SUMMARY
Volatile general anesthetics continue to be an important part of clinical anesthesia worldwide. The impact of volatile anesthetics on the immune system has been investigated at both mechanistic and clinical levels, but previous studies have returned conflicting findings due to varied protocols, experimental environments, and subject species. While many of these studies have focused on the immunosuppressive effects of volatile anesthetics, compelling evidence also exists for immunoactivation. Depending on the clinical conditions, immunosuppression and activation due to volatile anesthetics can be either detrimental or beneficial. This review provides a balanced perspective on the anesthetic modulation of innate and adaptive immune responses as well as indirect effectors of immunity. Potential mechanisms of immunomodulation by volatile anesthetics are also discussed. A clearer understanding of these issues will pave the way for clinical guidelines that better account for the impact of volatile anesthetics on the immune system, with the ultimate goal of improving perioperative management.
Keywords: volatile anesthetics, immune system, immune responses, immunomodulation, perioperative management
Introduction
In recent decades, the field of immunology has progressed substantially, elucidating many of the cellular and molecular mechanisms underlying human immune responses.1,2 During the same period, significant insights have been gained to better understand the action of general anesthetics.3–6 General anesthesia can be administered using inhalational anesthetics, intravenous medications, or most frequently a combination of both. All of these forms of anesthesia have been found to modulate the immune system and exert effects on innate and adaptive immunity.7–12 A comprehensive evaluation of the impact of anesthetic regimens on the immune system can help refine current perioperative management.
This review focuses on the effects of inhalational anesthetics, specifically volatile anesthetics, on the immune response. Volatile anesthetics play a significant role in clinical anesthesia throughout the world. Furthermore, the volatile nature of these compounds extends their influence to not only the immune system of the patients, but also that of the physicians, nurses, and other personnel in the perioperative setting.13 Both in vitro and in vivo studies9,14–17 have been conducted to answer various questions regarding how volatile anesthetics impact immunity. For example, what is the effect of anesthesia on postoperative infection? Is it the emotional and physical stress before and after the surgery or general anesthesia per se that predisposes patients to postoperative complications? Should the impact to immune system be part of the decision-making when choosing anesthesia regimens? Is there a benefit to immunosuppression in the ischemic setting? Do volatile anesthetics hasten the process of metastatic disease? Compared to in vitro and animal studies, there are fewer human studies due to the challenge in isolating a single variable in the clinical setting. Nevertheless, we sought to present a comprehensive review of in vitro studies and in vivo investigations with both small animals and humans. Figure 1 shows some of the direct immune modulations by volatile anesthetics. Direct modulation impacts innate and adaptive immunity, in which the majority of effector immune cells are natural killer (NK) cells, dendritic cells (DCs), neutrophils, macrophages, and lymphocytes. Table 1 summarizes the effects of some of the commonly used volatile anesthetics on these cells.7,9,18–44 In addition to the direct effects, volatile anesthetics also impact the neuroendocrine response from the hypothalamal-pituitary-adrenal axis, thereby indirectly influencing the immune response through the secretions of immunomodulator hormones such as catecholamines and glucocorticoids.45 Both direct and indirect modulations of immunity by volatile anesthetics are covered in this review.
Figure 1. Direct immune modulations by volatile anesthetics (VAs).
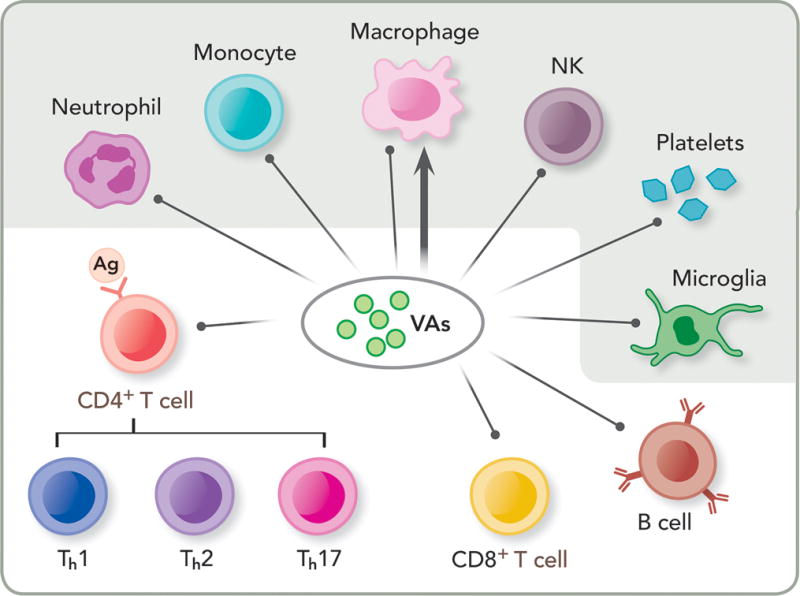
Depicted here are immune cells responsible for the innate (shaded) and adaptive (un-shaded) immunity. VAs have been shown to suppress innate immunity by impairing or suppressing neutrophil adhesion, monocytes, macrophages and natural killer cells (NK), and affecting resident cells in tissues, such as platelets and microglial cells. VAs also suppress adaptive immunity by decreasing lymphocyte proliferation, such as CD4+ and CD8+ T cells as well as B cells. Note that VAs can have both inhibition (shown as a line with a dot) and potentiation (shown as a line with an arrowhead) effects on macrophages, depending on the site of infection or inflammation. Ag: antigen; Th1, Th2, Th17: T helper cell type 1, 2, 17, respectively.
Table 1.
Immunosuppressive and immunoactivating effects of volatile anesthetics*
| Immune cell type | Effect | Volatile anesthetic |
|---|---|---|
| Neutrophil | Decreased cell number, adhesion | sevoflurane18–22 isoflurane19–21,23 halothane18–21 |
|
| ||
| Increase cell number | desflurane24 | |
|
| ||
| PBMC/Macrophage | Decreased cytokine release (IL-1B, TNF-a, IL-6, IL-8, IL-10) | sevoflurane25,26 isoflurane25,27 |
| Decreased phagocytosis, ROS, chemotaxis | halothane18 | |
| Decreased cell number | sevoflurane28 desflurane28 |
|
|
| ||
| Reversed N2O immune suppression | sevoflurane7 | |
| Enhanced nitrite production | isoflurane27 | |
| Increased cell number, respiratory burst | halothane29 | |
|
| ||
| NK cell | Decreased cytotoxicity | sevoflurane9,30–32 isoflurane9,30–33 halothane9,30–32 |
| Decreased response to IFN-γ | isoflurane25,30,34–36 halothane25,30,34–36 |
|
| Decreased cytokine release | sevoflurane34 | |
|
| ||
| Biphasic (increase then decrease cell number) | desflurane24 | |
|
| ||
| T lymphocyte | Decreased cell number, proliferation, change in Th1/Th2 ratio | isoflurane37,38 |
| Decreased Th1 | sevoflurane31 | |
|
| ||
| Increased Th1 | desflurane24 sevoflurane39 |
|
| Promoted cell-mediated immunity | sevoflurane39 | |
|
| ||
| B lymphocyte | Decreased cell number, increased B cell damage | Sevoflurane40,41 isoflurane40 desflurane40 |
|
| ||
| Other | Increased cortisol | isoflurane37 |
| Decreased platelet-immune cell adhesion | desflurane42 | |
| Decreased microglial cytokine release | isoflurane43 | |
| Decreased monocyte chemoattractant | isoflurane44 halothane44 |
|
|
| ||
| Increased platelet-immune cell adhesion | sevoflurane42 | |
Un-shaded and shaded entries are considered immunosuppressive and immunoactivating, respectively.
Innate immunity
The innate immune response is mediated by innate immune cells that are activated when protective barriers, such as the skin or other mucosa, have been compromised due to infection or injury. Cytokines, chemokines, and inflammatory mediators are secreted by both resident tissue cells and the recruited innate immune cells. They bring forth initial responders: neutrophils, monocytes, and NKs, as well as the complement pathways that enhance or amplify the immune responses. Surgery, sepsis, ischemia, and even the stress of being in the hospital or undergoing surgery can trigger reactions of the innate immune system. Volatile anesthetics have been found to exert a number of effects on innate immunity,9,18,46–48 mainly through neutrophils, DCs, NKs, and resident tissue macrophages.
Neutrophils
Neutrophils are the most abundant granulocytes and their numerous functions play a significant role in an inflammatory reaction.49 They are generally the first and most lethal effector cells recruited to an inflammation site. Phagocytosis and oxidative burst, which leads to rapid production of oxygen radicals, destroy foreign entities and damage native tissues. With this in mind, the impact of volatile anesthetics on this aspect of innate immunity can be viewed both positively and negatively.
The impaired function of neutrophils after exposure to volatile anesthetics was observed in several studies.18,26,47 Sevoflurane was found to decrease the number of reacting polymorphonuclear cells (PMNs).18 Reactive oxygen species (ROS) production and chemotaxis were affected after exposure to sevoflurane, desflurane, halothane and enflurane.18 Since halothane and enflurane are no longer used clinically, we will deemphasize their discussions in this review. Isoflurane and sevoflurane at clinical concentrations decreased neutrophil adhesion to human endothelial cells by inhibiting activation of PMNs.19 However, in the active state, PMNs are stimulated to roll and adhere to the endothelium of the vasculature within the inflamed tissue; thus, free neutrophils may not accurately reflect the active population.50 In contrast, the suppression of neutrophil adhesion after exposure to volatile anesthetics may positively affect the deleterious effects of PMNs in the ischemic setting. Isoflurane and sevoflurane have been shown to impair the post-ischemic adhesion of PMNs in the intact coronary system of isolated reperfused guinea pig hearts, and their inhibitory effects may be beneficial to cardiac function during general anesthesia.20,21
The results from in vivo studies largely parallel those from in vitro investigations. Exposing mice to 1.4% isoflurane before or after stimulation with lipopolysaccharide (LPS) for 30 minutes decreased PMN levels in the bronchial alveolar fluid.23 Neutrophils were noted to concentrate perivascularly, but were inhibited from migrating directly to the affected site. The neutrophil-attracting chemokines CXCL1 and CXCL2/3, which belong to the early signaling molecules for PMN recruitment in immune response, were also found to decrease in the same study. Mice injected with a sub-lethal dose of Influenza A showed fewer physical signs and symptoms of infection after exposure to halothane.51 A delay in appearance of neutrophils in lung tissue was demonstrated. This protective effect from halogenated volatile anesthetics was shown in a recent study52 to be the anesthetic-induced reduction in type I and type II interferon production. Similarly, in a rat model of liver transplantation, sevoflurane was found to attenuate neutrophil renal injury and decrease neutrophil infiltration, as well as decrease plasma tumor necrosis factor-α (TNF-α) and interleukin (IL)-6 levels.53 In a murine model of zymosan-induced peritonitis, isoflurane diminished the amplitude of PMN infiltration and down-regulated a panel of pro-inflammatory cytokines.54 In a human study, sevoflurane at 2 minimum alveolar concentration (MAC) induced leukocyte rolling, but decreased neutrophils in the peripheral blood samples.22 Despite the aforementioned evidence of the suppressive effects of volatile anesthetics on PMNs, contradictory data exist due to the immune complexity and variation inherent in the clinical setting. A study comparing sevoflurane and propofol in combination with fentanyl noted an overall similar inflammatory response, including increased IL-8, decreased IL-17, and decreased cellular adhesion.55 Additional research, particularly human studies, is necessary to determine the clinical impact of volatile anesthetic effects on neutrophil function.
