Abstract
Whereas few studies have dealt with the central sympathoexcitatory action of the inflammatory prostanoid prostaglandin E2 (PGE2), there is no information on the expression and cardiovascular function of different PGE2 (EP) receptors in one of the major cardiovascular-regulating nuclei, the rostral ventrolateral medulla (RVLM). The current study aimed at filling this knowledge gap as well as elucidating the implicated molecular mechanisms. To achieve these goals, we showed the expression of EP2, EP3, and EP4 receptors in the RVLM and investigated their cardiovascular roles in conscious rats, ex vivo as well as in cultured PC12 cells. Intra-RVLM PGE2 significantly increased blood pressure and sympathetic dominance (spectral analysis). Studies with selective EP receptor subtype agonists and antagonists showed that these PGE2-evoked responses were only replicated by intra-RVLM activation of the EP3 receptor with its agonist sulprostone. The RVLM of PGE2-treated rats exhibited increases in c-Fos expression and extracellular signal–regulated kinase 1/2 and neuronal nitric oxide synthase phosphorylation along with oxidative stress, and PGE2 increased l-glutamate release in PC12 cells (surrogates of RVLM neurons). Abrogation of the PGE2-evoked pressor and biochemical responses only occurred following EP3 receptor blockade (N-[(5-Bromo-2-methoxyphenyl)sulfonyl]-3-[2-(2-naphthalenylmethyl)phenyl]-2-propenamide, L-798106). These findings suggest the dependence of RVLM PGE2-mediated sympathoexcitation/pressor response on local EP3 receptor signaling in conscious rats, and highlight central EP3 receptor blockade as a potential therapeutic modality for hypertension management.
Introduction
Although central prostaglandins have been extensively studied for their inflammatory effects, few studies have focused on their role in central cardiovascular regulation. i.c.v. prostaglandin E2 (PGE2) or its microinjection into the hypothalamic paraventricular nucleus increases sympathetic activity and blood pressure (BP) in rats, and these effects are not observed with other prostaglandins (Ando et al., 1995; Ariumi et al., 2002; Murakami et al., 2002; Zhang et al., 2011). PGE2 also causes l-glutamate release in different central nervous system cell types (Bezzi et al., 1998; Wang et al., 2015) and increases reactive oxygen species (ROS) generation by activating NADPH oxidase (NOX) (Wang et al., 2013). Further, central (i.c.v.) injection of PGE2 or misoprostol, the prostaglandin EP receptor agonist, increases the expression of c-Fos, a marker of neuronal activity (Bullitt, 1990; Morgan and Curran, 1991) in different brain areas (Lacroix et al., 1996; Zhang et al., 2011). However, there is currently no information on the expression and function of different EP receptors in one of the major cardiovascular-regulating nuclei, the rostral ventrolateral medulla (RVLM).
The peripheral hypotensive and central hypertensive effects of PGE2 might be accounted for by the relative contribution of the different prostanoid (EP1, EP2, EP3, and EP4) receptor subtypes to the BP response (Narumiya et al., 1999; Yang and Du, 2012). In the periphery, the EP4 receptor mediates vasodilation (Hristovska et al., 2007), whereas EP3, and perhaps EP1 and EP2, receptors mediate vasoconstriction (Kennedy et al., 1999; Guan et al., 2007; Chen et al., 2012). It is worth noting that the central EP3 receptor also mediates BP elevation (Ariumi et al., 2002; Zhang et al., 2011). However, the role of the RVLM EP3 receptor in BP regulation remains unknown, perhaps due to a lack of information on EP3 receptor expression in the RVLM.
Reported studies linked l-glutamate release in the RVLM to local oxidative stress and subsequent sympathoexcitation and pressor responses elicited by different signaling pathways (Albrecht et al., 2010; Nishihara et al., 2012). Additional molecular events implicated in these responses are the activation (phosphorylation) of extracellular signal–regulated kinase 1/2 (ERK1/2) and neuronal nitric oxide synthase (nNOS) as well as NOX activation (Busnardo et al., 2010; Koriauli et al., 2015; Korotkov et al., 2015). Importantly, these biochemical responses have been implicated in PGE2-mediated inflammation/oxidative stress in different cell types (Chuang et al., 2006; Romero et al., 2011; Wang et al., 2013). Whether PGE2 triggers these biochemical responses in the RVLM and the implicated EP receptor subtype(s) have not been investigated.
In the present study, we tested the hypothesis that EP3 receptor activation by intra-RVLM PGE2 induces l-glutamate release and oxidative stress, which ultimately lead to sympathoexcitation and pressor response. To test our hypothesis, it was important to determine the expression of the different EP receptors in the RVLM and in PC12 cells before conducting the pharmacological and molecular studies. In the in vivo studies, we investigated the cardiovascular and autonomic (spectral analysis) responses elicited by intra-RVLM microinjection of the physiologic nonselective agonist PGE2 or the individual selective agonist for the EP2, EP3, and EP4 receptor in conscious rats. We also investigated the effects of prior selective EP2, EP3, or EP4 receptor blockade on the cardiovascular effects caused by intra-RVLM PGE2 or sulprostone. Finally, we conducted ex vivo and in vitro (PC12 cells) studies to elucidate the mechanistic role of EP3 receptor in the PGE2-evoked glutamate release, RVLM neuronal/sympathetic activity (c-Fos expression), and oxidative stress (enhanced NOX and reduced catalase activities).
Materials and Methods
Animal Preparation.
Male Sprague-Dawley rats (300–360 g, 12–13 weeks old; Charles River Laboratories, Raleigh, NC), housed two per cage, were used in this study. The rats were kept in a controlled environment at a constant temperature of 23 ± 1°C, humidity of 50 ± 10%, and a 12-hour light/dark cycle with food (Prolab Rodent Chow, Prolab RMH 3000; Granville Milling, Creedmoor, NC) and water provided ad libitum. Surgical and experimental procedures were performed in accordance with and approved by the Institutional Animal Care and Use Committee and in accordance with the Guide for the Care and Use of Laboratory Animals (Institute for Laboratory Animal Research, 2011).
Surgical Procedure.
Femoral artery catheterization and stereotaxic implantation of an RVLM guide cannula were performed under anesthesia (9 mg/100 g ketamine and 1 mg/100 g xylazine, i.p.) and appropriate analgesia (0.03 mg/kg buprenorphine) as detailed in our recent study (Rezq and Abdel-Rahman, 2016). Histologic verification of the RVLM position was done via intra-RVLM injection of fast green dye (EM Sciences, Cherry Hill, NJ).
Blood Pressure and Heart Rate Measurements.
Five days after the surgical procedures, an ML870 (PowerLab 8/30, AD Instruments, Colorado Springs, CO) system was used to measure BP and heart rate (HR) in conscious unrestrained rats. The data were analyzed and displayed using LabChart (version 7) prosoftware (AD Instruments, Colorado Springs, CO) as in our reported studies (Ibrahim and Abdel-Rahman, 2011; Nassar et al., 2011; Penumarti and Abdel-Rahman, 2014).
Spectral Analysis.
Spontaneous baroreflex sensitivity (BRS) was measured by the frequency domain analysis method as reported in studies, including ours (Parati et al., 1995; Shaltout and Abdel-Rahman, 2005), using the Nevrokard SA-BRS software package (Medistar, Ljubljana, Slovenia) for small animals. The power of inter-beat (RR) interval (RRI) and spectral density oscillations computed for two specific frequency bands, low-frequency (LF, 0.25–0.75 Hz) and high-frequency (HF, 0.75–3 Hz) domains, which reflect changes in sympathetic and vagal activity, respectively (Malliani et al., 1991), were used as indices for spontaneous BRS, whereas the sympathovagal balance index (LFRRI/HFRRI) was used as an index of heart rate variability.
Cell Culture.
A rat pheochromocytoma cell line (PC12 cells) purchased from American Type Culture Collection (Manassas, VA) was used according to the protocol detailed in our recent study (Rezq and Abdel-Rahman, 2016).
Western Blot.
Protocols from our recent study (Rezq and Abdel-Rahman, 2016) were followed. For EP receptor expression, equivalent amounts of proteins (20 μg/lane) were applied to 12% SDS-PAGE gel (Invitrogen, Carlsbad, CA), and then transfer was done using nitrocellulose membranes which were then incubated with anti-EP1–4 receptor polyclonal antibodies (1:500; Cayman, Ann Arbor, MI) at 4°C overnight. The band for EP3 receptor was verified using EP3 receptor antibody blocking peptide (Cayman). For ERK1/2 and nNOS measurements, nitrocellulose membranes were incubated overnight at 4°C with a mixture of rabbit anti–phospho-nNOS (Ser1417) antibody (1:500; Thermo Fisher Scientific, Waltham, MA) or rabbit anti-ERK1/2 antibody (1:500; Cell Signaling, Danvers, MA) and mouse polyclonal anti-nNOS antibody (1:500; BD Biosciences, San Jose, CA) or mouse anti–phosphorylated ERK1/2 antibody (1:500; Cell Signaling). Nitrocellulose membranes were washed four times with phosphate-buffered saline (PBS) containing 0.1% Tween 20, then incubated for 60 minutes with mixture containing IRDye680-conjugated goat anti-mouse and IRDye800-conjugated goat anti-rabbit (1:5000; LI-COR Biosciences, Lincoln, NE). Bands representing phosphorylated and total protein were detected simultaneously by using an Odyssey Infrared Imager and analyzed with Odyssey application software version .3 (LI-COR Biosciences). The data represent mean values of the integrated density ratio of phosphorylated nNOS or phosphorylated ERK1/2 normalized to the corresponding total nNOS or total ERK1/2, respectively, and expressed as a percentage of control.
