Abstract
Background: Evidence suggests that obesity in adulthood is associated with increased risk of “clinically significant” prostate cancer. However, studies of body mass index (BMI) across the adult life course and prostate cancer risks remain limited.
Methods: In a prospective cohort of 69 873 men in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial, we examined associations of prediagnostic BMI across the adult life course with risk of incident prostate cancer and fatal prostate cancer (prostate cancer–specific mortality). At 13 years of follow-up, we identified 7822 incident prostate cancer cases, of which 3078 were aggressive and 255 fatal. BMI trajectories were determined using latent-class trajectory modeling. Cox proportional hazards regression was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs).
Results: BMI at age 20 years, 50 years, and baseline questionnaire (mean age = 63 years) were associated with increased risks of fatal prostate cancer (HRs = 1.27–1.32 per five-unit increase). In five BMI trajectories identified, fatal prostate cancer risk was increased in men who had a normal BMI (HR = 1.95, 95% CI = 1.21 to 3.12) or who were overweight (HR = 2.65, 95% CI = 1.35 to 5.18) at age 20 years and developed obesity by baseline compared with men who maintained a normal BMI. Aggressive and nonaggressive prostate cancer were not associated with BMI, and modest inverse associations were seen for total prostate cancer.
Conclusions: Our results suggest that BMI trajectories during adulthood that result in obesity lead to an elevated risk of fatal prostate cancer.
The obesity epidemic in the United States has resulted in more than two-thirds of US men being classified as overweight or obese. The potential impact of obesity on cancer incidence and cancer-related mortality—particularly prostate cancer—remains to be fully elucidated (1,2). Previous studies of obesity and prostate cancer have found that risk varies by stage of disease, tumor grade, and cause-specific mortality (3). Recent systematic reviews and meta-analyses have indicated that greater body mass index (BMI) is associated with increased risks of aggressive/advanced prostate cancer and prostate cancer–specific mortality (4–6), but the relationships for total incident and nonaggressive prostate cancer remain inconclusive (6–9).
There is accumulating evidence that an earlier age at onset of obesity may be influential in the etiology of prostate cancer (10–14). Studies have investigated cumulative and per annum weight change during adulthood on risk of aggressive incident prostate cancer and prostate cancer–specific mortality (fatal prostate cancer), yet findings remain inconsistent (6,15). The influence of body size on prostate cancer development has provided important insights, yet no study to date has investigated both cumulative measures of BMI and latent trajectories of changes in BMI over multiple periods of adulthood on prostate cancer incidence and mortality.
The relationship between obesity, weight change, and prostate cancer is complex (16,17). Studies of the timing of obesity and changes in weight across the adult life course may help elucidate biological mechanisms that promote prostate carcinogenesis and that differentiate aggressive prostate cancer from nonaggressive disease. Using prospective cohort data from the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial, we assessed life course trajectories of BMI in relation to prostate cancer risks.
Methods
Study Population
The PLCO enrolled 76 683 men age 55 to 74 years (median age = 62 years) during 1993–2001 from 10 cancer screening centers across the United States. The PLCO design has been described previously (18). In brief, men were randomly assigned to usual care with opportunistic screening or to annual screening with prostate-specific antigen (PSA) testing at baseline and for a further six years (with optional annual digital rectal exam at baseline and for a further four years). Participants were followed for incident cancer diagnoses and cause-specific mortality. All men were uncompensated volunteers from the general population who no prior medical history of any cancer (except nonmelanoma skin cancer [NMSC]). Men were excluded for prior removal of their prostate gland, participation in another cancer screening or prevention trial, Finasteride use within six months prior to enrollment, and—starting in 1995 after a study protocol change—if they had received more than one PSA test in the three-year period prior to enrollment.
Of the 73 413 eligible men that completed the baseline questionnaire with informed consent, we excluded 1655 men who had prior history of cancer (excluding NMSC), 1715 men with missing height or weight, and 170 men with implausible BMIs (<15 or > 60 kg/m2), resulting in a final analytic cohort of 69 873 participants.
The study protocol was approved by the Institutional Review Board of the National Cancer Institute.
Exposure Ascertainment
At study entry, participants were asked their current height and body weight, as well as to recall such for ages 20 and 50 years. Height at study entry was used to derive BMI (calculated as weight [kg]/height [m2]) at each respective age. BMI categories were based on the World Health Organization (WHO) 2000 classifications: less than 18.5 (underweight), 18.5 to 24.9 (normal weight), 25.0 to 29.9 (overweight), and 30 or greater (obese). Mean BMIs during periods of adulthood were also assessed as an alternate method of examining the influence of the sustained or cumulative effects of excessive body weight. To examine trajectories of BMI change, we applied latent-class group-based trajectory models to identify and examine distinct groups of men with similar BMI patterns (19).
Outcome Ascertainment
Incident cancer diagnoses were histologically verified malignant adenocarcinoma of the prostate. Prostate cancer–specific death was ascertained by active follow-up using annual study update questionnaires and periodic linkage to the National Death Index (20). Aggressive prostate cancer was defined as biopsy Gleason score of 7 or higher, American Joint Committee on Cancer (AJCC) tumor node metastasis (TNM) clinical stage of III or higher, or prostate cancer–specific mortality; all remaining prostate cancers were classified as nonaggressive. Fatal prostate cancer was defined as prostate cancer–specific mortality (prostate cancer was the underlying cause of death).
Statistical Analysis
Cox proportional hazards regression models, with age in months as the time metric and baseline hazards stratified by calendar year of study entry (21), were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) of associations between anthropometric measures and prostate cancer risks (22,23). We standardized absolute risks within five-year age bands to the age distribution of all males in PLCO cohort (24). Follow-up started from the age at the baseline questionnaire until the age at prostate cancer diagnosis/death (event), or right-censored at age of study withdrawal (loss to follow-up), age at death from causes other than prostate cancer, and age at the end of follow-up (December 31, 2009), whichever occurred first. The proportional hazards assumption was assessed comparing models with and without a log(time)-interaction term using a likelihood ratio test.
Covariates that were associated (P < 0.1) with both the exposure and outcome of interest, or deemed a priori as potential confounders (screening arm, race, family history), were included in multivariable models. This resulted in adjusting all models for screening arm, family history of prostate cancer, race, study center, education, marital status, cigarette smoking status, diabetes, myocardial infarction, and PSA history during the three years prior to enrollment. Tests for trend (Ptrend) were modeled using medians of each category assessed. We tested for effect modification (Pinteraction) by covariates chosen a priori (study arm, race, diabetes, and smoking status) using a likelihood ratio test.
To examine BMI trajectories, we applied latent-class group-based trajectory models in order to define groups of men with similar patterns of BMI change during adulthood (25). We modeled BMI change using linear and quadratic polynomials with three to six trajectories permitted (with a minimum of 1% of participants per trajectory) using PROC TRAJ (SAS Institute, Inc., Cary, NC) (26). The optimal model (number of trajectory groups and pattern) was determined based on model fit using the change in Bayesian Information Criterion (BIC). Associations between trajectory categories and prostate cancer risks were assessed using Cox proportional hazards regression as previously described.