Macrophages
Peripheral blood mononuclear cells (PBMC) include both lymphocytes and monocytes, which become macrophages upon migration into a tissue. Macrophages are phagocytic scavengers of innate immunity, similar to neutrophils.49 As resident cells in the tissues, however, macrophages are often the first responders to infection, sending recruitment signals to other effector cells.
In vitro studies revealed suppressive effects of volatile anesthetics on peripheral blood mononuclear cells and macrophages. Sevoflurane and isoflurane at concentrations of 1.5–2.5 MAC suppressed the release of IL-1β and TNF-α from human peripheral mononuclear cells stimulated by natural killer sensitive tumor cells.25 Despite its potent inhibitory effect on inflammatory cytokines, sevoflurane does not reduce the proliferation of human PBMC.7 Interestingly, the same study suggested that sevoflurane might have a beneficial effect by alleviating the immunosuppressive effect of N2O, which inhibits the proliferation of PBMC.7
A number of in vivo studies also demonstrated that volatile anesthetics could be either detrimental or beneficial, depending on the setting of inflammation with or without an infection. In a ventilated pig model, sevoflurane and desflurane were shown to decrease macrophage levels in bronchial alveolar fluid, and the overall cellular infiltration was also reduced.28 Isoflurane at 1.0 MAC after LPS exposure decreased macrophage release of TNF-α and IL-1β.27 Sevoflurane decreased cytokine release, specifically IL-6, IL-8, and IL-10, in patients undergoing cardiac surgery.26 Decreased pulmonary sequestration of white blood cells was noted as well. In contrast to suppressions caused by volatile anesthetics, a study involving isoflurane and sevoflurane administered at 1.5 MAC over two hours showed significant increases in IL-1β, macrophage inflammatory protein-2 (MIP-2), interferon-γ (IFN-γ), and TNF-α in rat alveolar macrophages under mechanical ventilation.56 Post-exposure of 1.0 MAC isoflurane four hours after LPS-induced endotoxemia in rats attenuated the systemic release of TNF-α and IL-1β, but simultaneously enhanced the nitrite production in cultured alveolar macrophages.27 Altogether, it appears that the complexity of in vivo studies has resulted in uncertainties regarding the relationship between macrophage function and volatile anesthetic exposure. Table 2 summarizes some of the reported effects of volatile anesthetics on several pro-inflammatory and anti-inflammatory cytokines.22,24,43,52,57–109 Future in-depth mechanistic studies are needed to reconcile many of the observed discrepancies.
Table 2.
General Anesthetic Effects on Several Key Pro- and Anti-Inflammatory Cytokines*
| Anesthetics | Pro-Inflammatory Cytokines | Anti-Inflammatory Cytokines | ||||||
|---|---|---|---|---|---|---|---|---|
| IL-1β | IL-6 | TNF-α | INF-γ | IL-1α | IL-4 | IL-10 | TGF-β | |
| Isoflurane | ↑57–63 ↓43,63–68 |
↓61,64,65 | ↑59–61,63 ↓62,64–67 |
↑62,63 | – | – | ↑ 63,69,70 | ↑71–73 |
| Sevoflurane | ↓74–79 | ↑80–82 ↓22,76,79,83–86 |
↑81,82,87 ↓74–79,83,84,88–90 |
↓91 |
– | No effect92–94 | ↑74,77,87 ↓79,94 |
↑95,96 ↓88,97 |
| Desflurane | ↓89,98 No effect63 |
↑99 No effect82,98 |
↓89,98 | ↑100 | ↑101 | No effect24 | ↑102 ↓100 |
– |
| Halothane | ↑103 | ↑103 | ↑103,104 | ↑105 ↓52,106 |
– | ↑107,108 | No effect109 ↓106 |
↑73 |
Up-arrows indicate activation or potentiation, and down-arrows indicate suppression or inhibition.
Natural killer cells
NKs, unlike neutrophils and monocytes, are a component of innate immunity originating from the lymphoid lineage of white blood cells.49 They are large granular lymphocytes that play a critical role in the defense against viral infection as well as oncologic disease. Because surgery and general anesthesia are often necessary in the treatment of cancer, extensive research has been conducted to determine the effect of volatile anesthetics on the NK cell population.
In vitro studies show that isoflurane and sevoflurane suppress NK cell cytotoxicity and cytokine-associated NK cell activation.9,30–32 Isoflurane decreased the NK cells’ response to interferon; sevoflurane decreased cytokine release, specifically TNF-α.25,30,34 It was unclear whether NK cell functions could be fully restored post-operatively with supplemental interferon.32,110 An early human study indicated that NK cell activity decreased for several days post-operatively.47 A more recent in vivo study with dogs also showed a significant decrease in NK cytotoxic activity, measured by the percentage of NK cell-induced apoptosis and narcosis in canine thyroid adenocarcinoma cell line, 24 hours after isoflurane anesthesia compared to the baseline values and the control group without anesthesia.33 The decreased responses to interferon after exposure to isoflurane were also supported by other in vivo studies.30,31,34–36 A decrease in NK cell number as well as a shift in cell-mediated immunity away from NK cell promotion were reported.31,34 A recent meta-analysis on NK cell function and anesthetic exposure in 189 patients noted significant data heterogeneity without a conclusive association between anesthetic modulation and NK cell functions, and called for further clinical investigations.111
Other resident tissue cells
Resident cells in tissues, such as alveolar macrophages, platelets, and glial cells, can also be affected by volatile anesthetics, thereby affecting the immune response. Alveolar cells are in direct contact with volatile anesthetics. In rat alveolar type II cells in primary culture, isoflurane reduced cell secretions of IL-6, MIP-2, and monocyte chemoattractant protein-1 (MCP-1), but did not change total protein secretion.44 Although levels of IL-6 and MIP-2 were largely restored to baseline in 4–24 hours after anesthetic exposure, MCP-1 remained suppressed at 24 hours.44
Platelets also play a significant role in the immune response, as they are critical for cellular adhesion. After blood was incubated with 1 or 2 MAC sevoflurane for over an hour, the binding of platelets to lymphocytes, neutrophils and monocytes was enhanced and the expression of P-selectin on platelets increased.42 However, the same treatment with desflurane resulted in a reduction in lymphocyte-platelet, neutrophil-platelet and monocyte-platelet conjugates.42 Similar phenomena were also observed in an earlier study.112 Another independent study suggested that neither desflurane nor sevoflurane caused significant changes in ADP-stimulated platelets, even though sevoflurane increased the expression of P-selectin in un-stimulated platelets.113
Microglia, which are resident neural immune cells, were recognized recently to contribute to neuroinflammation and postoperative delirium and cognitive decline. The immune-activated microglia not only changed cell number, size, and shape, but also released the proinflammatory cytokines such as TNF-α, IL-1β, IL-6, and IFN-γ. In vivo experiments in young mice with repeated exposures to clinical concentrations of sevoflurane showed activation of microglia and accumulation of IL-6 and TNF-α, with associted cognitive impairment.82 The same study also found that these detrimental changes were absent in adult mice, suggesting selective vulnerability in a particular age group. Repeated exposures to desflurane did not lead to microglia activation and IL-6 and TNF-α accumulation in either young or old mice. In cultured H4 human neuroglioma cells, isoflurane was found to induce caspase-3 activation, cause mitochondrial dysfunction, promote ROS accumulation, induce apoptosis, and reduce cell viability.114–116 Strategies to target these isoflurane-induced events have been demonstrated.115,116
In other studies, beneficial effects to reduce neuroinflammation are noted from preconditioning with volatile anesthetics. Isoflurane suppressed the proinflammatory cytokine IL-1β in the mouse brain after intraperitoneal injection of LPS.43 In adult mice, exposure to isoflurane was found not to produce neuro-apoptosis but reduce astroglial processes.117 A more recent study64 showed that isoflurane preconditioning inhibited the upregulation of toll-like receptor 4 (TLR4), which is known to regulate microglia activation and microglia production of proinflammatory factors.
Overall, because of the complexity of various tissue responses to volatile anesthetics, the anesthetic effects on innate immunity remain an active area of investigation.
Adaptive immunity
Adaptive immune responses are distinct from innate immunity because they are generated by clonal selection of lymphocytes.118 There are two broad classes of adaptive immunity: humoral immune responses, which are mediated by macromolecules (such as antibodies and antimicrobial peptides) made by B lymphocytes, and cell-mediated immune responses, which are carried out mainly by T lymphocytes. Given the variety of lymphocytes and the multiple mechanisms involved in their recognition and response to antigens, investigations into the impact of volatile anesthetics on the adaptive immune system have been challenging. In general, volatile anesthetics induced a decrease in proliferation of lymphocytes or an increase in lymphocyte apoptosis.9,119–121
T lymphocytes
Cell-mediated immunity within the adaptive response includes T lymphocytes (T cells), distinguished from other lymphocytes by the T cell receptor (TCR), which can be modified and tailored for specific antigens.49 T cell precursors originate in the bone marrow and then travel in an immature state to the thymus to fully mature. From that point, they circulate in the blood and throughout the secondary lymphoid tissues, such as lymph nodes, in search of antigens sequestered there by antigen-presenting cells (APCs) that have traveled from infected sites. Upon activation, T cells proliferate and differentiate. T helper (Th) cells remain in the lymph nodes. There are three subsets of Th cells: Th1 cells that magnify inflammation via soluble protein secretion and macrophage stimulation; Th2 cells that stimulate B lymphocytes to mature and produce antibodies; and the more recently discovered Th17 cells that produce IL-17, IL-17F, and IL-22, and secrete IL-21 to communicate with the cells in the immune system.122,123
Different anesthetics have been found to produce varied effects on Th cells,37,100 even though exposure to volatile anesthetics has generally resulted in a decrease in the number and proliferation of T cells.9,47,124 It is often difficult to discern whether the decrease in the number and proliferation of T cells is related to a decrease in IFN-γ, an increase in cortisol, impaired antigen presentation, surgical insult, or a combination of all these factors. Patients exposed to isoflurane or propofol had drastically different T cell responses.37 Those exposed to isoflurane showed no change in their Th1/Th2 ratio, but did show an increase in cortisol, a known promoter of Th2 cells.37 A recent clinical trial on 40 breast cancer surgeries24 showed that desflurane can preserve Th1/Th2 ratio as well as the ratio of their cytokine products IL2/IL4. A separate study, however, reported a decrease in the Th1/Th2 ratio in patients who underwent isoflurane anesthesia.38 Another study compared patients who received spinal anesthesia and desflurane general anesthesia100 and showed that desflurane, but not bupivacaine, increased the Th1/Th2 ratio, mainly due to an increase of Th1 responses in patients.100 Exposure to sevoflurane alone was associated with a decrease in Th1 cells and in the Th1/Th2 ratio post-operatively.31 However, adding a spinal block to sevoflurane general anesthesia in surgery was noted to preserve the Th1/Th2 balance and thereby reduce the promotion of tumor metastasis in a mouse tumor model.31 For hepatocellular carcinoma patients, neuraxial anesthesia, specifically epidural, combined with general anesthesia, was found superior to general anesthesia alone in promoting anti-tumor Th polarization, including shifting the Th1/Th2 balance towards Th1 and decreasing Th17.39 Although further investigation is certainly needed to clarify the inconsistent effects of volatile anesthetics on the T cell-mediated immune responses, accumulating evidence seems to suggest that a proper selection of suitable anesthetic methods can mediate the balances of Th subsets or even benefit the balance of anti-tumor responses.
