Abstract
Worldwide, Salmonella spp. is a significant cause of disease for both humans and wildlife, with wild birds adapted to urban environments having different opportunities for pathogen exposure, infection, and transmission compared to their natural conspecifics. Food provisioning by people may influence these factors, especially when high-density mixed species flocks aggregate. White Ibises (Eudocimus albus), an iconic Everglades species in decline in Florida, are becoming increasingly common in urbanized areas of south Florida where most are hand-fed. We examined the prevalence of Salmonella shedding by ibises to determine the role of landscape characteristics where ibis forage and their behavior, on shedding rates. We also compared Salmonella isolated from ibises to human isolates to better understand non-foodborne human salmonellosis. From 2010–2013, 13% (n = 261) adult/subadult ibises and 35% (n = 72) nestlings sampled were shedding Salmonella. The prevalence of Salmonella shedding by ibises significantly decreased as the percent of Palustrine emergent wetlands and herbaceous grasslands increased, and increased as the proportion of open-developed land types (e.g. parks, lawns, golf courses) increased, suggesting that natural ecosystem land cover types supported birds with a lower prevalence of infection. A high diversity of Salmonella serotypes (n = 24) and strain types (43 PFGE types) were shed by ibises, of which 33% of the serotypes ranked in the top 20 of high significance for people in the years of the study. Importantly, 44% of the Salmonella Pulsed-Field Gel Electrophoresis patterns for ibis isolates (n = 43) matched profiles in the CDC PulseNet USA database. Of these, 20% came from Florida in the same three years we sampled ibis. Importantly, there was a negative relationship between the amount of Palustrine emergent wetland and the number of Salmonella isolates from ibises that matched human cases in the PulseNet database (p = 0.056). Together, our results indicate that ibises are good indicators of salmonellae strains circulating in their environment and they have both the potential and opportunity to transmit salmonellae to people. Finally, they may act as salmonellae carriers to natural environments where other more highly-susceptible groups (nestlings) may be detrimentally affected.
Introduction
The genus Salmonella has a worldwide distribution and is one of the most common causes of intestinal diseases for both people and animals [1]. Despite major public education efforts and improvements to food hygiene practices, salmonellosis remains a significant source of enteric disease for people in the United States and worldwide. Salmonella enterica accounts for 1.2 million illnesses and 450 deaths in the US each year [2]. While most Salmonella-associated illnesses had been associated with the consumption of contaminated beef and poultry products [2, 3], there is an increasing frequency of illnesses associated with produce [4–14]. Despite public health education about food handling practices, the incidence of salmonellosis in people has remained the same in the last 20 years, and there has been a shift in serotype distribution from foodborne-associated strains towards environmentally-acquired strains, the source of which is not always known [3, 15, 16].
Although salmonellae are important human pathogens, they can also cause small to large-scale mortalities in wildlife species. For example, throughout the world, Salmonella outbreaks caused by S. Typhimurium have been reported in passerine birds associated with bird feeders, and to a lesser extent, colony-nesting birds (e.g., egrets and herons) [17–25]. There is still much to learn about the epidemiology and impact of salmonellosis on wild birds as the prevalence of avian salmonellosis appear to be on the rise, in some cases causing catastrophic outbreaks, some of which have been implicated with population-level effects [25, 26, 27]. The increase in outbreaks over the last 25 years has caused some to consider it as an emergent disease that may be directly related to anthropogenic activities such as backyard feeding and the use of contaminated habitats [23, 24, 28, 29].
In general, the level and duration of infection and Salmonella shedding in wild birds and potential to develop clinical disease is probably similar to domestic poultry but there are some important epidemiological differences. Salmonella prevalence of chicks of some colonial nesting species such as herons and egrets is higher compared to adult birds [25]. The immature intestinal flora related to both age and diet, may explain this difference where the infection rate for adults is relatively low (1–2%), but this may also be due to differences in habitat use. For example, adult herring gulls can be transient carriers of Salmonella and may serve in its transport while foraging between contaminated and pristine environments [30]. Several studies illustrate a direct correlation between the prevalence of Salmonella shedding in wild birds and their proximity to anthropogenic habitats [21, 31–35]. Urbanized birds likely have unique opportunities for enteric pathogen exposure, infection, and transmission due to various factors such as the quality of food and water they consume, the species with which they have direct contact, their consistent aggregations in high numbers in small areas and comparatively sedentary lifestyles [36]. Urbanized species with a potential to be carriers of Salmonella should be of special interest because of their frequent contact with the public and their potential to contaminate environments used by people.
Human Salmonella cases have been linked to direct or indirect contact with wild birds in several independent reports [17, 18, 29, 35, 37–42]. The majority of those reports relied on post-outbreak surveys and few utilize molecular typing to investigate similarities between human and avian isolates [37]. Therefore, studies that elucidate the phylogenetic, spatial and temporal relationships between human and bird infections and contribute to understanding of the underlying ecological interactions ecological interactions associated with non-foodborne cases of salmonellosis in humans.
The American white ibis (Eudocimus albus) is a gregarious member of the Pelecaniformes that forms large nesting colonies in natural wetlands. Ibises feed by constantly probing the substrate with their beaks for aquatic invertebrates, frogs and fish in wetlands, and roost and nest in mixed-species flocks over water bodies [43]. Their reproductive success and population size is highly dependent on food availability and the hydrology of the Florida Everglades Ecosystem, where most ibis breed [44, 45]. In South Florida, the utilization of urban habitats by white ibis has been steadily increasing since the late 1990s [46]. It is likely that a suite of factors, including natural wetland loss, and a constant source of food and water in urban parks promoted through rapid urban expansion in the region is responsible for this new distribution [47, 48]. Ibises are now abundant in neighborhood parks, golf courses and other artificial wetlands, where they have become sedentary, habituated to consuming food provided by people and where they regularly interact directly and indirectly with humans and other urbanized avian species (e.g, various gull species, semi-domesticated and wild ducks such as Muscovy ducks (Cairina moschata), and mallards (Anas platyrhynchos) (Hernandez, pers observation). In Australia, a related species of ibis (Threskiornis molucca) has become similarly habituated to anthropogenic sources of food and has been shown to be a carrier of Salmonella enterica [32]. Thus, we consider the American white ibis a good model species for understanding the role of urbanization on Salmonella transmission between wildlife and humans. Specifically, we aimed to determine whether the prevalence of Salmonella infection of urbanized white ibises in South Florida was related to either ibis behavior or landscape characteristics, and the relationship between Salmonella isolated from urbanized white ibises and human salmonellosis cases. Finally, although urbanized ibises primarily forage in urbanized environments during their non-reproductive period (Hernandez, unpublished data), they disperse long distances to natural areas to breed; thus, we sought to understand the role ibises might play in the dissemination of urban-associated salmonellae to those areas.
Materials and Methods
Study Sites
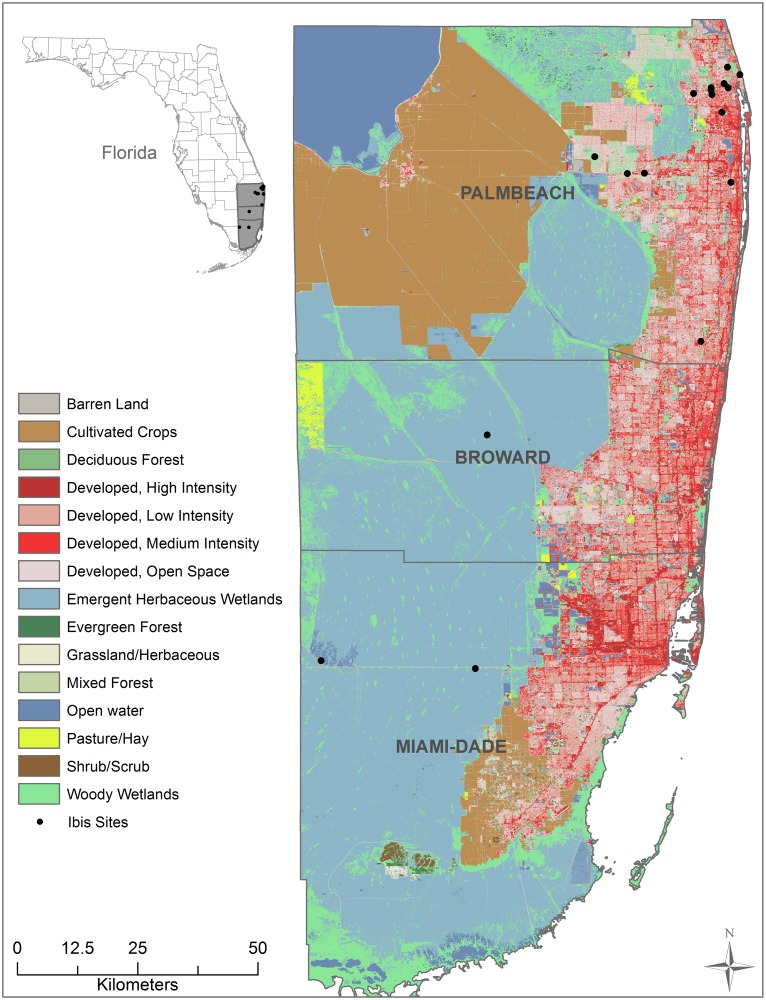
We collected fecal samples from both adult and subadult birds at 17 public access sites in Palm Beach County, Florida, USA, (26°43’N, 80°2’W) with fresh water bodies from 2010–2013. We defined subadults (and use the term juveniles synonymously) as birds that had left the nest and were less than three years of age, which were readily distinguished from adults by feather coloration. Sites were chosen based on accessibility and a repeated record of more than 10 ibises per site during daily visits. Two sites were within natural areas, whereas the remaining 15 were urban parks, zoos or neighborhoods. We also collected feces from nestlings at three ibis rookeries located within Broward (n = 1) and Miami-Dade (n = 2) counties (Fig 1).