Sensitivity Analyses
We assessed weight change in relation to prostate cancer risks with additional adjustment for 1) weight at the beginning of the period of interest and 2) mean weight during the period (which has a lower correlation with weight change than initial weight) (27). We examined three alternate definitions of aggressive prostate cancer (Supplementary Methods, available online) and conducted analyses within each trial arm and with a combined grouping for overweight/obese BMI. We examined a subcohort who completed a supplemental questionnaire (SQX) during 2006–2008 with expanded weight history (weight in each decade of adulthood), dietary history, and physical activity reported for the prior 12 months.
All analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC). All statistical tests were two-sided, and statistical significance was defined at a P value of less than .05.
Results
Participant characteristics by case status are shown in Table 1. The analytic cohort of 69 873 men contributed 695 205 person-years of follow-up time. There were 7822 incident prostate cancers, 3078 of which were aggressive, and 255 fatal cases, with a median follow-up of 11.5 years. Mean age at baseline was 62.6 years, and mean BMI was 27.5 kg/m2. A majority of men were non-Hispanic white (88.5%) and were former smokers (51.8%), and almost half (45.6%) had at least one PSA test in the three years prior to enrollment.
Table 1.
Characteristics of male participants in the PLCO Cancer Screening Trial by cancer incidence and mortality status
| Characteristic | All men | Cancer-free | Incident prostate cancer* |
Mortality | ||
|---|---|---|---|---|---|---|
| Total | Nonaggressive | Aggressive | Fatal prostate cancer | |||
| (n = 69 873) | (n = 62 051) | (n = 7822) | (n = 4587) | (n = 3078) | (n = 255) | |
| Trial arm, No. (%) | ||||||
| Usual care | 35 666 (51.0) | 31 493 (50.8) | 4173 (53.3) | 2556 (55.7) | 1530 (49.7) | 128 (50.2%) |
| Intervention | 34 207 (49.0) | 30 558 (49.2) | 3649 (46.7) | 2031 (44.3) | 1548 (50.3) | 127 (49.8%) |
| Age at study entry | ||||||
| 55–59 y, No. (%) | 22 447 (32.1) | 20 564 (33.1) | 1883 (24.1) | 1194 (26.0) | 652 (21.2) | 30 (11.8%) |
| 60–64 y, No. (%) | 22 023 (31.5) | 19 365 (31.2) | 2658 (34.0) | 1583 (34.5) | 1029 (33.4) | 71 (27.8%) |
| 65–69 y, No. (%) | 16 207 (23.2) | 14 042 (22.6) | 2165 (27.7) | 1247 (27.2) | 870 (28.3) | 75 (29.4%) |
| 70+ y, No. (%) | 9196 (13.2) | 8080 (13.0) | 1116 (14.3) | 563 (12.3) | 527 (17.1) | 79 (31.0%) |
| Mean (SD), y | 62.58 (5.33) | 62.58 (5.33) | 63.48 (5.11) | 63.14 (5.03) | 62.62 (5.31) | 65.92 (5.23) |
| Education, No. (%) | ||||||
| Less than high school | 5635 (8.1) | 5059 (8.2) | 576 (7.4) | 322 (7.0) | 243 (7.9) | 26 (10.2%) |
| High school graduate | 12 761 (18.3) | 11 334 (18.3) | 1427 (18.3) | 809 (17.7) | 589 (19.2) | 43 (16.9%) |
| Some college | 22 824 (32.7) | 20 340 (32.8) | 2484 (31.8) | 1482 (32.4) | 947 (30.8) | 81 (31.8%) |
| College graduate | 13 266 (19.0) | 11 772 (19.0) | 1494 (19.1) | 901 (19.7) | 569 (18.5) | 52 (20.4%) |
| Postgraduate | 15 249 (21.9) | 13 420 (21.7) | 1829 (23.4) | 1066 (23.3) | 725 (23.6) | 53 (20.8%) |
| Marital status, No. (%) | ||||||
| Married or cohabiting | 57 880 (83.0) | 51 181 (82.6) | 6699 (85.8) | 3959 (86.5) | 2612 (85.0) | 202 (79.2%) |
| Single | 11 865 (17.0) | 10 755 (17.4) | 1110 (14.2) | 619 (13.5) | 462 (15.0) | 53 (20.8%) |
| Race, No. (%) | ||||||
| White | 61 800 (88.5) | 54 849 (88.4) | 6951 (88.9) | 4142 (90.3) | 2666 (86.6) | 225 (88.2%) |
| Black | 3109 (4.5) | 2647 (4.3) | 462 (5.9) | 237 (5.2) | 214 (7.0) | 24 (9.4%) |
| Other† | 4926 (7.1) | 4520 (7.3) | 406 (5.2) | 206 (4.5) | 197 (6.4) | 6 (2.4%) |
| Family history of prostate cancer, No. (%) | ||||||
| No | 63 106 (92.6) | 56 302 (93.0) | 6804 (88.9) | 3983 (88.5) | 2679 (89.3) | 227 (90.4%) |
| Yes | 5079 (7.4) | 4228 (7.0) | 851 (11.1) | 516 (11.5) | 322 (10.7) | 24 (9.6%) |
| PSA test in last 3 y, No. (%) | ||||||
| No | 31 900 (45.7) | 28 550 (46.0) | 3350 (42.8) | 1892 (41.2) | 1402 (45.6) | 132 (51.8%) |
| Yes, once | 25 031 (35.8) | 22 233 (35.8) | 2798 (35.8) | 1691 (36.9) | 1048 (34.1) | 72 (28.2%) |
| Yes, more than once | 6876 (9.8) | 5818 (9.4) | 1058 (13.5) | 649 (14.1) | 375 (12.2) | 25 (9.8%) |
| Don't know | 6060 (8.7) | 5445 (8.8) | 615 (7.9) | 355 (7.7) | 252 (8.2) | 26 (10.2%) |
| Diabetes, No. (%) | ||||||
| No | 63 321 (91.1) | 56 038 (90.7) | 7283 (93.6) | 4304 (94.4) | 2833 (92.3) | 240 (94.5%) |
| Yes | 6221 (8.9) | 5719 (9.3) | 502 (6.4) | 257 (5.6) | 235 (7.7) | 14 (5.5%) |
| Myocardial infarction, No. (%) | ||||||