B lymphocytes and complement system
Similar to T cells, B lymphocytes (B cells) can modify their cell surface receptors or immunoglobulins to recognize specific pathogens.49 Data regarding the effects of volatile anesthetics on B cells, however, are relatively scarce. An early study implied that surgical trauma or associated perioperative conditions, not the specific anesthetic agent employed, was the dominant factor responsible for most postoperative specific humoral immunity impairment.47 A more recent study, however, seemed to suggest that isoflurane, sevoflurane, and desflurane could induce B cell damage due to calcium release from the endoplasmic reticulum.40 A study on mice also found that sevoflurane significantly decreased the level of splenic B cells.41
Complement-mediated immunity plays a role in both innate and adaptive immunity. It can act as an extension of the immunity provided by the B cells and the antibodies that they produce. To date, there are few reports concerning the effects of volatile anesthetics on the complement system. The combination of anesthesia and surgery was recognized as being associated with a decrease in complement levels, which may represent complement pathway activation.47 Patients exposed to halogenated volatile anesthetics developed specific IgG1 autoantibodies that were likely cleared by classical activation of the complement system, while anesthetic-induced hepatitis patients developed specific IgG4 autoantibodies that escaped clearance because of their small size or by direct inhibition of complement activation.125
Indirect effectors of immunity
Volatile anesthetics can indirectly affect immunity through their impact on stress hormone levels as well as other effectors of immunity. Stress is inherent in the perioperative setting and a known modulator of the immune system. The major stress hormones include endogenous glucocorticoid (e.g., cortisol in human and corticosterone in non-human animals) and catecholamines (e.g., epinephrine and norepinephrine), which can be released to result in systemic immune activations. Surgery-induced inflammatory response and alteration in cell-mediated immunity were found to be more pronounced after a balanced volatile anesthesia when compared to total intravenous anesthesia.120 The effects were attributed to the enhanced stress response in patients undergoing anesthesia with a volatile agent.120 Volatile anesthetics also often cause hypotension and transient hypoxia,126,127 which promote tissue inflammation and increase cellular adhesion. These can, in turn, depress the Th1 phenotype or promote cell-mediated immunity.46 Glycemic control in the perioperative environment is another topic of significant debate, and volatile anesthetics can exert direct effects on immunity by manipulating glucose control. Blood glucose levels were found to be higher in patients anesthetized with a combination of sevoflurane and fentanyl versus those anesthetized with propofol and fentanyl.55 Isoflurane was also noted to inhibit normal insulin production and produce a hyperglycemic response.128–130 The observed hyperglycemic response in isoflurane anesthesia was thought a consequence of both impaired glucose clearance and increased glucose production.129
Potential mechanisms of immunomodulation by volatile anesthetics
Although specific targets of volatile anesthetics in the immune system have not been well defined, molecular and cellular events involved in immune modulation by volatile anesthetics have been identified, including a reduction in the number of immune cells due to cell death and the suppression of immune activities (Figure 2). In reality, with the heterogeneity in immune responses, immunomodulation is likely more complicated than what is shown in Figure 2. For instance, cross talks may occur among different pathways, such as interactions between ROS and inducible nitric oxide synthase (iNOS). Understanding individual pathways and their relationships will facilitate mechanistic understanding of immune modulation by volatile anesthetics.
Figure 2. Potential mechanisms involved in the immunomodulation by volatile anesthetics (VAs).
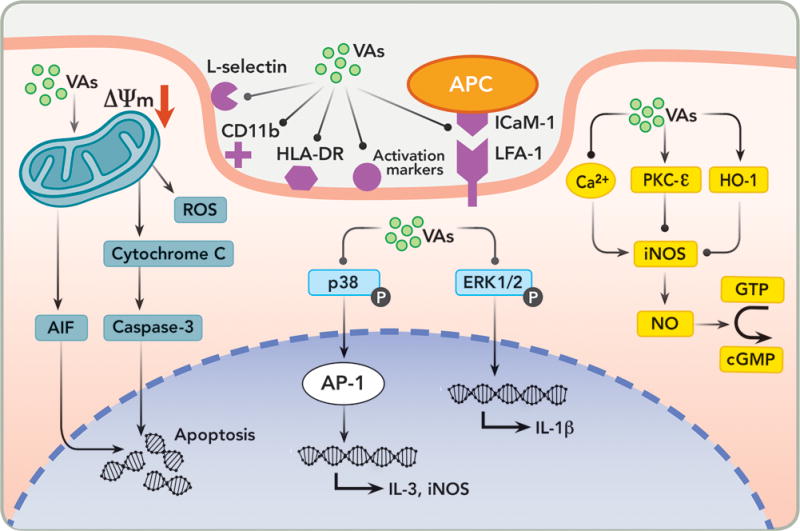
Depicted here are schematic representations of major immunomodulation pathways affected by VAs. The thick solid line shows the cytoplasmic membrane and the dashed line marks the nuclear membrane. The pink shaded areas are cytoplasmic and extracellular space, and the light purple shaded area is the cell nucleus. Lines with arrowheads and dots at the end represent “activation” and “inhibition”, respectively. ΔΨm = mitochondrial membrane potential; AIF = apoptosis inducing factor; ROS = reactive oxygen species; APC = antigen-presenting cell; HLA = human leukocyte antigen; DR = antigen D related; LFA-1 = lymphocyte function-associated antigen 1; ICAM-1 = intercellular adhesion molecule; AP-1 = activator protein 1; iNOS = inducible nitric oxide synthase; ERK = extracellular signal-regulated kinases; PKC = protein kinase C; HO-1 = heme oxygenase 1; GTP = guanosine triphosphate; cGMP = cyclic 3′,5′-guanosine monophosphate; and NO = nitric oxide.
Lymphocytes are more prone to apoptosis than other immune cells.131 Apoptosis is initiated by the mitochondria-triggered pathway (intrinsic pathway) or the death-receptor-triggered pathway (extrinsic pathway).132,133 Sevoflurane and isoflurane were found to decrease mitochondrial membrane potential (ΔΨm) in a dose-dependent manner, subsequently triggering the release of cytochrome C from the mitochondrial intermembrane space into the cytosol and eventually inducing apoptosis via activation of Caspase-3.14,121,134 The irreversible pan-caspase inhibitor Z-VAD-fmk was shown to block sevoflurane-induced apoptosis.14 Another distinct mitochondria-mediated molecule, apoptosis-inducing factor (AIF), also initiates apoptosis.132,133 AIF was originally identified as a mitochondrial flavoprotein that was released into the cytoplasm and subsequently entered the nucleus to cause cell death.132,133 A recent human study135 showed that sevoflurane increased AIF in cardiac surgery patients, who also exhibited decreased lymphocyte counts. ROS is another major signaling molecule in the mitochondrial pathway for apoptosis.70 Sevoflurane was shown to increase the production of intracellular ROS and promote lymphocyte apoptosis.134 Interestingly, the same study also suggested that propofol might attenuate the sevoflurane-induced mitochondria-related apoptosis.134 Compared to the mitochondria-triggered pathway, the death receptor-signaling pathway played little role in sevoflurane-induced lymphocyte apoptosis.14 Thus, it is reasonable to believe that mitochondria are central mediators of volatile anesthetic-associated apoptosis. In addition to apoptosis, cell necrosis could also contribute to the isoflurane-induced decrease in immune cell count.14
Adhesion molecules are important for immune cell recruitment and accumulation at inflammatory sites. The human leukocyte antigen (HLA) heterodimers are cell surface antigen for the TCR. Volatile anesthetics may interact directly with these molecules to modify their functions or reduce their expression. Immune cell trafficking and penetration depend predominantly on integrin lymphocyte function-associated antigen-1 (LFA-1).136 Isoflurane and sevoflurane bind to LFA-1 and allosterically block the coupling of LFA-1 to its major interaction partner intercellular adhesion molecule-1 (ICAM-1) found on APCs. As a result, immune cell adhesion is inhibited.16,17 It was found recently137 that isoflurane, but not sevoflurane, had the same inhibitory effect on Macrophage-1 antigen (MAC-1), a LFA-1 homologous protein. Structural biology approaches, combined with computational docking and mutations of key residues at the anesthetic binding site in LFA-1 and MAC-1, have shed new lights on how volatile anesthetic binding to a functionally important protein domain (the so-called “I domain” in LFA-1 and the homologous MAC-1) can allosterically change the binding pocket at a remote location on these immune signaling proteins to change their interaction with ICAM-1, thereby inhibiting the downstream events of leukocyte recruitment and migration.137,138 CD11b is another pivotal integrin on the surface of leukocytes. Isoflurane and sevoflurane at clinical concentrations abolished the upward regulation of CD11b on neutrophils and resulted in reduced neutrophil adhesion.19,21 L-selectin, a cell adhesion molecule belonging to the selectin family, can be found in most leukocytes. Sevoflurane decreased L-selectin expression by 25%, indicating an increased threshold for cellular activation.139
Volatile anesthetics mostly suppress, but in some cases up-regulate, iNOS expression and nitric oxide (NO) production.48,140 The suppressive effect is followed by the alteration of the NO-cyclic 3′,5′-guanosine monophosphate (NO-cGMP) system, which is a major signaling transduction pathway implicated in a wide range of physiologic functions.140,141 Evidence showed that volatile anesthetics interacted with several upstream mediators of iNOS, including calcium, protein kinase C (PKC), and heme oxygenase-1 (HO-1). Isoflurane and desflurane at clinically relevant concentrations mediated the inhibitory effect on iNOS expression by inhibiting mobilization of cytosolic free calcium, which occurred upon macrophage activation.48 Treatment or pretreatment with 2% isoflurane induced HO-1 protein expression and caused an induction of HO activity, which correlated with a decrease in iNOS expression and NO production in LPS-stimulated macrophages.142 Blockade of HO activity reversed these effects.142 Pretreatment with 2% isoflurane inhibited overexpression of iNOS and accumulation of nitrite induced by LPS and IFN-γ in macrophages.143 The isoflurane preconditioning effect may be mediated by isoform PKC-ε.143 It was noted that LPS stimulation in combination with IFN-γ resulted in increased nitrite release after exposure to isoflurane,48 indicating that supplementary IFN-γ is able to overcome any inhibition of normal macrophage function. The notion was further reinforced by the study showing the reversal of volatile anesthetic-induced impairment to macrophage chemotaxis and H2O2 production upon addition of IFN-γ.47
Mitogen-activated protein kinases (MAPKs), including extracellular signal-regulated kinase (ERK), c-Jun N-terminal protein kinase (JNK), and p38 MAPK, have been implicated in proinflammatory cytokine release.144 In isolated T cells, sevoflurane inhibited activation of the transcription factor Activator Protein-1 (AP-1), which was associated with the inhibition of p38 activity and resulted in a decreased IL-3 expression.15 Activation of p38 is known to regulate several inflammation-related genes, including TNF-α, IL-β, and IL-6. Isoflurane, but not halothane, can activate p38 MARK in a concentration-dependent manner.145 Most interestingly, both isoflurane and halothane can greatly enhance the proinflammatory cytokine-induced p38 activations, but have little effects on oxidative stress-induced p38 activations,145 suggesting that the anesthetic action might be upstream of phosphorylation of p38 MARK. Similarly, phosphorylation of ERK can activate the transcription factor cAMP response element-binding protein (CREB), which in turn modulates many CREB-targeted genes. In glial cells, particularly microglia, isoflurane suppressed LPS-induced phosphorylation of ERK 1/2 and the high expression of IL-1β mRNA and protein, but did not affect nuclear factor-kappa B (NF-κB) or AP-1 activation.43 More molecular biology investigations aimed at dissecting anesthetic effects on each of these pathways will help us better understand the molecular mechanisms of immunomodulation by volatile anesthetics.