Fig 1. Map of Sites.
Fecal samples were collected from adult/subadult and nestling white ibises in Palm Beach, Broward and Miami-Dade counties, FL. The land cover for a 1 km radius surrounding each sampling site was determined using land cover maps.
Using ArcGIS version 10.1, the land cover types were determined in a 1 km radius buffer around each site. Within each 1 km buffer, we quantified the percentages of each land cover class to examine the development of land and to classify sites by level of urbanization in each area. The publically-available land cover classes were developed by the Department of Commerce (DOC), National Oceanic and Atmospheric Administration (NOAA), National Ocean Service (NOS), Coastal Services Center (CSC) (http://gis.ncdc.noaa.gov/geoportal/catalog/search/resource/details.page?id=gov.noaa.ncdc:C00814). The NOAA classification originally contained 25 classes, and the accuracy of classification for this data set is 86.5% and 85.1%. Initially, the studied areas represented 18 land cover types ranging from urban areas, agriculture, forested areas, and wetlands (Table 1). However, because some land cover types were <1% of the total land cover for that site, we only included 12 land cover classes in the analysis and excluded Cultivated/Crops, Pasture/Grasslands and Bare Land. For clarification, we defined the four developed land cover types as follows: (1) Developed High Intensity- contained little or no vegetation, including heavily built-up urban centers as well as large constructed surfaces in suburban and rural areas-large buildings such as multiple family housing, hangars, and large barns, interstate highways, and runways, impervious surfaces accounted for 80–100 percent of the total cover (site range = 0 to 18%, Table 1); (2) Developed Medium Intensity- contained substantial amounts of constructed surface mixed with substantial amounts of vegetated surface, includes small buildings such as single family housing, farm outbuildings, and large sheds, impervious surfaces account for 50–79 percent of the total cover (site range = 0.1 to 49%, Table 1); (3) Developed Low Intensity- contained constructed surface mixed with vegetated surface, this class includes features seen in class 2, with the addition of streets and roads with associated trees and grasses, impervious surfaces account for 21–49 percent of the total cover (site range = 0.4 to 40%); and (4) Open Developed- included areas with a mixture of some constructed materials, but mostly vegetation in the form of lawn grasses, this subclass included parks, lawns, athletic fields, golf courses, and natural grasses occurring around airports and industrial sites, impervious surfaces accounted for less than 20 percent of total cover (site range = 0 to 20%).
Table 1. Percent land cover of selected land use categories within 1km radius circles surrounding sampling site.
| Burns | Donald Ross | Garden | Juno | San Marco | Lion Safari | Loxa-hatchee | PGA | Prosperity | Royal Palm | San Matera | Zoo | Seasons | Horse shoe | ICP | Kissimmee | Dreher | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Developed, High intensity | 15.79 | 2.67 | 7.65 | 5.90 | 17.94 | 0.46 | 0.43 | 3.47 | 16.34 | 2.75 | 14.76 | 8.48 | 18.23 | 4.24 | 4.41 | 0.00 | 7.05 |
| Developed, medium intensity | 44.20 | 31.44 | 36.20 | 26.05 | 29.24 | 2.87 | 13.56 | 29.18 | 44.51 | 30.84 | 43.31 | 46.00 | 36.00 | 25.34 | 49.16 | 0.14 | 46.29 |
| Developed, low intensity | 22.27 | 26.89 | 19.18 | 10.15 | 19.69 | 11.69 | 32.16 | 25.97 | 20.29 | 22.61 | 24.71 | 21.41 | 31.70 | 40.64 | 19.81 | 0.40 | 22.24 |
| Developed, open | 9.29 | 5.82 | 4.56 | 10.89 | 4.33 | 6.31 | 5.68 | 20.55 | 3.01 | 7.62 | 4.01 | 5.93 | 3.87 | 6.56 | 9.09 | 0.00 | 6.08 |
| Herbaceous grassland | 0.00 | 0.37 | 0.03 | 0.11 | 2.35 | 4.50 | 2.87 | 0.37 | 0.03 | 2.75 | 0.00 | 0.03 | 0.00 | 0.06 | 1.06 | 19.32 | 0.03 |
| Evergreen forest | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 0.69 | 0.49 | 0.03 | 0.09 | 0.03 | 0.00 | 0.03 | 0.00 | 0.03 | 0.03 | 0.11 | 0.03 |
| Scrub | 0.11 | 0.29 | 0.14 | 0.54 | 0.17 | 0.63 | 0.89 | 0.43 | 9.86 | 0.69 | 0.11 | 0.09 | 0.06 | 0.03 | 0.09 | 7.11 | 0.09 |
| Forest, palustrine | 5.93 | 20.81 | 22.84 | 17.03 | 8.37 | 63.57 | 34.48 | 12.35 | 0.92 | 15.85 | 9.26 | 10.52 | 8.46 | 20.84 | 4.82 | 20.04 | 10.32 |
| Scrub, palustrine | 0.66 | 2.35 | 1.49 | 2.01 | 1.32 | 5.68 | 3.15 | 0.57 | 1.00 | 2.04 | 0.63 | 1.18 | 0.43 | 0.72 | 1.75 | 32.04 | 1.26 |
| Emergent wetland, palustrine | 0.89 | 3.75 | 3.41 | 1.03 | 5.22 | 2.64 | 2.69 | 2.35 | 0.63 | 3.84 | 0.77 | 1.32 | 1.03 | 0.89 | 4.16 | 20.15 | 1.32 |
| Bare land | 0.14 | 0.09 | 3.41 | 0.66 | 5.07 | 0.23 | 2.49 | 0.00 | 0.00 | 8.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 |
| Open water | 0.26 | 4.67 | 0.72 | 24.39 | 5.50 | 0.03 | 0.00 | 3.90 | 3.24 | 2.04 | 2.29 | 4.61 | 0.20 | 0.69 | 4.64 | 0.03 | 4.70 |
Ibis behavior
Ibis behavior may be directly related to pathogen transmission among themselves and between ibis and people. For example, ibises that beg for food get very close to people and defecate in very close proximity to people or on structures used by people. Therefore, ibis flocks of adults/subadults were scored by their behavior based on direct observations on a habituation scale of 1–4, where 1 were the least habituated to humans and 4 were the most (Table 2). We based our scoring system on observations of ibis with the general public. Highly habituated flocks responded to people by moving towards them immediately and even tolerated hand feeding. This score was assigned by observing the flock for 30 min prior to any sampling or manipulation. Highly habituated flocks tended to be larger, were more consistently present at a particular urban site and had more opportunities for direct contact with people. In contrast, flocks considered not to be habituated at all did not tolerate the presence of people and flew away when people got within 10 m of the flock. regardless of whether people offered food or not, varied in numbers of individuals and were not found predictably at a particular site. Flock density could be related to behavior and was calculated by counting the number of individuals in the approximate area actively utilized by the flock.
Table 2. Habituation behavioral scores assigned to white ibises foraging in urban parks in south Florida.
| Score | Definition |
|---|---|
| 1 | Birds do not allow humans within 10 m and fly off if humans get within that distance |
| 2 | Birds allow humans within 10 m. but do not associate humans as a source of food, consume food provided by people or beg for food |
| 3 | Birds will allow humans within 10 m and will consume food provided by humans but do not allow humans within 10 ft. or beg for food |
| 4 | Birds allow humans within 3 m. and beg for food |
Fecal sample collection and Salmonella isolation
The collection of feces from ibises was achieved in one of two ways. In some cases, individual animals (adults/subadults) were observed until they defecated, at which point a sample from the center of the fecal pellet was immediately collected from the ground. Care was taken not to resample individuals by separating one individual from the rest of the flock with a food reward until it defecated. In some instances, as part of a larger project, all nestlings and some adult or subadult birds were individually captured and physically restrained for other procedures, during which time they defecated on a clean, water repellent cloth placed across the holder’s lap. Adult ibises were captured with leg lassos. Briefly, a leg lasso with a slip knot was constructed out of nylon monofilament and hidden in the substrate (i.e. grass). One person would hold the line approximately 6 m away, while a second person would bait a bird close with food, and encourage it to step into the lasso, at which point the line holder would swiftly tighten the lasso, capturing the bird by the leg. Both people would immediately physically restrain it and place it in a pillow case until it was processed. Juvenile ibis were captured prior to their ability to fly by visiting known nests, picking them, placing them in a pillow case and lowering them to the ground where they were immediately processed. In both cases, birds would defecate during handling and approximately 1 g of feces was collected with a sterile swab and immersed in 10 ml of dulcitol selenite broth (Becton, Dickinson and Company, Franklin Lakes, NJ), maintained at room temperature and shipped within 24 h to The Athens Diagnostic Laboratory at the University of Georgia. Salmonella enrichment and identification was performed as previously described [49]. Briefly, we performed a double selenite broth enrichment, followed by subculture on XLD and Brilliant Green agar plates (Remel). We selected 5–10 colonies from either selective plate that appeared to be Salmonella spp based on morphology. The number of colonies selected was determined based on the numbers of Salmonella suspect colonies present. Each colony was then serogrouped. One colony representative from each serogroup was selected and confirmed as Salmonella through the following biochemical tests: triple sugar iron (TSI), MIO, citrate, malonate, and phenylalanine. When isolates were confirmed, they were forwarded to the National Veterinary Service Laboratory (NVSL) at Ames, Iowa, for definitive serotyping. No animals were sacrificed as part of this research. All animal handling procedures were approved by the University of Georgia’s Institutional Animal Care and Use Committee (IACUC; A2011 08–018). This work required permits from both the Florida Fish and Wildlife Conservation Commission (LSSC-11-00119F) and the U. S. Fish and Wildlife Service (MB779238-0). Both permits required prior approval by an IACUC. We obtained a Special Use Permit from Arthur R. Marshall Loxahatchee National Wildlife Refuge (B15_09). In addition, for urban parks we obtained permission from the Palm Beach County Parks and Recreations Department. For all sampling on private lands, we obtained permission from the relevant authority. All permits were obtained for the specific procedures of this specific study.