| No | 60 247 (86.6) | 53 375 (86.4) | 6872 (88.3) | 4040 (88.7) | 2698 (88.0) | 219 (86.6%) |
| Yes | 9293 (13.4) | 8385 (13.6) | 908 (11.7) | 517 (11.3) | 368 (12.0) | 34 (13.4%) |
| Cigarette smoking, No. (%) | ||||||
| Never | 25 506 (36.5) | 22 300 (35.9) | 3206 (41.0) | 1893 (41.3) | 1258 (40.9) | 97 (38.0%) |
| Current | 8139 (11.6) | 7428 (12.0) | 711 (9.1) | 408 (8.9) | 288 (9.4) | 30 (11.8%) |
| Former | 36 218 (51.8) | 32 315 (52.1) | 3903 (49.9) | 2285 (49.8) | 1531 (49.8) | 128 (50.2%) |
| Aspirin use frequency, No. (%) | ||||||
| None | 32 101 (46.0) | 28 487 (46.0) | 3614 (46.3) | 2129 (46.5) | 1405 (45.8) | 116 (45.7%) |
| 1–3 times/mo | 7270 (10.4) | 6489 (10.5) | 781 (10.0) | 475 (10.4) | 296 (9.6) | 29 (11.4%) |
| 1–6 times/wk | 9241 (13.3) | 8119 (13.1) | 1122 (14.4) | 661 (14.4) | 437 (14.2) | 35 (13.8%) |
| 1 or more/d | 21 122 (30.3) | 18 834 (30.4) | 2288 (29.3) | 1315 (28.7) | 930 (30.3) | 74 (29.1%) |
| Height, cm | ||||||
| Mean (SD) | 177.8 (6.9) | 177.8 (7.0) | 178.0 (6.8) | 178.0 (6.8) | 177.9 (6.8) | 178.1 (6.4) |
| BMI, age 20 y, mean (SD), kg/m2 | 22.9 (2.9) | 23.0 (2.9) | 22.8 (3.0) | 22.8 (2.8) | 22.9 (2.8) | 23.1 (2.8) |
| BMI, age 50 y, mean (SD), kg/m2 | 26.6 (3.6) | 26.2 (3.3) | 26.2 (3.3) | 26.2 (3.3) | 26.3 (3.4) | 26.7 (3.8) |
| BMI, age at baseline, mean (SD), kg/m2 | 27.5 (4.1) | 27.6 (4.1) | 27.3 (3.8) | 27.2 (3.7) | 27.4 (4.0) | 27.9 (4.4) |
*One hundred fifty-seven prostate cancers lacked enough information to determine prostate cancer aggressiveness. Mean (SD) for continuous variables and counts, No. (%) for categorical variables. Study screening center (10 centers) is not shown. BMI = body mass index; PLCO = Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial.
†Other race includes Asian, Native Hawaiian or other Pacific Islander, American Indian, or Alaskan Native.
Height at study entry was modestly associated with total, nonaggressive, and aggressive prostate cancers (HRs = 1.02–1.04 per 5 cm), whereas the trend for the association between height and fatal prostate cancer was not statistically significant (Table 2). When modeled continuously, BMI at age 20 and 50 years and at baseline were associated with increased risks of fatal prostate cancer, with adjusted hazard ratios ranging from 1.27–1.32 per 5 kg/m2 increase (Table 2). Hazard ratios for each age-specific BMI category supported these associations, although some of the individual estimates did not reach the nominal statistical significance threshold of .05. Risk of fatal prostate cancer was highest in men with a BMI in the overweight category or higher at the onset of adulthood (HR = 1.53, 95% CI = 1.05 to 2.24) compared with men who were never overweight. Aggressive and nonaggressive prostate cancer was not associated with BMI at any age. Associations of BMI and risk of total prostate cancer showed modest inverse associations.
Table 2.
Adjusted hazard ratios and 95% confidence intervals for associations of height and age-specific body mass index in relation to prostate cancer incidence and mortality among men in the PLCO Cancer Screening Trial
| Characteristic | Incident prostate cancer* |
Mortality |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 7822) |
Nonaggressive (n = 4587) |
Aggressive (n = 3078) |
Fatal prostate cancer (n = 255) |
||||||||
| Cases | IR† | HR (95% CI)‡ | Cases | HR (95% CI)‡ | Cases | HR (95% CI)‡ | Cases | MR† | HR (95% CI)‡ | ||
| Height at baseline, cm | |||||||||||
| Q1: <172 | 2158 | 1059.2 | Reference | 1276 | Reference | 838 | Reference | 60 | 30.2 | Reference | |
| Q2: 175–177 | 2248 | 1131.7 | 1.03 (0.97 to 1.09) | 1308 | 0.98 (0.88 to 1.10) | 904 | 1.12 (1.01 to 1.23) | 82 | 45.1 | 1.33 (0.95 to 1.87) | |
| Q3: 180–182 | 2091 | 1184.1 | 1.08 (1.01 to 1.14) | 1216 | 1.02 (0.87 to 1.20) | 817 | 1.15 (1.04 to 1.28) | 76 | 47.5 | 1.45 (1.03 to 2.06) | |
| Q4: >185 | 1325 | 1196.1 | 1.06 (0.99 to 1.14) | 787 | 1.03 (0.81 to 1.29) | 519 | 1.16 (1.03 to 1.30) | 37 | 38.1 | 1.20 (0.79 to 1.82) | |
| Ptrend§ | .03 | .65 | .005 | .18 | |||||||
| Continuous, per 5 cm | 1.02 (1.00 to 1.04) | 1.03 (1.01 to 1.06) | 1.04 (1.01 to 1.06) | 1.06 (0.97 to 1.17) | |||||||
| BMI, age 20 y, kg/m2 | |||||||||||
| <18.5 | 351 | 1089.5 | 0.97 (0.87 to 1.09) | 207 | 0.96 (0.83 to 1.11) | 137 | 0.94 (0.79 to 1.12) | 5 | 15.6 | 0.42 (0.17 to 1.01) | |
| 18.5–25 | 5779 | 1144.1 | Reference | 3392 | Reference | 2266 | Reference | 185 | 39.3 | Reference | |
| 25–30 | 1578 | 1122.7 | 0.99 (0.94 to 1.05) | 917 | 1.03 (0.95 to 1.11) | 634 | 1.05 (0.96 to 1.15) | 58 | 46.8 | 1.29 (0.96 to 1.74) | |