Immunofluorescence.
The protocol used in our recent report (Rezq and Abdel-Rahman, 2016) was used for c-Fos-ir neuron studies in coronal sections containing the RVLM rostrally from −12.8 to −11.8 mm relative to the bregma (Paxinos et al., 1980). Frozen sections from brains of treated and control rats (n = 5–7) were incubated for 48 hours at 4°C in a rabbit polyclonal anti–c-Fos antibody (1:200; Santa Cruz Biotechnology, Santa Cruz, CA). The sections then were incubated for 2 hours in fluorescein isothiocyanate–conjugated donkey anti-rabbit (1:200; Jackson Immunoresearch Laboratories Inc., West Grove, PA). A Zeiss LSM 510 confocal microscope (Carl Zeiss Inc., Thornwood, NY) was used for the visualization, acquisition, and quantification of fluorescence intensity. Four to six sections per animal at the level of RVLM were examined. Fluorescence intensity was quantified using Zen Lite 2011 software (Carl Zeiss Microscopy, LLC, Thornwood, NY).
NOX Activity.
NOX activity was measured according to reported protocols (La Favor et al., 2013), with modification. For this assay, 5 μl of homogenate was incubated with 180 μl of a cocktail containing 10 μM Amplex Red (Molecular Probes, Eugene, OR), 2.0 U/ml horseradish peroxidase, 30 U/ml superoxide dismutase, and 100 μM NADPH (Sigma-Aldrich, St. Louis, MO) in PBS for 30 minutes at 37°C. Fluorescence intensity (530 nM excitation/590 nM emission) was then measured with a microplate fluorescence reader. Total NADPH-dependent H2O2 generated in the samples was used as an index of NOX activity. Activity was normalized to total protein content, as determined by Bradford assay (Bio-Rad, Hercules, CA).
Catalase Activity.
RVLM punches from injected sites were homogenized in 35 μl of lysis buffer [20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerol-phosphate, 1 mM activated sodium orthovanadate, and 1 μg/ml leupeptin] with protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN) followed by centrifugation at 14,000 rpm for 20 minutes at 4°C. The supernatant was separated and assayed for protein content (Bradford assay; Bio-Rad). Catalase activity was determined colorimetrically in 10 μg of protein using the Catalase Assay Kit (catalog no. CAT-100; Sigma-Aldrich) according to the manufacturer's instructions.
2′,7′-Dichlorofluorescein Diacetate.
A 20 mM stock solution of 2′,7′-dichlorofluorescein diacetate (DCFH-DA) (Molecular Probes, Grand Island, NY) in methanol was prepared and kept at −20°C in the dark. RVLM homogenate in PBS (50 mM, pH 7.4) from different groups was centrifuged at 14,000 rpm for 20 minutes at 4°C. The protein in the supernatant was quantified using a Bio-Rad protein assay system. Shortly before the experiment, DCFH-DA stock solution was freshly diluted with PBS to 150 µM working solution. The reaction was started by adding 10 µl RVLM homogenate supernatant in a 96-well plate for a final concentration of 25 µM DCFH-DA to generate fluorescent 2′,7′-dichlorofluorescein (DCF) followed by measuring fluorescence intensity using a microplate fluorescence reader at excitation 485 nm/emission 530 nm after 30-minute incubation at 37°C. DCF was used to generate the standard curve. ROS level was expressed in terms of relative fluorescence units of produced DCF, as detailed in our recent study (Rezq and Abdel-Rahman, 2016).
l-Glutamate Measurement.
l-Glutamate release in cultured PC12 cells was measured using the Amplex Red kit (Molecular Probes, Invitrogen) following the manufacturer’s instructions and as detailed in our recent study (Rezq and Abdel-Rahman, 2016).
Protocols and Experimental Groups.
In addition to determining the expression of the EP receptor subtypes in the RVLM and in its surrogate phenotype cell line (PC12 cells), we adopted two pharmacological approaches to elucidate the cardiovascular function of the EP receptor subtypes in the RVLM of conscious rats. In the first in vivo experiment, we investigated the cardiovascular and autonomic effects of intra-RVLM PGE2 (0.1 or 0.2 nmol) or the cardiovascular effects of a selective prostanoid EP2 (butaprost), EP3 (sulprostone), or EP4 (5-[(3S)-3-hydroxy-4-phenyl-1-buten-1-yl]1-[6-(2H-tetrazol-5R-yl)hexyl]-2-pyrrolidinone; CAY10598) receptor agonist (0.2 nmol). In the second experiment, we investigated the effects of prior selective EP2 ((2E)-N-[2-(2-Methyl-1H-indol-1-yl)ethyl]-3-(3,4,5-trimethoxyphenyl)-2-propenamide; TG4-155; 2 nmol), EP3 (N-[(5-Bromo-2-methoxyphenyl)sulfonyl]-3-[2-(2-naphthalenylmethyl)phenyl]-2-propenamide; L-798106; 0.1 nmol), or EP4 (N-[[4'-[[3-Butyl-1,5-dihydro-5-oxo-1-[2-(trifluoromethyl)phenyl]-4H-1,2,4-triazol-4-yl]methyl][1,1'-biphenyl]-2-yl]sulfonyl]-3-methyl-2-thiophenecarboxamide; L-161982; 2 nmol) receptor blockade on the cardiovascular and autonomic effects caused by intra-RVLM PGE2. Further, we investigated the effect of prior EP1 (8-Chloro-dibenzo(Z)[b,f][1,4]oxazepine-10(11H)-carboxylic acid, 2-acetylhydrazide; SC-19220; 2 nmol), EP2 (TG4-155; 2 nmol), or EP4 (L-161982; 2 nmol) receptor blockade on the cardiovascular responses elicited by intra-RVLM sulprostone, which also activates the EP1 receptor (Hide and Thiemermann, 1996), to determine their potential contribution to the sulprostone-evoked BP effects. It was important to determine, for the first time, the dose of L-798106 that optimally blocks the RVLM EP3 receptor in a pilot study (n = 3). The selected intra-RVLM PGE2 dose (0.2 nmol) was based on its ability to produce a pressor response when microinjected into the hypothalamus (Zhang et al., 2011). All other doses were based on a reported study (Zhang et al., 2011). Conscious unrestrained Sprague-Dawley rats (n = 5–7 each), prepared for intra-RVLM injections and BP measurements, as described in Materials and Methods, were used. BP and HR were allowed to stabilize at baseline, for at least 30 minutes, before intra-RVLM microinjections started. All injections (100 nl) were made unilaterally into the RVLM according to established protocol in our laboratory (Penumarti and Abdel-Rahman, 2014). Control rats received 100 nl of 1% dimethylsulfoxide in the artificial cerebrospinal fluid that was used as a vehicle. At the end of the BP recording period, animals were euthanized with a lethal dose of pentobarbital sodium (100 mg/kg), and their brains were rapidly removed and stored at −80°C. For the different biochemical measurements detailed earlier, a 0.75 micropunch instrument was used to collect unilateral micropunches (Stoelting Co., Wood Dale, IL) at −12.8 to −11.8 mm relative to the bregma (Paxinos et al., 1980) from the injected RVLM site (n = 5–7). For in vitro EP receptor expression (western blot), PGE2, and glutamate measurements, PC12 cells were used as surrogates of RVLM neurons. For l-glutamate assay, different concentrations of PGE2 (1.25–10 μM) were applied to PC12 cells. Finally, the same protocol was repeated in PC12 cells incubated with PGE2 (10 μM) in the absence or presence (added 30 minutes earlier) of individual selective EP receptor subtype blocker (10 μM); the selected concentration was based on reported studies (Bal-Price et al., 2002).
Drugs.
L-798106 was purchased from Sigma-Aldrich. All other drugs were purchased from Cayman (Ann Arbor, MI). Sterile saline was purchased from B. Braun Medical (Irvine, CA).
Statistical Analysis.
In vitro data were collected from three independent experiments, and in vivo data were collected from five to seven rats per group. Data are expressed as the mean ± S.E.M. Analysis of variance or repeated-measures analysis of variance followed by Bonferroni’s post hoc test and Student's t test were carried out using GraphPad Prism (GraphPad Software, La Jolla, CA) to state differences between groups.
Results
EP Receptor Expression in the RVLM and PC12 Cells.
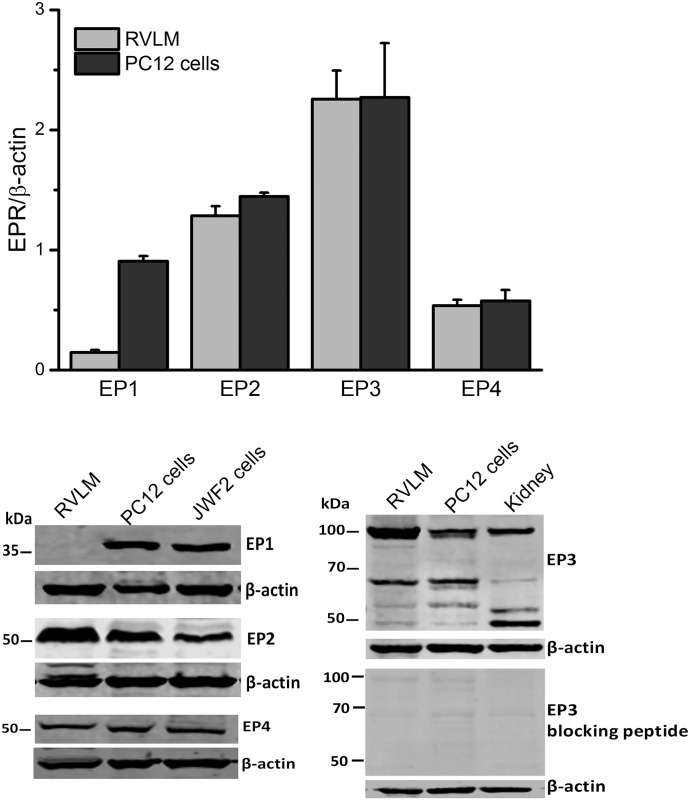
EP1, EP2, EP3, and EP4 receptors were expressed in the RVLM and in PC12 cells, but EP1 receptor expression in the RVLM was very scant (Fig. 1). Blots from the kidney or JWF2 cells, which express high levels of EP receptors, were used as positive controls (Kotani et al., 1995; Kiraly et al., 2016). Notably, the molecular weight of the EP3 receptor varies from 53 to 98 kDa (Fig. 1) according to the degree of its post-translational modification, and the average of all bands was used to determine its expression level, as reported (Osborne et al., 2009).
