Abstract
Aberrant β-adrenergic signaling and depressed calcium homeostasis, associated with an imbalance of protein kinase A and phosphatase-1 activities, are hallmarks of heart failure. Phosphatase-1 is restrained by its endogenous inhibitor, protein phosphatase inhibitor-1 (PPI-1). We assessed 352 normal subjects, along with 959 patients with heart failure and identified a polymorphism in PPI-1 (G147D) exclusively in black subjects. To determine whether the G147D variant could affect cardiac function, we infected adult cardiomyocytes with adenoviruses expressing D147 or wild-type (G147) PPI-1. Under basal conditions, there were no significant differences in fractional shortening or contraction or relaxation rates. However, the enhancement of contractile parameters after isoproterenol stimulation was significantly blunted in D147 compared with G147 and control myocytes. Similar findings were observed in calcium kinetics. The attenuated β-agonist response was associated with decreased (50%) phosphorylation of phospholamban (PLN) at serine 16, whereas phosphorylation of troponin I and ryanodine receptor was unaltered. These findings suggest that the human G147D PPI-1 can attenuate responses of cardiomyocytes to β-adrenergic agonists by decreasing PLN phosphorylation and therefore may contribute to deteriorated function in heart failure.—Chen, G., Zhou, X., Nicolaou, P., Rodriguez, P., Song, G., Mitton, B., Pathak, A., Zachariah, A., Fan, G.-C., Dorn, G. A., II., Kranias, E. G. A human polymorphism of protein phosphatase-1 inhibitor-1 is associated with attenuated contractile response of cardiomyocytes to β-adrenergic stimulation.
Keywords: cardiac function, phospholamban, heart failure
The molecular mechanisms underlying the pathogenesis of heart failure involve diverse and multiple interacting pathways. Among them, the β-adrenergic receptor signaling axis is one of the most prominent and well-characterized pathways in regulation of cardiac function, and disturbances of this cascade contribute to development and progression of heart failure (1, 2).
The stimulatory effects of β-adrenergic agonists on cardiomyocyte contractility are mediated by key intracellular target phosphoproteins (3). These include the ryanodine receptor (RyR) (4) and phospholamban (PLN) (5, 6) in sarcoplasmic reticulum, troponin I (6, 7) and C-protein (8, 9) in the myofilaments, and the plasma membrane L-type calcium channel (10, 11). Opposing the protein kinase A (PKA) axis are protein phosphatases that dephosphorylate these regulatory proteins and play an essential role in maintaining biochemical and functional phosphorylation homeostasis. However, in experimental and human heart failure, the adrenergic pathway is depressed, whereas phosphatase activity is elevated, tilting this fine balance in favor of dephosphorylation of key phosphoproteins (12–15). As a major protein phosphatase in the heart, protein phosphatase-1 (PP1), has emerged as an important player in cardiac function under physiological and pathological conditions. Thus, regulation of its activity has been suggested to constitute a potential target for the rescue of failing heart function (12, 16).
An additional layer of complexity to PKA-PP1 is added by regulation of this phosphatase activity by endogenous proteins. One such factor, inhibitor-1, is itself phosphorylated by PKA or β-adrenergic stimulation on threonine 35 and potently inhibits PP1 activity, allowing PKA phosphorylation to propagate and increase cardiac contractility in an unopposed manner (13, 17, 18). Indeed, expression of a truncated (amino acids 1–65) and constitutively phosphorylated form (T35D) of inhibitor-1 enhanced cardiac contractility by favoring specific phosphorylation of PLN and exhibited a higher resistance to cardiac remodeling and heart failure, induced by chronic pressure overload compared with wild-type controls (16). Furthermore, gene transfer of the active inhibitor-1 form in preexisting heart failure restored function and attenuated the progression of cardiac remodeling (16). In contrast, decreased activity and/or a reduced protein level of inhibitor-1 are associated with cardiac dysfunction in animal models and in human heart failure (12, 19), suggesting that defects in inhibitor-1 activity may contribute to depressed contractility.
To determine whether there exist genetic variants in human inhibitor-1 that may act as direct or conjunct factors in heart failure, we sequenced the inhibitor-1 gene in 352 normal subjects and 959 patients with heart failure. We identified a C-terminal polymorphism of inhibitor-1 (G147D), which had no effect on basal contractility but blunted the response of cardiomyocytes to β-adrenergic stimulation by interfering with phosphorylation of PLN. Thus, this novel human polymorphism of inhibitor-1 may contribute to cardiac dysfunction in heterozygous patients with heart failure.