| ≥30 | 114 | 913.4 | 0.82 (0.68 to 0.99) | 71 | 0.94 (0.73 to 1.20) | 41 | 0.78 (0.57 to 1.07) | 7 | 68.1 | 1.69 (0.75 to 3.82) | |
| Ptrend§ | .35 | .64 | .72 | .004 | |||||||
| Continuous, per 5 kg/m2 | 0.98 (0.94 to 1.02) | 1.02 (0.95 to 1.08) | 1.03 (0.97 to 1.10) | 1.27 (1.03 to 1.58) | |||||||
| BMI, age 50 y, kg/m2 | |||||||||||
| <18.5 | 11 | 623.1 | 0.60 (0.33 to 1.09) | 7 | 0.62 (0.29 to 1.31) | 4 | 0.53 (0.20 to 1.41) | 0 | 0.0 | – | |
| 18.5–25 | 2892 | 1198.2 | Reference | 1699 | Reference | 1125 | Reference | 91 | 38.0 | Reference | |
| 25–30 | 3971 | 1134.3 | 0.95 (0.91 to 1.00) | 2345 | 0.96 (0.88 to 1.05) | 1552 | 0.99 (0.91 to 1.07) | 124 | 39.2 | 1.05 (0.80 to 1.39) | |
| ≥30 | 948 | 979.9 | 0.87 (0.81 to 0.94) | 536 | 0.87 (0.73 to 1.04) | 397 | 0.96 (0.85 to 1.08) | 40 | 48.9 | 1.55 (1.05 to 2.29) | |
| Ptrend§ | .001 | .29 | .64 | .05 | |||||||
| Continuous, per 5 kg/m2 | 0.95 (0.92 to 0.99) | 0.90 (0.79 to 1.03) | 1.02 (0.96 to 1.07) | 1.32 (1.11 to 1.58) | |||||||
| BMI at baseline, kg/m2 | |||||||||||
| <18.5 | 14 | 801.9 | 0.65 (0.38 to 1.12) | 5 | 0.30 (0.11 to 0.79) | 9 | 1.12 (0.58 to 2.16) | 0 | 0.0 | – | |
| 18.5–25 | 2150 | 1173.0 | Reference | 1260 | Reference | 843 | Reference | 65 | 36.1 | Reference | |
| 25–30 | 4046 | 1150.0 | 0.98 (0.93 to 1.04) | 2397 | 1.08 (0.99 to 1.18) | 1563 | 1.00 (0.91 to 1.09) | 126 | 39.1 | 1.09 (0.81 to 1.48) | |
| ≥30 | 1612 | 1056.1 | 0.93 (0.87 to 1.00) | 925 | 1.12 (0.95 to 1.32) | 663 | 1.01 (0.91 to 1.12) | 64 | 47.5 | 1.46 (1.02 to 2.09) | |
| Ptrend§ | .06 | .13 | .99 | .04 | |||||||
| Continuous, per 5 kg/m2 | 0.97 (0.94 to 1.00) | 1.12 (0.99 to 1.26) | 1.02 (0.97 to 1.07) | 1.27 (1.09 to 1.49) | |||||||
| Time when BMI first exceeded 25 kg/m2 | |||||||||||
| Never | 1767 | 1187.1 | Reference | 1032 | Reference | 693 | Reference | 50 | 34.5 | Reference | |
| Baseline age | 1029 | 1199.8 | 1.02 (0.95 to 1.11) | 618 | 1.11 (1.00 to 1.23) | 388 | 0.98 (0.86 to 1.11) | 38 | 43.1 | 1.26 (0.82 to 1.94) | |
| Age 50 y | 3334 | 1101.2 | 0.95 (0.90 to 1.01) | 1949 | 1.03 (0.93 to 1.13) | 1322 | 0.99 (0.90 to 1.08) | 102 | 37.4 | 1.14 (0.80 to 1.61) | |
| Age 20 y | 1692 | 1104.7 | 0.96 (0.89 to 1.02) | 988 | 1.05 (0.93 to 1.19) | 675 | 1.02 (0.91 to 1.14) | 65 | 48.3 | 1.53 (1.05 to 2.24) | |
*One hundred fifty-seven prostate cancers lacked enough information to determine prostate cancer aggressiveness. Empty cells (–) are missing hazard ratios and 95% confidence intervals owing to 0 case counts for the respective category. BMI = body mass index; CI = confidence interval; IR = incidence rate; HR = hazard ratio; MR = mortality rate; PLCO = Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial.
†Age-standardized incidence rates and mortality rates per 100 000 person-years.
‡Hazard ratios (95% confidence intervals) from Cox regression models, with age (in months) as the underlying time metric stratified by year of diagnosis, were adjusted for trial arm, screening center, race (white, black, other), education (less than high school, high school graduate, some college, college graduate, postgraduate), married or cohabiting (yes, no), diabetes (yes, no), cigarette smoking (ever, former, never), prostate-specific antigen history during the three years prior to study entry (0, 1, 2+), family history of prostate cancer (yes, no), and myocardial infarction (yes, no).
§Ptrend was calculated using the median category values modeled as a continuous variable, excluding subjects whose change in weight for the given time period was negative. All P values are two-sided.
Mean BMI during early-to-mid adulthood (age 20 to 50 years) was associated with an increased risk of fatal prostate cancer (HR = 1.40, 95% CI = 1.13 to 1.75), with mean overweight (HR = 1.38, 95% CI = 1.06 to 1.80) and mean obese men (HR = 1.94, 95% CI = 1.12 to 3.33) at higher risk compared with men with a mean normal BMI (Ptrend = .002) (Table 3). Mean BMI of all ages during adulthood found similar results, all of which were statistically significant. Maximum BMI during early-to-mid adulthood (age 20 to 50 years) was associated with an increased risk of fatal prostate cancer (HR = 1.32, 95% CI = 1.11 to 1.58), and men whose maximum BMI was obese were at higher risk (HR = 1.54, 95% CI = 1.05 to 2.27), each compared with men with a maximum normal BMI (Ptrend = .06). Maximum BMI during all ages in adulthood showed similar associations. Mean and maximum BMI were not associated with aggressive or nonaggressive prostate cancer risk, yet modest inverse associations were observed for total prostate cancer.
Table 3.