Molecular typing of Salmonella isolates by pulsed-field gel electrophoresis
Ibis isolates were strain typed by Pulsed-Field Gel Electrophoresis (PFGE) to determine their genetic relatedness [49] of these isolates to themselves, to archived bird isolates (both wild and domestic), to isolates from other animal sources, to water isolates, and to human isolates deposited in the CDC PulseNet national database which contains >50,000 entries [50]. A master database of Salmonella PFGE patterns was generated in BioNumerics (Applied Maths; Austin, TX). This database consisted of 1,047 total PFGE entries for Salmonella isolated from water (n = 400) and various animal species (n = 674) and consisted primarily of S. enterica subsp. enterica (97%) isolates representing 58 serotypes. Comparisons were made between PFGE patterns using Dice coefficient and unweighted-pair group method using average linkage (UPGMA) clustering [51].
Statistical analysis
All data analyses were performed using R version 3.0.2 [52]. For the samples from adult/subadult ibises, generalized linear mixed models (GLMM) in the ‘lme4’ package of R [53]were used to analyze if Salmonella prevalence was influenced by the following fixed effects: (1) ibis flock density, (2) the behavioral variable ‘habituation score’, (3) sampling year, and (4) season. All models included site and sampling period as random variables, and used a binomial distribution. A likelihood ratio test was used to compare the GLMM models with and without the fixed effect, i.e. predictor variable, to test for the variable’s significance. In addition to adults and subadults, we also sampled nestlings for Salmonella, but in a more limited fashion, i.e. all nestlings were tested during the same season (April/May), and the majority of nestlings were sampled in 2013. Nestlings also did not flock and we were unable to rate habituation scores for adult birds at the rookeries; therefore, we did not test these fixed effects against all three age classes. We were able, however, to test whether there was a difference in Salmonella prevalence rates among the three age classes sampled: adults, subadults and nestlings using a GLMM with a binomial distribution. For those predictor variables showing a statistically significant relationship with Salmonella prevalence, we calculated the marginal R2, i.e. the R2 value for only the fixed effects in the model using the package MuMIn in R [54].
To determine the effect of land use/land cover on Salmonella prevalence in adult/subadult birds, GLMMs were also used. All models included site and sampling period as random variables, and used a binomial distribution. Land use/land cover categories tested as fixed effects included all twelve of the aforementioned. In addition, due to the lack of predictive power for many of the land cover variables when analyzed separately, we also analyzed the effect of the landscape composition on Salmonella prevalence by combining the 12 land cover types into a principal components analysis (PCA), using the function prcomp in R [55]. PCA collapsed the land cover variables into three PCs that explained 73% of the landscape composition (PC1 = 41%, PC2 = 20%, PC3 = 12%). PCA1 loaded negatively on the percentage of land in the four developed categories. PCA2 loaded negatively on the amount of forested areas (Palustrine forest and Evergreen forest) in a landscape (96%). PCA3 loaded strongly and positively on the amount of open water and the amount of open developed land cover. We used GLMMs with a binomial distribution and likelihood ratio tests to evaluate the relationship between the principal components metrics and two response variables: (1) the number of different serotypes and (2) Salmonella prevalence. For landscape variables showing a statistically significant relationship with Salmonella prevalence, we also calculated the marginal R2. We had an a priori expectation that there would be a significant difference in Salmonella prevalence between adult/subadult and nestlings; therefore, landscape analyses for prevalence did not include the sites from which nestlings were sampled.
To examine whether Salmonella prevalence displayed a spatial pattern among the sampled sites (excluding rookeries), we performed a Moran’s I test using a Euclidean distance matrix and the variable Salmonella prevalence, using the package spdep in R [56]. To test for patterns of serotype composition by site, we performed a distance based redundancy ordination using the function capscale in the vegan package of R [57]. We examined the various landscape and bird predictor variables (e.g. habituation score) in the ordination. Only sites with at least one ibis testing positive for Salmonella were used in this analysis. As we were unable to rate habituation scores for nestlings at the rookeries, these sites were also excluded from this analysis.
To evaluate the potential effects of land use/land cover on the number of isolates from white ibises that matched human cases in the PulseNet database we again used GLMMS with a binomial distribution and likelihood ratio tests. In this analysis we included isolates from nestlings, as we had no a priori reason to expect differences among age classes. Land use/land cover categories tested as fixed effects included all twelve of the aforementioned. Marginal R2 values were calculated for all significant land use/land cover predictors.
Results
Salmonella prevalence in Ibis
From January 2010 to July 2013 we collected and tested 261 fecal samples from birds at urban sites and 72 samples from nesting sites. We isolated Salmonella from 55 individual birds (33 adult/subadult birds, and 22 nestlings). The mean prevalence for birds (adult/subadult) by site was 13% (range 0–50%) and the mean prevalence for nestlings was 35% (range 6–50%) (Table 3). Salmonella prevalence was highest for nestlings, second highest for juvenile or subadult birds, and lowest in adult ibis (likelihood ratio test = 16.99, P < 0.001).
Table 3. Sampling sites, numbers of birds sampled and overall prevalence of Salmonella infection of white ibises in south Florida.
| Site | Dates Sampled | N | Salmonella prevalence % |
|---|---|---|---|
| Juno Beach urban park | 1/2010; 3/2012; 12/2012 | 45 | 16 |
| Horseshoe Acres neighborhood | 1/2010 | 9 | 0 |
| Dreher urban park | 1/2010; 3/2012 | 12 | 17 |
| Prosperity Oaks retirement community | 3/2010; 3/2012; 12/2012 | 26 | 15 |
| Garden Lakes business park | 3/2010 | 15 | 7 |
| Burns Rd urban park | 3/2010 | 5 | 40 |
| San Matera neighborhood | 3/2010 | 15 | 13 |
| San Marco Villas neighborhood | 3/2010 | 19 | 0 |
| Royal Palm urban park | 3/2010 | 10 | 0 |
| PGA golf community | 3/2010 | 5 | 40 |
| Donald Ross Rd empty lot | 3/2010 | 10 | 20 |
| Seasons 52 restaurant | 3/2010 | 10 | 0 |
| Indian Creek urban park | 12/2012 | 10 | 10 |
| Lion Country Safari park | 3/2010; 3/2012; 12/2012 | 29 | 14 |
| Palm Beach Zoo | 7/2010; 3/2012 | 13 | 8 |
| Loxahatchee National Wildlife Refuge | 3/2010 | 13 | 0 |
| Kissimmee Prairie Preserve State Park | 3/2013; 7/2013 | 15 | 27 |
| Tamiami West Colony | 4/2012; 4/2013 | 60 | 33 |
| Alley North Colony | 4/2014 | 15 | 6 |
| Ibis Colony | 4/2013 | 28 | 50 |
| 6th Bridge Colony | 4/2012; 5/2014 | 4 | 50 |
Combining all three years of the study, habituation score was not a significant predictor of Salmonella prevalence (likelihood ratio test = 0.17, P = 0.68). The prevalence in 2010 was significantly lower than in 2012 (likelihood ratio test = 8.01, P = 0.005), and although there was a trend towards a higher prevalence in 2013, the difference was not statistically significant. Although the majority of adult and subadult birds were sampled from December to March in both years, a few birds were sampled outside of this time period. We tested for an effect of season on Salmonella prevalence for adult/subadult birds and found no effect (likelihood ratio test = 0.20, P = 0.90). All nestlings were sampled in the same season, April/May. There was no relationship between prevalence and flock density (i.e. the number of white ibis per m2) (likelihood ratio test = 1.89, P = 0.17); however, for the density calculation we were not able to consider habitat quality.
