahmed and Koob, 1999). During abstinence, cocaine-escalated rats show persistent reward deficiency (Ahmed et al., 2002) very similar to depression and dysphoria during withdrawal reported by cocaine addicts (Markou and Koob, 1991). Thus, extended access to cocaine offers a unique animal model of cocaine dependence to investigate the consequence of chronic cocaine abuse in cocaine addicts.
Therefore, to shed light on the importance of Orx/Hcrt neurotransmission in the pPVT during cocaine seeking after dependence, the present study examined whether discrete administration of Orx-A/Hcrt-1 in the pPVT reinstates cocaine seeking in animals subjected to extended daily availability of cocaine. Knowing that Orx-A/Hcrt-1 binds to both Hcrt-r1 and Hcrt-r2 and that both receptors are expressed in the pPVT (Trivedi et al., 1998; Marcus et al., 2001), the present study also investigated the extent to which Hcrt-r1 and Hcrt-r2 are involved in Orx-A/Hcrt-1-induced cocaine seeking. A selective antagonist of either Hcrt-r1 or Hcrt-r2 was coadministered with Orx-A/Hcrt-1 and tested for its ability to block Orx-A/Hcrt-1–induced cocaine-seeking behavior.
Materials and Methods
Animals.
One hundred sixteen male Wistar rats (Charles River, Wilmington, MA), weighing 200–225 g upon arrival, were housed two per cage in a temperature- and humidity-controlled vivarium on a reverse 12 hour/12 hour light/dark cycle with ad libitum access to food and water throughout the duration of the experiment. The animals were separated only when placed in the operant chambers (one animal per operant chamber). All the procedures were conducted in strict adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Self-Administration and Extinction Training.
Rats that were designated for cocaine self-administration were surgically prepared with jugular catheters under 1.5%–2% isoflurane anesthesia 7 days before starting cocaine self-administration training in daily 6-hour sessions and treated with flunixin (2.5 mg/kg, s.c.) twice per day for postsurgery pain. Catheters were flushed daily with 0.2 ml of sterile antibiotic solution containing cefazolin (100 mg/ml; Hospira, San Diego, CA) until the test day. Every session was initiated by the extension of two retractable levers into the operant conditioning chambers (29 × 24 × 19.5 cm, Med Associates, St. Albans, VT). Responses at the active lever were reinforced on a fixed-ratio 1 (FR1) schedule by i.v. cocaine (National Institute on Drug Abuse, Bethesda, MD; 0.25 mg/0.1 ml per infusion), dissolved in 0.9% sodium chloride (Hospira, Lake Forest, IL) and infused over 4 seconds. Each reinforced response was followed by a 20-second timeout (TO20) period that was signaled by the illumination of a cue light above the active lever. Responses at the inactive lever were recorded but had no scheduled consequences.
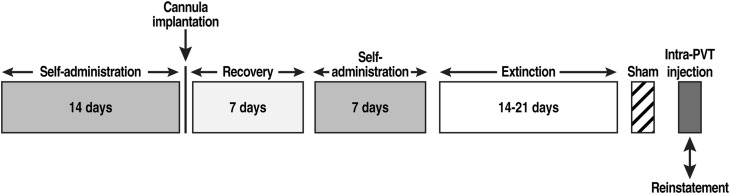
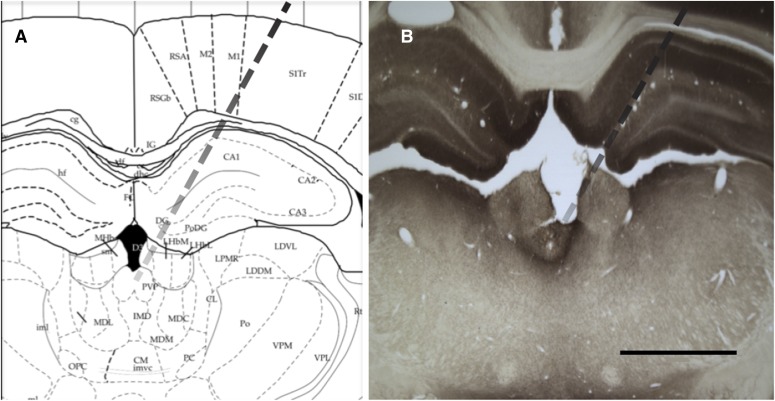
Fourteen days (Fig. 1) after beginning self-administration training, the rats were implanted with a guide cannula (23-gauge, 15-mm, Plastics One, Roanoke, VA) aimed at the pPVT (anterior/posterior, −3.3 mm; medial/lateral, ±2.72 mm from bregma; dorsal/ventral, −2.96 mm from dura, at an angle of 25°) (Paxinos and Watson, 1997) and positioned 3.5 mm above the target injection site (Fig. 2). After 7 days of recovery, the animals resumed self-administration training for an additional 7 days.
Fig. 1.
Behavioral procedure.
Fig. 2.
Injector placement in the pPVT (Paxinos and Watson, 1997). (A) Schematic representation. (B) Representative image of injector placement. Scale bar: 2000 µm.
Twenty-four hours after the last self-administration session, the rats were placed under extinction conditions until an extinction criterion of ≤10 lever presses over the last three sessions was reached (usually between 14 and 21 days, Fig. 1). All the extinction sessions lasted 2 hous and began with extension of the levers into the operant chambers. The extinction sessions followed the same schedule as the self-administration training (including the TO20 signaled by the illumination of a cue light above the active lever) but without reward (cocaine) delivery when the active lever was depressed. Responses at the inactive lever were recorded as well but had no scheduled consequences.
Intra-PVT Microinjection.