Additional Considerations
Oncologic considerations
Surgical intervention remains a primary treatment for cancer. Anesthetics used during the perioperative period may influence the immune systems, directly affect cancer cells, and ultimately modify oncological outcomes.146,147 Investigations on whether anesthetics affect the outcome and prognosis of cancer have been carried out on various types of cancer, including ovarian, colon, breast, prostate and rectal cancer. Several retrospective studies suggested that, in comparison to general anesthesia, regional anesthesia was associated with better outcomes.148–152 In one study, patients who had radical prostatectomy under general anesthesia using a combination of sevoflurane and N2O showed more than 20% higher mortality rate than those who received epidural anesthesia.148 A similar study also showed a decrease in time to tumor recurrence after primary cancer surgery or even to death when using an anesthetic regimen of sevoflurane alone compared to that of intraoperative neuraxial anesthesia combined with postoperative analgesia.153 However, conflicting data exist. The beneficial effect of regional anesthesia on cancer recurrence was not observed in all types of cancer.152,154,155 Some studies found no association between volatile anesthetics and death or length of cancer-free survival time.156,157 Thus, more prospective randomized controlled trials, with careful designs of various anesthetic regimens, are warranted. In addition, in order to optimize anesthesia management strategies for oncologic surgical patients, more mechanistic studies at a cellular level are also needed.7,158–161
Positive immune modulation by volatile anesthetics
Despite the suppressive effects of volatile anesthetics on the function of neutrophils, macrophages, dendritic cells, T cells, B cells, and NK cells, as reviewed above under the subheadings “Innate Immunity” and “Adaptive Immunity,” is it possible for volatile anesthetics to enhance the immune system or possibly generate therapeutic benefits? A recent review by Fukazawa and Lee presented compelling evidence of protective effects of volatile anesthetics against ischemic acute kidney injury (AKI) in both preclinical and clinical studies.162 The cellular mechanisms of volatile anesthetic-induced kidney protection lie in the anesthetic activation of multiple pathways synthesizing anti-inflammatory and cytoprotective signaling molecules, such as releasing TGF-β1, activating CD73 and inducing IL-11, generating adenosine, and producing SK and S1P.71,96,163–165 Anti-ischemic and anti-inflammatory effects of volatile anesthetics were also shown to affect other organs such as the heart, liver, and brain through either pretreatment before prolonged ischemia or after completion of an ischemic insult.166–169 The protective mechanisms on these organs, however, may differ from those observed in renal protection.170 Nevertheless, systematic investigations of protective effects of volatile anesthetics against AKI have led to an important discovery that volatile anesthetics can positively modulate immunity and provide off-label therapeutic effects, if dose and exposure time of volatile anesthetics are optimized.162
Closing Remarks
The majority of studies reported thus far show that volatile anesthetics have immunosuppressive effects. Whether a short period of immunosuppression has a prolonged effect on patient outcomes merits further investigation. Well-controlled randomized clinical trials are highly desirable, though isolating effects of volatile anesthetics from other factors in a perioperative setting remains challenging. Future studies should take into consideration the surgical procedures involved, the anesthetics and other medications used, and the time dependence in immune modulation and resolution. Because immunosuppression is in general detrimental for cancer patients, but potentially beneficial for septic patients, the choice of anesthesia regimens should be carefully evaluated in the overall planning for the perioperative care.
In vitro and in vivo level mechanistic studies focusing on how volatile anesthetics modulate various immune responses should continue. These studies not only can provide valuable clues to initiate more complex clinical trials, but also can identify useful biomarkers to detect the detrimental effects of volatile anesthetics.
Desirable off-label effects of volatile anesthetics have been demonstrated in a few areas, but on a large scale, they are underexplored.162 Whether and how volatile anesthetics positively modulate immune responses and subsequently generate therapeutic benefits to patients warrants further investigations.
Summary Statement.
This review provides a balanced perspective on the anesthetic modulation of innate and adaptive immune responses as well as indirect effectors of immunity. Potential mechanisms of immunomodulation by volatile anesthetics are also discussed.
Acknowledgments
Funding: L.M.S. was supported by a National Institute of Health (NIH, Bethesda, MA, USA) Ruth L. Kirschstein National Service Award (T32GM075770 to Y.X.). The research was supported by funding from the NIH (R01GM066358 and R01GM114851).
Footnotes
Conflicts of Interest: The authors disclose no conflicts of interest.
References
- 1.Satpathy AT, Wu X, Albring JC, Murphy KM. Re(de)fining the dendritic cell lineage. Nat Immunol. 2012;13:1145–54. doi: 10.1038/ni.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;16:343–53. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forman SA, Miller KW. Anesthetic sites and allosteric mechanisms of action on Cys-loop ligand-gated ion channels. Can J Anaesth. 2011;58:191–205. doi: 10.1007/s12630-010-9419-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–86. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- 5.Hemmings HC, Jr, Akabas MH, Goldstein PA, Trudell JR, Orser BA, Harrison NL. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci. 2005;26:503–10. doi: 10.1016/j.tips.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Tang P, Xu Y. Large-scale molecular dynamics simulations of general anesthetic effects on the ion channel in the fully hydrated membrane: the implication of molecular mechanisms of general anesthesia. Proc Natl Acad Sci U S A. 2002;99:16035–40. doi: 10.1073/pnas.252522299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneemilch CE, Hachenberg T, Ansorge S, Ittenson A, Bank U. Effects of different anaesthetic agents on immune cell function in vitro. Eur J Anaesthesiol. 2005;22:616–23. doi: 10.1017/s0265021505001031. [DOI] [PubMed] [Google Scholar]
- 8.Homburger JA, Meiler SE. Anesthesia drugs, immunity, and long-term outcome. Curr Opin Anaesthesiol. 2006;19:423–8. doi: 10.1097/01.aco.0000236143.61593.14. [DOI] [PubMed] [Google Scholar]
- 9.Kurosawa S, Kato M. Anesthetics, immune cells, and immune responses. J Anesth. 2008;22:263–77. doi: 10.1007/s00540-008-0626-2. [DOI] [PubMed] [Google Scholar]
- 10.Pirbudak Cocelli L, Ugur MG, Karadasli H. Comparison of effects of low-flow sevoflurane and desflurane anesthesia on neutrophil and T-cell populations. Curr Ther Res Clin Exp. 2012;73:41–51. doi: 10.1016/j.curtheres.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong L, Qin Q, Zhou L, Ouyang W, Li Y, Wu Y, Li Y. Effects of fentanyl anesthesia and sufentanil anesthesia on regulatory T cells frequencies. Int J Clin Exp Pathol. 2014;7:7708–16. [PMC free article] [PubMed] [Google Scholar]
- 12.Alsina E, Matute E, Ruiz-Huerta AD, Gilsanz F. The effects of sevoflurane or remifentanil on the stress response to surgical stimulus. Curr Pharm Des. 2014;20:5449–68. doi: 10.2174/1381612820666140325105723. [DOI] [PubMed] [Google Scholar]
- 13.Bargellini A, Rovesti S, Barbieri A, Vivoli R, Roncaglia R, Righi E, Borella P. Effects of chronic exposure to anaesthetic gases on some immune parameters. Sci Total Environ. 2001;270:149–56. doi: 10.1016/s0048-9697(00)00778-6. [DOI] [PubMed] [Google Scholar]
- 14.Loop T, Dovi-Akue D, Frick M, Roesslein M, Egger L, Humar M, Hoetzel A, Schmidt R, Borner C, Pahl HL, Geiger KK, Pannen BH. Volatile anesthetics induce caspase-dependent, mitochondria-mediated apoptosis in human T lymphocytes in vitro. Anesthesiology. 2005;102:1147–57. doi: 10.1097/00000542-200506000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Loop T, Scheiermann P, Doviakue D, Musshoff F, Humar M, Roesslein M, Hoetzel A, Schmidt R, Madea B, Geiger KK, Pahl HL, Pannen BH. Sevoflurane inhibits phorbol-myristate-acetate-induced activator protein-1 activation in human T lymphocytes in vitro: potential role of the p38-stress kinase pathway. Anesthesiology. 2004;101:710–21. doi: 10.1097/00000542-200409000-00020. [DOI] [PubMed] [Google Scholar]
- 16.Yuki K, Astrof NS, Bracken C, Soriano SG, Shimaoka M. Sevoflurane binds and allosterically blocks integrin lymphocyte function-associated antigen-1. Anesthesiology. 2010;113:600–9. doi: 10.1097/ALN.0b013e3181e89a77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, Astrof NS, Liu JH, Wang JH, Shimaoka M. Crystal structure of isoflurane bound to integrin LFA-1 supports a unified mechanism of volatile anesthetic action in the immune and central nervous systems. FASEB J. 2009;23:2735–40. doi: 10.1096/fj.09-129908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frohlich D, Rothe G, Schwall B, Schmid P, Schmitz G, Taeger K, Hobbhahn J. Effects of volatile anaesthetics on human neutrophil oxidative response to the bacterial peptide FMLP. Br J Anaesth. 1997;78:718–23. doi: 10.1093/bja/78.6.718. [DOI] [PubMed] [Google Scholar]
- 19.Mobert J, Zahler S, Becker BF, Conzen PF. Inhibition of neutrophil activation by volatile anesthetics decreases adhesion to cultured human endothelial cells. Anesthesiology. 1999;90:1372–81. doi: 10.1097/00000542-199905000-00022. [DOI] [PubMed] [Google Scholar]
- 20.Kowalski C, Zahler S, Becker BF, Flaucher A, Conzen PF, Gerlach E, Peter K. Halothane, isoflurane, and sevoflurane reduce postischemic adhesion of neutrophils in the coronary system. Anesthesiology. 1997;86:188–95. doi: 10.1097/00000542-199701000-00023. [DOI] [PubMed] [Google Scholar]