Land cover and Salmonella prevalence in Ibis
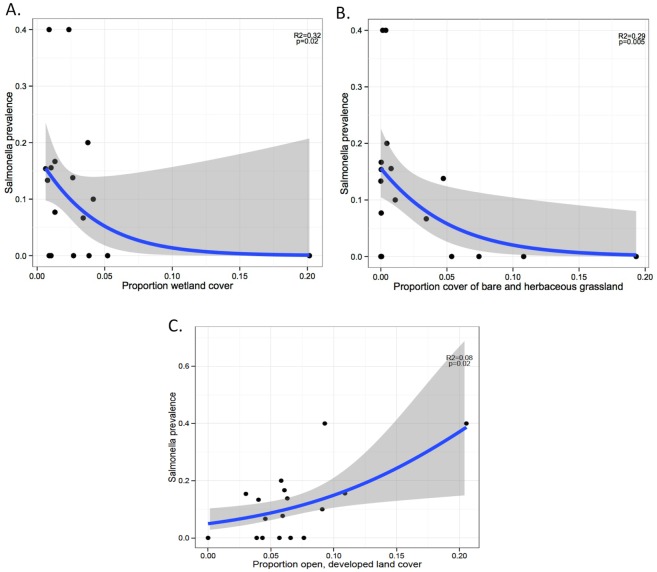
Landscape composition metrics determined from PCA were not significantly related to either Salmonella prevalence or serotype diversity (numbers of Salmonella serotypes isolated; PC1: prevalence, z = 1.04, P = 0.29; serotype diversity, z = -0.77, P = 0.44). No spatial pattern was found in Salmonella prevalence among the sampled sites (Moran’s I: observed = -0.04, expected = -0.06, P = 0.90). However, several land cover variables, when examined individually, showed a statistically significant relationship with the prevalence of Salmonella infection. As the percent cover of Palustrine wetlands and herbaceous grasslands in a 1km buffer surrounding the sampling site increased, the prevalence of Salmonella decreased (wetlands: LRT = 5.41, P = 0.02, grassland: LRT = 8.45, P = 0.004, Fig 2a, 2b and 2c). Palustrine wetlands are non-tidal wetlands dominated by trees, shrubs, and persistent emergent vegetation and low salinity. Within a 1 km radius around each capture point the amount cover of Palustrine emergent wetland ranged from 0.6% to 20% (Table 1). Conversely, the only developed land use type that was related to Salmonella prevalence, was the least intensive of the developed cover types, Open Developed, which was positively related to prevalence (Fig 2c, LRT = 5.51, P = 0.02).
Fig 2. Salmonella prevalence and habitat type.
Salmonella prevalence in white ibises is negatively influenced by cover of emergent wetlands (a) herbaceous grasslands (b) and positively influenced by open, developed land (c) in Palm Beach County, Florida.
Linkage between Salmonella strains in ibises and cases of human salmonellosis
A high diversity of Salmonella serotypes (24 serotypes) and strain types (43 PFGE types) were isolated from white ibises (Table 4). These 43 Salmonella strain types belonged to one of 28 clusters identified by PFGE (≥75% similarity; Fig 3). We used rarefaction analysis to determine our sampling effort of serotype diversity in white ibis, which predicted that if another 32 Salmonella-infected ibis were tested, between 0 and 22 new serotypes would be found (S1 Fig). Thirty-three percent of the ibis isolates were serotypes that ranked in the top 20 Salmonella serotypes associated with human cases in the U.S., as reported by the CDC for years 2010–2012 (S. Anatum, S. Bareilly, S. Braenderup, S. Javiana, S. Muenchen, S. Newport, S. Saint Paul, S. Typhimurium) [16]. Fifteen percent of our isolates were serotypes most frequently reported in the state of Florida for the years of this study (S. Javiana, IV 50:Z4,Z23:- (formerly S. Flint), S. Newport, S. Saintpaul, and S. Typhimurium).
Table 4. Salmonella serotype and strain diversity isolated from American white ibises in South Florida.
| Site a | Date | Source | Age d | Isolate | Serotype | PFGE Type | PulseNet | Matches h | Cases in FL h |
|---|---|---|---|---|---|---|---|---|---|
| Kissimmee Prairie Park | 07/13 | Ibis | Juvenile | KPP100 | Rubislaw | Rb2 | No Matches | ||
| Kissimmee Prairie Park | 07/13 | Ibis | Juvenile | KPP102 | Rubislaw | Rb7 | No Matches | ||
| Kissimmee Prairie Park | 07/13 | Ibis | Juvenile | KPP103 | Miami | Mi1 | TEAX01.0045 | 1 | 1 |
| Kissimmee Prairie Park | 07/13 | Ibis | Juvenile | KPP104 | Flint | Ft2 | No Matches | ||
| Indian Creek Park | 12/12 | Ibis | Adult | ICP301 | Bareilly (10)e | Ba1 | JAPX01.0064 | 8 | 1 |
| Donald Ross Lot | 03/10 | Ibis | Juvenile | DR001 | Baildon | Bl1 | TDEX01.0001g | 107 | 1 |
| Donald Ross Lot | 03/10 | Ibis | Juvenile | DR002 | Midway | Md1 | No Matches | 0 | 0 |
| Juno Beach | 01/10 | Ibis | Adult | JB003 | Rubislaw | Rb9 | JLPX01.0002g | 89 | 38 |
| Juno Beach | 01/10 | Ibis | Adult | JB001 | Rubislaw | Rb3 | No Matches | ||
| Juno Beach | 01/10 | Ibis | Adult | JB002 | Rubislaw | Rb9 | JLPX01.0002g | 89 | 38 |
| Juno Beach | 01/10 | Ibis | Adult | JB016 | Rubislaw | Rb1 | JLPX01.0059g | 86 | 24 |
| Juno Beach | 03/12 | Ibis | Adult | JB053 | Anatum (17)e | An2 | JAGX01.0001 | 104 | 3 |
| Juno Beach | 12/12 | Ibis | Adult | JB303 | San Diego | Sd3 | No Matches | ||
| Juno Beach | 12/12 | Ibis | Adult | JB308 | Newport (3)e | Np1 | JJPX01.0025 | 184 | 0 |
| San Matera | 03/10 | Ibis | Adult | SM004 | Typhimurium (2)e | Tm6 | JPXX01.0946 | 96 | 0 |
| San Matera | 03/10 | Ibis | Adult | SM009 | Reading | Rd1 | JLGX01.0012g | 7 | 0 |
| Garden Lake | 03/10 | Ibis | Adult | GL011 | Rubislaw | Rb1 | JLPX01.0059g | 86 | 24 |
| Prosperity Oaks | 03/12 | Ibis | Adult | PPO 080 | Florida | Fl1 | No Matches | ||
| Prosperity Oaks | 03/12 | Ibis | Juvenile | PPO 082 | Florida | Fl2 | No Matches | ||
| Prosperity Oaks | 03/12 | Ibis | Unknown | PPO 085 | Bareilly (10e | Ba1 | JAPX01.0064 | 8 | 1 |
| Prosperity Oaks | 12/12 | Ibis | Adult | PPO 306 | Muenchen (8)e | Mu2 | No Matches | ||
| PGA National | 03/10 | Ibis | Adult | PGA001 | Braenderup (11)e | Br2 | JBPX01.0008g | 3 | 1 |
| PGA National | 03/10 | Ibis | Adult | PGA004 | Saint Paul (8)e | NT | ND | ||
| Burns Road Park | 03/10 | Ibis | Adult | BR001 | San Diego | Sd2 | No Matches | ||
| Burns Road Park | 03/10 | Ibis | Adult | BR007 | San Diego | Sd2 | No Matches | ||
| Lion Country Safari | 03/10 | Ibis | Adult | LCS017 | Newport (3)e | Np5 | JJPX01.0714g | 0 | 0 |
| Lion Country Safari | 12/12 | Ibis | Adult | LCS040 | Anatum (17)e | An2 | JAGX01.0001 | 104 | 3 |
| Lion Country Safari | 12/12 | Ibis | Adult | LCS300 | Bareilly (10)e | Ba1 | JAPX01.0064 | 8 | 1 |
| Lion Country Safari | 12/12 | Ibis | Adult | LCS304 | Newport (3)e | Np2 | JJPX01.0880 | 2 | 0 |
| Palm Beach Zoo | 03/12 | Ibis | Adult | PBZ060.1 | Anatum (17)e | An2 | JAGX01.0001 | 104 | 3 |
| Palm Beach Zoo | 03/12 | Ibis | Adult | PBZ060.2 | Baildon | Bl1 | TDEX01.0001 | 107 | 1 |
| Dreher Park | 03/12 | Ibis | Adult | DP071 | Rubislaw | Rb1 | JLPX01.0059g | 86 | 24 |
| Dreher Park | 03/12 | Ibis | Juvenile | DP077 | Schwarzengrund | Sw3 | JM6X01.0143 | 1 | 0 |
| Ibis Colony, Greater Everglades b | 04/13 | Ibis | Nestling | IC003 | Rubislaw | Rb6 | No Matches | ||
| Ibis Colony, Greater Everglades b | 04/13 | Ibis | Nestling | IC008 | Tallahassee | Tl1 | No Matches | ||
| Ibis Colony, Greater Everglades b | 04/13 | Ibis | Nestling | IC009 | Litchfield | Li1 | No Matches | ||
| Ibis Colony, Greater Everglades b | 04/13 | Ibis | Nestling | IC010 | Gaminara | Ga1 | No Matches | ||
| Ibis Colony, Greater Everglades b | 04/13 | Ibis | Nestling | IC012 | Litchfield | Li4 | No Matches | ||
| Ibis Colony, Greater Everglades b | 03/13 | Ibis | Nestling | IC016 | Rubislaw | Rb5 | JLPX01.0273g | 3 | 0 |
| Ibis Colony, Greater Everglades b | 03/13 | Ibis | Nestling | IC019 | Hartford | Hf1 | JHAX01.0003g | 0 | 0 |
| Ibis Colony, Greater Everglades b | 03/13 | Ibis | Nestling | IC020 | Hartford | Hf3 | No Matches | ||
| Ibis Colony, Greater Everglades b | 03/13 | Ibis | Nestling | IC022 | Flint | Ft1 | TDHX01.0023 | 1 | 1 |
| Ibis Colony, Greater Everglades b | 05/13 | Ibis | Nestling | IC023 | Hartford | Hf2 | JHAX01.0080g | 0 | 0 |
| Ibis Colony, Greater Everglades b | 05/13 | Ibis | Nestling | IC024 | Litchfield | Li5 | No Matches | ||
| Ibis Colony, Greater Everglades b | 05/13 | Ibis | Nestling | IC026 | Anatum (17)f | An3 | JAGX01.0033 | 15 | 1 |
| Ibis Colony, Greater Everglades b | 05/13 | Ibis | Nestling | IC027 | Javiana (4)f | Jv4 | No Matches | ||
| Ibis Colony, Greater Everglades b | 05/13 | Ibis | Nestling | IC030 | Litchfield | Li3 | No Matches | ||
| Tamiami W Colony c | 03/13 | Ibis | Nestling | TW004A | Typhimurium (2)f | Tm1 | No Matches | ||
| Tamiami W Colony c | 03/13 | Ibis | Nestling | TW010 | Pensacola | Pn1 | No Matches | ||
| Tamiami W Colony c | 04/12 | Ibis | Nestling | TW034 | Litchfield | Li2 | No Matches | ||
| Tamiami W Colony c | 04/12 | Ibis | Nestling | TW039 | Muenchen(8)e | Mu1 | JJ6X01.0426g | 1 | 0 |
| Tamiami W Colony c | 04/13 | Ibis | Nestling | TW162-2 | Rubislaw | Rb8 | No Matches | ||
| Tamiami W Colony c | 04/13 | Ibis | Nestling | TW229B | Arechavelata | Ar1 | AREX01.0029 | 0 | 0 |
| Tamiami W Colony c | 04/13 | Ibis | Nestling | TW229C1 | Braenderup(11)f | Br4 | No Matches | ||
| Tamiami W Colony c | 04/13 | Ibis | Nestling | TW231 | Poona | Po1 | JL6X01.0678g | 1 | 1 |
a Sites are ordered in table corresponding to location from north (top) to south (bottom).