On the last day of extinction training, each rat received a sham injection for habituation to the microinjection (Fig. 1), and the rats were then divided into separate groups that were designated to receive either an intra-pPVT injection of Orx-A/Hcrt-1 or a combination of Orx-A/Hcrt-1 with an antagonist (between-subjects design). Twenty-four hours later, each animal received an intra-PVT microinjection of Orx-A/Hcrt-1 (0, 0.25, 0.5, 1, or 2 μg; American Peptide, Sunnyvale, CA) (Li et al., 2009; Li et al., 2010b) in 0.9% sodium chloride (Hospira, Lake Forest, IL) or a combination of 0.5 μg Orx-A/Hcrt-1 (i.e., the minimal dose able to induce cocaine-seeking behavior) and the Hcrt-r1 antagonist SB334867 (0, 5, 10, or 15 μg; Lilly Research Laboratories, Indianapolis, IN) (Li et al., 2011) in ≥99.5% dimethylsulfoxide (DMSO; Sigma Aldrich, St. Louis, MO) or the Hcrt-r2 antagonist TCSOX229 (Tocris Biosciences, Bristol, UK; 0, 5, 10, or 15 μg; Li et al., 2011) in 0.9% sodium chloride (saline; Hospira, Lake Forest, IL). Intra-pPVT microinjections were made using a microinfusion pump (Harvard 22 Syringe Pump, Holliston, MA) with injectors extending 3.5 mm beyond the guide cannula. The injection volume was 0.5 μl. The injections were made over 1 minute, and the injector was left in place for an additional minute before being removed. Following the injections, the rats were returned to their home cages for 2 minutes and then transferred to the operant chambers and tested under extinction conditions for 2 hours (Fig. 1).
Histology.
Upon completion of the reinstatement test (Fig. 1), the rats were euthanized by CO2 inhalation, and their brains were collected and snap frozen. The brains were then sliced in 40 μm coronal sections, and injector placements within the pPVT were verified (Fig. 2).
Statistical Analysis.
Cocaine intake (mg/kg) during the self-administration was analyzed with one-way repeated-measures analysis of variance (ANOVA) followed by Tukey post hoc tests. The dose-effect profile of Orx-A/Hcrt-1 or reversal effect profiles of SB334867 and TCSOX229 were analyzed using one-way ANOVA followed by Dunnett’s post hoc tests. The magnitude of the increase in responding (percent increase) that was induced by Orx-A/Hcrt-1 and the magnitude of the inhibitory effects of SB334867 or TCSOX229 (percent decrease) were analyzed using one-way ANOVA, followed by Dunnett’s post hoc tests to confirm significance.
Results
Six rats were lost (three to health complications, one for failure to acquire cocaine self-administration, and two because of off-target injections), reducing the total number of animals to 110 (n = 38 for Orx-A/Hcrt-1 dose effect; n = 38 for SB334867 test; n = 34 for TCSOX229 test).