Fig. 1.
Expression of EP1 (38 kDa), EP2 (52 kDa), EP3 (53–98 kDa), or EP4 (53 kDa) receptor in the rat RVLM and PC12 cells. JWF2 cells or kidney homogenates were used as positive controls; the wide range in molecular weight in EP3 receptor expression is ascribed to the different forms of the receptor (Osborne et al., 2009) and is verified using blots of rat RVLM, PC12 cells, or kidney when EP3 receptor primary antibody was preincubated with its blocking peptide. Quantification of blots from RVLM or PC12 cells is presented in the upper panel. Data are presented as integrated density ratio of the single EP receptor (EPR) to the corresponding β-actin values (n = 4) and are expressed as the mean ± S.E.M.
EP3 Receptor Mediates the Increases in BP and Central Sympathetic Tone Elicited by Intra-RVLM PGE2.
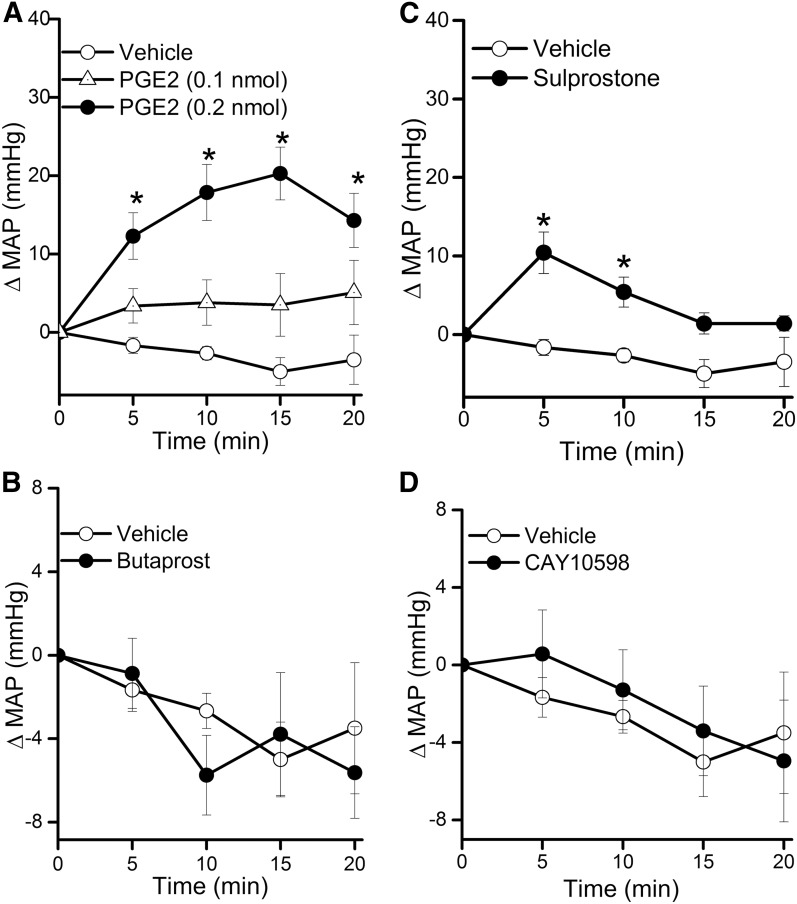
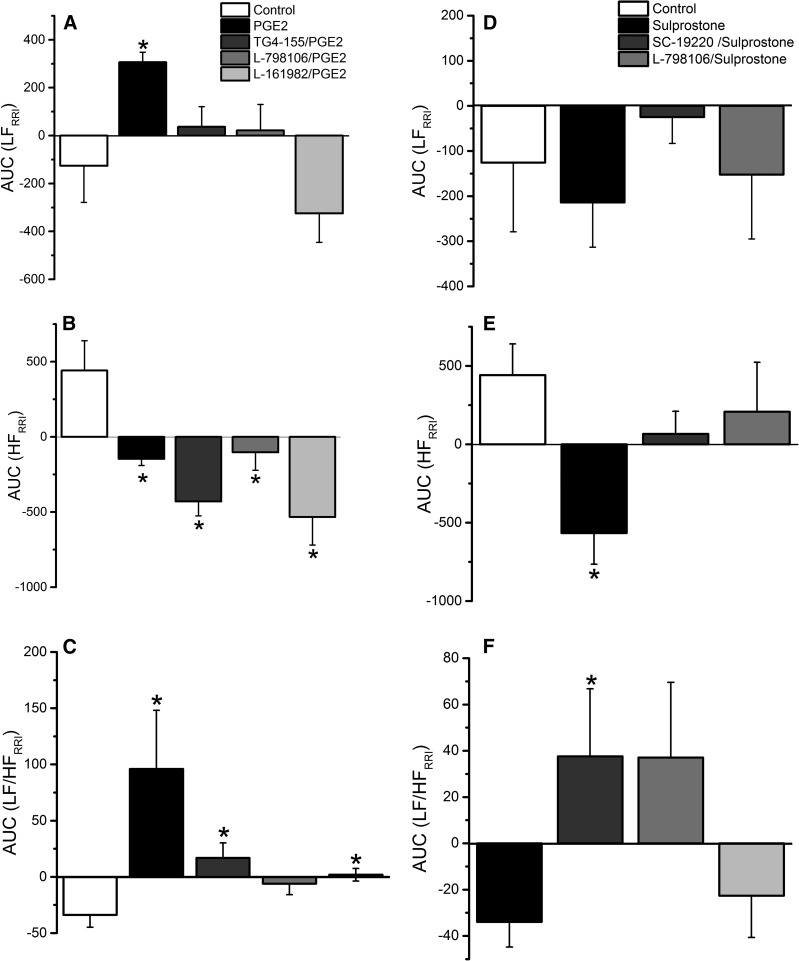
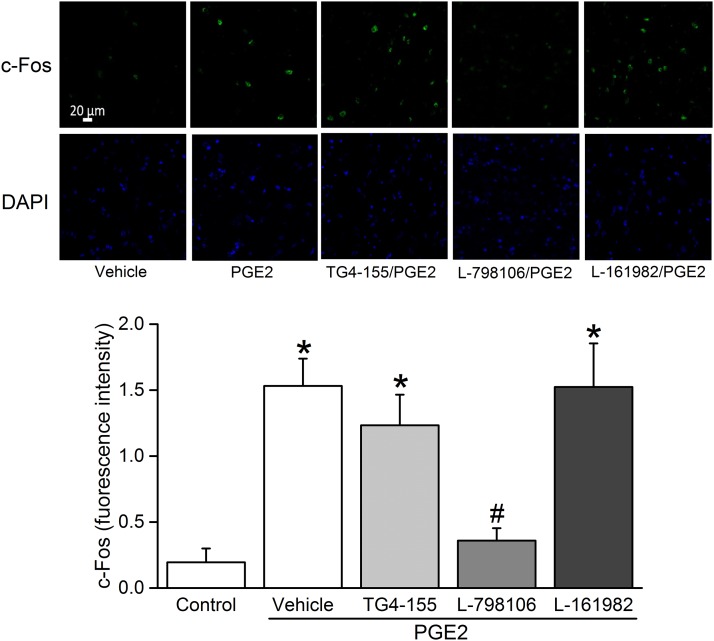
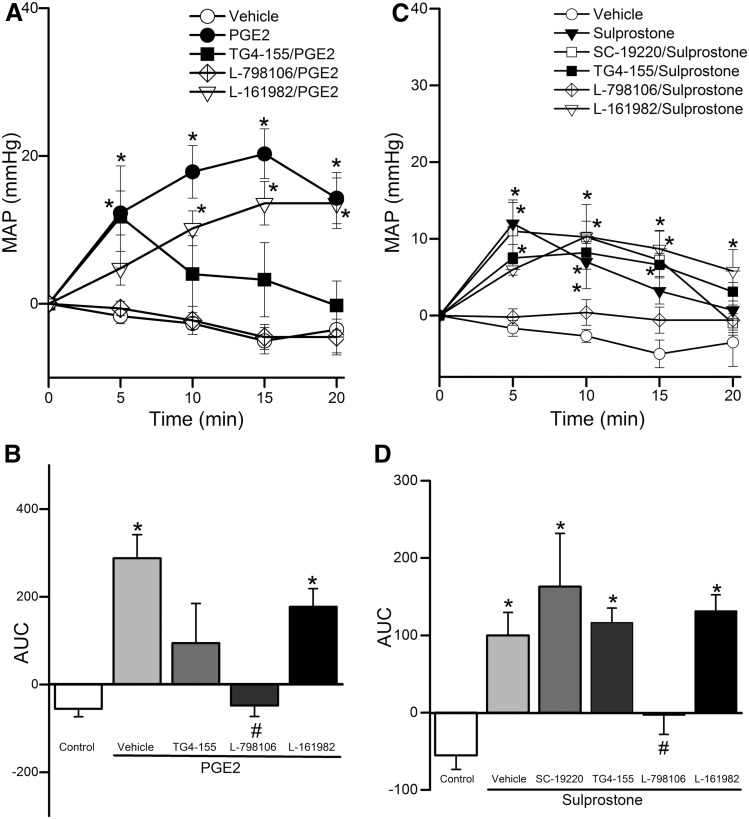
Due to sparse RVLM expression of the EP1 receptor (Fig. 1) and the lack of EP1 receptor expression on both the mRNA and protein levels in the whole brainstem (Candelario-Jalil et al., 2005), we focused on the roles of RVLM EP2, EP3, and EP4 receptors in the cardiovascular and autonomic effects caused by intra-RVLM administration of the endogenous ligand PGE2. There were no significant differences between the BP and HR values of all groups prior to drug or vehicle administration (Table 1). Intra-RVLM PGE2 (0.2 nmol) caused significant (P < 0.05; n = 7) increases in BP (Fig. 2), LF/HF ratio (index of increased sympathetic dominance) (Fig. 3), and c-Fos expression in RVLM neurons (index of sympathetic activation) (Fig. 4). In the latter experiment, the total numbers of RVLM neurons in the control and treatment group sections were similar, as illustrated with 4',6-diamidino-2-phenylindole (DAPI) (Fig. 4, lower panels). These responses were only replicated by RVLM EP3 receptor activation with sulprostone (Figs. 2–4). Further, the pressor effect and sympathetic dominance caused by intra-RVLM PGE2 or the selective EP1/EP3 agonist sulprostone (0.2 nmol) were abrogated by prior EP3 receptor blockade (0.1 nmol/100 nl L-798106; n = 5) (Figs. 3 and 5). EP1 (SC-19220, Figs. 3 and 5), EP2 (TG4-155), or EP4 (L-161982) receptor blockade (Fig. 5) had no effect on sulprostone-evoked responses. Finally, the EP2 receptor blocker TG4-155 (2 nmol) partially attenuated PGE2-evoked pressor response (Fig. 5), possibly due to its poor selectivity, because the selective EP2 agonist (butaprost) had no effect on BP or autonomic function (Figs. 2 and 3). BP values for the individual EP receptor blockers are shown in Table 2.