b Miami, Dade County.
c Ibis colony located in Broward County.
d Juveniles were identified by plumage color and were defined as birds < 3 years of age. Adults: >3 years of age. Nestlings were defined as birds that remained in the nest up to 25 days of age.
e Ranking of Salmonella serotype associated with human cases reporting for year of isolation in the U.S.
f Salmonella serotype in numerical ranking, in human cases for last year reported in in 2012.
g PFGE pattern not associated with any foodborne outbreaks for year of isolation.
h Matching entries for PFGE pattern for year of isolation.
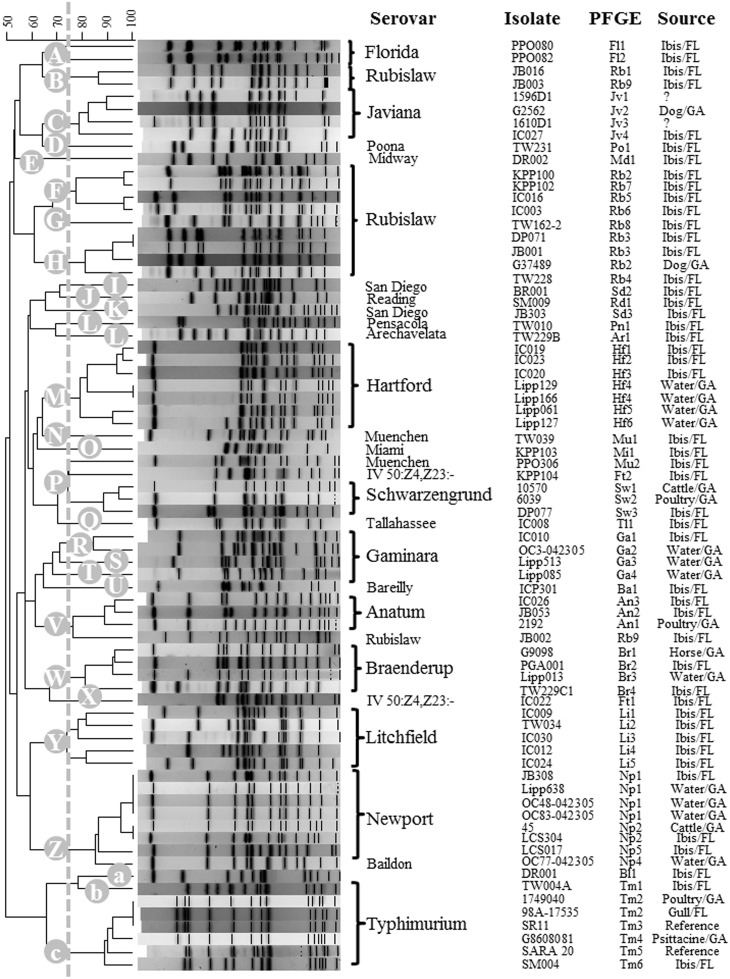
Fig 3. Cluter analysis of Salmonella.
Cluster analysis of Salmonella isolated from ibises by pulsed-field gel electrophoresis (PFGE) patterns generated with the restriction enzyme XbaI. Tiff images of Salmonella PFGE patterns were compared using DNA pattern recognition software, BioNumerics (Applied Maths, Austin, TX). Level of similarity was calculated using the band-based Dice similarity coefficient, and clustering of samples was performed using the unweighted pair-group method with arithmetic averaging (UPGMA). Salmonella PFGE patterns, generated in this study, were compared to a BioNumerics database of PFGE entries of Salmonella isolates from water and various animal species. PFGE patterns generated for ibis isolates were compared against an in-house database of Salmonella PFGE profiles for isolates from water or other animal species. A dendrogram was generated of PFGE patterns including those from isolates with the same or similar pattern (≥75% similarity). Clusters with >75% similarities were labeled A-Z, a-c on dendrogram tree on the left.
Salmonella serotypes Anatum, Braenderup, Hartford, Newport, Schwarzengrund, and Typhimurium from ibises had identical or highly similar PFGE patterns to archived isolates from water or other animal species [49, 58–60] (Fig 3). However, the PFGE patterns for Salmonella serotypes S. Bareilly, S. Muenchen, and S. Rubislaw isolated from ibises were distinct (<75% similarity) from PFGE profiles generated for the same serotypes isolated from other wildlife and water sources (data not shown).
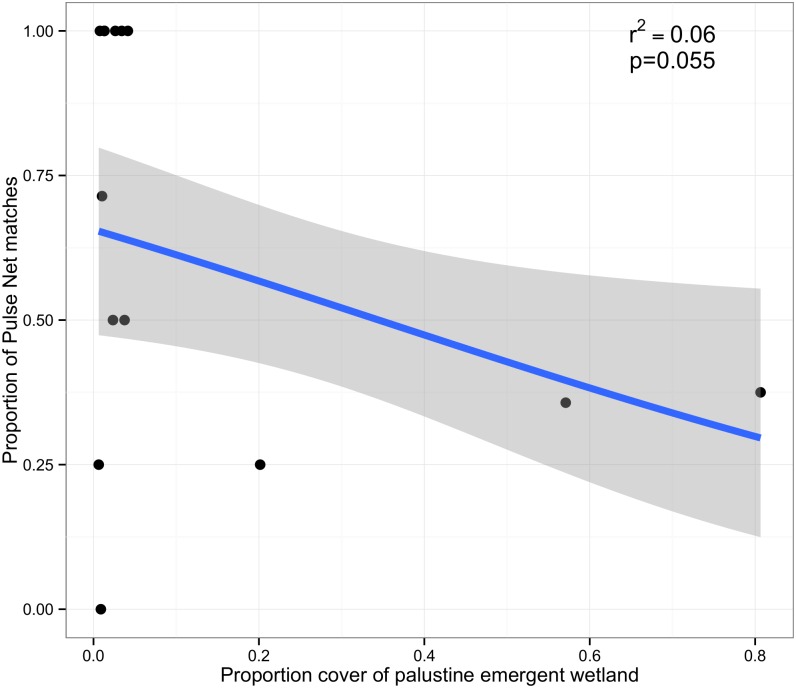
Additionally, forty-four percent of the Salmonella PFGE patterns for white ibis isolates (n = 43), matched profiles in the CDC PulseNet USA database. Several of these PulseNet patterns have not been associated with any foodborne outbreaks suggesting an environmental source (Table 4). Eighty percent of ibis isolates belonging to one of the top 20 Salmonella serotypes reported to the CDC (n = 15), had matching PFGE pattern with PulseNet. Twenty percent of entries reported to PulseNet, with matching patterns (n = 43), had reported human cases in Florida for the year we isolated the strain from ibis (n = 72, years 2010–2013). Florida had the most cases of any state associated with S. Rubislaw PulseNet patterns JLPX01.0002 and JLPX01.0059, and accounted for 86% human cases in Florida (n = 72). All Salmonella Rubislaw, isolated from ibises, produced unique PFGE patterns compared to PFGE patterns for S. Rubislaw isolated in Georgia [1]. Three of the eight white ibis S. Rubislaw PFGE patterns matched PFGE patterns for S. Rubislaw in PulseNet database of human cases. A non-random association was also observed in the distribution of Salmonella strain types from white ibises that matched human cases when examined by land cover type. There was a negative, but not statistically significant, relationship between Palustrine emergent wetland and the number of Salmonella isolates from white ibises that matched human cases in the PulseNet data base (p = 0.055; Fig 4). Furthermore, there was spatial and temporal overlap observed for Salmonella PFGE types isolated from ibises and humans in Florida (Table 5).
Fig 4. Salmonella matches and emergent wetland habitat type.
Salmonella isolates from white ibises in Florida that matched human isolates in the PulseNet database were negatively, and marginally statistically significantly related with the land cover type Palustrine emergent wetland.