Orx-A/Hcrt-1–Induced Reinstatement
Self-Administration and Extinction Training.
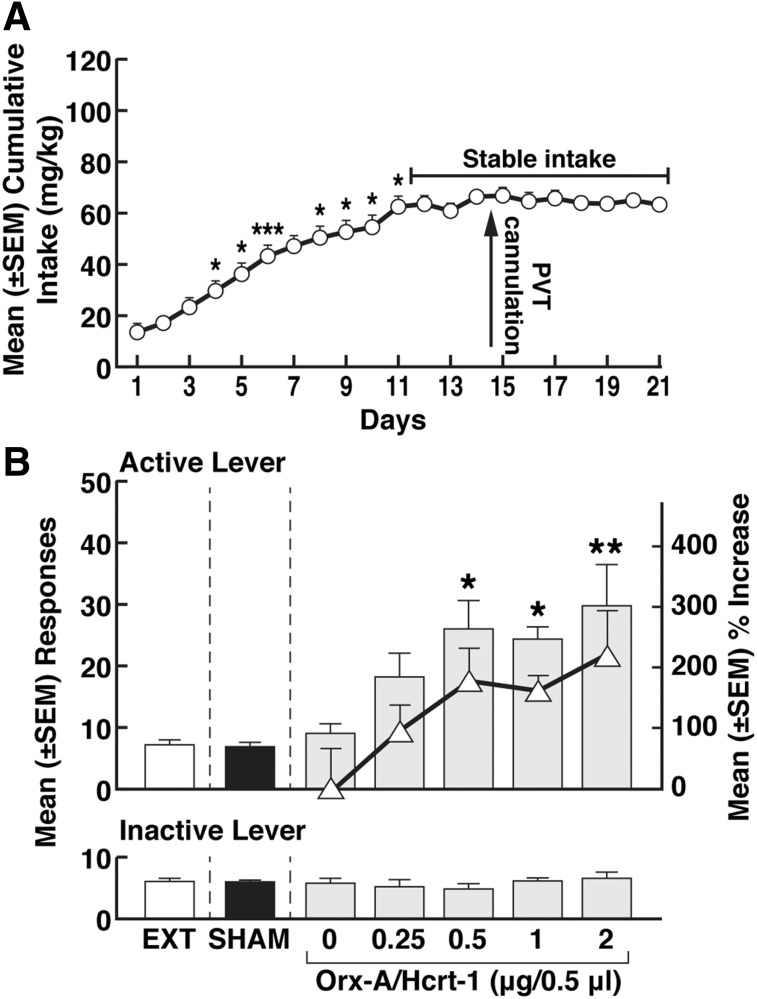
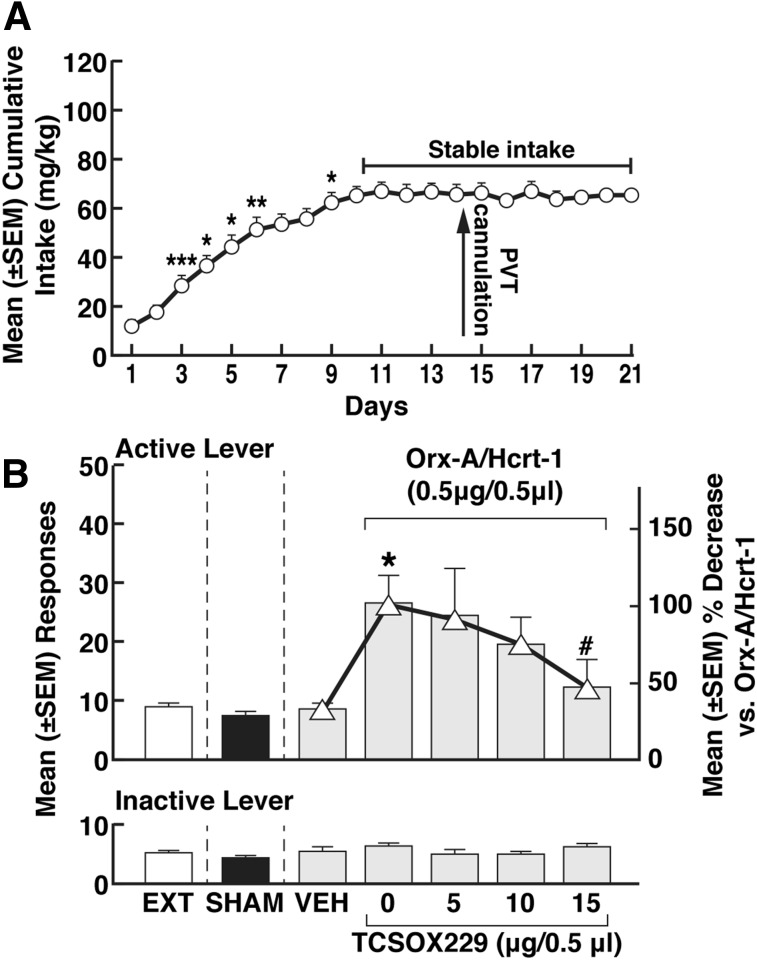
Cocaine intake gradually increased from day 1 (14.1 ± 2.2 mg/kg) to day 11 (63.0 ± 3.6 mg/kg) and then stabilized (repeated measures ANOVA; F37, 740 = 20.55, P < 0.001; Fig. 3A). At the end of extinction training (17 ± 1 days), the rats reached a 3-day average (± S.E.M.) number of responses of 7.5 ± 0.6 (Fig. 3B). The mean (± S.E.M.) number of responses during the extinction, sham, and vehicle sessions were statistically identical (F2,14 = 0.076, P > 0.05; Fig. 3B).
Fig. 3.
Priming effect of Orx-A/Hcrt-1. (A) Time course of cocaine self-administration over the 21 days of training. Tukey post hoc tests *P < 0.05; **P < 0.01; ***P < 0.001 vs. respective previous day (data shown depict the mean ± S.E.M.; n = 38). Intra-pPVT administration of Orx-A/Hcrt-1 induces cocaine-seeking behavior. *P < 0.05; **P < 0.01; vs. 0 μg (saline). ∆, % increase in responding): *P < 0.05; **P < 0.01 vs. 0 μg (saline). Asterisks (*, **) are referring to both the responses and the % increase. EXT, extinction; SHAM, sham injection (data shown depict the mean ± S.E.M; n = 38 for EXT and SHAM; n = 7–9 for Orx-A/Hcrt-1 doses).
Intra-PVT Microinjection of Orx-A/Hcrt-1.
After saline injection (0 μg), the mean (± S.E.M.) number of responses was negligible (9.2 ± 1.6; Fig. 3B), whereas increased doses of Orx-A/Hcrt-1 induced cocaine-seeking behavior. This observation was confirmed by a main effect of Orx-A/Hcrt-1 doses (one-way ANOVA; F4,33 = 3.626, P < 0.05). Dunnett’s post hoc tests showed that Orx-A/Hcrt-1 reinstated cocaine seeking at 0.5, 1, and 2 μg (P < 0.05, vs. 0 µg; Fig. 3B). Transformation of the data into percent increase in responding (Fig. 3B) induced by Orx-A/Hcrt-1 also confirmed that Orx-A/Hcrt-1 reinstated cocaine-seeking behavior at 0.5, 1, and 2 μg (Dunnett’s post hoc test after one-way ANOVA, F4,33 = 3.626, P < 0.05; Fig. 3B). Responses at the inactive lever were negligible and unaltered by Orx-A/Hcrt-1 (one-way ANOVA; F4,33 = 0.3367, P > 0.05) (Fig. 3B).
Blockade of Hcrt-r1 with SB334867
Self-Administration and Extinction Training.
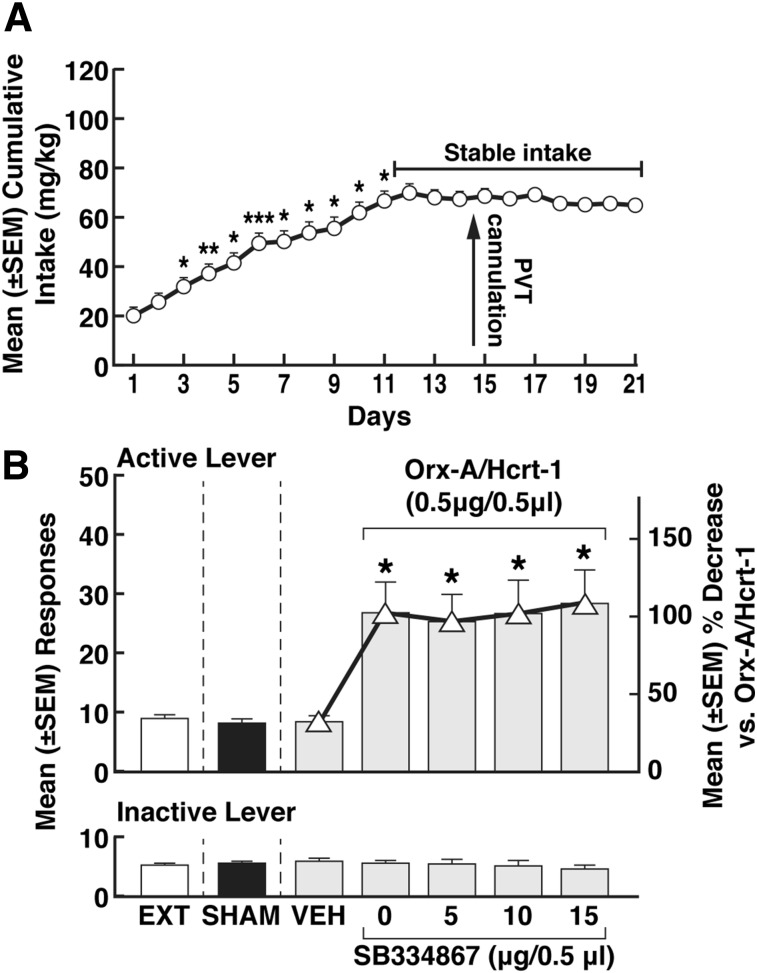
Cocaine intake gradually increased from day 1 (20 ± 3.3 mg/kg) to day 10 (62.4 ± 4.0 mg/kg) and then stabilized (repeated-measures ANOVA; F37, 740 = 19.00, P < 0.001) (Fig. 4A). By the end of extinction training (17 ± 2 days), the rats reached a 3-day average (± S.E.M.) number of responses of 9.1 ± 0.4 (Fig. 4B). The mean (± S.E.M.) number of responses during the extinction, sham, and vehicle sessions were statistically similar (F2,16 = 1.236, P > 0.05) (Fig. 4B).