TABLE 1.
Mean arterial pressure (MAP, mm Hg) and HR (beats/min) values immediately before intra-RVLM treatment with the indicated intervention or vehicle
Values are the mean ± S.E.M.
| Pretreatment/Treatment |
Rats per Group |
MAP |
HR |
|---|---|---|---|
| n | mm Hg | beats/min | |
| Vehicle | 6 | 128.0 ± 6.0 | 332.0 ± 8.5 |
| PGE2 (0.1 nmol) | 6 | 118.9 ± 6.6 | 361.9 ± 19.3 |
| PGE2 (0.2 nmol) | 7 | 118.4 ± 5.3 | 367.7 ± 23.3 |
| TG4-155/PGE2 | 5 | 118.0 ± 2.6 | 336.0 ± 25.5 |
| L-798106/PGE2 | 7 | 121.6 ± 7.6 | 336.1 ± 9.9 |
| L-161982/PGE2 | 5 | 117.6 ± 4.7 | 312.5 ± 16.9 |
| Sulprostone | 5 | 130.0 ± 5.1 | 335.0 ± 16.0 |
| SC-19220/sulprostone | 5 | 115.0 ± 1.6 | 291.4 ± 11.4 |
| TG4-155/sulprostone | 6 | 120.3 ± 6.6 | 339.1 ± 11.5 |
| L-798106/sulprostone | 5 | 138.6 ± 6.1 | 300.0 ± 14.6 |
| L-161982/sulprostone | 6 | 107.6 ± 5.0 | 312.2 ± 15.3 |
| Butaprost | 6 | 122.6 ± 3.2 | 308.2 ± 18.0 |
| CAY10598 | 5 | 124.8 ± 7.1 | 338.0 ± 14.9 |
| SC-19220 | 5 | 118.6 ± 3.8 | 310.0 ± 5.1 |
| TG4-155 | 5 | 125.8 ± 3.5 | 382.6 ± 4.9 |
| L-798106 | 5 | 135.1 ± 7.0 | 347.6 ± 13.4 |
| L-161982 | 5 | 117.5 ± 5.8 | 320.8 ± 21.1 |
Fig. 2.
Time-course changes in mean arterial pressure (MAP) caused by intra-RVLM PGE2 (0.1 or 0.2 nmol; n = 6–7) (A), the EP2 receptor agonist butaprost (0.2 nmol; n = 5) (B), the EP1/EP3 receptor agonist sulprostone (0.2 nmol; n = 5) (C), or the EP4 receptor agonist CAY10598 (0.2 nmol; n = 5) (D) compared with an equal volume of vehicle (100 nl; n = 6) in conscious male rats. Values are the mean change from baseline ± S.E.M. *P < 0.05 versus vehicle values.
Fig. 3.
Effect of intra-RVLM PGE2, sulprostone (0.2 nmol; n = 7) on LF component of spectral analysis of RRI (0.25–0.75 Hz), index of cardiac sympathetic tone (A and D); HF component of the spectral analysis of RRI (0.75–3 Hz), index of cardiac vagal tone (B and E); and LFRRI/HFRRI ratio as a measure of sympathovagal balance (C and F) in conscious male rats pretreated 10 minutes earlier with vehicle (100 nl; n = 6), EP1 (2 nmol SC-19220; n = 5), EP2 (2 nmol TG4-155; n = 5), EP3 (0.1 nmol L-798106; n = 7), or EP4 (2 nmol L-161982; n = 5) receptor blocker. Data represent area under the curve (AUC) values generated over the 30-minute BP recording period. Values are the mean ± S.E.M. *P < 0.05 versus vehicle values.
Fig. 4.
(Upper panel) Confocal images showing c-Fos immunoreactive cell nuclei (green) or the nuclear marker 4',6-diamidino-2-phenylindole (DAPI) (blue) in RVLM of rats treated (as described in Materials and Methods) with (from left to right) intra-RVLM vehicle, 0.2 nmol PGE2 pretreated 10 minutes earlier with vehicle (100 nl; n = 6), EP2 (2 nmol TG4-155; n = 5), EP3 (0.1 nmol L-798106; n = 7), or EP4 (2 nmol L-161982; n = 5) receptor blocker. Scale bar, 20 μm. Bar graphs represent the mean ± S.E.M. of data obtained from four to six coronal brain stem sections/animal (n = 5–7 rats/group) using one-way analysis of variance followed by Bonferroni comparison test. *P < 0.05 versus vehicle values; #P < 0.05 versus PGE2.
Fig. 5.
(A) Time-course changes in mean arterial pressure (MAP) caused by intra-RVLM PGE2 (0.2 nmol; n = 7) compared with an equal volume of vehicle in conscious male rats pretreated 10 minutes earlier with vehicle (100 nl; n = 6), EP2 (2 nmol TG4-155; n = 5), EP3 (0.1 nmol L-798106; n = 7), or EP4 (2 nmol L-161982; n = 5) receptor blocker. (C) Time-course changes in MAP caused by intra-RVLM sulprostone (0.2 nmol; n = 5) compared with an equal volume of vehicle in conscious male rats pretreated 10 minutes earlier with vehicle (100 nl; n = 6), EP1 (2 nmol SC-19220; n = 5), EP2 (2 nmol TG4-155; n = 6), EP3 (0.1 nmol L-798106; n = 7), or EP4 (2 nmol L-161982; n = 6) receptor blocker. (B and D) The area under the curve (AUC) data generated from (A) and (C), respectively, for different treatments. MAP data from the vehicle-, PGE2-, and sulprostone-treated groups are reproduced from Fig. 2. Values are the mean change from baseline ± S.E.M. *P < 0.05 versus control values; #P < 0.05 versus PGE2 or sulprostone values in vehicle-pretreated rats.
TABLE 2.
Mean arterial pressure (ΔMAP, mm Hg) changes produced by intra-RVLM injection of different treatments, compared with corresponding vehicle values
Values are mean ± S.E.M.