MATERIALS AND METHODS
Study subjects
The human study protocols were approved by the institutional review board of the University of Cincinnati, and subjects provided written informed consent. Subjects with heart failure were aged 18–80 yr and had New York Heart Association class II–IV heart failure.
Genotyping
Exons 1–6 of inhibitor-1 [according to National Center for Biotechnology Information, PPP1R1A protein phosphatase 1, regulatory (inhibitor) subunit 1A [Homo sapiens], Gene ID: 5502, in chromosome 12] were screened. Primer sequences, used for amplification and sequencing, included the following: for exon 1, 5′-CAAAACTCCGAGGACACTGAGGTATC (upstream fragment) and 5′-CAAAAGGGCGCACTGCTAAGGGAG (downstream fragment); for exon 2, 5′-GGCCTCCAGCTGCATTAACAT (upstream fragment) and 5′-GTTCTTCTTCCATCTAGCGCC (downstream fragment); for exon 3, 5′-CAAGGACGGGACTAGATGCAGAG (upstream fragment) and 5′-GTTCACTCATGCACATTTGGG (downstream fragment); for exon 4, 5′-TTGCTCACCATTGTAGTCTCC (upstream fragment) and 5′-CCCAGCTAGTCAGTGGCAAAA (downstream fragment); for exon 5, 5′-CCTCCTTGTTCAGATCTCAGTG (upstream fragment) and 5′-CCTTGAGACAGTTTTTGCC (downstream fragment); and for exon 6, 5′-CCCTGTTGGTCTTGCCTGATTG (upstream fragment) and CCTACTGCTCTCACCCATTCCA (downstream fragment). For polymorphism discovery and genotyping bidirectional automated sequencing on an Applied Biosystems ABI 3130-x1 Genetic Analyzer in 96-well formats was used. Sequences were confirmed in 98% of samples and were aligned with a reference sequence using Seqscape v2.5, individually verified by one of the investigators.
Adult rat ventricular myocyte isolation and culture
Adult rat ventricular myocytes were isolated and cultured as described previously (20). Briefly, hearts from adult male Sprague-Dawley rats (8 wk old) were perfused with modified Krebs-Henseleit buffer (KHB) (118 mM NaCl, 4.8 mM KCl, 25 mM HEPES, 1.25 mM K2HPO4, 1.25 mM MgSO4, 11 mM glucose, 5 mM taurine, and 10 mM butanedione monoxime; pH 7.4) for 5 min. Subsequently, hearts were perfused with an enzyme solution [KHB containing 0.7 mg/ml collagenase type II (263 U/mg), 0.2 mg/ml hyaluronidase, 0.1% BSA, and 25 μM Ca]. Ten and 15 min later, 25 and 50 μM Ca, respectively, were added to the perfusion buffer, so that the Ca concentration was raised to 100 μM, and perfusion was continued for another 5 min. Finally, left ventricular tissue was excised, minced, pipette dissociated, and filtered through a 240-μm screen. Cells were harvested and resuspended in KHB including 1 mM Ca and 1% BSA. After a brief centrifugation, the cells were resuspended in KHB including 1.8 mM Ca and 1% BSA, centrifuged briefly again, and resuspended in Dulbecco’s modified Eagle’s medium containing 2 mg/ml BSA, 2 mM L-carnitine, 5 mM creatine, 5 mM taurine, 1.8 mM Ca, 100 IU/ml penicillin, and 100 μg/ml streptomycin. Cells were then counted and plated on laminin-coated glass coverslips or dishes.
Adenovirus-mediated gene transfer
Recombinant adenovirus carrying empty vector, wild-type (WT) G147 inhibitor-1, or D147 inhibitor-1 was generated by using the Adeno-X expression system kit, as described before (21). Two hours after isolation and culture, myocytes seeded on coverslips or dishes were infected with adenovirus in diluted media, at a multiplicity of infection of 500, for 2 h before the addition of a suitable volume of culture media. Twenty-four hours later, the myocytes were washed in KHB including 1 mM Ca and harvested for quantitative immunoblotting or use in the experiments outlined below.