Fig. 4.
Effect of SB334867 Orx-A/Hcrt-1’s priming effect. (A) Time course of cocaine self-administration over the 21 days of training. Tukey post hoc tests *P < 0.05; **P < 0.01; ***P < 0.001 vs. respective previous day (data shown depict the mean ± S.E.M; n = 38). (B) Lack of effect of SB334867 on Orx-A/Hcrt-1 (0.5 µg) priming effect: *P < 0.05, vs. VEH (DMSO): ∆: % decrease in responding: *P < 0.05, vs. VEH (DMSO). Asterisks (*) refer to both the responses and the % decrease. EXT, extinction; SHAM, sham injection; VEH, vehicle (data shown depict the mean ± S.E.M; n = 38 for EXT and SHAM; n = 9 for VEH; n = 7–9 for SB334867 doses).
Coadministration of SB334867 with Orx-A/Hcrt-1.
After vehicle (DMSO) injection, the mean (±S.E.M.) number of responses was negligible (8.2 ± 1.0) (Fig. 4B). Coadministration of SB334867 (5, 10, and 15 µg) with Orx-A/Hcrt-1 (0.5 µg) did not prevent reinstatement (one-way ANOVA, F4,33 = 3.490, P < 0.05) (Fig. 4B). Transformation of the data into percent decrease of responding versus Orx-A/Hcrt-1 (Fig. 4B) confirmed the lack of effect of SB334867 on the reinstating effects of Orx-A/Hcrt-1 (one-way ANOVA, F4,33 = 3.490, P < 0.05) (Fig. 4B). Responses at the inactive lever were negligible and unaltered by SB334867 (one-way ANOVA; F4,33 = 0.1038, P > 0.05, Fig. 4B).
Blockade of Hcrt-r2 with TCSOX229
Self-Administration and Extinction Training.
Cocaine intake gradually increased from day 1 (11.9 ± 1.7 mg/kg) to day 9 (62.4 ± 3.8 mg/kg) and then stabilized (repeated measures ANOVA; F32,640 = 17.63, P < 0.001) (Fig. 5A). At the end of extinction training (16 ± 2 days), the rats reached a mean (±S.E.M.) 3-day average number of responses of 8.5 ± 0.7 (Fig. 5B). The mean (±S.E.M.) number of responses during the extinction, sham, and vehicle sessions were statistically comparable (F2,16 = 1.413, P > 0.05) (Fig. 5B).
Fig. 5.
Effect of TCSOX229 Orx-A/Hcrt-1’s priming effect (A) Cocaine intake over the self-administration days. Tukey post hoc tests: *P < 0.05; **P < 0.01 vs. respective previous day (data shown depict the mean ± S.E.M; n = 34). (B) Reversal of Orx-A/Hcrt-1 (0.5 µg) priming effect by TCSOX229. *P < 0.05; **P < 0.01 vs. VEH (saline); #P < 0.05, vs. 0 μg (Orx-A/Hcrt-1 alone). ∆: % decrease responding: *P < 0.05, vs. VEH (saline); #P < 0.05, vs. 0 μg (Orx-A/Hcrt-1 alone). Symbols (*, #) refers to both the responses and the % decrease. EXT, extinction; SHAM, sham injection; VEH, vehicle (data shown depict the mean ± S.E.M; n = 34 for EXT and SHAM; n = 9 for VEH; n = 6 to 7 for TCSOX229 doses).
Coadministration of TCSOX229 with Orx-A/Hcrt-1.
After vehicle (saline) injection, the mean (±S.E.M.) number of responses remained low (8.3 ± 1.0) (Fig. 5B). Coadministration of TCSOX229 (5, 10, and 15 µg) with Orx-A/Hcrt-1 (0.5 µg) prevented cocaine-seeking behavior at 15 µg (P < 0.05, Dunnett’s post hoc test after one-way ANOVA, F4,29 = 2.844; P < 0.05) (Fig. 5B). Transformation of the data into percent decrease of responding (% decrease vs. Orx-A/Hcrt-1) confirmed that TCSOX229 completely blocked the effect of Orx-A/Hcrt-1 at 15 µg (one-way ANOVA followed by Dunnett’s post hoc test, F4,29 = 2.844, P < 0.05) (Fig. 5B). Responses at the inactive lever were negligible and unaltered by TCSOX229 (one-way ANOVA; F4,29 = 0.3701, P > 0.05) (Fig. 5B).