- 21.Heindl B, Reichle FM, Zahler S, Conzen PF, Becker BF. Sevoflurane and isoflurane protect the reperfused guinea pig heart by reducing postischemic adhesion of polymorphonuclear neutrophils. Anesthesiology. 1999;91:521–30. doi: 10.1097/00000542-199908000-00027. [DOI] [PubMed] [Google Scholar]
- 22.Morisaki H, Aoyama Y, Shimada M, Ochiai R, Takeda J. Leucocyte distribution during sevoflurane anaesthesia. Br J Anaesth. 1998;80:502–3. doi: 10.1093/bja/80.4.502. [DOI] [PubMed] [Google Scholar]
- 23.Reutershan J, Chang D, Hayes JK, Ley K. Protective effects of isoflurane pretreatment in endotoxin-induced lung injury. Anesthesiology. 2006;104:511–7. doi: 10.1097/00000542-200603000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Woo JH, Baik HJ, Kim CH, Chung RK, Kim DY, Lee GY, Chun EH. Effect of Propofol and Desflurane on Immune Cell Populations in Breast Cancer Patients: A Randomized Trial. J Korean Med Sci. 2015;30:1503–8. doi: 10.3346/jkms.2015.30.10.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitsuhata H, Shimizu R, Yokoyama MM. Suppressive effects of volatile anesthetics on cytokine release in human peripheral blood mononuclear cells. Int J Immunopharmacol. 1995;17:529–34. doi: 10.1016/0192-0561(95)00026-x. [DOI] [PubMed] [Google Scholar]
- 26.Cho EJ, Yoon JH, Hong SJ, Lee SH, Sim SB. The effects of sevoflurane on systemic and pulmonary inflammatory responses after cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2009;23:639–45. doi: 10.1053/j.jvca.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 27.Flondor M, Hofstetter C, Boost KA, Betz C, Homann M, Zwissler B. Isoflurane inhalation after induction of endotoxemia in rats attenuates the systemic cytokine response. Eur Surg Res. 2008;40:1–6. doi: 10.1159/000107614. [DOI] [PubMed] [Google Scholar]
- 28.Kalimeris K, Christodoulaki K, Karakitsos P, Batistatou A, Lekka M, Bai M, Kitsiouli E, Nakos G, Kostopanagiotou G. Influence of propofol and volatile anaesthetics on the inflammatory response in the ventilated lung. Acta Anaesthesiol Scand. 2011;55:740–8. doi: 10.1111/j.1399-6576.2011.02461.x. [DOI] [PubMed] [Google Scholar]
- 29.Colucci D, Harvey G, Gayol MC, Elena G, Puig N. Halothane anesthesia in mice: effect on the phagocytic activity and respiratory burst of peritoneal macrophages. Neuroimmunomodulation. 2011;18:11–8. doi: 10.1159/000313367. [DOI] [PubMed] [Google Scholar]
- 30.Melamed R, Bar-Yosef S, Shakhar G, Shakhar K, Ben-Eliyahu S. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: mediating mechanisms and prophylactic measures. Anesth Analg. 2003;97:1331–9. doi: 10.1213/01.ANE.0000082995.44040.07. [DOI] [PubMed] [Google Scholar]
- 31.Wada H, Seki S, Takahashi T, Kawarabayashi N, Higuchi H, Habu Y, Sugahara S, Kazama T. Combined spinal and general anesthesia attenuates liver metastasis by preserving TH1/TH2 cytokine balance. Anesthesiology. 2007;106:499–506. doi: 10.1097/00000542-200703000-00014. [DOI] [PubMed] [Google Scholar]
- 32.Welden B, Gates G, Mallari R, Garrett N. Effects of anesthetics and analgesics on natural killer cell activity. AANA J. 2009;77:287–92. [PubMed] [Google Scholar]
- 33.Miyata T, Kodama T, Honma R, Nezu Y, Harada Y, Yogo T, Hara Y, Tagawa M. Influence of general anesthesia with isoflurane following propofol-induction on natural killer cell cytotoxic activities of peripheral blood lymphocytes in dogs. J Vet Med Sci. 2013;75:917–21. doi: 10.1292/jvms.12-0436. [DOI] [PubMed] [Google Scholar]
- 34.Markovic SN, Knight PR, Murasko DM. Inhibition of interferon stimulation of natural killer cell activity in mice anesthetized with halothane or isoflurane. Anesthesiology. 1993;78:700–6. doi: 10.1097/00000542-199304000-00013. [DOI] [PubMed] [Google Scholar]
- 35.Sessler DI. Does regional analgesia reduce the risk of cancer recurrence? A hypothesis. Eur J Cancer Prev. 2008;17:269–72. doi: 10.1097/CEJ.0b013e3282f0c005. [DOI] [PubMed] [Google Scholar]
- 36.Brand JM, Kirchner H, Poppe C, Schmucker P. The effects of general anesthesia on human peripheral immune cell distribution and cytokine production. Clin Immunol Immunopathol. 1997;83:190–4. doi: 10.1006/clin.1997.4351. [DOI] [PubMed] [Google Scholar]
- 37.Ren XF, Li WZ, Meng FY, Lin CF. Differential effects of propofol and isoflurane on the activation of T-helper cells in lung cancer patients. Anaesthesia. 2010;65:478–82. doi: 10.1111/j.1365-2044.2010.06304.x. [DOI] [PubMed] [Google Scholar]
- 38.Inada T, Yamanouchi Y, Jomura S, Sakamoto S, Takahashi M, Kambara T, Shingu K. Effect of propofol and isoflurane anaesthesia on the immune response to surgery. Anaesthesia. 2004;59:954–9. doi: 10.1111/j.1365-2044.2004.03837.x. [DOI] [PubMed] [Google Scholar]
- 39.Zhou D, Gu FM, Gao Q, Li QL, Zhou J, Miao CH. Effects of anesthetic methods on preserving anti-tumor T-helper polarization following hepatectomy. World J Gastroenterol. 2012;18:3089–98. doi: 10.3748/wjg.v18.i24.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang H, Liang G, Hawkins BJ, Madesh M, Pierwola A, Wei H. Inhalational anesthetics induce cell damage by disruption of intracellular calcium homeostasis with different potencies. Anesthesiology. 2008;109:243–50. doi: 10.1097/ALN.0b013e31817f5c47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puig NR, Ferrero P, Bay ML, Hidalgo G, Valenti J, Amerio N, Elena G. Effects of sevoflurane general anesthesia: immunological studies in mice. Int Immunopharmacol. 2002;2:95–104. doi: 10.1016/s1567-5769(01)00151-5. [DOI] [PubMed] [Google Scholar]
- 42.Horn NA, de Rossi L, Robitzsch T, Hecker KE, Hutschenreuter G, Rossaint R. The effects of sevoflurane and desflurane in vitro on platelet-leukocyte adhesion in whole blood. Anaesthesia. 2003;58:312–9. doi: 10.1046/j.1365-2044.2003.03076.x. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka T, Kai S, Matsuyama T, Adachi T, Fukuda K, Hirota K. General anesthetics inhibit LPS-induced IL-1beta expression in glial cells. PLoS One. 2013;8:e82930. doi: 10.1371/journal.pone.0082930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giraud O, Molliex S, Rolland C, Lecon-Malas V, Desmonts JM, Aubier M, Dehoux M. Halogenated Anesthetics reduce interleukin-1 beta-induced cytokine secretion by rat alveolar type II cells in primary culture. Anesthesiology. 2003;98:74–81. doi: 10.1097/00000542-200301000-00015. [DOI] [PubMed] [Google Scholar]
- 45.Marana E, Annetta MG, Meo F, Parpaglioni R, Galeone M, Maussier ML, Marana R. Sevoflurane improves the neuroendocrine stress response during laparoscopic pelvic surgery. Can J Anaesth. 2003;50:348–54. doi: 10.1007/BF03021031. [DOI] [PubMed] [Google Scholar]
- 46.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–65. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stevenson GW, Hall SC, Rudnick S, Seleny FL, Stevenson HC. The Effect of Anesthetic Agents on the Human Immune-Response. Anesthesiology. 1990;72:542–552. doi: 10.1097/00000542-199003000-00024. [DOI] [PubMed] [Google Scholar]
- 48.Tschaikowsky K, Ritter J, Schroppel K, Kuhn M. Volatile anesthetics differentially affect immunostimulated expression of inducible nitric oxide synthase: role of intracellular calcium. Anesthesiology. 2000;92:1093–102. doi: 10.1097/00000542-200004000-00028. [DOI] [PubMed] [Google Scholar]
- 49.Parham P. The immune system. 2nd. New York: Garland Science; 2005. [Google Scholar]
- 50.Leonard SA, Redmond HP. Effects of volatile and intravenous anesthetic agents on neutrophil function. Int Anesthesiol Clin. 2003;41:21–9. doi: 10.1097/00004311-200341010-00004. [DOI] [PubMed] [Google Scholar]
- 51.Penna AM, Johnson KJ, Camilleri J, Knight PR. Alterations in influenza A virus specific immune injury in mice anesthetized with halothane or ketamine. Intervirology. 1990;31:188–96. doi: 10.1159/000150153. [DOI] [PubMed] [Google Scholar]
- 52.MacDonald BA, Chakravarthy KV, Davidson BA, Mullan BA, Alluri R, Hakansson AP, Knight PR. Halothane modulates the type i interferon response to influenza and minimizes the risk of secondary bacterial pneumonia through maintenance of neutrophil recruitment in an animal model. Anesthesiology. 2015;123:590–602. doi: 10.1097/ALN.0000000000000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kong HY, Zhu SM, Wang LQ, He Y, Xie HY, Zheng SS. Sevoflurane protects against acute kidney injury in a small-size liver transplantation model. Am J Nephrol. 2010;32:347–55. doi: 10.1159/000319623. [DOI] [PubMed] [Google Scholar]
- 54.Chiang N, Schwab JM, Fredman G, Kasuga K, Gelman S, Serhan CN. Anesthetics impact the resolution of inflammation. PLoS One. 2008;3:e1879. doi: 10.1371/journal.pone.0001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tylman M, Sarbinowski R, Bengtson JP, Kvarnstrom A, Bengtsson A. Inflammatory response in patients undergoing colorectal cancer surgery: the effect of two different anesthetic techniques. Minerva Anestesiol. 2011;77:275–82. [PubMed] [Google Scholar]
- 56.Kotani N, Takahashi S, Sessler DI, Hashiba E, Kubota T, Hashimoto H, Matsuki A. Volatile anesthetics augment expression of proinflammatory cytokines in rat alveolar macrophages during mechanical ventilation. Anesthesiology. 1999;91:187–97. doi: 10.1097/00000542-199907000-00027. [DOI] [PubMed] [Google Scholar]
- 57.Zhang J, Tan H, Jiang W, Zuo Z. The choice of general anesthetics may not affect neuroinflammation and impairment of learning and memory after surgery in elderly rats. J Neuroimmune Pharmacol. 2015;10:179–89. doi: 10.1007/s11481-014-9580-y. [DOI] [PubMed] [Google Scholar]
- 58.Kong FJ, Ma LL, Zhang HH, Zhou JQ. Alpha 7 nicotinic acetylcholine receptor agonist GTS-21 mitigates isoflurane-induced cognitive impairment in aged rats. J Surg Res. 2015;194:255–61. doi: 10.1016/j.jss.2014.09.043. [DOI] [PubMed] [Google Scholar]
- 59.Yang C, Zhu B, Ding J, Wang ZG. Isoflurane anesthesia aggravates cognitive impairment in streptozotocin-induced diabetic rats. Int J Clin Exp Med. 2014;7:903–10. [PMC free article] [PubMed] [Google Scholar]