Contractile parameter measurements
As described previously (21), myocytes that adhered to the coverslips were bathed in temperature (37°C) -equilibrated KHB containing 1 mM Ca for 20 min. The myocyte suspension was then placed in a Plexiglas chamber, which was positioned on the stage of an inverted epifluorescence microscope (Diaphot 200; Nikon, Tokyo, Japan). Myocyte contraction was field-stimulated by a Grass S5 stimulator (0.5 Hz, square waves; Grass Technologies, An Astro-Med, Inc., West Warwick, RI, USA), and contractions were videotaped and digitized on a computer. A video edge motion detector (Crescent Electronics, Windsor, ON, Canada) was used to measure myocyte length and cell shortening, from which the percent fractional shortening (% FS) and maximal rates of contraction and relaxation (±dL/dt) were calculated (22). To investigate the response of adult rat cardiomyocytes to isoproterenol, a maximal concentration of isoproterenol (100 nM) was added to the KHB (including 1 mM Ca) in the Plexiglas chamber, and the above-mentioned measurements were repeated. All data were analyzed using software from Felix 1.1 software (Photon Technology International, Birmingham, NJ, USA) and IonWizard (IonOptix Corp., Milton, MA, USA).
Immunoblot analysis
As described previously (23), proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. After incubation in 5% dried milk for 1 h, membranes were probed overnight at 4°C with primary antibody. A peroxidase-labeled secondary antibody (Amersham Biosciences Corp., Piscataway, NJ, USA) was used in combination with an enhanced chemiluminescent detection system (Amersham Biosciences Corp.) to visualize the primary antibodies. The optical density of the bands was analyzed by ImageQuant 5.2 software (GE Healthcare, Little Chalfont, UK).
Statistics
Results are expressed as means ± SE. Analysis of variance was used to compare data between WT and G147D groups in the isolated myocyte studies. In all analyses, P < 0.05 was considered statistically significant.
RESULTS
Identification of a genetic variant in the human inhibitor-1 gene
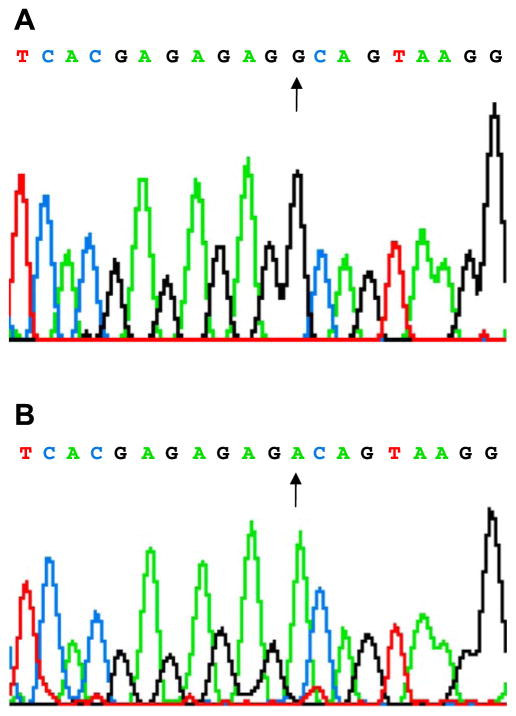
The PP1 inihibitor-1 gene was sequenced in 963 unrelated patients with heart failure recruited from the University Hospital and Cincinnati Heart Failure/Transplant Program, and we identified a single nucleotide transition from G>A at position 440 (c.440G>A), which resulted in the transition of glycine to aspartic acid at position 147 in exon 6 (p.147G>D) (Fig. 1). Interestingly, the G147D genetic variant of inhibitor-1 was identified only in black subjects with an allele frequency of 5–6% in either the population with heart failure (n=288) or the normal (n=40) population (all heterozygotes with one homozygote). This variant was not observed in 671 white patients although 1 white normal subject of the 312 who were screened exhibited the G147D polymorphism.
Figure 1.
Identification of a single nucleotide transition from G>A at position 440, which resulted in the transition of glycine to aspartic acid at position 147 in exon 6 of inhibitor-1.