Discussion
The present findings indicate that a microinjection of Orx-A/Hcrt-1 into the pPVT primed cocaine seeking in animals with a history of cocaine dependence. Surprisingly, however, coadministration of SB334867 with Orx-A/Hcrt-1 did not interfere with cocaine seeking, whereas TCSOX229 fully prevented Orx-A/Hcrt-1-induced cocaine seeking. The data indicate that the priming effects of an Orx-A/Hcrt-1 injection into the pPVT are mediated by Hcrt-r2, making this receptor subtype a potential target for cocaine relapse prevention.
Before considering the implications of these findings, it is important to discuss the possibility of nonspecific locomotor effects from the injection of Orx-A/Hcrt-1, SB334867, and TCSOX229 into the pPVT. For instance, one can argue that the priming effect of Orx-A/Hcrt-1 is due to a general nonspecific locomotor activation rather than specific cocaine-seeking behavior. This is inconsistent with the inactive lever data showing no increased activity (Fig. 3B). The same observation was made with the administration of SB334867 and TCSOX229 (Figs. 4B and 5B) where the responses on the inactive lever stayed low and unaffected. Therefore the present data, consistent with earlier published findings, suggest that injection of Orx-A/Hcrt-1, SB334867, and TCSOX229 into the pPVT at the doses range used do not induce any nonspecific locomotor effect and further suggest that Orx-A/Hcrt-1 act on the PVT to produce arousal independent of general locomotor activation (Li et al., 2009, 2010b, 2011; Barson et al., 2015).
Another possible behavioral confound after microinjection into the pPVT could be due to the close position of the PVT to the third ventricle and therefore the possibility of Orx-A/Hcrt-1, SB334867, and TCSOX229 to diffuse through the ventricles and act nonspecifically at other brain regions. This interpretation is inconsistent with the data obtained with SB334867. If nonspecific diffusion in other brain regions occurred, a reduction in cocaine seeking by SB334867 should have been measured similar to what was observed when SB334867 was injected peripherally (Martin-Fardon and Weiss, 2014), but this was not the case. Further, the absence of effect of SB334867 in the pPVT confirmed the observation by others (James et al., 2011) that intra-PVT administration of SB334867 did not affect cocaine-conditioned reinstatement (another animal model of relapse), suggesting a specific role for pPVT Hcrt-r2 signaling in cocaine-seeking behavior.
Demonstration that discrete injection of Orx-A/Hcrt-1 into the pPVT primed cocaine-seeking behavior strongly supports the implication of the Orx/Hcrt projection to the PVT in the control of drug craving and relapse. It has been hypothesized that LH Orx/Hcrt neurons may regulate the activation of the ventral striatum via a relay through the PVT (Kelley et al., 2005a,b; Parsons et al., 2006). This interpretation is consistent with earlier findings showing that the PVT is heavily innervated by Orx/Hcrt fibers that originate from the LH and perifornical nucleus, with the densest innervation in the pPVT (Kirouac et al., 2005). Moreover, a previous study has shown that both Orx/Hcrt neurons in the LH and PVT were activated by alcohol-related stimuli (Dayas et al., 2008), suggesting that LH Orx/Hcrt projections to the PVT are involved in drug-seeking behavior. Further anatomic evidence supports this hypothesis, as it was shown that Orx/Hcrt fibers juxtapose to PVT neurons and project to the nucleus accumbens shell (NACsh; Parsons et al., 2006), a brain region known to be involved in the reinstatement of ethanol-, cocaine-, and sucrose-seeking behavior (Dayas et al., 2007; Cruz et al., 2014; Guercio et al., 2015).
A potential explanation of Orx-A/Hcrt-1’s priming effects could be related to the role of the Orx/Hcrt system in arousal states. In fact, it is known that Orx/Hcrt controls general arousal (Sakurai et al., 2010); for instance, it has been described that expectation of food rewards induces the activation of neurons that contain Orx/Hcrt receptors within the PVT (Choi et al., 2010). Most neurons in the PVT are sensitive to Orx-A/Hcrt-1 and Orx-B/Hcrt-2, and the prefrontal cortex is an important target of Orx/Hcrt-activated PVT neurons (Ishibashi et al., 2005; Huang et al., 2006). The present results suggest that Orx/Hcrt inputs to the PVT facilitate cortical activation that is linked to general arousal (Sato-Suzuki et al., 2002), which could explain the reinstatement of cocaine seeking. Moreover, Orx-A/Hcrt-1 administration in the PVT significantly increased dopamine levels in the nucleus accumbens (Choi et al., 2012), suggesting that the PVT is a key relay for Orx/Hcrt’s effects on the mesolimbic dopamine system and reward-seeking behavior.
Another possible mechanism by which injection of Orx-A/Hcrt-1 into the PVT induces cocaine-seeking behavior could be related to PVT’s function in mediating anxiety- and stress-like responses, which are known factors to precipitate cocaine seeking in abstinent individuals. For example, the PVT sends projections to the dorsolateral bed nucleus of the stria terminalis and central nucleus of the amygdala that contains neurons densely expressing dynorphin and corticotropin releasing factor (Li and Kirouac, 2008), which are peptides associated with the manifestation of negative emotional states and response to stress (Davis, 1998; Heinrichs and Koob, 2004; Shirayama and Chaki, 2006; Davis et al., 2010). In addition, Orx/Hcrt neurons become activated (i.e., increased Fos) after exposure to stress (Espana et al., 2003; Furlong et al., 2009), which indicates that the Orx/Hcrt system is involved in the expression of behavioral and physiologic responses to stressful events. Supporting this hypothesis, both Orx-A/Hcrt-1 and Orx-B/Hcrt-2, when injected in the PVT, produce anxiety-like behavior in rats observed in the open field (Li et al., 2010a) or in the elevated plus maze (Li et al., 2010b), suggesting that Orx/Hcrt can act as a stressor and can therefore precipitate cocaine-seeking behavior.