- 60.Li XM, Zhou MT, Wang XM, Ji MH, Zhou ZQ, Yang JJ. Resveratrol pretreatment attenuates the isoflurane-induced cognitive impairment through its anti-inflammation and -apoptosis actions in aged mice. J Mol Neurosci. 2014;52:286–93. doi: 10.1007/s12031-013-0141-2. [DOI] [PubMed] [Google Scholar]
- 61.Wu X, Lu Y, Dong Y, Zhang G, Zhang Y, Xu Z, Culley DJ, Crosby G, Marcantonio ER, Tanzi RE, Xie Z. The inhalation anesthetic isoflurane increases levels of proinflammatory TNF-alpha, IL-6, and IL-1beta. Neurobiol Aging. 2012;33:1364–78. doi: 10.1016/j.neurobiolaging.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang HL, Ma RH, Fang H, Xue ZG, Liao QW. Impaired Spatial Learning Memory after Isoflurane Anesthesia or Appendectomy in Aged Mice is Associated with Microglia Activation. J Cell Death. 2015;8:9–19. doi: 10.4137/JCD.S30596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schlapfer M, Piegeler T, Dull RO, Schwartz DE, Mao M, Bonini MG, Z’Graggen BR, Beck-Schimmer B, Minshall RD. Propofol increases morbidity and mortality in a rat model of sepsis. Crit Care. 2015;19:45. doi: 10.1186/s13054-015-0751-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiang HF, Cao DH, Yang YQ, Wang HQ, Zhu LJ, Ruan BH, Du J, Wang MC. Isoflurane protects against injury caused by deprivation of oxygen and glucose in microglia through regulation of the Toll-like receptor 4 pathway. J Mol Neurosci. 2014;54:664–70. doi: 10.1007/s12031-014-0373-9. [DOI] [PubMed] [Google Scholar]
- 65.Wang H, Wang L, Li NL, Li JT, Yu F, Zhao YL, Wang L, Yi J, Wang L, Bian JF, Chen JH, Yuan SF, Wang T, Lv YG, Liu NN, Zhu XS, Ling R, Yun J. Subanesthetic isoflurane reduces zymosan-induced inflammation in murine Kupffer cells by inhibiting ROS-activated p38 MAPK/NF-kappaB signaling. Oxid Med Cell Longev. 2014;2014:851692. doi: 10.1155/2014/851692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liang Y, Li Z, Mo N, Li M, Zhuang Z, Wang J, Wang Y, Guo X. Isoflurane preconditioning ameliorates renal ischemia-reperfusion injury through antiinflammatory and antiapoptotic actions in rats. Biol Pharm Bull. 2014;37:1599–605. doi: 10.1248/bpb.b14-00211. [DOI] [PubMed] [Google Scholar]
- 67.Altay O, Suzuki H, Hasegawa Y, Ostrowski RP, Tang J, Zhang JH. Isoflurane on brain inflammation. Neurobiol Dis. 2014;62:365–71. doi: 10.1016/j.nbd.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Faller S, Strosing KM, Ryter SW, Buerkle H, Loop T, Schmidt R, Hoetzel A. The volatile anesthetic isoflurane prevents ventilator-induced lung injury via phosphoinositide 3-kinase/Akt signaling in mice. Anesth Analg. 2012;114:747–56. doi: 10.1213/ANE.0b013e31824762f0. [DOI] [PubMed] [Google Scholar]
- 69.Tomihari M, Nishihara A, Shimada T, Yanagawa M, Miyoshi M, Miyahara K, Oishi A. A comparison of the immunological effects of propofol and isoflurane for maintenance of anesthesia in healthy dogs. J Vet Med Sci. 2015;77:1227–33. doi: 10.1292/jvms.14-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Canakci CF, Cicek Y, Canakci V. Reactive oxygen species and human inflammatory periodontal diseases. Biochemistry (Mosc) 2005;70:619–28. doi: 10.1007/s10541-005-0161-9. [DOI] [PubMed] [Google Scholar]
- 71.Kim M, Ham A, Kim JY, Brown KM, D’Agati VD, Lee HT. The volatile anesthetic isoflurane induces ecto-5′-nucleotidase (CD73) to protect against renal ischemia and reperfusion injury. Kidney Int. 2013;84:90–103. doi: 10.1038/ki.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim M, Park SW, Kim M, D’Agati VD, Lee HT. Isoflurane post-conditioning protects against intestinal ischemia-reperfusion injury and multiorgan dysfunction via transforming growth factor-beta1 generation. Ann Surg. 2012;255:492–503. doi: 10.1097/SLA.0b013e3182441767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee HT, Kim M, Kim J, Kim N, Emala CW. TGF-beta1 release by volatile anesthetics mediates protection against renal proximal tubule cell necrosis. Am J Nephrol. 2007;27:416–24. doi: 10.1159/000105124. [DOI] [PubMed] [Google Scholar]
- 74.Rancan L, Huerta L, Cusati G, Erquicia I, Isea J, Paredes SD, Garcia C, Garutti I, Simon C, Vara E. Sevoflurane prevents liver inflammatory response induced by lung ischemia-reperfusion. Transplantation. 2014;98:1151–7. doi: 10.1097/TP.0000000000000408. [DOI] [PubMed] [Google Scholar]
- 75.Qiao S, Xie H, Wang C, Wu X, Liu H, Liu C. Delayed anesthetic preconditioning protects against myocardial infarction via activation of nuclear factor-kappaB and upregulation of autophagy. J Anesth. 2013;27:251–60. doi: 10.1007/s00540-012-1494-3. [DOI] [PubMed] [Google Scholar]
- 76.Ferrando C, Aguilar G, Piqueras L, Soro M, Moreno J, Belda FJ. Sevoflurane, but not propofol, reduces the lung inflammatory response and improves oxygenation in an acute respiratory distress syndrome model: a randomised laboratory study. Eur J Anaesthesiol. 2013;30:455–63. doi: 10.1097/EJA.0b013e32835f0aa5. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Y, Zhang FG, Meng C, Tian SY, Wang YX, Zhao W, Chen J, Zhang XS, Liang Y, Zhang SD, Xing YJ. Inhibition of sevoflurane postconditioning against cerebral ischemia reperfusion-induced oxidative injury in rats. Molecules. 2012;17:341–54. doi: 10.3390/molecules17010341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bedirli N, Bagriacik EU, Emmez H, Yilmaz G, Unal Y, Ozkose Z. Sevoflurane and isoflurane preconditioning provides neuroprotection by inhibition of apoptosis-related mRNA expression in a rat model of focal cerebral ischemia. J Neurosurg Anesthesiol. 2012;24:336–44. doi: 10.1097/ANA.0b013e318266791e. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Y, Tian SY, Li YW, Zhang L, Yu JB, Li J, Chen YY, Wang YX, Liang Y, Zhang XS, Wang WS, Liu HG. Sevoflurane preconditioning improving cerebral focal ischemia-reperfusion damage in a rat model via PI3K/Akt signaling pathway. Gene. 2015;569:60–5. doi: 10.1016/j.gene.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 80.Sun Z, Satomoto M, Makita K. Therapeutic effects of intravenous administration of bone marrow stromal cells on sevoflurane-induced neuronal apoptosis and neuroinflammation in neonatal rats. Korean J Anesthesiol. 2015;68:397–401. doi: 10.4097/kjae.2015.68.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qiao Y, Feng H, Zhao T, Yan H, Zhang H, Zhao X. Postoperative cognitive dysfunction after inhalational anesthesia in elderly patients undergoing major surgery: the influence of anesthetic technique, cerebral injury and systemic inflammation. BMC Anesthesiol. 2015;15:154. doi: 10.1186/s12871-015-0130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shen X, Dong Y, Xu Z, Wang H, Miao C, Soriano SG, Sun D, Baxter MG, Zhang Y, Xie Z. Selective anesthesia-induced neuroinflammation in developing mouse brain and cognitive impairment. Anesthesiology. 2013;118:502–15. doi: 10.1097/ALN.0b013e3182834d77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Romero A, Moreno A, Garcia J, Sanchez C, Santos M, Garcia J. Effects of sevoflurane on ventilator induced lung injury in a healthy lung experimental model. Rev Esp Anestesiol Reanim. 2016;63:22–28. doi: 10.1016/j.redar.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 84.Otsuki T, Ishikawa M, Hori Y, Goto G, Sakamoto A. Volatile anesthetic sevoflurane ameliorates endotoxin-induced acute lung injury via microRNA modulation in rats. Biomed Rep. 2015;3:408–412. doi: 10.3892/br.2015.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Luo C, Yuan D, Zhao W, Chen H, Luo G, Su G, Hei Z. Sevoflurane ameliorates intestinal ischemia-reperfusion-induced lung injury by inhibiting the synergistic action between mast cell activation and oxidative stress. Mol Med Rep. 2015;12:1082–90. doi: 10.3892/mmr.2015.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu QB, Li HM, Li LL, Wang SY, Wu YB. Sevoflurane downregulates interleukin-6 and interleukin-8 levels in patients after cardiopulmonary bypass surgery: a meta-analysis. Genet Mol Res. 2015;14:19016–27. doi: 10.4238/2015.December.29.9. [DOI] [PubMed] [Google Scholar]
- 87.Shen QY, Fang L, Wu HM, Wu L, He F, Liu RY. Effect of Toll-like receptor 2 on the inhibition role of sevoflurane on airway inflammation in asthmatic mice. Zhonghua Yi Xue Za Zhi. 2016;96:138–41. doi: 10.3760/cma.j.issn.0376-2491.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 88.Burburan SM, Silva JD, Abreu SC, Samary CS, Guimaraes IH, Xisto DG, Morales MM, Rocco PR. Effects of inhalational anaesthetics in experimental allergic asthma. Anaesthesia. 2014;69:573–82. doi: 10.1111/anae.12593. [DOI] [PubMed] [Google Scholar]
- 89.Schilling T, Kozian A, Senturk M, Huth C, Reinhold A, Hedenstierna G, Hachenberg T. Effects of volatile and intravenous anesthesia on the alveolar and systemic inflammatory response in thoracic surgical patients. Anesthesiology. 2011;115:65–74. doi: 10.1097/ALN.0b013e318214b9de. [DOI] [PubMed] [Google Scholar]
- 90.Lai J, Zhang L, Wang H, Lin P, Chen W. Effects of sevoflurane preconditioning on cardiomyocyte apoptosis and myocardial inflammation in rats with sepsis. Nan Fang Yi Ke Da Xue Xue Bao. 2014;34:1680–3. [PubMed] [Google Scholar]
- 91.Polak PE, Dull RO, Kalinin S, Sharp AJ, Ripper R, Weinberg G, Schwartz DE, Rubinstein I, Feinstein DL. Sevoflurane reduces clinical disease in a mouse model of multiple sclerosis. J Neuroinflammation. 2012;9:272. doi: 10.1186/1742-2094-9-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cata JP, Bauer M, Sokari T, Ramirez MF, Mason D, Plautz G, Kurz A. Effects of surgery, general anesthesia, and perioperative epidural analgesia on the immune function of patients with non-small cell lung cancer. J Clin Anesth. 2013;25:255–62. doi: 10.1016/j.jclinane.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 93.Kvarnstrom AL, Sarbinowski RT, Bengtson JP, Jacobsson LM, Bengtsson AL. Complement activation and interleukin response in major abdominal surgery. Scand J Immunol. 2012;75:510–6. doi: 10.1111/j.1365-3083.2012.02672.x. [DOI] [PubMed] [Google Scholar]