G147D inhibitor-1 attenuated contractility of cardiomyocytes in response to β-adrenergic activation
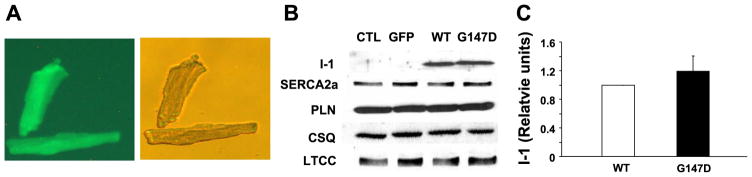
The inhibitory effects of activated inhibitor-1 on PP1 have been demonstrated to be important in maintaining normal cardiac contractile function via regulation of phosphorylation of PLN and other key targets. Recently, the C-terminal region of inhibitor-1, including the 147 amino acid site, was found to be necessary for its function (24). To assess the potential functional significance of this inhibitor-1 genetic variant in cardiac function, we generated adenoviruses carrying green fluorescent protein (GFP), as a control, WT G147 inhibitor-1, or D147 inhibitor-1. The human isoform of inhibitor-1 was used in these studies because of the variation in the C-terminal region of this protein among species (23). All of the adenoviruses also expressed GFP as an indicator of transfection efficiency. Adult rat cardiomyocytes were infected with the viruses, and GFP fluorescence indicated >90% infectivity (Fig. 2A). Quantitative immunoblotting revealed that cardiomyocytes expressed comparable levels of WT inhibitor-1 and G147D inhibitor-1 (Fig. 2B, C) at 24 h posttransfection. Importantly, overexpression of inhibitor-1 or its mutant did not alter the levels of the major regulatory proteins related to cardiac calcium homeostasis (Fig. 2B), indicating minimal compensatory changes in the adult cardiomyocytes.
Figure 2.
Isolated adult rat cardiomyocytes were infected with adenoviruses expressing GFP as control (CTL, empty), G147 inhibitor-1 (WT) or D147 inhibitor-1 (G147D). A) Images of infected cardiomyocytes and GFP expression. B) Representative Western blots display expression levels of inhibitor-1 (I-1), sarcoplasmic reticulum Ca-ATPase 2a isoform (SERCA 2a), PLN, calsequestrin (CSQ), and L-type calcium channels (LTCC). C) Quantitation of the expressional levels of WT inhibitor-1 and G147D inhibitor-1. Cardiomyocytes expressed comparable levels of WT inhibitor-1 and G147D inhibitor-1. Overexpression of inhibitor-1 or its variant did not alter the levels of the major regulatory proteins related to cardiac calcium homeostasis. Data are presented as mean values. Values are normalized to WT. Error bars indicate SEM values. n represents at least three independent experiments.
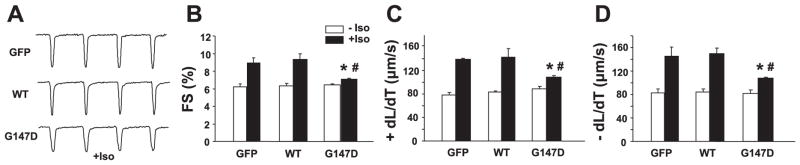
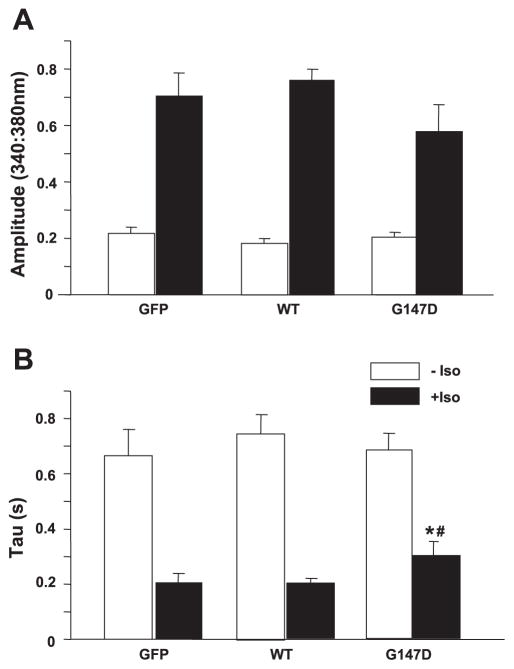
Mechanical properties of the infected cardiomyocytes indicated that overexpression of WT inhibitor-1 or the G147D mutant did not alter cellular contractility, as revealed by fractional shortening, contracting velocity, and relaxing velocity under basal conditions (Fig. 3). On stimulation with 100 nM isoproterenol, cardiomyocytes infected with control or WT inhibitor-1 viruses displayed enhanced contractility, demonstrated by increased fractional shortening (Fig. 3B), accelerated contraction (Fig. 3C), and enhanced relaxation (Fig. 3D). However, the effect of isoproterenol was significantly blunted in cardiomyocytes with overexpression of D147 inhibitor-1 (Fig. 3). Although the contractile parameters in D147 cells were significantly increased by isoproterenol treatment, these values remained depressed by 24–28% compared with WT inhibitor-1-stimulated cells. Similar findings were observed in calcium kinetic parameters of the G147D inhibitor-1-stimulated cardiomyocytes (Fig. 4). Furthermore, a lower isoproterenol dose (10 nM) revealed differences similar to those observed in 100 nM as described above. Thus, the G147 inhibitor-1 variant attenuated the stimulatory responses of isoproterenol on calcium kinetics and mechanics of adult cardiomyocytes.