Adjusted hazard ratios and 95% confidence intervals for associations of mean and maximum body mass index in relation to prostate cancer incidence and mortality among men in the PLCO Cancer Screening Trial
| Characteristic | Incident prostate cancer* |
Mortality |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 7822) |
Nonaggressive (n = 4587) |
Aggressive (n = 3078) |
Fatal prostate cancer (n = 255) |
||||||||
| Cases | IR† | HR (95% CI)‡ | Cases | HR (95% CI)‡ | Cases | HR (95% CI)‡ | Cases | MR† | HR (95% CI)‡ | ||
| Mean BMI, age 20 and 50 y, kg/m2 | |||||||||||
| <18.5 | 24 | 893.9 | 0.81 (0.54 to 1.21) | 15 | 0.79 (0.47 to 1.32) | 9 | 0.73 (0.38 to 1.41) | 0 | 0.0 | – | |
| 18.5–25 | 4766 | 1160.3 | Reference | 2819 | Reference | 1845 | Reference | 142 | 36.0 | Reference | |
| 25–30 | 2741 | 1117.6 | 0.98 (0.93 to 1.03) | 1582 | 0.98 (0.91 to 1.07) | 1108 | 1.06 (0.98 to 1.14) | 97 | 45.1 | 1.38 (1.06 to 1.80) | |
| ≥30 | 291 | 874.5 | 0.82 (0.73 to 0.93) | 171 | 0.90 (0.74 to 1.10) | 116 | 0.87 (0.71 to 1.05) | 16 | 58.1 | 1.94 (1.12 to 3.33) | |
| Ptrend§ | 0.02 | .52 | .76 | .002 | |||||||
| Continuous, per 5 kg/m2 | 0.96 (0.92 to 1.00) | 0.99 (0.89 to 1.10) | 1.03 (0.96 to 1.10) | 1.40 (1.13 to 1.75) | |||||||
| Mean BMI, all ages, kg/m2 | |||||||||||
| <18.5 | 7 | 518.9 | 0.48 (0.23 to 1.01) | 4 | 0.42 (0.15 to 1.12) | 3 | 0.51 (0.16 to 1.57) | 0 | 0.0 | – | |
| 18.5–25 | 3815 | 1174.3 | Reference | 2258 | Reference | 1469 | Reference | 114 | 36.1 | Reference | |
| 25–30 | 3461 | 1120.8 | 0.96 (0.92 to 1.01) | 2020 | 0.97 (0.89 to 1.06) | 1380 | 1.04 (0.96 to 1.12) | 116 | 41.9 | 1.24 (0.95 to 1.62) | |
| ≥30 | 539 | 974.4 | 0.88 (0.80 to 0.97) | 305 | 0.93 (0.76 to 1.14) | 226 | 1.00 (0.86 to 1.15) | 25 | 56.9 | 1.81 (1.15 to 2.85) | |
| Ptrend§ | .02 | .51 | .56 | .008 | |||||||
| Continuous, per 5 kg/m2 | 0.96 (0.92 to 1.00) | 1.05 (0.87 to 1.26) | 1.03 (0.97 to 1.10) | 1.42 (1.15 to 1.75) | |||||||
| Maximum BMI, age 20 and 50 y, kg/m2 | |||||||||||
| <18.5 | 8 | 670.9 | 0.70 (0.35 to 1.41) | 5 | 0.72 (0.30 to 1.74) | 3 | 0.63 (0.20 to 1.94) | 0 | 0.0 | – | |
| 18.5–25 | 2788 | 1196.8 | Reference | 1645 | Reference | 1078 | Reference | 88 | 37.8 | Reference | |
| 25–30 | 4050 | 1137.8 | 0.96 (0.91 to 1.01) | 2384 | 0.96 (0.88 to 1.05) | 1589 | 1.01 (0.93 to 1.09) | 126 | 39.1 | 1.05 (0.80 to 1.39) | |
| ≥30 | 976 | 979.6 | 0.87 (0.81 to 0.94) | 553 | 0.87 (0.74 to 1.03) | 408 | 0.97 (0.86 to 1.09) | 41 | 49.2 | 1.54 (1.04 to 2.27) | |
| Ptrend§ | .001 | .19 | .80 | .06 | |||||||
| Continuous, per 5 kg | 0.95 (0.92 to 0.99) | 0.94 (0.83 to 1.06) | 1.01 (0.96 to 1.07) | 1.32 (1.11 to 1.58) | |||||||
| Maximum BMI, kg, all ages | |||||||||||
| < 18.5 | 5 | 839.0 | 0.77 (0.32 to 1.86) | 2 | 0.48 (0.12 to 1.94) | 3 | 1.13 (0.36 to 3.50) | 0 | 0.0 | – | |
| 18.5–25 | 1762 | 1188.5 | Reference | 1030 | Reference | 690 | Reference | 50 | 34.6 | Reference | |
| 25–30 | 4244 | 1159.7 | 0.98 (0.93 to 1.04) | 2522 | 1.05 (0.95 to 1.15) | 1634 | 0.99 (0.90 to 1.09) | 130 | 38.4 | 1.13 (0.81 to 1.57) | |
| ≥ 30 | 1811 | 1034.7 | 0.91 (0.85 to 0.98) | 1033 | 1.01 (0.85 to 1.19) | 751 | 0.99 (0.89 to 1.10) | 75 | 48.2 | 1.59 (1.10 to 2.31) | |
| Ptrend§ | .009 | .74 | .79 | .01 | |||||||
| Continuous, per 5 kg | 0.96 (0.93 to 0.99) | 1.01 (0.87 to 1.18) | 1.02 (0.97 to 1.07) | 1.30 (1.11 to 1.51) | |||||||
*One hundred fifty-seven prostate cancers lacked enough information to determine prostate cancer aggressiveness. Empty cells (–) are missing hazard ratios and 95% confidence intervals owing to 0 case counts for the respective category. BMI = body mass index; CI = confidence interval; IR = incidence rate; HR = hazard ratio; MR = mortality rate; PLCO = Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial.
†Age-standardized incidence rates and mortality rates per 100 000 person-years.
‡Hazard ratios (95% confidence intervals) from Cox regression models, with age (in months) as the underlying time metric stratified by year of diagnosis, were adjusted for covariates previously specified in Table 2.
§Ptrend was calculated using the median category values modeled as a continuous variable, excluding subjects whose change in weight for the given time period was negative. All P values are two-sided.
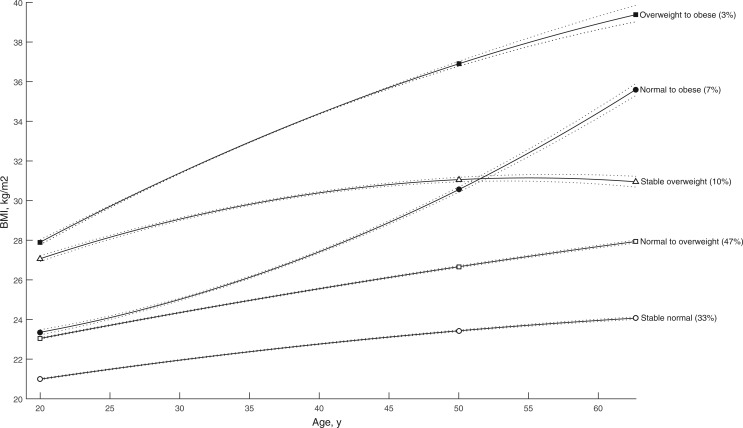
In trajectory analyses, we identified five distinct trajectories (quadratic polynomials) of BMI. Risk of fatal prostate cancer was increased in men who were normal BMI (HR = 1.95, 95% CI = 1.21 to 3.12) or overweight (HR = 2.65, 95% CI = 1.35 to 5.18) at age 20 years and became obese by baseline, compared with men who maintained a steady normal BMI (Figure 1 and Table 4). No associations were seen for aggressive or nonaggressive prostate cancer, and total prostate cancer risk showed slight inverse associations among men who were overweight at age 20 years and remained overweight or became obese.
Figure 1.
Prediagnostic body mass index trajectories among men in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Each trajectory was modeled using quadratic polynomials. Solid lines indicate the trajectory, dashed lines indicate the lower and upper bound 95% confidence intervals of the trajectory. BMI = body mass index.
Table 4.