Surprisingly, Hcrt-r1 blockade did not prevent Orx-A/Hcrt-1 prime-induced reinstatement. This was unexpected considering the large number of studies that reported efficient blockade of drug-seeking behavior when SB334867 was administered peripherally (Richards et al., 2008; Zhou et al., 2012; Martin-Fardon and Weiss, 2014; Bentzley and Aston-Jones, 2015) and the significant expression of Hcrt-r1 in the pPVT (Trivedi et al., 1998; Marcus et al., 2001). One possible explanation of a lack of SB334867effect may be that the doses used were not behaviorally relevant. This interpretation is unlikely, however, because it has been shown by others that SB334867 at doses even lower than the ones tested in the present study attenuated physical symptoms of morphine withdrawal as well as cocaine seeking-behavior when injected in the locus coeruleus and in the ventral tegmental area, respectively (Azizi et al., 2010; James et al., 2011). Another more acceptable hypothesis is that the effect of a peripheral administration (i.e., not site specific) of SB334867 may be attributable to its action in brain regions other than the PVT. Supporting this hypothesis, it has been shown that SB334867 decreased conditioned reinstatement of cocaine-seeking behavior when injected into the ventral tegmental area but not PVT (James et al., 2011).
TCSOX229, in contrast to SB334867, prevented the priming effects of Orx-A/Hcrt-1. It is important to note that most studies in the drug addiction field have prioritized on investigating the role of Hcrt-r1, with only a few reports implicating Hcrt-r2 in drug-seeking behavior. As described for Hctrt-r1, Hcrt-r2 is highly expressed in the PVT (Trivedi et al., 1998; Marcus et al., 2001), and the literature suggests that Hcrt-r2 blockade can attenuate drug intake and drug seeking when Hcrt-r2 antagonists are administered peripherally. For example, peripheral injection of an Hcrt-r2 antagonist attenuated ethanol self-administration, reduced the acquisition of ethanol-induced CPP, prevented ethanol-induced expression and reinstatement of CPP (Shoblock et al., 2011), reduced cue-induced reinstatement of nicotine seeking (Uslaner et al., 2014), and attenuated heroin self-administration in escalated animals (Schmeichel et al., 2015). Further, when administered into the PVT, TCSOX229 prevented footshock-elicited anxiety-like behavior (Li et al., 2010b), implying that Hcrt-r2 signaling in the PVT is important in the mediation of stress-related behavior such as cocaine-seeking behavior.
In summary, the data showed that discrete administration of Orx-A/Hcrt-1 in the pPVT elicited a priming effect that reinstated cocaine-seeking behavior in dependent animals. The blockade of Hcrt-r1 did not prevent reinstatement induced by Orx-A/Hcrt-1, whereas Hcrt-r2 blockade reversed Orx-A/Hcrt-1-induced reinstatement, suggesting that Hcrt-r2 in the pPVT mediates Orx-A/Hcrt-1-induced cocaine seeking. Future studies are guaranteed to assess molecular changes (i.e., Orx/Hcrt and Hcrt-r production) attributable to cocaine dependence and long periods of abstinence. Such findings may shed light on valuable targets for the treatment of persistent vulnerability to relapse associated with cocaine dependence.
Acknowledgments
This is publication number 29322 from The Scripps Research Institute. The authors thank B. N. Leos for technical assistance and M. Arends for assistance with preparation of the manuscript.
Abbreviations
- aPVT
anterior paraventricular nucleus of thalamus
- Hcrt
hypocretin
- LH
lateral hypothalamus
- Orx
orexin
- PVT
paraventricular nucleus of thalamus
- pPVT
posterior paraventricular nucleus of thalamus
- SB334867
N-(2-methyl-6-benzoxazolyl)-N ′-1,5-ne th-aphthyridin-4-yl urea
- TCSOX229
(2S)-1-(3,4-dihydro-6,7-dimethoxy-2(1H)-isoquinolinyl)-3,3-dimethyl-2-[(4-pyridinylmethyl)amino]-1-butanone hydrochloride
Authorship Contributions
Participated in research design: Matzeu, Kerr, Weiss, Martin-Fardon.
Conducted experiments: Matzeu, Kerr, Martin-Fardon.
Performed data analysis: Matzeu, Martin-Fardon.
Wrote or contributed to writing the manuscript: Matzeu, Martin-Fardon.
Footnotes
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA033344 to R.M.F., DA08467 and DA07348 to F.W.].