Figure 3.
Contractile parameters were measured in infected adult rat cardiomyocytes. A) Representative traces for contractility. B) Fractional shortening (FS %). C) Contracting velocity (+dL/dT). D) Relaxing velocity (−dL/dT). On stimulation with 100 nM isoproterenol (Iso), cardiomyocytes infected with control or WT inhibitor-1 viruses displayed enhanced contractility, demonstrated by increased fractional shortening, accelerated contraction and enhanced relaxation; #P < 0.05, +Iso vs. −Iso. However, the effect of isoproterenol was significantly blunted in cardiomyocytes with overexpression of G147D inhibitor-1; *P < 0.05, G147D vs. GFP or WT. n = 4 hearts/group; 20–40 cells/group. Values represent means ± SE.
Figure 4.
Calcium kinetics were measured in infected adult rat cardiomyocytes. A) Calcium transient amplitude. B) Tau for calcium transient decay. Overexpression of G147D inhibitor-1 did not significantly alter calcium amplitude of cardiomyocytes in the presence of isoproterenol (Iso), but caused a significant delay of calcium transient decay. *P < 0.05, G147D vs. GFP or WT; #P < 0.05, +Iso vs. −Iso. n = 4 hearts/group; 20 – 40 cells/group. Values represent means ± SE.
G147D inhibitor-1 decreased PLN phosphorylation in response to β-adrenergic activation
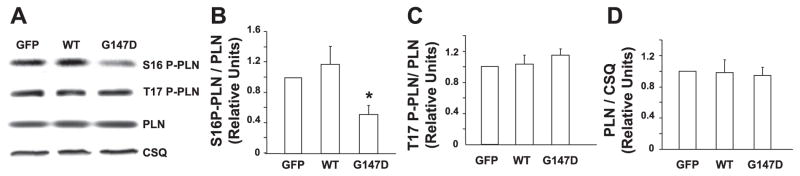
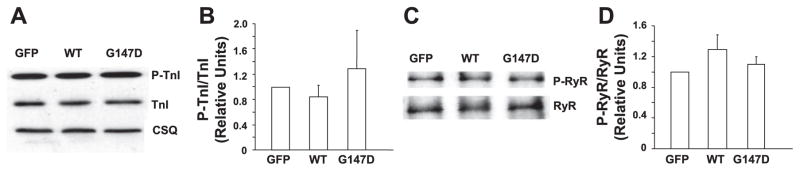
The effect of activated inhibitor-1 on regulation of protein phosphorylation is critical in the stimulatory effects of β-agonists in the heart. To explore the underlying mechanism by which G147D inhibitor-1 blunts the response of cardiomyocytes to β-adrenergic stimulation, we examined whether this polymorphism affected phosphorylation of the major phosphoproteins. Under basal conditions, phosphorylation of PLN was not detectable in isoproterenol-treated adult rat cardiomyocytes, consistent with our previous findings (23). In the presence of isoproterenol, phosphorylation of PLN was similarly increased in both WT inhibitor-1-expressing and control cells, in agreement with the data for contractility. However, the level of phosphorylated PLN on serine 16 was depressed by ~50% in the G147 inhibitor-1-infected myocytes (Fig. 5). Interestingly, the levels of PLN phosphorylation on threonine 17 were similar among the three groups. In addition, phosphorylation levels of troponin I (serine 22/23) and RyR (serine 2809) were also examined. As expected, the phosphorylation levels of these two proteins were increased by isoproterenol treatment (date not shown). However, there were no significant differences observed between the three groups in the presence of isoproterenol (Fig. 6). Thus, the attenuated stimulatory responses to isoproterenol were associated mainly with decreased PKA phosphorylation of PLN in the G147D inhibitor-1-infected cardiomyocytes.
Figure 5.