Table 5. Salmonella PFGE patterns from American white ibis isolates that matched CDC Pulsenet database and human cases reported in Florida.
| Serotype | PulseNet Pattern | Date of Isolation | Florida County | ||
|---|---|---|---|---|---|
| Ibis | Human | Ibis | Human | ||
| Anatum | JAGX01.0001 | 03/2012;12/2012 | 02/24/2014 | Palm Beach | Lee |
| 05/22/2012 | Palm Beach | Broward | |||
| 11/20/2012 | Palm Beach | Indian River | |||
| JAGX01.0033 | 05/2013 | 12/28/2012 | Dade | Hillsborough | |
| 06/23/2013 | Dade | Putnam | |||
| Baildon | TDEX01.0001 | 03/2012 | 08/07/2010 | Palm Beach | Broward |
| Bareilly | JAPX01.0064 | 12/2012 | 08/20/2012 | Palm Beach | Broward |
| Braenderup | JBPX01.0008 | 03/2010 | 02/12/2010 | Palm Beach | Unknown |
| IV 50:Z4,Z23:- (formerly Flint) | TDHX01.0023 | 03/2013 | 10/31/2013 | Greater Everglades | Volusia |
| Lomalinda | TDEX01.0001 | 3/2010 | 08/07/2010 | Palm Beach | Broward |
| Miami | TEAX01.0045 | 07/2013 | 06/08/2013 | Okeechobee | Pasco |
| Rubislaw | JLPX01.0059 | 01/2010; 03/2010 | 01/14/2010 | Palm Beach | Lee |
| 02/16/2010 | Palm Beach | ||||
| 03/13/2010 | Broward | ||||
| 04/11/2010 | Pinellas | ||||
| 04/24/2010 | Palm Beach | ||||
| 05/08/2010 | Orange | ||||
| 05/29/2010 | Orange | ||||
| 06/12/2010 | Palm Beach | ||||
| 06/16/2010 | Broward | ||||
| 06/27/2010 | Broward | ||||
| 07/08/2010 | Broward | ||||
| 07/13/2010 | Orange | ||||
| 07/14/2010 | Hillsborough | ||||
| 07/18/2010 | Palm Beach | ||||
| 07/30/2010 | Broward | ||||
| 08/02/2010 | Orange | ||||
| 08/19/2010 | Brevard | ||||
| 08/19/2010 | Sarasota | ||||
| 08/23/2010 | Broward | ||||
| 08/28/2010 | Hillsborough | ||||
| 08/31/2010 | Pinellas | ||||
| 09/17/2010 | Brevard | ||||
| 09/21/2010 | Hillsborough | ||||
| 10/08/2010 | Seminole | ||||
| 10/09/2010 | Seminole | ||||
| 10/09/2010 | Lake | ||||
| 11/11/2010 | Hillsborough | ||||
| 11/15/2010 | Hillsborough | ||||
| 12/31/2010 | Hillsborough | ||||
| 3/2012 | 02/14/2012 | Palm Beach | Lee | ||
| 02/23/2012 | Palm Beach | ||||
| 03/30/2012 | Broward | ||||
| 04/24/2012 | Unknown | ||||
| 06/07/2012 | Hillsborough | ||||
| 06/09/2012 | Pinellas | ||||
| 06/30/2012 | Hillsborough | ||||
| 07/01/2012 | Pinellas | ||||
| 08/09/2012 | Polk | ||||
| 08/18/2012 | Lee | ||||
| 08/19/2012 | Lake | ||||
| 08/21/2012 | Hillsborough | ||||
| 09/03/2012 | Broward | ||||
| 09/10/2012 | Hillsborough | ||||
| 09/14/2012 | Palm Beach | ||||
| 10/03/2012 | Sarasota | ||||
| 10/06/2012 | Broward | ||||
| 10/15/2012 | Polk | ||||
| 10/20/2012 | Pasco | ||||
| 11/01/2012 | Lee | ||||
| 11/04/2012 | Palm Beach | ||||
| 11/23/2012 | Broward | ||||
| 12/12/2012 | Unknown | ||||
| 12/28/2012 | Palm Beach | ||||
Discussion
Previous reports have documented isolated and limited outbreaks of salmonellosis of colony-nesting birds, particularly during die-offs of nestlings [19, 39, 61–63]; however, this is the first report on the prevalence of Salmonella spp. in healthy, American white ibises. We found that the prevalence of Salmonella in ibises decreased as the percent of Palustrine wetlands and herbaceous grasslands increased, suggesting that land cover types that are closer to natural ecosystems support birds with a lower prevalence of carriage. White ibises are more highly dispersed in these habitats and wetlands/herbaceous grasslands provide high quality foraging habitat. Conversely, the prevalence of Salmonella shedding increased with the Open Developed land cover type. Ibises, as well as other peridomestic species known to carry Salmonella, seem to be highly attracted to open, developed areas because they are an optimal mixture of anthropogenic materials with high levels of vegetation that promote both foraging in grasses (particularly recently irrigated lawns and golf courses) and receiving handouts in parks and fields [64]. Urban parks in particular, where white ibises congregate in search of food provisions, promote the prolonged use of these sites by birds at high densities of individuals, likely resulting in cycles of Salmonella infection within those populations. Congregating around food sources, especially anthropogenic sources, is a well-known risk for transmission of pathogens, including Salmonella, among numerous wildlife species [65]. Persistently contaminated environments are a result of both birds that are carriers intermittently excreting salmonellae into the environment, and the environmental persistence of Salmonella spp [23, 66]. We found a significantly higher in prevalence in 2012, when compared to 2010, which may be attributed to hydrological conditions and its effect on the foraging behavioral patterns of white ibis. Years of unusually high or low precipitation create situations in natural wetlands that promote the utilization of anthropogenic food sources by white ibises [67] and the year 2012 was among the driest of our sampling period and one that would be consistent with increased urban foraging. While we did not find a positive association between the habituation score (as an indicator of dependency to urban habitats) and Salmonella prevalence, it is important to note that our comparison is based a small sample of animals that were not habituated and that behavior among birds may be site-specific.
The high diversity of Salmonella serotypes and strains found in white ibises suggests a lack of host-specificity and indicates that white ibises are likely to acquire Salmonella from their environment. Similarly, field and experimental studies of adult herring gulls (Larus smithonianus) and other urbanized birds demonstrate rapid elimination of Salmonella from the intestines, and a clear relationship between human/domestic animal waste and Salmonella colonization. This suggests that like these birds, ibis may be transiently colonized by Salmonella and may simply serve as a mechanical transport mechanism for the movement of salmonellae ingested from contaminated environments (for example, see Cizek et al.1994 [68], Reche et al 2003 [34] but for a review, see Tizard, 2004 [24]). Of the most frequently isolated serotypes from ibises, S. Litchfield, S. Rubislaw and S. Miami were specifically correlated with wetland land cover types—serotypes that are not among the most frequently reported in humans in Florida or US-wide (S2 Fig).
Twenty-five of the PFGE patterns detected in ibis matched human clinical cases in the PulseNet database. Of those, 72% (n = 18) matched human cases from the state of Florida. There was a negative but only marginally statistically significant relationship between the number of isolates that matched human cases and emergent wetlands. At first glance, it might appear that age influenced this result, as nestlings made up a large proportion of the isolates from that land cover type and had the highest prevalence of infection; however, the nestlings we sampled were still fed solely by their parents who are foraging in nearby available habitat, confirming that the isolates from ibises in natural environments are dissimilar from human isolates.
The Florida Department of Health reports that the majority of the 5,000–6,000 human salmonellosis cases reported every year are sporadic in nature, or not associated with a food outbreak, and the most significant sources of these infections have not been identified to date (Florida Dept of Health, 2012 [69]). In urban parks in Palm Beach County, people actively feed white ibises at very close range (birds take food from people’s hands) and it is common to see people, especially children and the elderly, leaning or sitting on surfaces contaminated with white ibis feces. Specifically in Florida, the state that houses the largest breeding colonies of white ibises in the US, there are over 5,000 cases of salmonellosis every year, of which nearly half are children less than 5 years of age for which consequences of infection can be severe (Florida Bureau of Environmental Public Health Medicine, Division of Environmental Health web 2011 [70]). Ibises have always been a common sight in southern Florida, but their adaption to urban habitats due to wetland loss and the increasing urbanization in Palm Beach County may increase the interaction between ibises and people [71]. Recently, urbanized ibis flocks have been noted in other urban centers in south and central Florida (Hernandez, personal observations). In Australia, highly urbanized ibises of a different species but with similar ecology are described as a nuisance and considered a threat public health threat, in part due to their carriage with Salmonella [32, 47]
These results indicate that ibises are likely acquiring salmonellae from environmental sources, may be good indicators of salmonellae circulating in their environment, and have the potential and opportunity to transmit salmonellae to people. Future testing of environmental samples is recommended. Additionally, our preliminary work of radio tagged ibises suggests that ibises tagged at urban sites move among several urban foraging sites on a weekly basis, and exhibit high site fidelity to urban habitats during the non-breeding season (using urban parks on a daily basis), yet fly long distances to return to natural areas to roost at night, such as the Loxahatchee National Wildlife Refuge or back and forth to nest and acquire food for nestlings between March-July in the Greater Everglades Ecosystem (Hernandez, unpublished data) where they can disseminate salmonellae acquired in urban areas. It is at these remote breeding areas that nestling mortality is likely to occur but least likely to be detected. Further research is needed to elucidate the relationship between habitat type, and the rate and persistence of Salmonella infection, and strain types.
Supporting Information
Rarefaction prediction for serotype diversity of Salmonella isolated from white ibises in Palm Beach, Florida.