References
- Ahmed SH, Kenny PJ, Koob GF, Markou A. (2002) Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci 5:625–626. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. (1998) Transition from moderate to excessive drug intake: change in hedonic set point. Science 282:298–300. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. (1999) Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology (Berl) 146:303–312. [DOI] [PubMed] [Google Scholar]
- Azizi H, Mirnajafi-Zadeh J, Rohampour K, Semnanian S. (2010) Antagonism of orexin type 1 receptors in the locus coeruleus attenuates signs of naloxone-precipitated morphine withdrawal in rats. Neurosci Lett 482:255–259. [DOI] [PubMed] [Google Scholar]
- Baimel C, Bartlett SE, Chiou LC, Lawrence AJ, Muschamp JW, Patkar O, Tung LW, Borgland SL. (2015) Orexin/hypocretin role in reward: implications for opioid and other addictions. Br J Pharmacol 172:334–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo BA, Daniel RA, Berridge CW, Kelley AE. (2003) Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol 464:220–237. [DOI] [PubMed] [Google Scholar]
- Barson JR, Ho HT, Leibowitz SF. (2015) Anterior thalamic paraventricular nucleus is involved in intermittent access ethanol drinking: role of orexin receptor 2. Addict Biol 20:469–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. (2008) Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron 57:432–441. [DOI] [PubMed] [Google Scholar]
- Bentzley BS, Aston-Jones G. (2015) Orexin-1 receptor signaling increases motivation for cocaine-associated cues. Eur J Neurosci 41:1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. (1985) Toxicity associated with long-term intravenous heroin and cocaine self-administration in the rat. JAMA 254:81–83. [PubMed] [Google Scholar]
- Brown EE, Robertson GS, Fibiger HC. (1992) Evidence for conditional neuronal activation following exposure to a cocaine-paired environment: role of forebrain limbic structures. J Neurosci 12:4112–4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning JR, Jansen HT, Sorg BA. (2014) Inactivation of the paraventricular thalamus abolishes the expression of cocaine conditioned place preference in rats. Drug Alcohol Depend 134:387–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Fitzgerald ME, Benoit SC. (2010) The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats. Neuroscience 167:11–20. [DOI] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Magrisso IJ, Fitzgerald ME, Lipton JW, Benoit SC. (2012) Orexin signaling in the paraventricular thalamic nucleus modulates mesolimbic dopamine and hedonic feeding in the rat. Neuroscience 210:243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, Babin KR, Leao RM, Goldart EM, Bossert JM, Shaham Y, Hope BT. (2014) Role of nucleus accumbens shell neuronal ensembles in context-induced reinstatement of cocaine-seeking. J Neurosci 34:7437–7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. (1998) Are different parts of the extended amygdala involved in fear versus anxiety? Biol Psychiatry 44:1239–1247. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L and Grillon C (2010) Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology 35:105–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, Liu X, Simms JA, Weiss F. (2007) Distinct patterns of neural activation associated with ethanol seeking: effects of naltrexone. Biol Psychiatry 61:979–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F. (2008) Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry 63:152–157. [DOI] [PubMed] [Google Scholar]
- de Lecea L. (2012) Hypocretins and the neurobiology of sleep-wake mechanisms. Prog Brain Res 198:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, et al. (1998) The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA 95:322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España RA, Valentino RJ, Berridge CW. (2003) Fos immunoreactivity in hypocretin-synthesizing and hypocretin-1 receptor-expressing neurons: effects of diurnal and nocturnal spontaneous waking, stress and hypocretin-1 administration. Neuroscience 121:201–217. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. (2001) The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev 36:129–138. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Druhan JP. (2000) Expression of Fos-related antigens in the nucleus accumbens and associated regions following exposure to a cocaine-paired environment. Eur J Neurosci 12:2097–2106. [DOI] [PubMed] [Google Scholar]
- Freedman LJ, Cassell MD. (1994) Relationship of thalamic basal forebrain projection neurons to the peptidergic innervation of the midline thalamus. J Comp Neurol 348:321–342. [DOI] [PubMed] [Google Scholar]
- Furlong TM, Vianna DM, Liu L, Carrive P. (2009) Hypocretin/orexin contributes to the expression of some but not all forms of stress and arousal. Eur J Neurosci 30:1603–1614. [DOI] [PubMed] [Google Scholar]
- Guercio LA, Schmidt HD, Pierce RC. (2015) Deep brain stimulation of the nucleus accumbens shell attenuates cue-induced reinstatement of both cocaine and sucrose seeking in rats. Behav Brain Res 281:125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, Choi EA, McNally GP. (2009) Paraventricular thalamus mediates context-induced reinstatement (renewal) of extinguished reward seeking. Eur J Neurosci 29:802–812. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. (2005) A role for lateral hypothalamic orexin neurons in reward seeking. Nature 437:556–559. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Koob GF. (2004) Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J Pharmacol Exp Ther 311:427–440. [DOI] [PubMed] [Google Scholar]
- Huang H, Ghosh P, van den Pol AN. (2006) Prefrontal cortex-projecting glutamatergic thalamic paraventricular nucleus-excited by hypocretin: a feedforward circuit that may enhance cognitive arousal. J Neurophysiol 95:1656–1668. [DOI] [PubMed] [Google Scholar]
- Ishibashi M, Takano S, Yanagida H, Takatsuna M, Nakajima K, Oomura Y, Wayner MJ, Sasaki K. (2005) Effects of orexins/hypocretins on neuronal activity in the paraventricular nucleus of the thalamus in rats in vitro. Peptides 26:471–481. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Robbins TW, Everitt BJ. (2002) Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J Neurosci 22:6247–6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Charnley JL, Jones E, Levi EM, Yeoh JW, Flynn JR, Smith DW, Dayas CV. (2010) Cocaine- and amphetamine-regulated transcript (CART) signaling within the paraventricular thalamus modulates cocaine-seeking behaviour. PLoS One 5:e12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Charnley JL, Levi EM, Jones E, Yeoh JW, Smith DW and Dayas CV (2011) Orexin-1 receptor signalling within the ventral tegmental area, but not the paraventricular thalamus, is critical to regulating cue-induced reinstatement of cocaine-seeking. Int J neuropsychopharmacology 14:684–690. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Balster RL, Bonese K. (1976) Self-administration of psychomotor stimulant drugs: the effects of unlimited access. Pharmacol Biochem Behav 4:45–51. [DOI] [PubMed] [Google Scholar]