- 94.Deegan CA, Murray D, Doran P, Moriarty DC, Sessler DI, Mascha E, Kavanagh BP, Buggy DJ. Anesthetic technique and the cytokine and matrix metalloproteinase response to primary breast cancer surgery. Reg Anesth Pain Med. 2010;35:490–5. doi: 10.1097/AAP.0b013e3181ef4d05. [DOI] [PubMed] [Google Scholar]
- 95.Lee HT, Kim M, Song JH, Chen SW, Gubitosa G, Emala CW. Sevoflurane-mediated TGF-beta1 signaling in renal proximal tubule cells. Am J Physiol Renal Physiol. 2008;294:F371–8. doi: 10.1152/ajprenal.00277.2007. [DOI] [PubMed] [Google Scholar]
- 96.Lee HT, Chen SW, Doetschman TC, Deng C, D’Agati VD, Kim M. Sevoflurane protects against renal ischemia and reperfusion injury in mice via the transforming growth factor-beta1 pathway. Am J Physiol Renal Physiol. 2008;295:F128–36. doi: 10.1152/ajprenal.00577.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee HJ, Kwon JY, Shin SW, Baek SH, Choi KU, Jeon YH, Kim WS, Bae JH, Choi HJ, Kim HK, Baik SW. Effects of sevoflurane on collagen production and growth factor expression in rats with an excision wound. Acta Anaesthesiol Scand. 2010;54:885–93. doi: 10.1111/j.1399-6576.2009.02190.x. [DOI] [PubMed] [Google Scholar]
- 98.Boost KA, Hofstetter C, Flondor M, Betz C, Homann M, Pfeilschifter J, Muehl H, Zwissler B. Desflurane differentially affects the release of proinflammatory cytokines in plasma and bronchoalveolar fluid of endotoxemic rats. Int J Mol Med. 2006;17:1139–44. [PubMed] [Google Scholar]
- 99.Baki ED, Aldemir M, Kokulu S, Koca HB, Ela Y, Sivaci RG, Ozturk NK, Emmiler M, Adali F, Uzel H. Comparison of the effects of desflurane and propofol anesthesia on the inflammatory response and s100beta protein during coronary artery bypass grafting. Inflammation. 2013;36:1327–33. doi: 10.1007/s10753-013-9671-6. [DOI] [PubMed] [Google Scholar]
- 100.Koksoy S, Sahin Z, Karsli B. Comparison of the effects of desflurane and bupivacaine on Th1 and Th2 responses. Clin Lab. 2013;59:1215–20. doi: 10.7754/clin.lab.2013.120413. [DOI] [PubMed] [Google Scholar]
- 101.Sheen MJ, Yang CP, Liu YC, Borel CO, Wong CS, Ho ST, Wu CT. Comparing the effects of minimal low-flow desflurane with that of semi-close high flow desflurane on perioperative cytokine response in patients undergoing gastrectomy. Acta Anaesthesiol Taiwan. 2006;44:5–10. [PubMed] [Google Scholar]
- 102.Sun Z, Lv J, Zhu Y, Song D, Zhu B, Miao C. Desflurane preconditioning protects human umbilical vein endothelial cells against anoxia/reoxygenation by upregulating NLRP12 and inhibiting non-canonical nuclear factor-kappaB signaling. Int J Mol Med. 2015;36:1327–34. doi: 10.3892/ijmm.2015.2335. [DOI] [PubMed] [Google Scholar]
- 103.You Q, Cheng L, Reilly TP, Wegmann D, Ju C. Role of neutrophils in a mouse model of halothane-induced liver injury. Hepatology. 2006;44:1421–31. doi: 10.1002/hep.21425. [DOI] [PubMed] [Google Scholar]
- 104.Dugan CM, MacDonald AE, Roth RA, Ganey PE. A mouse model of severe halothane hepatitis based on human risk factors. J Pharmacol Exp Ther. 2010;333:364–72. doi: 10.1124/jpet.109.164541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dugan CM, Fullerton AM, Roth RA, Ganey PE. Natural killer cells mediate severe liver injury in a murine model of halothane hepatitis. Toxicol Sci. 2011;120:507–18. doi: 10.1093/toxsci/kfr005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kobayashi E, Kobayashi M, Tsuneyama K, Fukami T, Nakajima M, Yokoi T. Halothane-induced liver injury is mediated by interleukin-17 in mice. Toxicol Sci. 2009;111:302–10. doi: 10.1093/toxsci/kfp165. [DOI] [PubMed] [Google Scholar]
- 107.Chakraborty M, Fullerton AM, Semple K, Chea LS, Proctor WR, Bourdi M, Kleiner DE, Zeng X, Ryan PM, Dagur PK, Berkson JD, Reilly TP, Pohl LR. Drug-induced allergic hepatitis develops in mice when myeloid-derived suppressor cells are depleted prior to halothane treatment. Hepatology. 2015;62:546–57. doi: 10.1002/hep.27764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Proctor WR, Chakraborty M, Fullerton AM, Korrapati MC, Ryan PM, Semple K, Morrison JC, Berkson JD, Chea LS, Yang Q, Li AP, Spolski R, West EE, Rochman Y, Leonard WJ, Bourdi M, Pohl LR. Thymic stromal lymphopoietin and interleukin-4 mediate the pathogenesis of halothane-induced liver injury in mice. Hepatology. 2014;60:1741–52. doi: 10.1002/hep.27169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Simeonova GP, Slavov E, Usunov R, Halacheva K, Dinev DN. Increased apoptosis of peripheral blood mononuclear cells (PBMC) during general and epidural anaesthesia in dogs. Vet Res Commun. 2008;32:619–26. doi: 10.1007/s11259-008-9063-9. [DOI] [PubMed] [Google Scholar]
- 110.Kutza J, Gratz I, Afshar M, Murasko DM. The effects of general anesthesia and surgery on basal and interferon stimulated natural killer cell activity of humans. Anesth Analg. 1997;85:918–23. doi: 10.1097/00000539-199710000-00037. [DOI] [PubMed] [Google Scholar]
- 111.Conrick-Martin I, Kell MR, Buggy DJ. Meta-analysis of the effect of central neuraxial regional anesthesia compared with general anesthesia on postoperative natural killer T lymphocyte function. J Clin Anesth. 2012;24:3–7. doi: 10.1016/j.jclinane.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 112.Frohlich D, Rothe G, Schmitz G, Hansen E. Volatile anaesthetics induce changes in the expression of P-selectin and glycoprotein Ib on the surface of platelets in vitro. Eur J Anaesthesiol. 1998;15:641–8. doi: 10.1097/00003643-199811000-00004. [DOI] [PubMed] [Google Scholar]
- 113.Koroglu A, Cicek M, Toprak HI, Karakoc Y, Noyan F, Ersoy OM. Comparison of the effects of desflurane and sevoflurane on the expression of platelet surface glycoproteins in unstimulated and adenosine diphosphate-induced platelets in vitro. J Clin Anesth. 2007;19:328–33. doi: 10.1016/j.jclinane.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 114.Zhang Y, Zhen Y, Dong Y, Xu Z, Yue Y, Golde TE, Tanzi RE, Moir RD, Xie Z. Anesthetic propofol attenuates the isoflurane-induced caspase-3 activation and Abeta oligomerization. PLoS One. 2011;6:e27019. doi: 10.1371/journal.pone.0027019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sun Y, Zhang Y, Cheng B, Dong Y, Pan C, Li T, Xie Z. Glucose may attenuate isoflurane-induced caspase-3 activation in H4 human neuroglioma cells. Anesth Analg. 2014;119:1373–80. doi: 10.1213/ANE.0000000000000383. [DOI] [PubMed] [Google Scholar]
- 116.Cheng B, Zhang Y, Wang A, Dong Y, Xie Z. Vitamin C Attenuates Isoflurane-Induced Caspase-3 Activation and Cognitive Impairment. Mol Neurobiol. 2015;52:1580–9. doi: 10.1007/s12035-014-8959-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dallasen RM, Bowman JD, Xu Y. Isoflurane does not cause neuroapoptosis but reduces astroglial processes in young adult mice. Med Gas Res. 2011;1:27. doi: 10.1186/2045-9912-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Janeway C. Immunobiology : the immune system in health and disease. 6th. New York: Garland Science; 2005. [Google Scholar]
- 119.Picq CA, Clarencon D, Sinniger VE, Bonaz BL, Mayol JF. Impact of Anesthetics on Immune Functions in a Rat Model of Vagus Nerve Stimulation. PLoS One. 2013;8:e67086. doi: 10.1371/journal.pone.0067086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schneemilch CE, Ittenson A, Ansorge S, Hachenberg T, Bank U. Effect of 2 anesthetic techniques on the postoperative proinflammatory and anti-inflammatory cytokine response and cellular immune function to minor surgery. J Clin Anesth. 2005;17:517–27. doi: 10.1016/j.jclinane.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 121.Matsuoka H, Kurosawa S, Horinouchi T, Kato M, Hashimoto Y. Inhalation anesthetics induce apoptosis in normal peripheral lymphocytes in vitro. Anesthesiology. 2001;95:1467–72. doi: 10.1097/00000542-200112000-00028. [DOI] [PubMed] [Google Scholar]
- 122.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 123.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–98. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 124.Hamra JG, Yaksh TL. Halothane inhibits T cell proliferation and interleukin-2 receptor expression in rats. Immunopharmacol Immunotoxicol. 1996;18:323–36. doi: 10.3109/08923979609052739. [DOI] [PubMed] [Google Scholar]
- 125.Njoku DB, Mellerson JL, Talor MV, Kerr DR, Faraday NR, Outschoorn I, Rose NR. Role of CYP2E1 immunoglobulin G4 subclass antibodies and complement in pathogenesis of idiosyncratic drug-induced hepatitis. Clin Vaccine Immunol. 2006;13:258–65. doi: 10.1128/CVI.13.2.258-265.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rossi A, Falzetti G, Donati A, Orsetti G, Pelaia P. Desflurane versus sevoflurane to reduce blood loss in maxillofacial surgery. J Oral Maxillofac Surg. 2010;68:1007–12. doi: 10.1016/j.joms.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 127.Dal D, Celiker V, Ozer E, Basgul E, Salman MA, Aypar U. Induced hypotension for tympanoplasty: a comparison of desflurane, isoflurane and sevoflurane. Eur J Anaesthesiol. 2004;21:902–6. doi: 10.1017/s0265021504000262. [DOI] [PubMed] [Google Scholar]
- 128.van den Brom CE, Bulte CS, Loer SA, Bouwman RA, Boer C. Diabetes, perioperative ischaemia and volatile anaesthetics: consequences of derangements in myocardial substrate metabolism. Cardiovasc Diabetol. 2013;12:42. doi: 10.1186/1475-2840-12-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lattermann R, Schricker T, Wachter U, Georgieff M, Goertz A. Understanding the mechanisms by which isoflurane modifies the hyperglycemic response to surgery. Anesth Analg. 2001;93:121–7. doi: 10.1097/00000539-200107000-00026. [DOI] [PubMed] [Google Scholar]
- 130.Desborough JP, Jones PM, Persaud SJ, Landon MJ, Howell SL. Isoflurane inhibits insulin secretion from isolated rat pancreatic islets of Langerhans. Br J Anaesth. 1993;71:873–6. doi: 10.1093/bja/71.6.873. [DOI] [PubMed] [Google Scholar]
- 131.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6:813–22. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 132.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–6. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 133.Jaattela M, Tschopp J. Caspase-independent cell death in T lymphocytes. Nat Immunol. 2003;4:416–23. doi: 10.1038/ni0503-416. [DOI] [PubMed] [Google Scholar]