(TIF)
Ordination analysis for serotype diversity and sampling site of Salmonella isolated from white ibises in Palm Beach, Florida. The size of the circles varies in size based on the proportion of wetland land cover within a 1 km radius from the sampling site.
(TIFF)
Acknowledgments
We truly appreciate the cooperation of the veterinarians Drs. Mark Cunningham and Dan Wolf with the Florida Wildlife Conservation Commission, staff at the Lion Country Safari, the Palm Beach Zoo, Kissimmee State Park, and the Solid Waste Authority, and the towns of Juno Beach and Palm Beach for permission and help in accessing urban ibises. Similarly, we appreciate the participation of several private landowners that allowed sampling. We thank Henry Adams for technical assistance with this manuscript. We sincerely appreciate the help of various graduate and undergraduate students who participated in the collection of samples and handling of ibises including Shaylin Duncan, Karen Christ, Henry Adams, Sarah Coker, Sebastian Ortiz and others.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was funded by National Institutes of Health 1R15AI089565-01 to Erin K Lipp; American Association of Zoo Veterinarians to Dr Sonia M. Hernandez; University of Georgia to Dr Sonia Maria Hernandez; and National Science Foundation EEID 1518611 to Dr. Sonia M. Hernandez. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Thorns CJ. Bacterial food-borne zoonoses. Revue scientifique et technique-International Office of Epizootics. 2000;19: 226–239. [DOI] [PubMed] [Google Scholar]
- 2.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, et al. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis. 2011. January;17(1):7–15 10.3201/eid1701.P11101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vugia D, Cronquist A, Hadler J, Tobin-D'angelo M, Blythe D, Smith K, et al. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food—10 states, United States, 2005. MMWR. Morb & Mort Weekly Rep. 2006;55: 392–395. [PubMed] [Google Scholar]
- 4.CDC. Multistate outbreaks of Salmonella serotype Poona infections associated with eating Cantaloupe from Mexico—United States and Canada. MMWR. Morb & Mort Weekly Rep. 2002;51:1044–1047. [PubMed] [Google Scholar]
- 5.CDC. Outbreak of Salmonella serotype Enteritidis infections associated with raw almonds. 2004;53:484–487. [PubMed] [Google Scholar]
- 6.CDC. Multistate outbreaks of Salmonella infections associated with Raw Tomatoes eaten in Restraurants—United States, 2005–2006. MMWR. Morb. Mort. Weekly Rep. 2007b;56:909–911. [PubMed] [Google Scholar]
- 7.CDC. Salmonella Annual Summary, 2006. Atlanta: US Department of Health and Human Services, CDC; 2008 [Google Scholar]
- 8.CDC. Outbreak of Salmonella serotype Saintpaul infections associated with eating alfalfa sprouts—United States, 2009. MMWR. Morb Mort Weekly Rep. 2009;58:500–503. [PubMed] [Google Scholar]
- 9.CDC. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food—10 states, 2010. MMWR. Morb Mort Weekly Rep. 2010;59(14);418–422. [PubMed] [Google Scholar]
- 10.Greene SK, Daly ER, Talbot EA, Demma LJ, Holzbauer S, Patel NJ, et al. Recurrent multistate outbreak of Salmonella Newport associated with tomatoes from contaminated fields, 2005. Epidemiol Infect. 2008. February 1;136(02):157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hedberg CW, Angulo FJ, White KE, Langkop CW, Schell WL, Stobierski MG, et al. Outbreaks of salmonellosis associated with eating uncooked tomatoes: implications for public health. Epidemiol Infect. 1999;122:385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lynch M, Painter J, Woodruff R, Braden C. Surveillance for foodborne-disease outbreaks—United States, 1998–2002. MMWR. Morb Mort Weekly Rep. 2006;55:1–34. [PubMed] [Google Scholar]
- 13.Mohle-Boetani JC, Farrar J, Bradley P, Barak JD, Miller M, Mandrell R, et al. Salmonella infections associated with mung bean sprouts: epidemiological and environmental investigations. Epidemiol Infect. 2009;137:357–366. 10.1017/S0950268808000411 [DOI] [PubMed] [Google Scholar]
- 14.Proctor ME, Hamacher M, Tortorello ML, Archer JR, Davis JP. Multistate outbreak of Salmonella serotype Muenchen infections associated with alfalfa sprouts grown from seeds pretreated with calcium hypochlorite. J Clin Microbiol. 2001;39:3461–3465. 10.1128/JCM.39.10.3461-3465.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olsen SJ, Bishop R, Brenner FW, Roels TH, Bean N, Tauxe RV, et al. The Changing Epidemiology of Salmonella: Trends in Serotypes Isolated from Humans in the United States, 1987–1997. J Infect Dis. 2001;183: 753–761. 10.1086/318832 [DOI] [PubMed] [Google Scholar]
- 16.CDC. National Salmonella Surveillance Annual Report, 2012. Atlanta: US Department of Health and Human Services, CDC; 2014. [Google Scholar]
- 17.Tauni MA, Österlund A. Outbreak of Salmonella typhimurium in cats and humans associated with infection in wild birds. J Small Anim Prac. 2000;41: 339–341. [DOI] [PubMed] [Google Scholar]
- 18.Alley MR, Connolly JH, Fenwick DG, Mackereth GF, Leyland MJ, Rogers LE, et al. An epidemic of salmonellosis caused by Salmonella Typhimurium DT160 in wild birds and humans in New Zealand. NZ Vet J. 2002;50: 170–176. [DOI] [PubMed] [Google Scholar]
- 19.Refsum T, Vikoren T, Handeland K, Kapperud G, Holstad G. Epidemiologic and pathologic aspects of Salmonella typhimurium infection in passerine birds in Norway. J Wild Dis. 2003;39: 64–72. [DOI] [PubMed] [Google Scholar]
- 20.Pennycott TW, Park A, Mather HA. Isolation of different serotypes of Salmonella enterica from wild birds in Great Britain between 1995 and 2003. Vet Rec. 2006;158:817–820. [DOI] [PubMed] [Google Scholar]
- 21.Une Y, Sanbe A, Suzuki S, Niwa T, Kawakami K, Kurosawa R, et al. Salmonella enterica serotype Typhimurium infection causing mortality in eurasian tree sparrows (Passer montanus) in Hokkaido. Jpn. J Infect Dis. 2008;61:166–167. [PubMed] [Google Scholar]
- 22.Fukui D, Takahashi K, Kubo M, Une Y, Kato Y, Izumiya H. Mass mortality of eurasian tree sparrows (Passer montanus) from Salmonella typhimurium DT40 in Japan, winter 2008–2009. J Wild Dis. 2014;50(3), 2014, pp. 484–495 [DOI] [PubMed] [Google Scholar]
- 23.Friend M, Franson JC. Salmonellosis. Field manual of wildlife diseases. General field procedures and diseases of birds. No. ITR-1999-001. Geological Survey Madison, WI Biological Resources Div; 1999.