- Jupp B, Krstew E, Dezsi G, Lawrence AJ. (2011) Discrete cue-conditioned alcohol-seeking after protracted abstinence: pattern of neural activation and involvement of orexin₁ receptors. Br J Pharmacol 162:880–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. (2005) The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 162:1403–1413. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE. (2005a) A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol 493:72–85. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. (2005b) Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav 86:773–795. [DOI] [PubMed] [Google Scholar]
- Kirouac GJ, Parsons MP, Li S. (2005) Orexin (hypocretin) innervation of the paraventricular nucleus of the thalamus. Brain Res 1059:179–188. [DOI] [PubMed] [Google Scholar]
- Li S, Kirouac GJ. (2008) Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J Comp Neurol 506:263–287. [DOI] [PubMed] [Google Scholar]
- Li Y, Li S, Sui N, Kirouac GJ. (2009) Orexin-A acts on the paraventricular nucleus of the midline thalamus to inhibit locomotor activity in rats. Pharmacol Biochem Behav 93:506–514. [DOI] [PubMed] [Google Scholar]
- Li Y, Li S, Wei C, Wang H, Sui N, Kirouac GJ. (2010a) Changes in emotional behavior produced by orexin microinjections in the paraventricular nucleus of the thalamus. Pharmacol Biochem Behav 95:121–128. [DOI] [PubMed] [Google Scholar]
- Li Y, Li S, Wei C, Wang H, Sui N, Kirouac GJ. (2010b) Orexins in the paraventricular nucleus of the thalamus mediate anxiety-like responses in rats. Psychopharmacology (Berl) 212:251–265. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang H, Qi K, Chen X, Li S, Sui N, Kirouac GJ. (2011) Orexins in the midline thalamus are involved in the expression of conditioned place aversion to morphine withdrawal. Physiol Behav 102:42–50. [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. (2001) Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 435:6–25. [DOI] [PubMed] [Google Scholar]
- Markou A and Koob GF (1991) Postcocaine anhedonia: an animal model of cocaine withdrawal. Neuropsychopharmacology 4:17–26. [PubMed] [Google Scholar]
- Martin-Fardon R, Weiss F. (2014) Blockade of hypocretin receptor-1 preferentially prevents cocaine seeking: comparison with natural reward seeking. Neuroreport 25:485–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Zorrilla EP, Ciccocioppo R, Weiss F. (2010) Role of innate and drug-induced dysregulation of brain stress and arousal systems in addiction: focus on corticotropin-releasing factor, nociceptin/orphanin FQ, and orexin/hypocretin. Brain Res 1314:145–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzeu A, Cauvi G, Kerr TM, Weiss F, Martin-Fardon R. (2015a) The paraventricular nucleus of the thalamus is differentially recruited by stimuli conditioned to the availability of cocaine versus palatable food. Addict Biol [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzeu A, Weiss F, Martin-Fardon R. (2015b) Transient inactivation of the posterior paraventricular nucleus of the thalamus blocks cocaine-seeking behavior. Neurosci Lett 608:34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. (2001) The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 21:8655–8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieda M, Yanagisawa M. (2002) Sleep, feeding, and neuropeptides: roles of orexins and orexin receptors. Curr Opin Neurobiol 12:339–345. [DOI] [PubMed] [Google Scholar]
- Otake K. (2005) Cholecystokinin and substance P immunoreactive projections to the paraventricular thalamic nucleus in the rat. Neurosci Res 51:383–394. [DOI] [PubMed] [Google Scholar]
- Parsons MP, Li S, Kirouac GJ. (2006) The paraventricular nucleus of the thalamus as an interface between the orexin and CART peptides and the shell of the nucleus accumbens. Synapse 59:480–490. [DOI] [PubMed] [Google Scholar]
- Paxinos G and Watson C (1997) The Rat Brain in Stereotaxic Coordinates, 3rd ed., Academic Press, San Diego, CA.
- Perry CJ, McNally GP. (2013) A role for the ventral pallidum in context-induced and primed reinstatement of alcohol seeking. Eur J Neurosci 38:2762–2773. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ryabinin AE, Crabbe JC. (2005) Patterns of brain activation associated with contextual conditioning to methamphetamine in mice. Behav Neurosci 119:759–771. [DOI] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, Bartlett SE. (2008) Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology (Berl) 199:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, et al. (1998) Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92:573–585. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Mieda M, Tsujino N. (2010) The orexin system: roles in sleep/wake regulation. Ann N Y Acad Sci 1200:149–161. [DOI] [PubMed] [Google Scholar]
- Sato-Suzuki I, Kita I, Seki Y, Oguri M, Arita H. (2002) Cortical arousal induced by microinjection of orexins into the paraventricular nucleus of the rat. Behav Brain Res 128:169–177. [DOI] [PubMed] [Google Scholar]
- Schmeichel BE, Barbier E, Misra KK, Contet C, Schlosburg JE, Grigoriadis D, Williams JP, Karlsson C, Pitcairn C, Heilig M, et al. (2015) Hypocretin receptor 2 antagonism dose-dependently reduces escalated heroin self-administration in rats. Neuropsychopharmacology 40:1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama Y, Chaki S. (2006) Neurochemistry of the nucleus accumbens and its relevance to depression and antidepressant action in rodents. Curr Neuropharmacol 4:277–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoblock JR, Welty N, Aluisio L, Fraser I, Motley ST, Morton K, Palmer J, Bonaventure P, Carruthers NI, Lovenberg TW, et al. (2011) Selective blockade of the orexin-2 receptor attenuates ethanol self-administration, place preference, and reinstatement. Psychopharmacology (Berl) 215:191–203. [DOI] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW. (2011) Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev 63:348–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JG, de Lecea L. (2000) The hypocretins: excitatory neuromodulatory peptides for multiple homeostatic systems, including sleep and feeding. J Neurosci Res 62:161–168. [DOI] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. (1998) Distribution of orexin receptor mRNA in the rat brain. FEBS Lett 438:71–75. [DOI] [PubMed] [Google Scholar]
- Uslaner JM, Winrow CJ, Gotter AL, Roecker AJ, Coleman PJ, Hutson PH, Le AD, Renger JJ. (2014) Selective orexin 2 receptor antagonism blocks cue-induced reinstatement, but not nicotine self-administration or nicotine-induced reinstatement. Behav Brain Res 269:61–65. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB. (2008) Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. J Comp Neurol 508:212–237. [DOI] [PubMed] [Google Scholar]
- Wedzony K, Koros E, Czyrak A, Chocyk A, Czepiel K, Fijal K, Mackowiak M, Rogowski A, Kostowski W, Bienkowski P. (2003) Different pattern of brain c-Fos expression following re-exposure to ethanol or sucrose self-administration environment. Naunyn Schmiedebergs Arch Pharmacol 368:331–341. [DOI] [PubMed] [Google Scholar]
- Zhou L, Smith RJ, Do PH, Aston-Jones G, See RE. (2012) Repeated orexin 1 receptor antagonism effects on cocaine seeking in rats. Neuropharmacology 63:1201–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]