Adjusted hazard ratios and 95% confidence intervals for associations between BMI trajectories and prostate cancer incidence and mortality among men in the PLCO Cancer Screening Trial
| BMI trajectory | Incident prostate cancer* |
Mortality |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 7822) |
Nonaggressive (n = 4587) |
Aggressive (n = 3078) |
Fatal prostate cancer (n = 255) |
|||||||
| Cases | IR† | HR (95% CI)‡ | Cases | HR (95% CI)‡ | Cases | HR (95% CI)‡ | Cases | MR† | HR (95% CI)‡ | |
| Stable normal | 2744 | 1183.1 | Reference | 1606 | Reference | 1077 | Reference | 86 | 37.1 | Reference |
| Normal to overweight | 3752 | 1133.4 | 0.96 (0.91 to 1.01) | 2246 | 0.97 (0.88 to 1.07) | 1430 | 0.96 (0.89 to 1.05) | 108 | 36.0 | 0.99 (0.74 to 1.32) |
| Normal to obese | 514 | 1153.9 | 0.98 (0.89 to 1.08) | 282 | 0.92 (0.74 to 1.14) | 224 | 1.14 (0.98 to 1.32) | 24 | 65.5 | 1.95 (1.21 to 3.12) |
| Stable overweight | 657 | 1014.1 | 0.91 (0.83 to 0.99) | 371 | 0.86 (0.71 to 1.05) | 276 | 1.02 (0.89 to 1.17) | 26 | 47.0 | 1.51 (0.96 to 2.36) |
| Overweight to obese | 155 | 858.5 | 0.79 (0.67 to 0.93) | 82 | 0.73 (0.51 to 1.05) | 71 | 0.95 (0.74 to 1.22) | 11 | 73.5 | 2.65 (1.35 to 5.18) |
*One hundred fifty-seven prostate cancers lacked enough information to determine prostate cancer aggressiveness. BMI = body mass index; CI = confidence interval; IR = incidence rate; HR = hazard ratio; MR = mortality rate; PLCO = Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial.
†Age-standardized incidence rates and mortality rates per 100 000 person-years.
‡Hazard ratios (95% confidence intervals) from Cox regression models, with age (in months) as the underlying time metric stratified by year of diagnosis, were adjusted for covariates previously specified in Table 2.
In all Cox models, the proportional hazards assumption was not violated (Ps > .05). There was no evidence of interaction between BMI exposures and study arm, race, history of diabetes, or smoking status (Ps > .10). In sensitivity analyses, weight change was not associated with prostate cancer risks, although there were suggestions of associations between large gains in weight and fatal prostate cancer (Supplementary Tables 1 and 2, available online), and these associations were stronger (Pinteraction = .08) and statistically significant in men who were overweight/obese at age 20 years (Supplementary Table 3, available online). Adjustment for initial weight or mean weight did not materially influence the results. Akin to the main results, sensitivity analyses that used three alternate definitions of aggressive prostate cancer provided mostly null results (Supplementary Table 4, available online). Stratified analyses by trial arm showed overall similar results (Supplementary Tables 5 and 6, available online), although results for fatal prostate cancer were slightly attenuated in the screening arm. Analyses with a combined category for overweight/obese BMI did not drastically differ from the main results (Supplementary Table 7, available online). In a subcohort analysis (41% of the analytic cohort), additional adjustment for energy consumption and physical activity did not materially affect the results (data not shown).
Discussion
In this large, prospective study, we found evidence that higher age-specific and mean BMI and life course trajectories of BMI that result in obesity were associated with greater risk of fatal prostate cancer. Risk of fatal disease was also increased among men whose BMI was overweight at the onset of adulthood compared with men who maintained a steady normal BMI throughout adulthood, suggesting that the timing of initial overweight/obesity may be influential. Conversely, risk of incident nonaggressive and aggressive prostate cancer appeared unrelated to body size, while total prostate cancer showed some modest inverse associations with higher BMI.
Prior studies of cumulative or per annum weight gain between early adulthood and mid-to-late adulthood in relation to prostate cancer risks vary by subtype, and evidence is mixed (6). Previous studies have predominantly modeled changes in weight or BMI using cumulative or per annum measures over time, which is limited in differentiating between specific body weight patterns in populations and may potentially be overly simplistic. Differing methodology may also account for variability in results, as adjusting for starting or ending values induces mathematical coupling (28,29) and regression to the mean (30,31). Recent research suggests that trajectory modeling offers an alternative and, arguably, more robust risk prediction method compared with cumulative measures of body weight change (32).
The only study to date that has examined trajectories of body weight in relation to prostate cancer has done so using body shape (somatotype), and only in relation to advanced (AJCC TNM stage ≥ III) prostate cancer (33). Men with “heavy stable/increase” body shapes were at lower risk of advanced prostate cancer (relative risk [RR] = 0.67, 95% CI = 0.47 to 0.95) compared with men who maintained a lean-stable body shape. In contrast, our BMI trajectory analysis demonstrated strong and statistically significant positive associations between excessive weight gain that resulted in obesity and risk of fatal prostate cancer. These differences in study results may be attributable to the different exposures and outcomes assessed. The BMI trajectory risk estimates from our study were stronger when compared with our—and other—risk estimates associated with weight change (34–36), the latter of which may be viewed as a weaker metric of weight patterns (37).
The only prior study to have assessed prostate cancer risk and mean BMI during adulthood was a case-control study that found null associations for all prostate cancer subtypes assessed, including fatal disease (38). In our study, we found a consistent positive association between mean BMI and risk of fatal prostate cancer, whether examined during early-to-mid adulthood (age 20 to 50 years) or across the adult life course (age 20 years to baseline). Our use of a prospective cohort study design, which reduces the likelihood of recall bias, could account for these different associations between prostate cancer risks and mean BMIs.
In age-specific BMI analyses, obesity at different ages during adulthood has been associated with poorer prostate cancer outcomes in a majority of prospective studies (3,4,39), as evidenced by a recent meta-analysis that showed a 15% increase in risk of fatal prostate cancer per 5 kg/m2 increase in BMI (5). Our study found that self-reported BMIs at all ages, including early adulthood (age 20 years), were associated with increased risks of fatal disease, which is contrary to prior findings from the Health Professionals Follow-up Study that—as per the body shape analysis in this cohort—reported inverse associations between earlier adulthood obesity and advanced prostate cancer (40–42). Differences in exposure categorization, outcome assessed, and study design could account for these differences of associations between prostate cancer risks and age-specific BMIs. Meta-analyses to date show a slight positive association (RRs = 1.01–1.05) between obesity and total prostate cancer, while slight inverse or null associations are seen for nonaggressive disease (3,9,43). Our findings suggest no association for nonaggressive disease and modest inverse associations for total prostate cancer with BMI at age 50 years and baseline, but no association at age 20 years. These slight inverse associations could be explained by reduced prostate cancer detection in larger men because of PSA hemodilution and/or larger prostate organ volumes.