| Treatment | Time (min) |
|||
|---|---|---|---|---|
| 5 | 10 | 15 | 20 | |
| SC-19220 | 2.8 ± 1.7 | −0.3 ± 1.7 | −1.0 ± 1.8 | −2.0 ± 3.4 |
| TG4-155 | −1.0 ± 3.1 | 2.8 ± 4.7 | 1.3 ± 3.6 | −1.3 ± 2.6 |
| L-798106 | 1.3 ± 1.3 | −0.8 ± 1.3 | −1.0 ± 1.2 | −1.8 ± 1.9 |
| L-161982 | 7.0 ± 2.8 | 5.6 ± 4.1 | 4.6 ± 5.3 | 2.2 ± 5.0 |
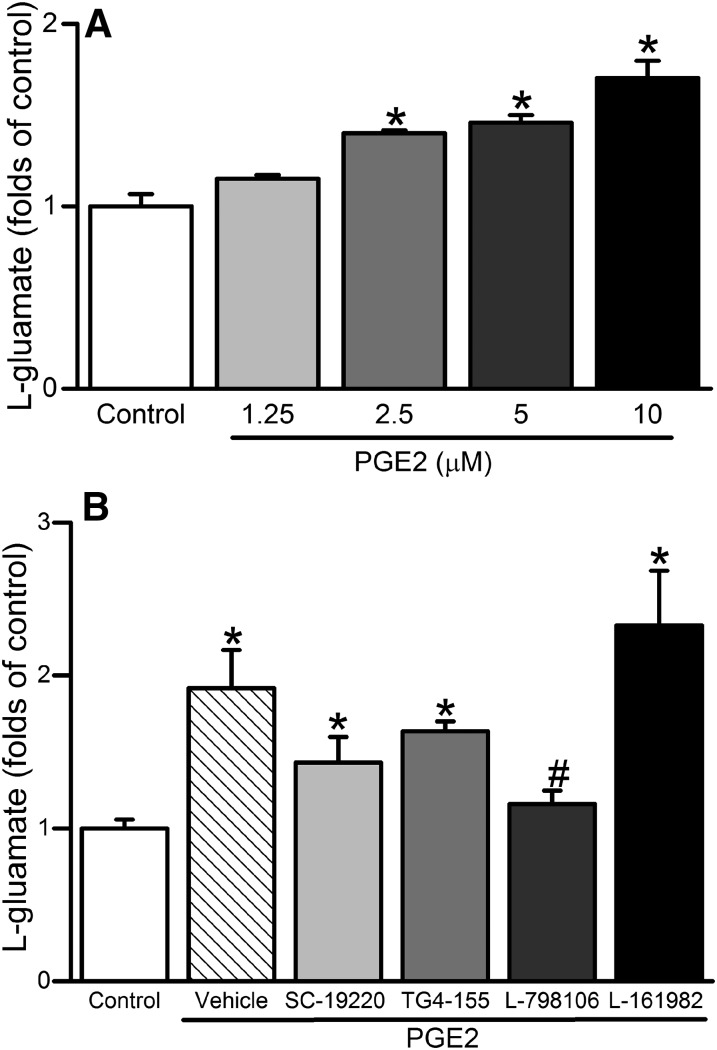
EP3 Receptor Mediates the PGE2-Evoked Increase in Glutamate Levels.
PGE2 caused concentration-dependent increases in l-glutamate levels in cultured PC12 cells (Fig. 6A), and such response was virtually abolished by prior incubation with the selective EP3 receptor blocker L-798106 (10 µM), but not with EP1, EP2, or EP4 receptor blocker (Fig. 6B).
Fig. 6.
(A) Effect of 5-minute exposure of PC12 cells to PGE2 (1.25–10 μM) on l-glutamate levels (folds increase of control). (B) Effect of 30-minute prior incubation with EP1 (SC-19220), EP2 (TG4-155), EP3 (L-798106), or EP4 (L-161982) receptor blocker (10 μM) on PGE2-mediated l-glutamate release in cultured PC12 cells. Data are expressed as the mean ± S.E.M. of three independent experiments. *P < 0.05 versus control values; #P < 0.05 versus PGE2 values.
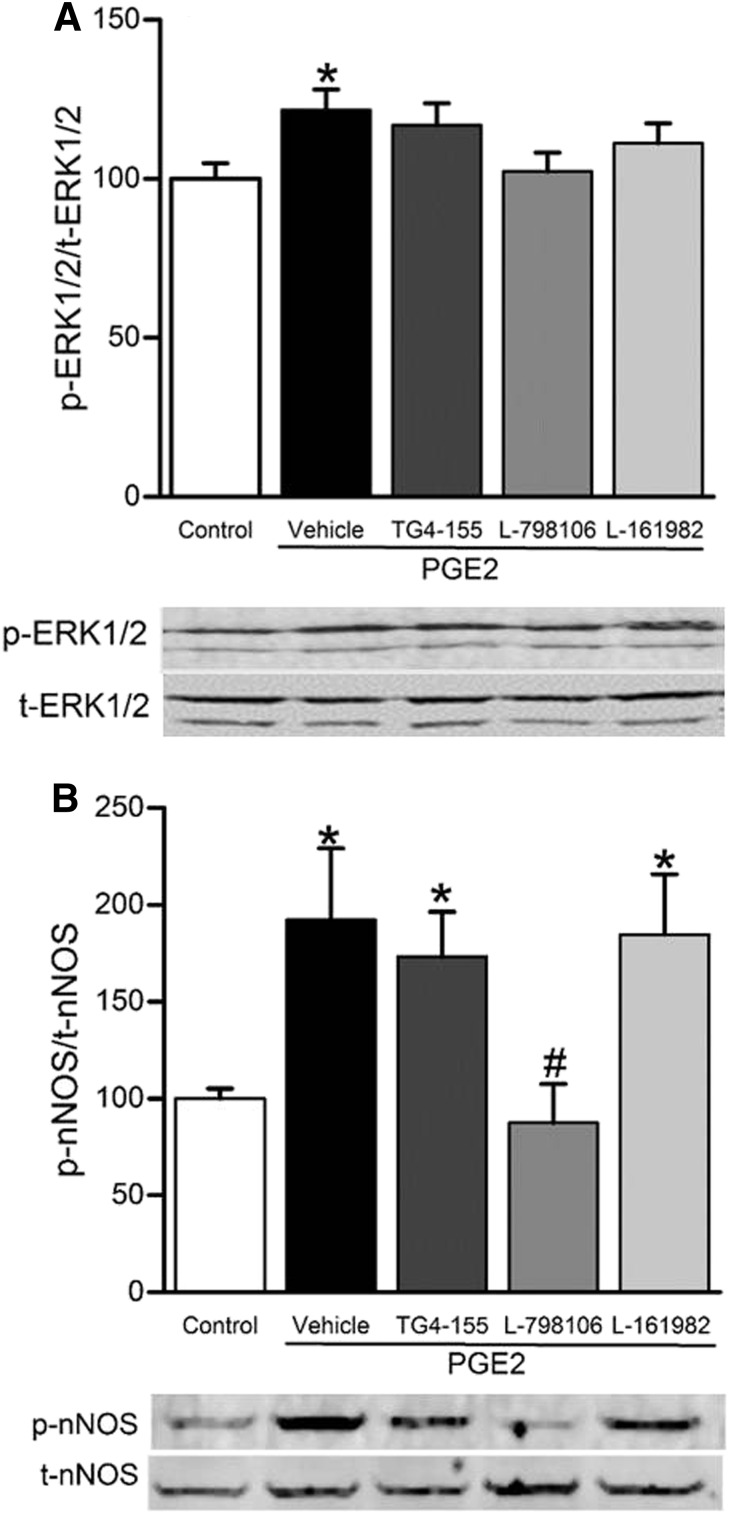
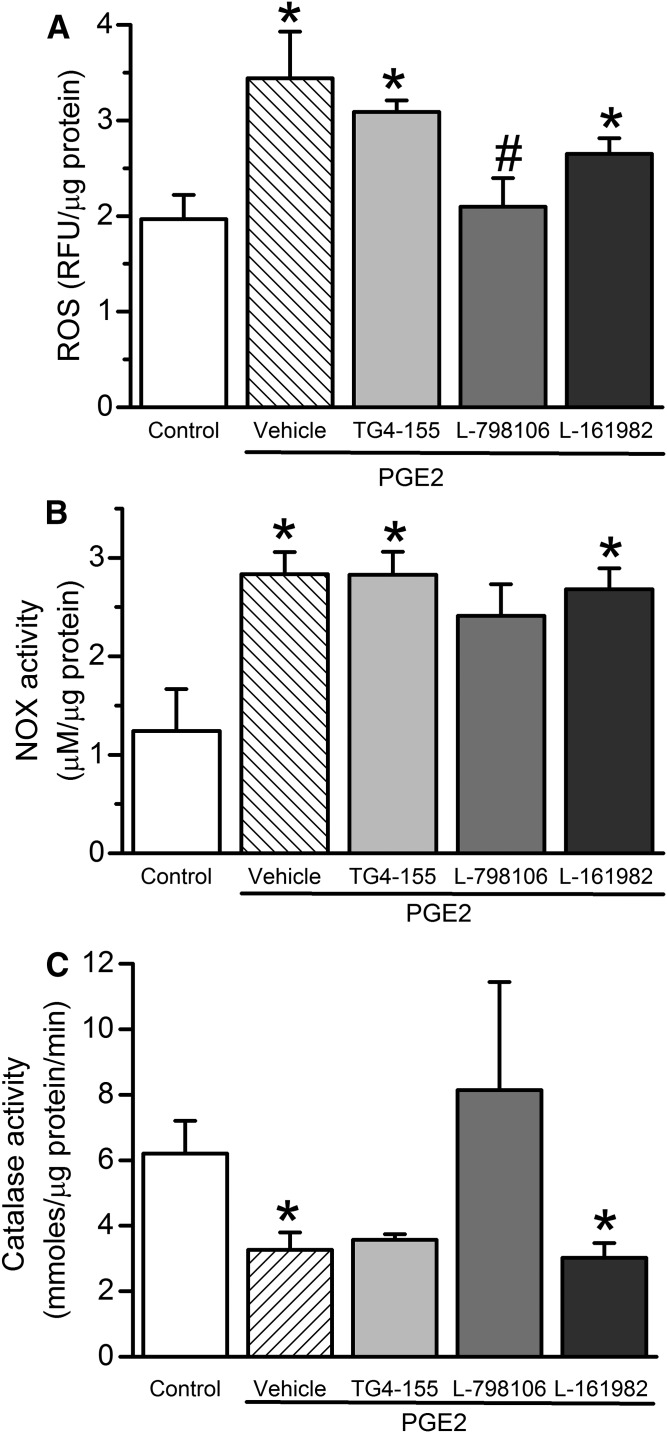
EP3 Receptor Blockade Abrogated the Increases in RVLM ERK1/2-nNOS Phosphorylation and NOX Activity and the Decrease in Catalase Activity Caused by Intra-RVLM PGE2.