- 134.Zhou Y, Li E, Li Y, Liu S. Attenuating sevoflurane-induced cellular injury of human peripheral lymphocytes by propofol in a concentration-dependent manner. Arch Pharm Res. 2011;34:1535–43. doi: 10.1007/s12272-011-0916-3. [DOI] [PubMed] [Google Scholar]
- 135.Jia L, Dong R, Zhang F, Wang W, Lu H, Luo Y, Xue Q, Yu B. Propofol Provides More Effective Protection for Circulating Lymphocytes Than Sevoflurane in Patients Undergoing Off-Pump Coronary Artery Bypass Graft Surgery. J Cardiothorac Vasc Anesth. 2015;29:1172–9. doi: 10.1053/j.jvca.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 136.Evans R, Patzak I, Svensson L, De Filippo K, Jones K, McDowall A, Hogg N. Integrins in immunity. J Cell Sci. 2009;122:215–25. doi: 10.1242/jcs.019117. [DOI] [PubMed] [Google Scholar]
- 137.Jung S, Yuki K. Differential effects of volatile anesthetics on leukocyte integrin macrophage-1 antigen. J Immunotoxicol. 2016;13:148–56. doi: 10.3109/1547691X.2015.1019596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yuki K, Bu W, Shimaoka M, Eckenhoff R. Volatile anesthetics, not intravenous anesthetic propofol bind to and attenuate the activation of platelet receptor integrin alphaIIbbeta3. PLoS One. 2013;8:e60415. doi: 10.1371/journal.pone.0060415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lucchinetti E, Aguirre J, Feng J, Zhu M, Suter M, Spahn DR, Harter L, Zaugg M. Molecular evidence of late preconditioning after sevoflurane inhalation in healthy volunteers. Anesth Analg. 2007;105:629–40. doi: 10.1213/01.ane.0000278159.88636.aa. [DOI] [PubMed] [Google Scholar]
- 140.Toda N, Toda H, Hatano Y. Anesthetic modulation of immune reactions mediated by nitric oxide. J Anesth. 2008;22:155–62. doi: 10.1007/s00540-007-0590-2. [DOI] [PubMed] [Google Scholar]
- 141.Vulliemoz Y. The nitric oxide-cyclic 3′,5′-guanosine monophosphate signal transduction pathway in the mechanism of action of general anesthetics. Toxicol Lett. 1998:100–101. 103–8. doi: 10.1016/s0378-4274(98)00172-6. [DOI] [PubMed] [Google Scholar]
- 142.Li QF, Zhu YS, Jiang H, Xu H, Sun Y. Heme oxygenase-1 mediates the anti-inflammatory effect of isoflurane preconditioning in LPS-stimulated macrophages. Acta Pharmacol Sin. 2009;30:228–34. doi: 10.1038/aps.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xu X, Feng J, Zuo Z. Isoflurane preconditioning reduces the rat NR8383 macrophage injury induced by lipopolysaccharide and interferon gamma. Anesthesiology. 2008;108:643–50. doi: 10.1097/ALN.0b013e318167aeb4. [DOI] [PubMed] [Google Scholar]
- 144.Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–83. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 145.Itoh T, Hirota K, Hisano T, Namba T, Fukuda K. The volatile anesthetics halothane and isoflurane differentially modulate proinflammatory cytokine-induced p38 mitogen-activated protein kinase activation. J Anesth. 2004;18:203–9. doi: 10.1007/s00540-004-0237-5. [DOI] [PubMed] [Google Scholar]
- 146.Xie Z. Cancer prognosis: can anesthesia play a role? Anesthesiology. 2013;119:501–3. doi: 10.1097/ALN.0b013e31829e4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gottschalk A, Sharma S, Ford J, Durieux ME, Tiouririne M. Review article: the role of the perioperative period in recurrence after cancer surgery. Anesth Analg. 2010;110:1636–43. doi: 10.1213/ANE.0b013e3181de0ab6. [DOI] [PubMed] [Google Scholar]
- 148.Lin L, Liu C, Tan H, Ouyang H, Zhang Y, Zeng W. Anaesthetic technique may affect prognosis for ovarian serous adenocarcinoma: a retrospective analysis. Br J Anaesth. 2011;106:814–22. doi: 10.1093/bja/aer055. [DOI] [PubMed] [Google Scholar]
- 149.Christopherson R, James KE, Tableman M, Marshall P, Johnson FE. Long-term survival after colon cancer surgery: a variation associated with choice of anesthesia. Anesth Analg. 2008;107:325–32. doi: 10.1213/ane.0b013e3181770f55. [DOI] [PubMed] [Google Scholar]
- 150.Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105:660–4. doi: 10.1097/00000542-200610000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Biki B, Mascha E, Moriarty DC, Fitzpatrick JM, Sessler DI, Buggy DJ. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: a retrospective analysis. Anesthesiology. 2008;109:180–7. doi: 10.1097/ALN.0b013e31817f5b73. [DOI] [PubMed] [Google Scholar]
- 152.Gupta A, Bjornsson A, Fredriksson M, Hallbook O, Eintrei C. Reduction in mortality after epidural anaesthesia and analgesia in patients undergoing rectal but not colonic cancer surgery: a retrospective analysis of data from 655 patients in central Sweden. Br J Anaesth. 2011;107:164–70. doi: 10.1093/bja/aer100. [DOI] [PubMed] [Google Scholar]
- 153.de Oliveira GS, Jr, Ahmad S, Schink JC, Singh DK, Fitzgerald PC, McCarthy RJ. Intraoperative neuraxial anesthesia but not postoperative neuraxial analgesia is associated with increased relapse-free survival in ovarian cancer patients after primary cytoreductive surgery. Reg Anesth Pain Med. 2011;36:271–7. doi: 10.1097/AAP.0b013e318217aada. [DOI] [PubMed] [Google Scholar]
- 154.Gottschalk A, Ford JG, Regelin CC, You J, Mascha EJ, Sessler DI, Durieux ME, Nemergut EC. Association between epidural analgesia and cancer recurrence after colorectal cancer surgery. Anesthesiology. 2010;113:27–34. doi: 10.1097/ALN.0b013e3181de6d0d. [DOI] [PubMed] [Google Scholar]
- 155.Ismail H, Ho KM, Narayan K, Kondalsamy-Chennakesavan S. Effect of neuraxial anaesthesia on tumour progression in cervical cancer patients treated with brachytherapy: a retrospective cohort study. Br J Anaesth. 2010;105:145–9. doi: 10.1093/bja/aeq156. [DOI] [PubMed] [Google Scholar]
- 156.Myles PS, Peyton P, Silbert B, Hunt J, Rigg JR, Sessler DI, Investigators ATG. Perioperative epidural analgesia for major abdominal surgery for cancer and recurrence-free survival: randomised trial. BMJ. 2011;342:d1491. doi: 10.1136/bmj.d1491. [DOI] [PubMed] [Google Scholar]
- 157.Fleischmann E, Marschalek C, Schlemitz K, Dalton JE, Gruenberger T, Herbst F, Kurz A, Sessler DI. Nitrous oxide may not increase the risk of cancer recurrence after colorectal surgery: a follow-up of a randomized controlled trial. BMC Anesthesiol. 2009;9:1. doi: 10.1186/1471-2253-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Huang H, Benzonana LL, Zhao H, Watts HR, Perry NJ, Bevan C, Brown R, Ma D. Prostate cancer cell malignancy via modulation of HIF-1alpha pathway with isoflurane and propofol alone and in combination. Br J Cancer. 2014;111:1338–49. doi: 10.1038/bjc.2014.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Benzonana LL, Perry NJ, Watts HR, Yang B, Perry IA, Coombes C, Takata M, Ma D. Isoflurane, a commonly used volatile anesthetic, enhances renal cancer growth and malignant potential via the hypoxia-inducible factor cellular signaling pathway in vitro. Anesthesiology. 2013;119:593–605. doi: 10.1097/ALN.0b013e31829e47fd. [DOI] [PubMed] [Google Scholar]
- 160.Tavare AN, Perry NJ, Benzonana LL, Takata M, Ma D. Cancer recurrence after surgery: direct and indirect effects of anesthetic agents. Int J Cancer. 2012;130:1237–50. doi: 10.1002/ijc.26448. [DOI] [PubMed] [Google Scholar]
- 161.Buckley A, McQuaid S, Johnson P, Buggy DJ. Effect of anaesthetic technique on the natural killer cell anti-tumour activity of serum from women undergoing breast cancer surgery: a pilot study. Br J Anaesth. 2014;113(Suppl 1):i56–62. doi: 10.1093/bja/aeu200. [DOI] [PubMed] [Google Scholar]
- 162.Fukazawa K, Lee HT. Volatile anesthetics and AKI: risks, mechanisms, and a potential therapeutic window. J Am Soc Nephrol. 2014;25:884–92. doi: 10.1681/ASN.2013111215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ham A, Kim M, Kim JY, Brown KM, Yeh J, D’Agati VD, Lee HT. Critical role of interleukin-11 in isoflurane-mediated protection against ischemic acute kidney injury in mice. Anesthesiology. 2013;119:1389–401. doi: 10.1097/ALN.0b013e3182a950da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bakar AM, Park SW, Kim M, Lee HT. Isoflurane protects against human endothelial cell apoptosis by inducing sphingosine kinase-1 via ERK MAPK. Int J Mol Sci. 2012;13:977–93. doi: 10.3390/ijms13010977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kim M, Kim M, Park SW, Pitson SM, Lee HT. Isoflurane protects human kidney proximal tubule cells against necrosis via sphingosine kinase and sphingosine-1-phosphate generation. Am J Nephrol. 2010;31:353–62. doi: 10.1159/000298339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pagel PS. Postconditioning by volatile anesthetics: salvaging ischemic myocardium at reperfusion by activation of prosurvival signaling. J Cardiothorac Vasc Anesth. 2008;22:753–65. doi: 10.1053/j.jvca.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 167.Lv X, Yang L, Tao K, Liu Y, Yang T, Chen G, Yu W, Lv H, Wu F. Isoflurane preconditioning at clinically relevant doses induce protective effects of heme oxygenase-1 on hepatic ischemia reperfusion in rats. BMC Gastroenterol. 2011;11:31. doi: 10.1186/1471-230X-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xiong L, Zheng Y, Wu M, Hou L, Zhu Z, Zhang X, Lu Z. Preconditioning with isoflurane produces dose-dependent neuroprotection via activation of adenosine triphosphate-regulated potassium channels after focal cerebral ischemia in rats. Anesth Analg. 2003;96:233–7. doi: 10.1097/00000539-200301000-00047. table of contents. [DOI] [PubMed] [Google Scholar]
- 169.Warltier DC, al-Wathiqui MH, Kampine JP, Schmeling WT. Recovery of contractile function of stunned myocardium in chronically instrumented dogs is enhanced by halothane or isoflurane. Anesthesiology. 1988;69:552–65. doi: 10.1097/00000542-198810000-00016. [DOI] [PubMed] [Google Scholar]
- 170.Lee HT, Ota-Setlik A, Fu Y, Nasr SH, Emala CW. Differential protective effects of volatile anesthetics against renal ischemia-reperfusion injury in vivo. Anesthesiology. 2004;101:1313–24. doi: 10.1097/00000542-200412000-00011. [DOI] [PubMed] [Google Scholar]