There is a complex array of biological mechanisms through which obesity may influence prostate carcinogenesis and metastasis, including hyperinsulinemia, elevated insulin-like growth factor (IGF) hormone levels, dysregulation of sex steroid hormones, altered levels of adipokines, and chronic inflammation (3,44). Hyperinsulinemia has been associated with increased circulating levels of IGF-1, which have been found to promote carcinogenesis, including within the prostate (45–47). Obesity is also associated with chronic inflammation and biomarkers of inflammation in the body (48,49), such as higher levels of C-reactive protein (50), which have been associated with prostate cancer–specific mortality (39). Obese men have been shown to exhibit reduced levels of androgens (51), and there is evidence that men with lower levels of testosterone have more aggressive tumors at clinical presentation (52,53). The influence of early-life exposures is suspected to be important in prostate cancer etiology (13) as carcinogenic processes have been seen within prostatic tissue in men in their 20s (14). The complexity of adipose effects on carcinogenesis necessitates further research if we are to confirm whether the timing and duration of obesity influences the development and progression of prostate cancer.
Strengths of this study include its large sample size with extended follow-up. The study attained nearly complete follow-up of all participants with histologic verification of cancers and registered underlying causes of death. BMI trajectory modeling enabled the identification of men with distinct BMI changes across the adult life course.
This study has limited generalizability because of being based within a screening trial that was predominantly comprised of white, well-educated participants. Body weight and height were self-reported, which can lead to misclassification of exposure and could have artifactually affected our risk estimates (54). However, similar cohorts have found strong correlations (rs > .8) between measured anthropometrics and those self-reported for both current and historical weight/height (55–58). Mean BMI at study entry among men in our cohort was also in line with mean BMI measurements from US population-based National Health and Nutrition Examination Survey (NHANES) collected during similar periods (59). Another limitation is that detection of prostate cancer in obese men may differ from nonobese men because of PSA hemodilution and larger prostate volumes, leading to increased sampling error. Trajectory groups were only based on three BMI time points, thereby limiting pattern sensitivity. Finally, only 10% of our population exhibited BMI patterns that resulted in obesity at baseline, and our results require further replication in other large cohorts.
In conclusion, BMI trajectory modeling coupled with the clinically relevant outcome of fatal prostate cancer has demonstrated that excessive weight gain during the adult life course results in an elevated risk of fatal prostate cancer. Moreover, early-onset overweight/obesity appears to be an influential factor for risk of fatal prostate cancer. Life course assessment of BMI may aid future predictive algorithms to help identify men who are at greater risk of developing fatal forms of prostate cancer. The clinical utility of BMI necessitates further study of this metric across the life course, including how it is related to progressive forms of prostate cancer.
Funding
This work was supported by the Intramural Program of the National Cancer Institute at the National Institutes of Health and Department of Health and Human Services.
Notes
There are no financial disclosures from any of the authors. The study sponsor had no role in the design of the study, the data collection, the analysis or interpretation of the data, the writing of the article, or the decision to submit for publication.
Supplementary Material
References
- 1.Flegal KM, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA . 2012;307(5):491–497. [DOI] [PubMed] [Google Scholar]
- 2.Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377(9765):557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: Weighing the evidence. Eur Urol. 2013;63(5):800–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao Y, Ma J. Body Mass Index, Prostate cancer–specific mortality, and biochemical recurrence: A systematic review and meta-analysis. Cancer Prev Res. 2011;4(4):486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhong S, Yan X, Wu Y, et al. Body mass index and mortality in prostate cancer patients: A dose-response meta-analysis. Prostate Cancer Prostatic Dis. 2016;19(2):122–31. [DOI] [PubMed] [Google Scholar]
- 6.Chen Q, Chen T, Shi W, et al. Adult weight gain and risk of prostate cancer: A dose-response meta-analysis of observational studies. Int J Cancer. 2016;138(4):866–874. [DOI] [PubMed] [Google Scholar]
- 7.Freedland SJ, Platz EA. Obesity and prostate cancer: Making sense out of apparently conflicting data. Epidemiol Rev. 2007;29:88–97. [DOI] [PubMed] [Google Scholar]
- 8.Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3(9):565–574. [DOI] [PubMed] [Google Scholar]
- 9.MacInnis RJ, English DR. Body size and composition and prostate cancer risk: Systematic review and meta-regression analysis. Cancer Causes Control. 2006;17(8):989–1003. [DOI] [PubMed] [Google Scholar]
- 10.Andersson SO, Baron J, Wolk A, et al. Early-life risk-factors for prostate-cancer - A population-based case-control study in Sweden. Cancer Epidemiol Biomarkers Prev. 1995;4(3):187–192. [PubMed] [Google Scholar]
- 11.Robinson WR, Stevens J, Gammon MD, et al. Obesity before age 30 years and risk of advanced prostate cancer. Am J Epidemiol. 2005;161(12):1107–1114. [DOI] [PubMed] [Google Scholar]
- 12.Robinson WR, Poole C, Godley PA. Systematic review of prostate cancer's association with body size in childhood and young adulthood. Cancer Causes Control. 2008;19(8):793–803. [DOI] [PubMed] [Google Scholar]
- 13.Sutcliffe S, Colditz GA. Prostate cancer: Is it time to expand the research focus to early-life exposures? Nat Rev Cancer. 2013;13(3):208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakr WA, Haas GP, Cassin BF, et al. The frequency of carcinoma and intraepithelial neoplasia of the prostate in young male-patients. J Urol. 1993;150(2):379–385. [DOI] [PubMed] [Google Scholar]
- 15.Keum N, Greenwood DC, Lee DH, et al. Adult weight gain and adiposity-related cancers: A dose-response meta-analysis of prospective observational studies. J Natl Cancer Inst. 2015;107(2). doi: 10.1093/jnci/djv088. [DOI] [PubMed] [Google Scholar]
- 16.Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology. 2007;132(6):2208–2225. [DOI] [PubMed] [Google Scholar]
- 17.Renehan AG, Roberts DL, Dive C. Obesity and cancer: Pathophysiological and biological mechanisms. Arch Physiol Biochem. 2008;114(1):71–83. [DOI] [PubMed] [Google Scholar]
- 18.Prorok PC, Andriole GL, Bresalier RS, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Controlled Clinical Trials. 2000;21(6):273s–309s. [DOI] [PubMed] [Google Scholar]
- 19.Nagin DS. Analyzing developmental trajectories: A semiparametric, group-based approach. Psychol Methods. 1999;4(2):139–157. [DOI] [PubMed] [Google Scholar]