PGE2 (0.2 nmol; n = 5), compared with its vehicle (n = 5), significantly (P < 0.05) increased ERK1/2 (Fig. 7A) and nNOS (Fig. 7B) phosphorylation. The RVLM of PGE2-treated rats also exhibited significant (P < 0.05) increases in ROS level (Fig. 8A) and NOX activity (Fig. 8B), along with significant (P < 0.05) reduction in catalase activity (Fig. 8C). These neurochemical responses were significantly attenuated by prior EP3 receptor blockade with L-798106 (0.1 nmol/100 nl) in PGE2-treated rats (Figs. 7 and 8), which paralleled the abrogation of the pressor and sympathoexcitatory responses in the same rats (Figs. 3–5).
Fig. 7.
Changes in rat RVLM ERK1/2 (A) and nNOS (B) phosphorylation following intra-RVLM PGE2 (0.2 nmol; n = 5) with or without prior (10-minute) treatment with vehicle (100 nl; n = 5), EP2 (2 nmol TG4-155; n = 5), EP3 (0.1 nmol L-798106; n = 5), or EP4 (2 nmol L-161982; n = 5) receptor blocker. Data are presented as the integrated density ratio of phosphorylated (p-ERK1/2 or p-nNOS) to the corresponding total (t-ERK1/2 or t-nNOS) protein, respectively, and are expressed as a percentage of control. Values are the mean ± S.E.M. of five per group. *P < 0.05 versus vehicle values; #P < 0.05 versus PGE2.
Fig. 8.
(A) Effect of intra-RVLM PGE2 (0.2 nmol; n = 5) with or without prior (10-minute) treatment with vehicle (100 nl; n = 5), EP2 (2 nmol TG4-155; n = 5), EP3 (0.1 nmol L-798106; n = 5), or EP4 (2 nmol L-161982; n = 5) receptor blocker on DCFH-DA measured ROS levels in terms of relative fluorescence units (RFU) of produced DCF in the RVLM. NOX activity presented as the concentration of produced H2O2 (μM/μg protein) (B) or catalase activity (μmoles/μg protein/min) (C). Values are the mean ± S.E.M. *P < 0.05 versus vehicle values; #P < 0.05 versus PGE2.
Discussion
The present study contributes new knowledge on the role of RVLM PGE2 and its receptors in BP regulation. Our most important in vivo (in conscious rats) and ex vivo/in vitro findings are as follows: 1) EP2, EP3, and EP4 receptors are expressed in the RVLM; 2) intra-RVLM PGE2 causes increases in BP, indices of sympathetic activity, and RVLM oxidative stress (enhanced NOX and reduced catalase activities); 3) the PGE2-evoked responses are replicated only following RVLM EP3 receptor activation (sulprostone), and are abrogated following local EP3 receptor blockade (L-798106); and 4) EP3 receptor mediates the PGE2-evoked elevations in l-glutamate levels (PC12 cells), c-Fos expression, and ERK1/2 and nNOS phosphorylation in the RVLM. We present the first evidence for EP3 receptor–dependent sympathoexcitatory and pressor responses elicited by PGE2 in the RVLM, at least partly, via tipping the local redox balance toward oxidative stress.
Although there is growing interest in the central effects of prostaglandins, particularly the PGE2-evoked sympathoexcitation via EP3 receptor activation within the hypothalamus (Ariumi et al., 2002; Zhang et al., 2011; Ando et al., 2015), the molecular mechanisms involved in this action are not clear. Equally important, there are no studies on a potential BP role for PGE2-EP3 receptor signaling in the RVLM, a major brain stem cardiovascular regulatory area. We reasoned that, if PGE2-evoked increases in NOX activity (Wang et al., 2013), c-Fos expression (Lacroix et al., 1996; Zhang et al., 2011), and ERK1/2 pathway activation (Chuang et al., 2006) occur in the RVLM, they may uncover a new role for RVLM PGE2, because triggering these molecular mechanisms in the RVLM causes sympathoexcitation and BP increase (Chalmers et al., 1994; Minson et al., 1994; Ibrahim and Abdel-Rahman, 2012; Chen et al., 2013). Therefore, we used a multilevel experimental approach to investigate these novel and interrelated possibilities.
In the absence of any reports on the roles of PGE2 or its different EP receptor subtypes in the RVLM, it was important to first determine the expression of EP receptors in the RVLM. We showed, and verified, the expression of EP2, EP3, and EP4 receptors in the RVLM as well as in PC12 cells, which are used as a surrogate cell line for biochemical studies that are not feasible in the RVLM (Fig. 1). Thereafter, our study was also the first to show that intra-RVLM PGE2 (0.2 nmol) elicited a pressor response (Fig. 2), which agrees with a similar effect when PGE2 is microinjected into the hypothalamic pressor area (Zhang et al., 2011). Two findings confirmed that the intra-RVLM sympathoexcitatory effect of PGE2 is EP3 receptor–dependent. First, only sulprostone, the EP1/EP3 receptor agonist, replicated the sympathoexcitatory effect of PGE2 (Fig. 2). It is highly unlikely, however, that the EP1 receptor contributed to the pressor effect of PGE2 or the dual agonist sulprostone because the EP1 receptor is not expressed in the RVLM (Fig. 1), which agrees with a lack of EP1 receptor expression in the brain stem (Candelario-Jalil et al., 2005). Second, only EP3 receptor blockade with its selective antagonist, L-798106, abrogated the pressor response caused by intra-RVLM PGE2 or sulprostone (Fig. 5). Equally important, failure of the selective EP1 (SC-19220), EP2 (TG4-155), or EP4 (L-161982) receptor blockade to influence sulprostone-evoked pressor response (Fig. 5) reaffirms EP3 receptor mediation of the latter response. A partial attenuation of PGE2-evoked pressor response by the EP2 receptor blocker TG4-155 (Fig. 5) might be attributed to its poor selectivity (Jiang and Dingledine, 2013). Despite this limitation, the PGE2-evoked pressor responses in the absence or presence of TG4-155 were statistically similar (Fig. 5B). Equally important, the selective EP2 receptor agonist butaprost had no effect on BP (Fig. 2B).
We considered l-glutamate contribution to the PGE2/EP3 receptor–mediated pressor response because l-glutamate release within the RVLM causes pressor response (Ito et al., 2003; Rezq and Abdel-Rahman, 2016), and because PGE2: 1) produces Ca+2-dependent l-glutamate release in cultured astrocytes and neurons (Bezzi et al., 1998; Wang et al., 2015); 2) enhances glutamatergic neurotransmission in primary microcultures of the rat hypothalamic structures (Simm et al., 2016); 3) augments excitotoxicity produced by N-methyl-D-aspartate in mice (Iadecola et al., 2001); and 4) augments glutamate-induced excitotoxicity in hippocampal slices via EP3 receptor activation (Ikeda-Matsuo, 2013). Given the unfeasibility to measure l-glutamate level in RVLM tissue, and building on the aforementioned in vitro studies, we showed that PGE2 caused a concentration-dependent increase in glutamate release in PC12 cells (Fig. 6A). Among the different selective EP receptor subtype blockers, only EP3 receptor blockade abrogated PGE2-evoked glutamate release (Fig. 6B), which lends further support to the premise that EP3 receptor mediates the sympathoexcitatory effect of intra-RVLM PGE2.
We present evidence that PGE2 exerts central sympathoexcitatory action within the RVLM because it increased c-Fos expression in the RVLM (Fig. 4), which reflects activation of RVLM presympathetic neurons (Bullitt, 1990). This finding is further supported by the sympathetic dominance revealed by spectral analysis in the same rats (Fig. 3). These inter-related effects exerted by PGE2 are EP3 receptor–dependent because they were all abrogated by selective EP3 receptor blockade (L-798106), and not by EP2 receptor or EP4 receptor blockade (Figs. 3 and 4). It was important, however, to elucidate the molecular mechanisms of this PGE2/EP3 receptor–mediated neuronal activation in the RVLM.
A number of studies, including ours, directly linked RVLM ERK1/2 phosphorylation to central sympathoexcitation, at least partly, via activation of nNOS (Chan et al., 2010; Ibrahim and Abdel-Rahman, 2012), because nNOS-derived nitric oxide inhibits RVLM GABAergic neurotransmission and induces sympathoexcitation in conscious rats (Martins-Pinge et al., 2007; Ibrahim and Abdel-Rahman, 2011). Further, nNOS-derived nitric oxide contributes to ROS generation by interacting with the superoxide radical to produce peroxynitrite (Beckman et al., 1990), and to l-glutamate release (McNaught and Brown, 1998; Bal-Price et al., 2002). The involvement of the ERK1/2 pathway in PGE2-EP3 receptor–mediated signaling in other models (Chuang et al., 2006; Nicola et al., 2008) presented a rationale for investigating this pathway in the current study. Consistent with our hypothesis, intra-RVLM PGE2 increased local ERK1/2 and nNOS phosphorylation (Fig. 7). It is noteworthy that the PGE2-evoked elevation in l-glutamate level (Fig. 6) could also contribute to the observed higher nNOS phosphorylation, which plays an important role in the glutamatergic neurotransmission and sympathoexcitation (Garthwaite et al., 1988; Dawson et al., 1993). These findings implicate direct or indirect nNOS phosphorylation in PGE2-evoked sympathoexcitation, and suggest a pivotal role for the RVLM EP3 receptor in mediating these molecular events because they were only abrogated by prior EP3 receptor blockade (Figs. 6 and 7).