Phosphorylation levels of PLN on serine 16 and threonine 17 were examined in the three groups of infected adult rat cardiomyocytes in the presence of isoproterenol. A) Representative Western blots. B) Ratio of phosphorylated serine 16 PLN (S16 P-PLN)/total PLN. C) Ratio of phosphorylated threonine 17 PLN (T17 P-PLN)/total PLN. D) Ratio of total PLN/calsequestrin (CSQ). The level of phosphorylated PLN on serine 16 but not that on threonine 17 was depressed by ~50% in the G147D inhibitor-1-infected myocytes on exposure to isoproterenol. *P < 0.05, G147D vs. GFP or WT. Data are presented as mean values. Values are normalized to GFP. Error bars indicate SEM values. n represents at least three independent experiments.
Figure 6.
Phosphorylation levels of troponin I (TnI) and RyR were examined in infected adult rat cardiomyocytes in the presence of isoproterenol. Representative Western blots are shown for phosphorylated and total troponin I (A) and RyR (C). The ratios of phosphorylated/total troponin I (B) and phosphorylated/total RyR (D) were calculated. There were no significant differences in the phosphorylation levels of TnI or RyR observed between the three groups in the presence of isoproterenol. Data are presented as mean values. Values are normalized to GFP. Error bars indicate SEM values. n represents at least three independent experiments.
DISCUSSION
We identified a previously uncharacterized genetic variant, entailing substitution of aspartic acid for glycine at amino acid position 147 in inhibitor-1, which was almost exclusively present in blacks. The functional significance of this newly identified variant was elucidated through acute adenoviral infection of cultured adult rat cardiomyocytes with human WT or mutant (G147D) inhibitor-1 forms. Although there were no differences under basal conditions, the mutant inhibitor-1 suppressed the β-agonist stimulatory effects on contractile parameters and calcium kinetics. The functional significance of such attenuated β-agonist responses by the D147 mutant becomes especially salient in the context of heart failure, in which disruptions in the kinase/phosphatase balance have been shown to render the cardiomyocyte unable to adequately cope with stress. Among these, attenuation of signaling through the β-adrenergic receptor/cAMP pathway, which may occur as a result of receptor down-regulation, receptor desensitization, and receptor uncoupling, is a primary insult (1, 10). This alteration is associated with reduced PKA phosphorylation of intra-cellular targets, such as PLN, and concomitantly reduced cardiac contractility (7, 18). Importantly, the activity of PP1 is increased in the failing heart, which further enables dephosphorylation of important regulatory phosphoproteins, leading to overall decreased function (6).
Indeed, the reduced contractile and calcium-cycling responses of cardiomyocytes expressing the D147 inhibitor-1 to isoproterenol appeared to specifically reflect depressed phosphorylation of PLN at serine 16, without alterations in the phosphorylation levels of threonine 17. These findings are similar to our recent observations, using forskolin stimulation of cardiomyocytes overexpressing an inhibitor-1 form with constitutive phosphorylation of a new phosphorylated site, threonine 75 (T75D) (19). Thus, the apparent specificity of inhibitor-1 for the Ser-16 site on PLN may reflect the differences between the cAMP-PKA and calcium-calmodulin cascades, triggering phosphorylation of Ser-16 and Thr-17, respectively. Nevertheless, because it has been demonstrated that phosphorylation of serine 16 is critical in mediating the heart’s responses to β-agonists (22), the specific inhibition of this site’s phosphorylation by D147 inhibitor-1 correlates with the reduced cardiomyocyte responses to isoproterenol stimulation. Most importantly, there were no differences in the phosphorylation levels of the RyR or troponin-I between D147 and G147-inhibitor-1 cells. These paradoxical findings are in agreement with our previous studies (16), suggesting an apparent specificity of the inhibitor-1 for the PLN phosphatase, which may involve its specific anchoring subunit and the relative affinity between the catalytic and binding subunits of this enzyme in the various macromolecular complexes (25).
Notably, this inhibitor-1 variant, G147D, is located close to the C terminus of the protein, which may be important for its activity (24). Thus, activation of this variant by the cAMP-pathway may be compromised during β-agonist stimulation. Note that the endogenous inhibitor-1 was sufficient to fully inhibit PP1 and there was no further enhancement by overexpression of WT inhibitor-1. However, overexpression of the G147D variant, which may compete with the endogenous inhibitor-1 by mass action, prevents full inhibition of PP1. Alternatively, the G147D variant may decrease the ability of endogenous inhibitor-1 to be phosphorylated by β-adrenergic agonists. Unfortunately, the low levels of endogenous inhibitor-1 (Fig. 2B) and the lack of effective antibodies against the phosphorylated form of inhibitor-1 in cardiomyocytes preclude testing of these hypotheses.