- 24.Tizard I. Salmonellosis in wild birds. Seminars in Avian and Exotic Pet Medicine. 2004;13:50–66 [Google Scholar]
- 25.Daoust PY, Prescott JF. Salmonellosis In: Hunter DB, Thomas NJ, editors. Infectious diseases of wild birds. Ames: Blackwell Publishing; 2007;270–88. [Google Scholar]
- 26.Daoust PY, Busby DG, Ferns L, Goltz J, McBurney S, Poppe C, et al. Salmonellosis in songbirds in the Canadian Atlantic provinces during winter-summer 1997–98. Can Vet J. 2000;41:54–59. [PMC free article] [PubMed] [Google Scholar]
- 27.Hall AJ, Saito EK. Avian wildlife mortality events due to salmonellosis in the United States, 1985–2004. J Wildl Dis. 2008. July;44:585–593. 10.7589/0090-3558-44.3.585 [DOI] [PubMed] [Google Scholar]
- 28.Lawson B, Howard T, Kirkwood JK, Macgregor SK, Perkins M, Robinson RA, et al. Epidemiology of Salmonellosis in Garden Birds in England and Wales, 1993 to 2003. Ecohealth. 2010;7(3): 294–306 10.1007/s10393-010-0349-3 [DOI] [PubMed] [Google Scholar]
- 29.Lawson B, de Pinna E, Horton RA, Macgregor ES, John SK, Chantrey J, et al. Epidemiological Evidence That Garden Birds Are a Source of Human Salmonellosis in England and Wales. PloS one. 2014. Feb 1;9(2):e88968 10.1371/journal.pone.0088968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Girdwood RW, Fricker CR, Munro D, Shedden CB, Monaghan P. The incidence and significance of salmonella carriage by gulls (Larus spp.) in Scotland. J Hyg. 1985. October 1;95(02):229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daisuke F. Infectious Diseases Associated with Relation between Humans and Wildlife—Consideration on Wild Bird Mortality and Bird-Feeding. Jpn J Zoo & Wild Med. 2013;18(2):41–48 [Google Scholar]
- 32.Epstein JH, McKee J, Shaw P, Hicks V, Micalizzi G, Daszak P, et al. The Australian white ibis (Threskiornis molucca) as a reservoir of zoonotic and livestock pathogens. EcoHealth. 2006. December 1;3(4):290–8. [Google Scholar]
- 33.Pedersen K, Clark L, Andelt WF, Salman MD. Prevalence of shiga toxin-producing Escherichia coli and Salmonella enterica in rock pigeons captured in Fort Collins, Colorado. J Wildl Dis. 2006;42:46–55. 10.7589/0090-3558-42.1.46 [DOI] [PubMed] [Google Scholar]
- 34.Reche MP, Jiménez PA, Alvarez F, García de los Rios JE, Rojas AM, de Pedro P. Incidence of salmonellae in captive and wild free-living raptorial birds in central Spain. J Vet Med B Infect Dis. Vet. Public Health. 2003;50:42–44. [DOI] [PubMed] [Google Scholar]
- 35.Refsum T, Heir E, Kapperud G, Vardund T, Holstad G. Molecular epidemiology of Salmonella enterica serovar typhimurium isolates determined by pulsed-field gel electrophoresis: comparison of isolates from avian wildlife, domestic animals, and the environment in Norway. Appl & Environ Microbiol. 2002;68: 5600–5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bradley CA, Altizer S. Urbanization and the ecology of wildlife diseases. Trends Eco & Evo. 2007;22: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hernandez SM, Keel K, Sanchez S, Trees E, Gerner-Smidt P, Adams JK, et al. Epidemiology of a Salmonella enterica subsp. enterica serovar Typhimurium strain associated with a songbird outbreak. Appl Enviro Microbiol. 2012;78:7290–7298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hauser E, Hühn S, Junker E, Jaber M, Schroeter A, Helmuth R, et al. Characterization of a phenotypic monophasic variant belonging to Salmonella enterica subsp. enterica serovar Typhimurium from wild birds and its possible transmission to cats and humans. Berl Munch Tierarztl Wochenschr. 2012;122:169–177. [PubMed] [Google Scholar]
- 39.Kapperud G, Stenwig H, Lassen J. Epidemiology of Salmonella typhimurium O:4–12 infection in Norway: evidence of transmission from an avian wildlife reservoir. Am J Epi. 1998;147: 774–782. [DOI] [PubMed] [Google Scholar]
- 40.Nesse LL, Refsum T, Heir E, Nordby K, Vardund T, Holstad G. Molecular epidemiology of Salmonella spp. isolates from gulls, fish-meal factories, feed factories, animals and humans in Norway based on pulsed-field gel electrophoresis. Epidemiol Infect 2005;133:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palmgren H, Aspán A, Broman T, Bengtsson K, Blomquist L, Bergström S, et al. Salmonella in Black-headed gulls (Larus ridibundus); prevalence, genotypes and influence on Salmonella epidemiology. Epidemiol Infect 2006;134:635–644. 10.1017/S0950268805005261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Penfold JB, Amery HC, Peet PJ. Gastroenteritis associated with wild birds in a hospital kitchen. Brit Med J. 1979;29: 802. [PMC free article] [PubMed] [Google Scholar]
- 43.Kushlan JA. Feeding Ecology and Prey Selection in the White Ibis. The Condor. 1979;81: 376–389. [Google Scholar]
- 44.Bildstein KL, Post W, Johnston J, Frederick P. Freshwater Wetlands, Rainfall, and the Breeding Ecology of White Ibises in Coastal South Carolina. The Wilson Bulletin. 1990;102: 84–98. [Google Scholar]
- 45.Gawlik DE. The Effects of Prey Availability on the Numerical Response of Wading Birds. Eco Mono. 2002;72: 329–346. [Google Scholar]
- 46.Kushlan JA. Supplemental Information for the White Ibis: Biological Status Review Report. Tallahassee: Florida Fish and Wildlife Conservation Commission; 2011; pp. 3. [Google Scholar]
- 47.Martin JM, French K, Ross GA, Major RE. Foraging distances and habitat preferences of a recent urban coloniser: The Australian white ibis. Lan & Urb Plan. 2011;102: 65–72. [Google Scholar]
- 48.Tiner RW. Wetlands of the United States: current status and recent trends. United Fish and Wildlife Service.1984. Accessed: http://repositories.tdl.org/tamug-ir/bitstream/handle/1969.3/21208/3386-Wetlands%20of%20the%20United%20States-Current%20Status%20and%20Recent%20Trends.pdf?sequence=1
- 49.Maurer JJ, Martin G, Hernandez SM, Cheng Y, Gerner-Smidt P, Hise KB, et al. Diversity and persistence of Salmonella enterica strains in rural landscapes in the Southeastern United States. PloS one. 2015;10(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gerner-Smidt P, Hise K, Kincaid J, Hunter S, Rolando S, Hyytia-Trees E, et al. PulseNet USA: a five-year update. Food Pathog Dis. 2006;3:9–19. [DOI] [PubMed] [Google Scholar]
- 51.Zamperini K, Soni V, Waltman D, Sanchez S, Theriault EC, Bray J, et al. Molecular characterization reveals Salmonella enterica serovar 4,[5],12:i:- from poultry is a variant Typhimurium serovarserotype. Avian Dis. 2007;51:958–964. 10.1637/7944-021507-REGR.1 [DOI] [PubMed] [Google Scholar]
- 52.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2016; Available: http://www.R-project.org/ [Google Scholar]
- 53.Bates D, Maechler M, Bolker B, Walker S, Christensen RHB, Singmann H, et al. Linear mixed-effects models using ‘Eigen’ and S4, ver 1.1–8. 2015; Available at http://lme4.r-forge.r-project.org/
- 54.Bartoń K. Model selection and model averaging based on information criteria (AICc and alike), ver 1.15.6. 2016; Available at https://cran.r-project.org/web/packages/MuMIn/MuMIn.pdf
- 55.Mardia KV, Kent JT, Bibby JM. Multivariate Analysis. 1st ed London: Academic Press; 1979 [Google Scholar]
- 56.Bivand R. Spatial Dependence: Weighting Schemes, Statistics and Models, ver 0.6–5. 2016; Available: https://cran.r-project.org/web/packages/spdep/spdep.pdf
- 57.Oksanen J, Guillaume Blanchet F, Friendly M, Kindt R, Legendre P, McGlinn D, et al. Community Ecology package. Ver 2.4–0. Available at https://cran.r-project.org/web/packages/vegan/vegan.pdf
- 58.Liljebjelke KA, Hofacre CL, Liu T, White DG, Ayers S, Young S, et al. Vertical and horizontal transmission of Salmonella within integrated broiler production system. Food Path & Dis. 2005. Mar 1;2(1):90–102. [DOI] [PubMed] [Google Scholar]
- 59.Meinersmann RJ, Berrang ME, Jackson CR, Fedorka-Cray P, Ladely S, Little E, et al. Salmonella, Campylobacter and Enterococcus spp.: Their antimicrobial resistance profiles and their spatial relationships in a synoptic study of the Upper Oconee River basin. Micro Eco. 2008. Apr 1;55(3):444–52 [DOI] [PubMed] [Google Scholar]
- 60.Haley BJ, Cole DJ, Lipp EK. Distribution, diversity, and seasonality of waterborne salmonellae in a rural watershed. Appl & Environ Microbiol. 2009. March 1;75(5):1248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Phalen DN, Drew ML, Simpson B, Roset K, Dubose K, Mora M. Salmonella enterica subsp. Enterica in Cattle Egret (Bubulcus ibis) chicks from central Texas: prevalence, serotypes, pathogenicity, and epizootic potential. J of Wild Dis. 2010;46: 379–389. [DOI] [PubMed] [Google Scholar]
- 62.Velarde R, Porrero MC, Serrano E, Marco I, García M, Téllez S, et al. Septicemic Salmonellosis aused by salmonella hessarek in wintering and migrating Song Thrushes (Turdus philomelos) in Spain. J of Wild Dis. 2012;48: 113–121. [DOI] [PubMed] [Google Scholar]
- 63.Locke LN, Ohlendorf HM, Shillinger RB, Jareed T. Salmonellosis in a captive heron colony. J Wild Dis. 1974. April;10(2):143–5. [DOI] [PubMed] [Google Scholar]
- 64.Oliver AJ, Hong-Wa C, Devonshire J, Olea KR, Rivas GF, Gahl MK. Avifauna richness enhanced in large, isolated urban parks. Landscape and Urban Planning. 2011. Sept 1;102(4): 215–225. [Google Scholar]
- 65.Miller S, Zieger U, Ganser C, Satterlee SA, Bankovich B, Amadi V. Influence of land use and climate on salmonella carrier status in the small Indian mongoose (Herpestes auropunctatus) in Grenada, West Indies. J Wild Dis. 2015. January;51(1):60–8. [DOI] [PubMed] [Google Scholar]
- 66.Wales A, Davies RH. Environmental Aspects of Salmonella In: Barrow PA, Methner U, editors. Salmonella in Domestic Animals. Oxfordshire: CABI International; 2013. pp 399–426. [Google Scholar]
- 67.Dorn NJ, Cook MI, Herring G, Boyle B, Nelson J, Gawlik DE. Aquatic prey switching and urban foraging by the White Ibis Eudocimus albus are determined by wetland hydrological conditions. Ibis. 2011;153, 323–335. [Google Scholar]
- 68.Cizek A, Literak I, Hejlicek K, Treml F, Smola J. Salmonella contamination of the environment and its incidence in wild birds. J Vet Med B. 1994;41(5): 320–327 [DOI] [PubMed] [Google Scholar]
- 69.Dept Florida. Health 2012.
- 70.Florida Bureau of Enviro 2011.
- 71.Mitsch WJ, Gosselink JG. Wetlands. 4th ed Hoboken: John Wiley & Sons; 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Rarefaction prediction for serotype diversity of Salmonella isolated from white ibises in Palm Beach, Florida.
(TIF)
Ordination analysis for serotype diversity and sampling site of Salmonella isolated from white ibises in Palm Beach, Florida. The size of the circles varies in size based on the proportion of wetland land cover within a 1 km radius from the sampling site.
(TIFF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.