It is important to comment on increased RVLM ROS formation as a viable mechanistic link between the EP3 receptor–dependent ERK1/2-nNOS activation and the sympathoexcitation caused by intra-RVLM PGE2. This premise gains credence from the parallel increases in RVLM ROS level and NOX activity (Fig. 8) and sympathoexcitation (increased c-Fos expression; Fig. 6) caused by intra-RVLM PGE2, and by the ability of NOX-derived superoxide anion to activate the ERK pathway leading to increases in c-Fos expression and BP (Chan et al., 2005, 2007). These findings are also consistent with local PGE2 activation of NOX following central angiotensin II injection, which also leads to sympathoexcitation and pressor response (Wang et al., 2013). Our conclusion is bolstered by the ability of EP3 receptor blockade (L-798106) to abrogate the increases in nNOS phosphorylation, c-Fos, ROS, and NOX activity, and to restore the reduced catalase activity in PGE2-treated rats (Figs. 4, 6, 7, and 8). It is also noteworthy that reported studies, including ours, confirmed a mechanistic role for enhanced RVLM nNOS phosphorylation in sympathoexcitation and pressor response caused by intra-RVLM microinjection of other pharmacologic interventions, because such responses were abrogated by prior inhibition of nNOS with N(ω)-propyl-L-arginine (Tavares et al., 2007; Ibrahim and Abdel-Rahman, 2012).
In conclusion, this study yields new insight into the expression and function of EP receptor subtypes in the RVLM, and provides evidence that EP3 receptor activation mediates the sympathoexcitation and pressor response caused by intra-RVLM PGE2 in conscious rats. We present novel findings on molecular events implicated in EP3 receptor mediation of neuronal oxidative stress in the RVLM, which ultimately lead to sympathoexcitation and pressor response. The present pharmacologic and molecular findings highlight central EP3R blockade as a potential therapeutic modality for hypertension management.
Acknowledgments
The authors thank Kui Sun, Dr. Fanrong Yao, and Dr. Mohamed Fouda for technical assistance.
Abbreviations
- BP
blood pressure
- BRS
baroreflex sensitivity
- CAY10598
5-[(3S)-3-hydroxy-4-phenyl-1-buten-1-yl]1-[6-(2H-tetrazol-5R-yl)hexyl]-2-pyrrolidinone
- DCFH-DA
2′,7′-dichlorofluorescein diacetate
- ERK1/2
extracellular signal–regulated kinase 1/2
- HF
high frequency
- HR
heart rate
- L-161982
N-[[4'-[[3-butyl-1,5-dihydro-5-oxo-1-[2-(trifluoromethyl)phenyl]-4H-1,2,4-triazol-4-yl]methyl][1,1'-biphenyl]-2-yl]sulfonyl]-3-methyl-2-thiophenecarboxamide
- L-798106
N-[(5-bromo-2-methoxyphenyl)sulfonyl]-3-[2-(2-naphthalenylmethyl)phenyl]-2-propenamide
- LF
low frequency
- nNOS
neuronal nitric oxide synthase
- NOX
NADPH oxidase
- PBS
phosphate-buffered saline
- PGE2
prostaglandin E2
- ROS
reactive oxygen species
- RRI
inter-beat interval
- RVLM
rostral ventrolateral medulla
- SC-19220
8-chloro-dibenzo(Z)[b,f][1,4]oxazepine-10(11H)-carboxylic acid, 2-acetylhydrazide
- TG4-155
(2E)-N-[2-(2-methyl-1H-indol-1-yl)ethyl]-3-(3,4,5-trimethoxyphenyl)-2-propenamide
Authorship Contributions
Participated in research design: Rezq, Abdel-Rahman.
Conducted experiments: Rezq.
Performed data analysis: Rezq.
Wrote or contributed to the writing of the manuscript: Rezq, Abdel-Rahman.
Footnotes
This research was supported by the Department of Pharmacology and Toxicology at the Brody School of Medicine, East Carolina University; in part by the Zagazig Faculty of Pharmacy via a scholarship provided by the Egyptian Government (Scholarship Missions Program, Ministry of Higher Education); and the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism (Grant 2R01-AA01444-09).
References
- Albrecht P, Lewerenz J, Dittmer S, Noack R, Maher P, Methner A. (2010) Mechanisms of oxidative glutamate toxicity: the glutamate/cystine antiporter system xc- as a neuroprotective drug target. CNS Neurol Disord Drug Targets 9:373–382. [DOI] [PubMed] [Google Scholar]
- Ando K, Kondo F, Yamaguchi N, Tachi M, Fukayama M, Yoshikawa K, Gosho M, Fujiwara Y, Okada S. (2015) Centrally administered isoproterenol induces sympathetic outflow via brain prostaglandin E2-mediated mechanisms in rats. Auton Neurosci 189:1–7. [DOI] [PubMed] [Google Scholar]
- Ando T, Ichijo T, Katafuchi T, Hori T. (1995) Intracerebroventricular injection of prostaglandin E2 increases splenic sympathetic nerve activity in rats. Am J Physiol 269:R662–R668. [DOI] [PubMed] [Google Scholar]
- Ariumi H, Takano Y, Masumi A, Takahashi S, Hirabara Y, Honda K, Saito R, Kamiya HO. (2002) Roles of the central prostaglandin EP3 receptors in cardiovascular regulation in rats. Neurosci Lett 324:61–64. [DOI] [PubMed] [Google Scholar]
- Bal-Price A, Moneer Z, Brown GC. (2002) Nitric oxide induces rapid, calcium-dependent release of vesicular glutamate and ATP from cultured rat astrocytes. Glia 40:312–323. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. (1990) Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA 87:1620–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. (1998) Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature 391:281–285. [DOI] [PubMed] [Google Scholar]
- Bullitt E. (1990) Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J Comp Neurol 296:517–530. [DOI] [PubMed] [Google Scholar]
- Busnardo C, Crestani CC, Tavares RF, Resstel LB, Correa FM. (2010) Cardiovascular responses to L-glutamate microinjection into the hypothalamic paraventricular nucleus are mediated by a local nitric oxide-guanylate cyclase mechanism. Brain Res 1344:87–95. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E, Slawik H, Ridelis I, Waschbisch A, Akundi RS, Hull M, Fiebich BL. (2005) Regional distribution of the prostaglandin E2 receptor EP1 in the rat brain: accumulation in Purkinje cells of the cerebellum. J Mol Neurosci 27:303–310. [DOI] [PubMed] [Google Scholar]
- Chalmers J, Arnolda L, Llewellyn-Smith I, Minson J, Pilowsky P, Suzuki S. (1994) Central neurons and neurotransmitters in the control of blood pressure. Clin Exp Pharmacol Physiol 21:819–829. [DOI] [PubMed] [Google Scholar]
- Chan SH, Hsu KS, Huang CC, Wang LL, Ou CC, Chan JY. (2005) NADPH oxidase-derived superoxide anion mediates angiotensin II-induced pressor effect via activation of p38 mitogen-activated protein kinase in the rostral ventrolateral medulla. Circ Res 97:772–780. [DOI] [PubMed] [Google Scholar]
- Chan SH, Sun EY, Chang AY. (2010) Extracellular signal-regulated kinase 1/2 plays a pro-life role in experimental brain stem death via MAPK signal-interacting kinase at rostral ventrolateral medulla. J Biomed Sci 17:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SH, Wang LL, Tseng HL, Chan JY. (2007) Upregulation of AT1 receptor gene on activation of protein kinase Cbeta/nicotinamide adenine dinucleotide diphosphate oxidase/ERK1/2/c-fos signaling cascade mediates long-term pressor effect of angiotensin II in rostral ventrolateral medulla. J Hypertens 25:1845–1861. [DOI] [PubMed] [Google Scholar]
- Chen J, Xia C, Wang J, Jiang M, Zhang H, Zhang C, Zhu M, Shen L, Zhu D. (2013) The effect of orexin-A on cardiac dysfunction mediated by NADPH oxidase-derived superoxide anion in ventrolateral medulla. PLoS One 8:e69840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Miao Y, Zhang Y, Dou D, Liu L, Tian X, Yang G, Pu D, Zhang X, Kang J, et al. (2012) Inactivation of the E-prostanoid 3 receptor attenuates the angiotensin II pressor response via decreasing arterial contractility. Arterioscler Thromb Vasc Biol 32:3024–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang PC, Sun HS, Chen TM, Tsai SJ. (2006) Prostaglandin E2 induces fibroblast growth factor 9 via EP3-dependent protein kinase Cdelta and Elk-1 signaling. Mol Cell Biol 26:8281–8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson VL, Dawson TM, Bartley DA, Uhl GR, Snyder SH. (1993) Mechanisms of nitric oxide-mediated neurotoxicity in primary brain cultures. J Neurosci 13:2651–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J, Charles SL, Chess-Williams R. (1988) Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature 336:385–388. [DOI] [PubMed] [Google Scholar]
- Guan Y, Zhang Y, Wu J, Qi Z, Yang G, Dou D, Gao Y, Chen L, Zhang X, Davis LS, et al. (2007) Antihypertensive effects of selective prostaglandin E2 receptor subtype 1 targeting. J Clin Invest 117:2496–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hide EJ, Thiemermann C. (1996) Sulprostone-induced reduction of myocardial infarct size in the rabbit by activation of ATP-sensitive potassium channels. Br J Pharmacol 118:1409–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristovska AM, Rasmussen LE, Hansen PB, Nielsen SS, Nüsing RM, Narumiya S, Vanhoutte P, Skøtt O, Jensen BL. (2007) Prostaglandin E2 induces vascular relaxation by E-prostanoid 4 receptor-mediated activation of endothelial nitric oxide synthase. Hypertension 50:525–530. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Niwa K, Nogawa S, Zhao X, Nagayama M, Araki E, Morham S, Ross ME. (2001) Reduced susceptibility to ischemic brain injury and N-methyl-D-aspartate-mediated neurotoxicity in cyclooxygenase-2-deficient mice. Proc Natl Acad Sci USA 98:1294–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim BM, Abdel-Rahman AA. (2011) Role of brainstem GABAergic signaling in central cannabinoid receptor evoked sympathoexcitation and pressor responses in conscious rats. Brain Res 1414:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim BM, Abdel-Rahman AA. (2012) Enhancement of rostral ventrolateral medulla neuronal nitric-oxide synthase-nitric-oxide signaling mediates the central cannabinoid receptor 1-evoked pressor response in conscious rats. J Pharmacol Exp Ther 341:579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda-Matsuo Y. (2013) [The role of prostaglandin E2 in stroke-reperfusion injury]. Yakugaku Zasshi 133:947–954. [DOI] [PubMed] [Google Scholar]
- Institute for Laboratory Animal Research (2011) Guide for the Care and Use of Laboratory Animals, 8th ed, National Research Council, Washington, DC.