In summary, our data suggest that a genetic variant of inhibitor-1, G147D, which is predominantly identified in blacks, is associated with depressed phosphorylation of PLN and attenuation of the cardiac responses to β-adrenergic signaling. Thus, inhibitor-1 is an important molecule in maintaining normal heart function and disturbances in its function may lead to aberrant calcium handling, which may contribute to contractile dysfunction in failing cardiomyocytes.
Acknowledgments
This work was supported by U.S. National Institutes of Health grants HL-26507, HL-64018, and HL-77101; by the Leducq Foundation; and by American Heart Association postdoctoral fellowship 0525435B (to G.C.).
References
- 1.Marian AJ. β-Adrenergic receptors signaling and heart failure in mice, rabbits and humans. J Mol Cell Cardiol. 2006;41:11–13. doi: 10.1016/j.yjmcc.2006.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldman DS, Carnes CA, Abraham WT, Bristow MR. Mechanisms of disease: β-adrenergic receptors—alterations in signal transduction and pharmacogenomics in heart failure. Nat Clin Pract Cardiovasc Med. 2005;2:475–483. doi: 10.1038/ncpcardio0309. [DOI] [PubMed] [Google Scholar]
- 3.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 4.Valdivia HH, Kaplan JH, Ellis-Davies GC, Lederer WJ. Rapid adaptation of cardiac ryanodine receptors: modulation by Mg2+ and phosphorylation. Science. 1995;267:1997–2000. doi: 10.1126/science.7701323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kranias EG. Regulation of Ca2+ transport by cyclic 3′,5′-AMP-dependent and calcium-calmodulin-dependent phosphorylation of cardiac sarcoplasmic reticulum. Biochim Biophys Acta. 1985;844:193–199. doi: 10.1016/0167-4889(85)90090-4. [DOI] [PubMed] [Google Scholar]
- 6.Kranias EG, Solaro RJ. Phosphorylation of troponin I and phospholamban during catecholamine stimulation of rabbit heart. Nature. 1982;298:182–184. doi: 10.1038/298182a0. [DOI] [PubMed] [Google Scholar]
- 7.Solaro RJ, Moir AJ, Perry SV. Phosphorylation of troponin I and the inotropic effect of adrenaline in the perfused rabbit heart. Nature. 1976;262:615–617. doi: 10.1038/262615a0. [DOI] [PubMed] [Google Scholar]
- 8.Gautel M, Zuffardi O, Freiburg A, Labeit S. Phosphorylation switches specific for the cardiac isoform of myosin binding protein-C: a modulator of cardiac contraction? EMBO J. 1995;14:1952–1960. doi: 10.1002/j.1460-2075.1995.tb07187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofmann PA, Lange JH., 3rd Effects of phosphorylation of troponin I and C protein on isometric tension and velocity of unloaded shortening in skinned single cardiac myocytes from rats. Circ Res. 1994;74:718–726. doi: 10.1161/01.res.74.4.718. [DOI] [PubMed] [Google Scholar]
- 10.Hartzell HC. Regulation of cardiac ion channels by catecholamines, acetylcholine and second messenger systems. Prog Biophys Mol Biol. 1988;52:165–247. doi: 10.1016/0079-6107(88)90014-4. [DOI] [PubMed] [Google Scholar]
- 11.Haase H, Karczewski P, Beckert R, Krause EG. Phosphorylation of the L-type calcium channel β subunit is involved in β-adrenergic signal transduction in canine myocardium. FEBS Lett. 1993;335:217–222. doi: 10.1016/0014-5793(93)80733-b. [DOI] [PubMed] [Google Scholar]
- 12.Carr AN, Schmidt AG, Suzuki Y, del Monte F, Sato Y, Lanner C, Breeden K, Jing SL, Allen PB, Greengard P, Yatani A, Hoit BD, Grupp IL, Hajjar RJ, DePaoli-Roach AA, Kranias EG. Type 1 phosphatase, a negative regulator of cardiac function. Mol Cell Biol. 2002;22:4124–4135. doi: 10.1128/MCB.22.12.4124-4135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta RC, Neumann J, Watanabe AM, Lesch M, Sabbah HN. Evidence for presence and hormonal regulation of protein phosphatase inhibitor-1 in ventricular cardiomyocyte. Am J Physiol. 1996;270:H1159–H1164. doi: 10.1152/ajpheart.1996.270.4.H1159. [DOI] [PubMed] [Google Scholar]