- 20.Miller AB, Yurgalevitch S, Weissfeld JL, et al. Death review process in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Controlled Clinical Trials. 2000;21(6):400s–406s. [DOI] [PubMed] [Google Scholar]
- 21.Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: Choice of the time-scale. Am J Epidemiol. 1997;145(1):72–80. [DOI] [PubMed] [Google Scholar]
- 22.Cox DR. Regression models and life-tables. J Royal Stat Soc Series B Stat Methodol. 1972;34(2):187–220. [Google Scholar]
- 23.Gail MH, Graubard B, Williamson DE, et al. Comments on “Choice of time scale and its effect on significance of predictors in longitudinal studies.” Stat Med. 2009;28(8):1315–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breslow NE, Day NE. Statistical Methods in Cancer Research, Vol. II—The Design and Analysis of Cohort Studies. Lyon, France: International Agency for Research on Cancer; 1987. [PubMed] [Google Scholar]
- 25.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–138. [DOI] [PubMed] [Google Scholar]
- 26.Jones BL, Nagin DS. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Soc Methods Res. 2007;35(4):542–571. [Google Scholar]
- 27.Oldham PD. A note on analysis of repeated measurements of same subjects. J Chron Dis. 1962;15(Oct):969–977. [DOI] [PubMed] [Google Scholar]
- 28.Gilthorpe MS, Tu YK. Mathematical coupling: A multilevel approach. Int J Epidemiol. 2004;33(6):1399–1400. [DOI] [PubMed] [Google Scholar]
- 29.Tu YK, Baelum V, Gilthorpe MS. The problem of analysing the relationship between change and initial value in oral health research. Eur J Oral Sci. 2005;113(4):271–278. [DOI] [PubMed] [Google Scholar]
- 30.Blomqvist N. On the bias caused by regression toward the mean in studying the relation between change and initial value. J Clin Periodontol. 1987;14(1):34–37. [DOI] [PubMed] [Google Scholar]
- 31.Glymour MM, Weuve J, Berkman LF, et al. When is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am J Epidemiol. 2005;162(3):267–278. [DOI] [PubMed] [Google Scholar]
- 32.Zheng H, Tumin D, Qian Z. Obesity and mortality risk: new findings from body mass index trajectories. Am J Epidemiol. 2013;178(11):1591–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song M, Willett WC, Hu FB, et al. Trajectory of body shape across the lifespan and cancer risk. Int J Cancer. 2016;138(10):2383–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bassett JK, Severi G, Baglietto L, et al. Weight change and prostate cancer incidence and mortality. Int J Cancer. 2012;131(7):1711–1719. [DOI] [PubMed] [Google Scholar]
- 35.Hernandez BY, Park SY, Wilkens LR, et al. Relationship of body mass, height, and weight gain to prostate cancer risk in the multiethnic cohort. Cancer Epidemiol Biomarkers Prev. 2009;18(9):2413–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright ME, Chang SC, Schatzkin A, et al. Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer. 2007;109(4):675–684. [DOI] [PubMed] [Google Scholar]
- 37.Tilling K, Howe LD, Ben-Shlomo Y. Commentary: Methods for analysing life course influences on health-untangling complex exposures. Int J Epidemiol. 2011;40(1):250–252. [DOI] [PubMed] [Google Scholar]
- 38.Moller E, Adami HO, Mucci LA, et al. Lifetime body size and prostate cancer risk in a population-based case-control study in Sweden. Cancer Causes Control. 2013;24(12):2143–2155. [DOI] [PubMed] [Google Scholar]
- 39.Ma J, Li HJ, Giovannucci E, et al. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer–specific mortality in men with prostate cancer: A long-term survival analysis. Lancet Oncol. 2008;9(11):1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giovannucci E, Rimm EB, Stampfer MJ, et al. Height, body weight, and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1997;6(8):557–563. [PubMed] [Google Scholar]
- 41.Giovannucci E, Rimm EB, Liu Y, et al. Body mass index and risk of prostate cancer in US health professionals. J Natl Cancer Inst. 2003;95(16):1240–1244. [DOI] [PubMed] [Google Scholar]
- 42.Moller E, Wilson KM, Batista JL, et al. Body size across the life course and prostate cancer in the Health Professionals Follow-up Study. Int J Cancer. 2016;138(4):853–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. [DOI] [PubMed] [Google Scholar]
- 44.Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: New mechanistic insights from epidemiology. Nature Reviews Cancer. 2015;15(8):484–498. [DOI] [PubMed] [Google Scholar]
- 45.Hsing AW, Gao YT, Chua S, et al. Insulin resistance and prostate cancer risk. J Natl Cancer Inst. 2003;95(1):67–71. [DOI] [PubMed] [Google Scholar]
- 46.Albanes D, Weinstein SJ, Wright ME, et al. Serum insulin, glucose, indices of insulin resistance, and risk of prostate cancer. J Natl Cancer Inst. 2009;101(18):1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rowlands MA, Gunnell D, Harris R, et al. Circulating insulin-like growth factor peptides and prostate cancer risk: A systematic review and meta-analysis. Int J Cancer. 2009;124(10):2416–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Marzo AM, Platz EA, Sutcliffe S, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7(4):256–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Platz EA, De Marzo AM. Epidemiology of inflammation and prostate cancer. J Urol. 2004;171(2 Pt 2):S36–S40. [DOI] [PubMed] [Google Scholar]
- 50.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18(3):363–374. [DOI] [PubMed] [Google Scholar]
- 51.Williams G. Aromatase up-regulation, insulin and raised intracellular oestrogens in men, induce adiposity, metabolic syndrome and prostate disease, via aberrant ER-alpha and GPER signalling. Mol Cell Endocrinol. 2012;351(2):269–278. [DOI] [PubMed] [Google Scholar]
- 52.Schnoeller T, Jentzmik F, Rinnab L, et al. Circulating free testosterone is an independent predictor of advanced disease in patients with clinically localized prostate cancer. World J Urol. 2013;31(2):253–259. [DOI] [PubMed] [Google Scholar]
- 53.Theoret MR, Ning YM, Zhang JJ, et al. The risks and benefits of 5 alpha-reductase inhibitors for prostate cancer prevention. N Engl J Med. 2011;365(2):97–99. [DOI] [PubMed] [Google Scholar]
- 54.Gorber SC, Tremblay M, Moher D, et al. Diagnostic in obesity comorbidities - A comparison of direct vs. self-report measures for assessing height, weight and body mass index: A systematic review. Obes Rev. 2007;8(4):307–326. [DOI] [PubMed] [Google Scholar]
- 55.Rimm EB, Stampfer MJ, Colditz GA, et al. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1(6):466–473. [DOI] [PubMed] [Google Scholar]
- 56.Dahl AK, Hassing LB, Fransson EI, et al. Agreement between self-reported and measured height, weight and body mass index in old age-a longitudinal study with 20 years of follow-up. Age Ageing. 2010;39(4):445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dahl AK, Reynolds CA. Accuracy of recalled body weight-a study with 20-years of follow-up. Obesity. 2013;21(6):1293–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spencer EA, Appleby PN, Davey GK, et al. Validity of self-reported height and weight in 4808 EPIC-Oxford participants. Public Health Nutr. 2002;5(4):561–565. [DOI] [PubMed] [Google Scholar]
- 59.Flegal KM, Troiano RP. Changes in the distribution of body mass index of adults and children in the US population. Int J Obes. 2000;24(7):807–818. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.