- Ito S, Hiratsuka M, Komatsu K, Tsukamoto K, Kanmatsuse K, Sved AF. (2003) Ventrolateral medulla AT1 receptors support arterial pressure in Dahl salt-sensitive rats. Hypertension 41:744–750. [DOI] [PubMed] [Google Scholar]
- Jiang J, Dingledine R. (2013) Role of prostaglandin receptor EP2 in the regulations of cancer cell proliferation, invasion, and inflammation. J Pharmacol Exp Ther 344:360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy CR, Zhang Y, Brandon S, Guan Y, Coffee K, Funk CD, Magnuson MA, Oates JA, Breyer MD, Breyer RM. (1999) Salt-sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor. Nat Med 5:217–220. [DOI] [PubMed] [Google Scholar]
- Kiraly AJ, Soliman E, Jenkins A, Van Dross RT. (2016) Apigenin inhibits COX-2, PGE2, and EP1 and also initiates terminal differentiation in the epidermis of tumor bearing mice. Prostaglandins Leukot Essent Fatty Acids 104:44–53. [DOI] [PubMed] [Google Scholar]
- Koriauli S, Natsvlishvili N, Barbakadze T, Mikeladze D. (2015) Knockdown of interleukin-10 induces the redistribution of sigma1-receptor and increases the glutamate-dependent NADPH-oxidase activity in mouse brain neurons. Biol Res 48:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkov AA, Glazova MV, Nikitina LS, Dorofeeva NA, Kirillova OD, Chernigovskaya EV. (2015) [Molecular mechanisms of Erk1/2 kinases regulation in the glutamate- and gaba-ergic neurons during seizure expression in Krushinsky-Molodkina Rats]. Ross Fiziol Zh Im I M Sechenova 101:1135–1149. [PubMed] [Google Scholar]
- Kotani M, Tanaka I, Ogawa Y, Usui T, Mori K, Ichikawa A, Narumiya S, Yoshimi T, Nakao K. (1995) Molecular cloning and expression of multiple isoforms of human prostaglandin E receptor EP3 subtype generated by alternative messenger RNA splicing: multiple second messenger systems and tissue-specific distributions. Mol Pharmacol 48:869–879. [PubMed] [Google Scholar]
- Lacroix S, Valliéres L, Rivest S. (1996) C-fos mRNA pattern and corticotropin-releasing factor neuronal activity throughout the brain of rats injected centrally with a prostaglandin of E2 type. J Neuroimmunol 70:163–179. [DOI] [PubMed] [Google Scholar]
- La Favor JD, Anderson EJ, Dawkins JT, Hickner RC, Wingard CJ. (2013) Exercise prevents Western diet-associated erectile dysfunction and coronary artery endothelial dysfunction: response to acute apocynin and sepiapterin treatment. Am J Physiol Regul Integr Comp Physiol 305:R423–R434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malliani A, Pagani M, Lombardi F, Cerutti S. (1991) Cardiovascular neural regulation explored in the frequency domain. Circulation 84:482–492. [DOI] [PubMed] [Google Scholar]
- Martins-Pinge MC, Garcia MR, Zoccal DB, Crestani CC, Pinge-Filho P. (2007) Differential influence of iNOS and nNOS inhibitors on rostral ventrolateral medullary mediated cardiovascular control in conscious rats. Auton Neurosci 131:65–69. [DOI] [PubMed] [Google Scholar]
- McNaught KS, Brown GC. (1998) Nitric oxide causes glutamate release from brain synaptosomes. J Neurochem 70:1541–1546. [DOI] [PubMed] [Google Scholar]
- Minson JB, Llewellyn-Smith IJ, Arnolda LF, Pilowsky PM, Oliver JR, Chalmers JP. (1994) Disinhibition of the rostral ventral medulla increases blood pressure and Fos expression in bulbospinal neurons. Brain Res 646:44–52. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. (1991) Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci 14:421–451. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Okada S, Nishihara M, Yokotani K. (2002) Roles of brain prostaglandin E2 and thromboxane A2 in the activation of the central sympatho-adrenomedullary outflow in rats. Eur J Pharmacol 452:289–294. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. (1999) Prostanoid receptors: structures, properties, and functions. Physiol Rev 79:1193–1226. [DOI] [PubMed] [Google Scholar]
- Nassar NN, Li G, Strat AL, Abdel-Rahman AA. (2011) Enhanced hemeoxygenase activity in the rostral ventrolateral medulla mediates exaggerated hemin-evoked hypotension in the spontaneously hypertensive rat. J Pharmacol Exp Ther 339:267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola C, Chirpac A, Lala PK, Chakraborty C. (2008) Roles of Rho guanosine 5′-triphosphatase A, Rho kinases, and extracellular signal regulated kinase (1/2) in prostaglandin E2-mediated migration of first-trimester human extravillous trophoblast. Endocrinology 149:1243–1251. [DOI] [PubMed] [Google Scholar]
- Nishihara M, Hirooka Y, Matsukawa R, Kishi T, Sunagawa K. (2012) Oxidative stress in the rostral ventrolateral medulla modulates excitatory and inhibitory inputs in spontaneously hypertensive rats. J Hypertens 30:97–106. [DOI] [PubMed] [Google Scholar]
- Osborne NN, Li GY, Ji D, Andrade da Costa BL, Fawcett RJ, Kang KD, Rittenhouse KD. (2009) Expression of prostaglandin PGE2 receptors under conditions of aging and stress and the protective effect of the EP2 agonist butaprost on retinal ischemia. Invest Ophthalmol Vis Sci 50:3238–3248. [DOI] [PubMed] [Google Scholar]
- Parati G, Frattola A, Di Rienzo M, Castiglioni P, Pedotti A, Mancia G. (1995) Effects of aging on 24-h dynamic baroreceptor control of heart rate in ambulant subjects. Am J Physiol 268:H1606–H1612. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson CR, Emson PC. (1980) AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. J Neurosci Methods 3:129–149. [DOI] [PubMed] [Google Scholar]
- Penumarti A, Abdel-Rahman AA. (2014) The novel endocannabinoid receptor GPR18 is expressed in the rostral ventrolateral medulla and exerts tonic restraining influence on blood pressure. J Pharmacol Exp Ther 349:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezq S, Abdel-Rahman AA. (2016) Central GPR109A Activation Mediates Glutamate-Dependent Pressor Response in Conscious Rats. J Pharmacol Exp Ther 356:456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero TR, Resende LC, Duarte ID. (2011) The neuronal NO synthase participation in the peripheral antinociception mechanism induced by several analgesic drugs. Nitric Oxide 25:431–435. [DOI] [PubMed] [Google Scholar]
- Shaltout HA, Abdel-Rahman AA. (2005) Mechanism of fatty acids induced suppression of cardiovascular reflexes in rats. J Pharmacol Exp Ther 314:1328–1337. [DOI] [PubMed] [Google Scholar]
- Simm B, Ott D, Pollatzek E, Murgott J, Gerstberger R, Rummel C, Roth J. (2016) Effects of prostaglandin E2 on cells cultured from the rat organum vasculosum laminae terminalis and median preoptic nucleus. Neuroscience 313:23–35. [DOI] [PubMed] [Google Scholar]
- Tavares RF, Resstel LB, Corrêa FM. (2007) Interaction between glutamatergic and nitrergic mechanisms mediating cardiovascular responses to L-glutamate injection in the diagonal band of Broca in anesthetized rats. Life Sci 81:855–862. [DOI] [PubMed] [Google Scholar]
- Wang D, Wang P, Jiang J, Lv Q, Zeng X, Hong Y. (2015) Activation of Mas Oncogene-Related G Protein-Coupled Receptors Inhibits Neurochemical Alterations in the Spinal Dorsal Horn and Dorsal Root Ganglia Associated with Inflammatory Pain in Rats. J Pharmacol Exp Ther 354:431–439. [DOI] [PubMed] [Google Scholar]
- Wang G, Sarkar P, Peterson JR, Anrather J, Pierce JP, Moore JM, Feng J, Zhou P, Milner TA, Pickel VM, et al. (2013) COX-1-derived PGE2 and PGE2 type 1 receptors are vital for angiotensin II-induced formation of reactive oxygen species and Ca(2+) influx in the subfornical organ. Am J Physiol Heart Circ Physiol 305:H1451–H1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Du Y. (2012) Distinct roles of central and peripheral prostaglandin E2 and EP subtypes in blood pressure regulation. Am J Hypertens 25:1042–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZH, Yu Y, Wei SG, Nakamura Y, Nakamura K, Felder RB. (2011) EP₃ receptors mediate PGE₂-induced hypothalamic paraventricular nucleus excitation and sympathetic activation. Am J Physiol Heart Circ Physiol 301:H1559–H1569. [DOI] [PMC free article] [PubMed] [Google Scholar]