- 14.Neumann J, Eschenhagen T, Jones LR, Linck B, Schmitz W, Scholz H, Zimmermann N. Increased expression of cardiac phosphatases in patients with end-stage heart failure. J Mol Cell Cardiol. 1997;29:265–272. doi: 10.1006/jmcc.1996.0271. [DOI] [PubMed] [Google Scholar]
- 15.Gupta RC, Mishra S, Rastogi S, Imai M, Habib O, Sabbah HN. Cardiac SR-coupled PP1 activity and expression are increased and inhibitor 1 protein expression is decreased in failing hearts. Am J Physiol. 2003;285:H2373–H2381. doi: 10.1152/ajpheart.00442.2003. [DOI] [PubMed] [Google Scholar]
- 16.Pathak A, del Monte F, Zhao W, Schultz JE, Lorenz JN, Bodi I, Weiser D, Hahn H, Carr AN, Syed F, Mavila N, Jha L, Qian J, Marreez Y, Chen G, McGraw DW, Heist EK, Guerrero JL, DePaoli-Roach AA, Hajjar RJ, Kranias EG. Enhancement of cardiac function and suppression of heart failure progression by inhibition of protein phosphatase 1. Circ Res. 2005;96:756–766. doi: 10.1161/01.RES.0000161256.85833.fa. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad Z, Green FJ, Subuhi HS, Watanabe AM. Autonomic regulation of type 1 protein phosphatase in cardiac muscle. J Biol Chem. 1989;264:3859–3863. [PubMed] [Google Scholar]
- 18.Endo S, Zhou X, Connor J, Wang B, Shenolikar S. Multiple structural elements define the specificity of recombinant human inhibitor-1 as a protein phosphatase-1 inhibitor. Biochemistry. 1996;35:5220–5228. doi: 10.1021/bi952940f. [DOI] [PubMed] [Google Scholar]
- 19.El-Armouche A, Pamminger T, Ditz D, Zolk O, Eschenhagen T. Decreased protein and phosphorylation level of the protein phosphatase inhibitor-1 in failing human hearts. Cardiovasc Res. 2004;61:87–93. doi: 10.1016/j.cardiores.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Fan GC, Gregory KN, Zhao W, Park WJ, Kranias EG. Regulation of myocardial function by histidine-rich, calcium-binding protein. Am J Physiol. 2004;287:H1705–H1711. doi: 10.1152/ajpheart.01211.2003. [DOI] [PubMed] [Google Scholar]
- 21.Fan GC, Chu G, Mitton B, Song Q, Yuan Q, Kranias EG. Small heat-shock protein Hsp20 phosphorylation inhibits β-agonist-induced cardiac apoptosis. Circ Res. 2004;94:1474–1482. doi: 10.1161/01.RES.0000129179.66631.00. [DOI] [PubMed] [Google Scholar]
- 22.Chu G, Lester JW, Young KB, Luo W, Zhai J, Kranias EG. A single site (Ser16) phosphorylation in phospholamban is sufficient in mediating its maximal cardiac responses to β-agonists. J Biol Chem. 2000;275:38938–38943. doi: 10.1074/jbc.M004079200. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez P, Mitton B, Nicolaou P, Chen G, Kranias EG. Phosphorylation of human inhibitor-1 at Ser67 and/or Thr75 attenuates stimulatory effects of protein kinase A signaling in cardiac myocytes. Am J Physiol. 2007;293:H762–H769. doi: 10.1152/ajpheart.00104.2007. [DOI] [PubMed] [Google Scholar]
- 24.Weiser DC, Sikes S, Li S, Shenolikar S. The inhibitor-1 C terminus facilitates hormonal regulation of cellular protein phosphatase-1: functional implications for inhibitor-1 isoforms. J Biol Chem. 2004;279:48904–48914. doi: 10.1074/jbc.M404416200. [DOI] [PubMed] [Google Scholar]
- 25.Depaoli-Roach AA. Protein phosphatase 1 binding proteins. In: Bradshaw RA, editor. Handbook of Cell Signaling. Elsevier Science; New York: 2003. pp. 613–619. [Google Scholar]